Abstract
Multiple sclerosis (MS) is a chronic autoimmune demyelinating disease of the CNS. Oligodendrocytes, the myelin forming cells of the central nervous system (CNS), are target cells in MS. Although the etiology of MS is poorly known, new insights suggest oligodendrocyte apoptosis as one of the critical events followed by glial activation and infiltration of lymphocytes and macrophages. A major breakthrough in delineation of the mechanism of cell death, perivascular cuffing and glial activation came from elucidation of the sphingolipid signal transduction pathway. The sphingolipid signal transduction pathway induces apoptosis, differentiation, proliferation, and growth arrest depending upon cell and receptor types, and downstream targets. Sphingomyelin, a major component of myelin membrane formed by mature oligodendrocytes, is abundant in the CNS and ceramide, its primary catabolic product released by activation of either neutral or acidic sphingomyelinase, serves as a potential lipid second messenger or mediator molecule modulating diverse cellular signaling pathways. Similarly, under certain conditions, sphingosine produced from ceramide by ceramidase is phosphorylated by sphingosine kinases to sphingosine-1 phosphate, another potent second messenger molecule. Both ceramide and sphingosine-1 phosphate regulate life and death of many cell types including brain cells and participate in pathogenic processes of MS. In this review, we have made an honest attempt to compile recent findings made by others and us relating to the role of sphingolipids in the disease process of MS.
Index Entries: Sphingomyelinases, Ceramide, Sphingosine-1 phosphate, Signal transduction, Oligodendroglial apoptosis, Glial activation, Perivascular cuffing
Introduction
Sphingolipids are ubiquitous components of all eukaryotic cell membranes, especially the plasma membrane, and is particularly abundant in the nervous system primarily due to galactosylceramide (GalC) in myelin, the fatty insulation wrapped around nerve cells produced by either Schwann cells in the peripheral nervous system (PNS) or oligodendrocytes in the CNS. Structurally, it is composed of a long chain sphingoid base, an amide linked fatty acid and a polar head group at the position 1. Depending on the head group, sphingolipids are classified into three groups- ceramide, sphingomyelin and glycosphingolipids. Ceramide has a hydroxyl group at 1 position, sphingomyelin has phosphorylcholine while glycosphingolipids contain carbohydrate head group. Glycosphingolipids are further subdivided into neutral (cerebrosides) or acidic fractions (gangliosides and sulfatides).
Apart from being a structural component of cell membrane it plays a role in signal transduction. Sphingolipids and its derivatives play important role both in physiological and pathophysiological processes including MS. The sphingomyelin (SM) pathway is triggered by a diverse range of endogenous and exogenous stimulants including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), Fas ligands (FasL), anticancer drugs, glucocorticoids, antibody cross-linking, heat shock, ionizing and ultraviolet radiation, serum deprivation, and infection by viruses and bacteria (Kolesnick & Golde, 1994, Hannun, 1994, Zhang & Kolesnick, 1995, Hannun & Obeid, 2008, Spiegel & Milstien, 2003). A transient hydrolysis of sphingomyelin, by a specific phospholipase C enzyme known as sphingomyelinases, with concomitant generation of ceramide is observed in response to these apoptosis-inducing stimuli. Although sphingomyelin hydrolysis is the major pathway of ceramide generation, cellular ceramide levels could also be regulated by other mechanisms (e.g. de novo synthesis of ceramide, conversion to complex sphingolipids, phosphorylation by ceramide kinase, and hydrolysis by ceramidases) (Bartke & Hannun, 2009). Accordingly, another critical second messenger sphingosine-1-phosphate (S1P) is formed from ceramide by the sequential actions of ceramidases and sphingosine kinases. Usually S1P levels in cells are low and tightly regulated by the balance between its synthesis and degradation. The degradation of S1P is mediated by reversible dephosphorylation back to sphingosine by specific S1P phosphatases and irreversible breakdown by a pyridoxal phosphate-dependent S1P lyase (Spiegel & Milstien, 2003, Hannun & Obeid, 2008). Both ceramide and S1P serve to modulate diverse signaling pathways in various cells ranging from proliferation and differentiation to cell cycle arrest and apoptosis (Hannun, 1996, Hannun & Obeid, 2008, Spiegel & Milstien, 2003). The decision to enter these disparate pathways determining a diverse neurobiological outcome depends on levels and molecular species of lipid second messenger formed, cell types and age, expression of specific receptors, and selective targets coupled to specific signaling pathways.
Multiple sclerosis (MS)
Pathological mechanisms involved in MS
Cytokine imbalance
Multiple studies have coupled ceramide accumulation to the action of several extracellular cytokines and stress responses. There are substantial data from both animal models and clinical studies that cytokines including TNF-α, IL-1β, IL-2, LTα, LTβ, and IFNγ are up-regulated in cerebrospinal fluid (CSF) and brain tissue of MS patients (Kunz & Ibrahim, 2009, Matei & Matei, 2002). A number of studies have demonstrated that some of these cytokines modulate phospholipid and sphingolipid metabolism (Plo et al., 1999, Singh et al., 1998).
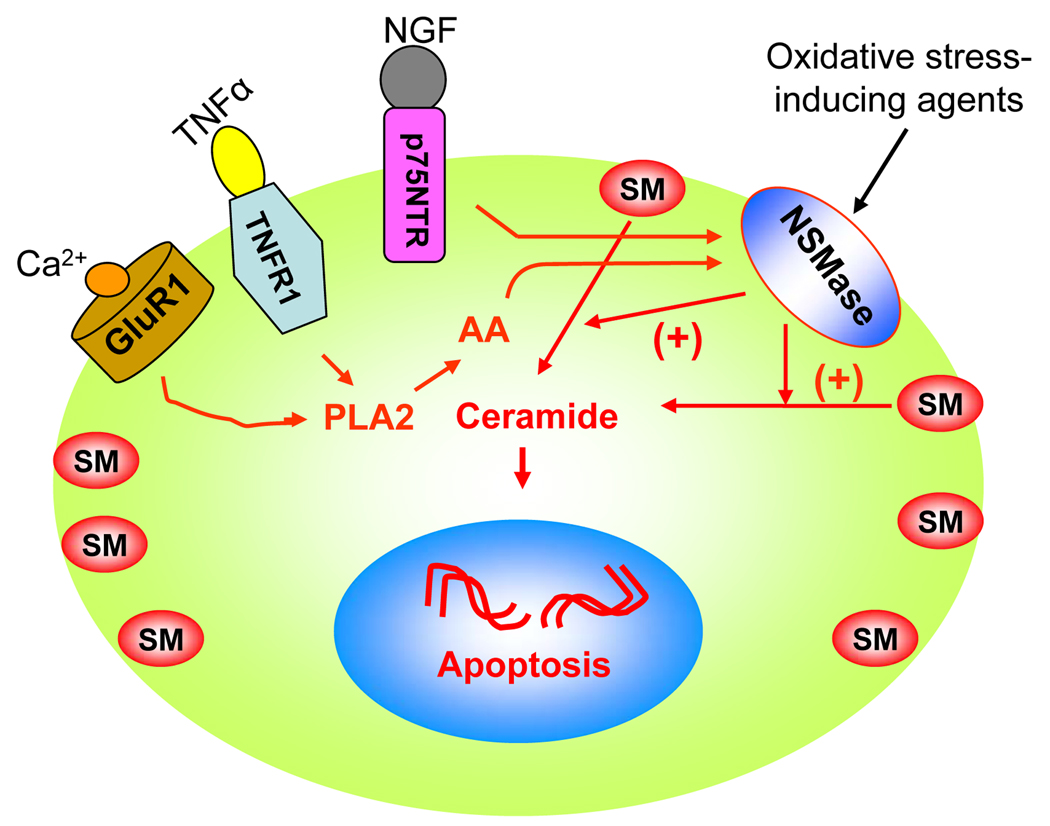
Among all proinflammatory cytokines studied to date with respect to the modulation of sphingolipid metabolism, TNF-α is definitely the most important one. Many second messenger pathways have been implicated in TNF-mediated signaling, including activation of phospholipase A2 (PLA2) (Fig. 1). Blocking PLA2 with PLA2 inhibitor CHEC-9 shows a remarkable decrease in both the onset and progression of experimental allergic encephalomyelitis (EAE), an animal model of MS (Cunningham et al., 2006, Marusic et al., 2005) and this has been further corroborated in PLA2 (−/−) mice (Marusic et al., 2008). Activation of PLA2 causes the release of arachidonic acid (AA) leading to the activation of sphingomyelinases (SMases) that cleave sphingomyelin to form ceramide (Jayadev et al., 1997). Possible mechanisms of activation of NSMase, the predominant form in oligodendrocytes, include binding of NSMase to the TNF-α receptor–TRADD–FAN (factor that activates NSMase)–caspase 8 complex (Segui et al., 2001) or posttranslational modification of NSMase to increase its affinity for rafts (Tani & Hannun, 2007) or transcriptional regulation of NSMase. Therefore, MS-associated proinflammatory cytokines are capable of activating the sphingomyelin cycle in oligodendrocytes, myelin-synthesizing cells in the CNS.
Fig. 1. Model showing the possible involvement of NSMase-ceramide pathway in cell death signaling in oligodendrocytes in MS.
Oxidative stress-inducing agents directly activate the NSMase-ceramide pathway. On the other hand, NGF requires p75NTR while TNFα and Ca2+ use the phospholipase (PLA2) – arachidonic acid (AA) pathway to activate NSMase in oligodendrocytes.
Oxidative stress
During MS, immune cells attack multiple areas of the brain and spinal cord with the release of large amounts of reactive oxygen species (ROS) leading to oxidative stress. ROS are implicated as mediators of demyelination and axonal damage in both MS and EAE, an animal model of MS (Gilgun-Sherki et al., 2004). This increase in ROS level depletes the antioxidant defense potential within the lesion area (Smith et al., 1999). ROS may damage protein, lipids and nucleic acids ultimately leading to cell death (Gilgun-Sherki et al., 2004). Further, studies from our lab have shown that ROS plays a crucial role in cytokine-induced down-regulation of myelin genes in human primary oligodendrocytes (Jana & Pahan, 2005). In addition, ROS might be responsible for trafficking of monocytes through BBB via upregulation of adhesion molecules (Van der Goes et al., 2001).
Oligodendrocytes are particularly susceptible to oxidative stress. Predisposing factors include their high metabolic rate and ATP requirement for the synthesis of large amounts of myelin membrane, large intracellular stores of iron, abundance of hydrogen peroxide, and the presence of high levels of polyunsaturated fatty acid in myelin sheath, and lower levels of antioxidant molecules (McTigue & Tripathi, 2008). These are discussed below.
a) Iron content of oligodendrocytes
Iron, an essential component in brain tissue, serves as a vital cofactor in many biological pathways including the synthesis of neurotransmitters and myelin (Zecca et al., 2004). Myelin synthesis and maintenance requires relatively high and constant supply of iron. Subsequently, inadequate iron levels are thought to impair myelination and leads to mental retardation (Beard, 2007). Histochemical stains for iron demonstrate that oligodendrocytes are the predominant cell type in the brain that stains for iron (Zhang et al., 2005, Connor & Menzies, 1996, LeVine, 1991). This differential staining arises from the fact that oligodendrocytes have a relatively high metabolic rate and the activity of the enzymes associated with metabolic activity is iron dependent (Beard et al., 2003, Juurlink et al., 1998). In addition, ferritin, the major protein involved in iron storage, is abundant in oligodendrocytes (Bartzokis, 2004, Zecca et al., 2004). This high iron content in oligodendrocytes generate highly toxic hydroxyl radicals by reacting with peroxides through the non-enzymatic Fenton reaction that act on many cellular macromolecules such as DNA, proteins and lipids (Ferriero, 2001). Furthermore, research findings have revealed high serum level of soluble transferrin receptor in MS patients compared with control subjects (Abo-Krysha & Rashed, 2008). Together, these results suggest that iron overload and upregulation of iron-handling proteins may be responsible for increased susceptibility of oligodendrocytes to oxidative stress.
b) Polyunsaturated fatty acid of myelin sheath
Myelin sheath of oligodendrocytes contains polyunsaturated fatty acids (PUFA) and is extremely susceptible to lipid peroxidation. Studies have shown that PUFA react with peroxides and hydroxyl radical triggering a cascade of oxidative damage leading to cell death probably via NSMase activation (Halliwell, 1992, Jana & Pahan, 2007). In addition, it has been reported that PUFA enhance iron uptake by modulating iron transporters, and accelerate apoptotic death (Schonfeld et al., 2007, Brand et al., 2008). Similar to oligodendrocytes, neurons also have high content of easily oxidizable polyunsaturated membrane lipids (Bazan, 2003). Docosahexaenoic acid (DHA), a major PUFA in the CNS, is used continuously for the biogenesis and maintenance of neuronal membrane and is a target for lipid peroxidation (Bazan, 2003). Therefore, free radical attack of PUFA may be responsible for increased sensitivity of oligodendrocytes to oxidative stress.
c) Lower levels of oxidative defense molecules
Transcriptional activation of antioxidant enzymes is mediated by antioxidant responsive element (ARE). The transcription factor nuclear factor-erythroid 2-related factor 2 (Nrf2), a basic leucine zipper transcription factor, regulates genetic expression of many protective antioxidant enzymes by binding to the ARE. Under physiological conditions, Nrf2 is bound by actin-bound Keap1 and sequestered in the cytoplasm but when oxidative stress sets in; Nrf2 translocates in the nucleus and binds to ARE–regulated genes including superoxide dismutases, catalase and glutathione peroxidase (Itoh et al., 2003, McCord & Edeas, 2005, Dringen et al., 2005, Thimmulappa et al., 2002). Activation of this pathway protects cells from oxidative stress-induced cell death. Studies have shown that Nrf2-knock out (Nrf2-KO) mice are more susceptible to EAE suggesting that activation of Nrf2 may attenuate pathogenesis of autoimmune diseases such as MS. Further, in MS plaques and in the serum of MS patient reduced concentrations of antioxidant enzymes have been reported (van Meeteren et al., 2005, Gilgun-Sherki et al., 2004). In addition, oligodendrocytes have low concentration of the antioxidant glutathione (McTigue & Tripathi, 2008) and this may predispose these myelin-synthesizing cells to the accumulation of intracellular hydrogen peroxide and subsequent damage.
d) Presence of Ca2+ permeable receptors
There is growing evidence about the role of glutamate, the principal excitatory neurotransmitter, in the pathology of MS and EAE (Bolton & Paul, 2006). The glutamate levels are significantly higher in CSF of MS patients and correlate with disease severity. Interestingly, the expression of glutamate receptor (GluR1), a Ca2+-permeable ionotropic AMPA receptor subunit, was up-regulated on oligodendrocytes in active MS lesion borders, but Ca2+-impermeable AMPA GluR2 subunit levels remain unchanged (Newcombe et al., 2008). High extracellular glutamate leads to receptor over-activation resulting in Ca2+ entry which activates Ca2+-dependent proteases, phospholipases, kinases, nitric oxide synthase, and endonucleases (Alberdi et al., 2002). These enzymes mediate proteolysis, lipid peroxidation and free-radical generation leading to oligodendrocyte death. In addition, high levels of extracellular glutamate limit the cellular availability of cysteine resulting in glutathione depletion and finally oxidative stress-mediated oligodendrocyte death (Schubert & Piasecki, 2001). Therefore, altered glutamate metabolism may be responsible for increased oxidative stress in oligodendrocytes.
Abnormal sphingolipid metabolism
Sphingolipid metabolism is essential for proper myelination since sphingolipids are crucial for synthesis and proper maintenance of the myelin membrane. Disordered sphingolipid content, therefore, leads to decompaction and destabilization of myelin structure. Different pathological signatures of MS such as proinflammatory cytokines, oxidative stress and GluR1 are potent activators of the sphingomyelin cycle in oligodendrocytes (Fig. 1). For example, TNF-α and IL-1β decrease intracellular GSH and induce the degradation of sphingomyelin to ceramide in rat primary oligodendrocytes and this effect is blocked by pretreatment of the cells with antioxidants like N-acetyl cysteine (NAC) (Singh et al., 1998). Accordingly, there are several evidences of disruptions of sphingolipid metabolism in MS brain that may perturb membrane asymmetry and myelin structure:
Ceramide levels increase in regions surrounding plaques (Singh et al., 1998).
Sphingosine (sph) content of NAWM (normal appearing white matter) is increased in MS (Moscatelli & Isaacson, 1969).
Increased hydroxylated sulfatide in MS white matter (Marbois et al., 2000).
Evidence of increased polyunsaturated fatty acid and phosphatidyl serine in EAE (Ohler et al., 2004).
Increased lipid peroxidation products in the form of 4-HNE modified lysine and histidine residues in the white matter of MS (Wheeler et al., 2008)
Physiological role of sphingolipids
Receptor clustering
Receptor clustering is a key event in transmembrane signaling in which ceramide serves to initiate fusion of preexisting sphingolipid-rich membrane microdomains into large ceramide-enriched signaling platforms including CD40L (Grassme et al., 2002b). B cell express CD40 and T cell express CD40L. The interaction between the CD40 ligand with its cognate receptor, CD40, is one of the key events of B-cell activation and is responsible for Ig class switching upon antigenic stimulation. Studies have shown that stimulation of lymphocytes via CD40 ligation results in p53-dependent activation and translocation of ASMase from intracellular stores and the formation of ceramide-enriched lipid raft on the extracellular surface of the plasma membrane (Grassme et al., 2002a). Genetic deficiency of ASMase or neutralization of surface ceramide or destruction of sphingolipid rich raft prevents CD40 clustering and CD40-initiated cell signaling (Grassme et al., 2002a, Grassme et al., 2002b).
In addition, this unique property of ceramide is also responsible for removal of activated T and B cells that would otherwise lead to autoimmune disease. Upon activation, the expression of two key cell surface proteins increases on the surface of T cells. These are Fas and FasL, which are responsible for T cell apoptosis. The importance of Fas and FasL in the removal of activated T cell is underscored in lpr/lpr mice that carry non-functional Fas and spontaneously develop autoimmune disease (Dittel, 2000). Within seconds of Fas engagement by ligand or agonistic antibody, ASMase translocates from an intracellular pool and hydrolyze sphingomyelin to release ceramide that facilitates Fas clustering and recruitment of Fas-associated death domain (FADD) followed by procaspase 8. The association of FADD with procaspase 8 initiates a proteolytic cascade leading to cell death (Cremesti et al., 2002, Grassme et al., 2001). Therefore, via inducing receptor clustering, ceramide may cause cell death in activated B and T lymphocytes.
Oligodendrocytes differentiation
Among different brain cells, the development of myelin-producing cells oligodendrocytes has been well-characterized. Oligodendrocytes arise from oligodendrocytes progenitor cells (OPC) that originate from subventricular zones of the brain and migrate along axonal tracts to various regions, where they differentiate into immature and mature oligodendrocytes (Miller, 2002, Pfeiffer et al., 1993). Differentiated oligodendrocytes synthesize large amounts of myelin that is enriched in GalC and its sulphated form sulfatide. These two lipids appear in oligodendrocytes at the stage of transition from proliferative prooligodendrocyte stage to nonproliferative immature oligodendrocyte stage marking a key step in the differentiation of oligodendrocytes (Bansal et al., 1988). Differentiation of oligodendrocytes is a critical event during brain development culminating in elaboration of the myelin sheath that insulate the axons and regulate nerve conduction. Impaired myelination leads to serious neurological disorders, and mutation or myelin gene deletion has been extensively used to examine this (Greenfield et al., 1977, Trapp & Nave, 2008). Another approach has been to reversibly inhibit oligodendrocytes progenitor cell differentiation by specific monoclonal anti-glycolipid antibody that perturbs myelination: following antibody removal, the oligodendrocytes re-differentiate (Bansal & Pfeiffer, 1989). Together, sphingolipids play a pivotal role in oligodendrocyte differentiation and hence myelination.
Controlling immunogenicity
The glycolipid GalC is a major component of CNS myelin. Besides, its hydrophilic head group extends from the myelin sheath and is vulnerable to antibody recognition thereby serving as a candidate autoantigen in MS. Furthermore, glycosphingolipid autoreactivity can be induced by bacterial infection as it displays broad structural similarities to the Borrelia burgdorferi glycolipid antigen BbGL-2. Interestingly, GalC is also structurally similar to natural killer T (NKT) cell ligand alpha-galactosylceramide, a Sphingomonas antigen (Blewett, 2008). It has been shown that MS patients have elevated levels of anti-GalC antibodies compared to healthy controls (Kasai et al., 1986). Accordingly, anti-GalC antibodies can cause demyelination and degrade myelin basic protein (Raine et al., 1981, Menon et al., 1997). Lipid antigens are recognized by MHC class1 like molecules CD1 found on microglia and some B cells that then present these lipids to T-cell receptors. Studies by Kanter et al. have shown increased lipid reactivity in the CSF of MS patients compared to controls (Kanter et al., 2006).
In order to suppress the autoimmune response, the immunogenic property of sphingolipids plays a vital role. For example, NKT cells recognize lipid antigen in the context of CD1 molecule and secrete copious amount of IL-4 upon TCR engagement. Experimental findings have shown that prior stimulation of NKT cells with α-galactosylceramide may protect against EAE (Jahng et al., 2001). This protection against EAE may be due to shift in the encephalitogenic immune response from Th1 to Th2 mode and an increase in the expression of GATA-3, a key regulator of Th2 cytokines, and a decrease in the expression of T-bet, a key regulator of Th1 cytokines. In addition to GalC, immune responses to lipids including sulfatide, oxidized phosphocholine, oxidized cholesterol, sphingomyelin and asialo-GM1 also contribute to the pathogenesis of MS (Podbielska & Hogan, 2009). Taken together, sphingolipids are important modulators of immunogenicity.
Stabilization of myelin
The insulating sheath or myelin that surrounds the core of a nerve fiber or axon is produced in the CNS by oligodendrocytes. This sheath acts like a conduit in an electrical system, ensuring that messages sent by axons are not lost on the way thereby allowing efficient conduction of action potentials down the axon. In addition to this, myelin contains numerous enzymes. Myelin lipids accounts for 70–80% lipid by weight and serves as the matrix in which the myelin proteins are embedded. There are significant hydrophobic interactions between lipids and proteins that facilitates in the maintenance of the myelin structure. In addition, cis or trans interactions between two glycosphingolipids GalC and sulfatide both of which are present in high concentrations in the multilayered myelin sheath is responsible for stabilization of the myelin sheath. This type of interaction between the lipids, cis or trans, was investigated using fluorescent and spin-label probes and anti-glycolipid antibodies (Boggs et al., 2000). Hence changes in sphingolipid composition may disrupt the membrane organization and lead to breakdown of axon insulation and loss of salutatory conditions.
Facilitation of fast nerve conduction
The presence of the myelin sheath facilitates fast saltatory conduction of the action potential. This property of myelin sheath depends on both the insulating nature of myelin and the clustering of voltage-gated sodium (Na+) channels at the nodes of Ranvier. Deficiency of glycolipid including GalC, sulfatide and galactodiglyceride causes breakdown of axon insulation and loss of salutatory conduction (Stoffel & Bosio, 1997). It has been suggested that ablation of the ceramide galactosyltransferase gene in mice may be a promising approach in unraveling the role of glycolipids in the above process (Stoffel & Bosio, 1997).
Pathophysiological role of sphingolipids
CNS Inflammation
Neuroinflammatory disorders including MS is associated with chronic neuroinflammation and elevated levels of proinflammatory cytokines, cell adhesion molecules, chemokines, proinflammatory enzymes such as inducible nitric-oxide synthase (iNOS) and cyclooxygenase in CNS lesions of patients with MS and animals with EAE (Benveniste, 1997, Martin et al., 1992). Chronic neuroinflammation results in sustained release of inflammatory mediators that works to perpetuate the inflammatory cycle that is more glial activation and further release of inflammatory factors. Recently we have shown that NSMase-NF-κB pathway is responsible for the release of inflammatory mediators from activated glia (unpublished report). Interestingly, knockdown of NSMase, bu t n o t ASMase, by either antisense oligonucleotides or chemical inhibitor prevented the induction of proinflammatory molecules (TNF-α, iNOS, IL-1β, and IL-6) and the activation of NF-κB in human primary astroglia. Persistent glial activation and increased proinflammatory cytokine levels may then lead to blood brain barrier disruption, transvascular leakage and lymphocyte migration through upregulation of cell adhesion molecules (ICAM-I, VCAM-I, and Selectin) expressed on the endothelium of BBB.
Gliosis
Glial activation has been implicated in the pathogenesis of a variety of neurodegenerative diseases including MS. Although activated glia secrete different neurotrophic factors for neuronal survival, it is believed that rapid and severe activation initiates an inflammatory response, leading to neuronal and oligodendrocyte death (Tani et al., 1996, Carson, 2002). We have recently found that NSMase activation is involved in gliosis (unpublished data). Consequently, antisense knockdown of NSMase, but not ASMase, decreased the activation of glia in vivo in the mouse cortex. Earlier Pahan et al (Pahan et al., 1998) have shown that ceramide stimulates the activation of NF-κB and the expression of iNOS in primary rat astrocytes. Recently findings from our lab have demonstrated NO is an important signal for the upregulation of GFAP in astrocytes (Brahmachari et al., 2006). In addition to the NSMase-ceramide pathway, according to Wu et al (Wu et al., 2008), the sphingosine kinase 1 - S1P receptor signaling also regulates gliosis (Fig. 2). Deletion of either sphingosine kinase 1 or S1P receptor reduces astroglial proliferation and gliosis. Therefore, it appears that the NSMase – ceramide pathway and/or sphingosine kinase – S1P pathway are important mediators of astroglial activation and gliosis.
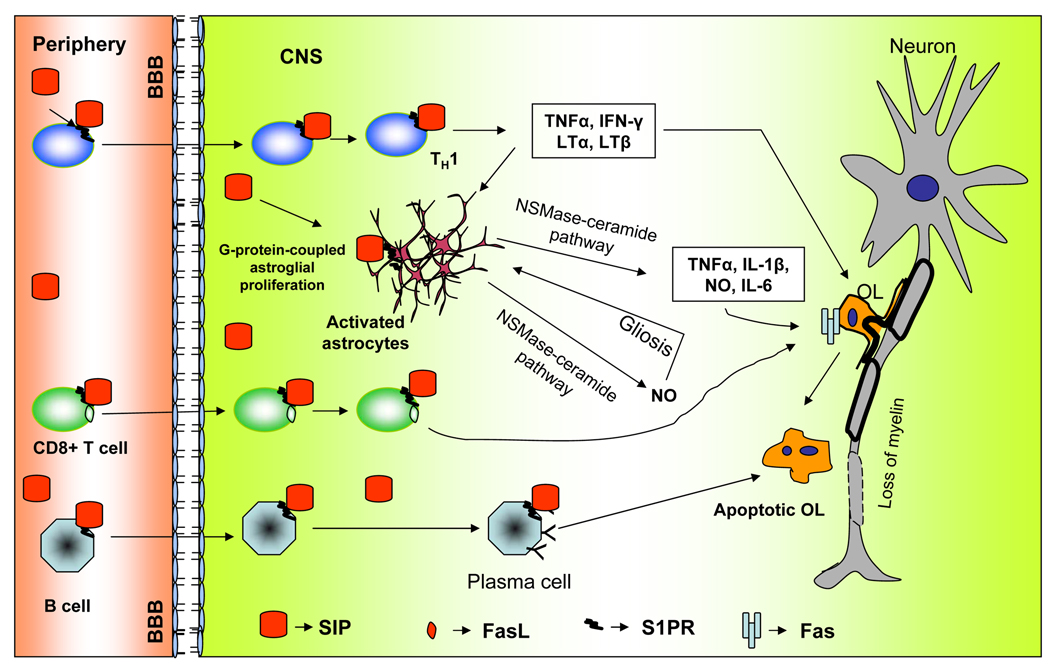
Fig. 2. Schematic diagram showing the proposed role of S1P in MS.
Activated T cells (Th1) expressing S1P receptor interacts with S1P found in blood during inflammation and enters the CNS where they produce proinflammatory cytotoxic products that promote oligodendrocytes death and demyelination. While CD8+ T cell directly kill oligodendrocytes through Fas and FasL interaction via ceramide pathway. Similarly B cells expressing S1PR enters the CNS and become plasma cells killing oligodendrocytes through ADCC pathway (antibody-dependant cell-mediated cytotoxicity). Astrocytes expressing S1PR undergoes gliosis via NSMase-ceramide pathway and utilizes the same pathway to release more proinflammatory cytotoxic products.
Regulation of T cell trafficking
The CNS has long been considered to be an immunoprivileged site and exhibited limited immunological responses. While multiple mechanisms contribute to immune privilege, restriction of peripheral leukocyte trafficking from the periphery to the CNS through the BBB is the most critical one. Under physiological conditions leukocyte entry into the healthy CNS across the BBB is kept at a low level since autoreactive naive T cells undergo negative selection and die by apoptosis. On the other hand in MS or its prototype animal model EAE some of these autoreactive cells escape the negative selection and become activated encephalitogenic effector cells that have the potential to initiate immune attack into the CNS. Induction of autoimmune responses against myelin components in the CNS is believed to occur through mechanisms such as molecular mimicry, bystander activation and epitope spreading (Vanderlugt & Miller, 2002, Fujinami et al., 2006). The activated T cells then proliferate and secrete pro-inflammatory cytokines, which in turn stimulate microglia, macrophages and astrocytes, and recruit B cells (Fig. 2), ultimately resulting in damage to myelin, oligodendrocytes and axons (Zamvil & Steinman, 2003).
There are mounting evidences to show that S1P receptor (S1PR1) is essential for lymphocyte recirculation egression from both thymus and peripheral lymphoid organs (Rosen & Goetzl, 2005). High levels of S1P are found in blood and lymph than in lymphoid organs (Rosen & Goetzl, 2005). Accordingly, the egress of lymphocytes from lymphoid organs into the blood is dependant on the interaction between S1P with S1PR1. Interestingly, FTY720, a S1PR1 modulator, down-regulates S1PR1 in lymphocytes and causes lymphocyte sequestration. The molecular basis for the mode of action of the drug FTY720 has been established recently. FTY720, after phosphorylation by sphingosine kinase, forms the active moiety FTY720-phosphate that acts as a high-affinity agonist for G protein-coupled S1PR1 receptor on thymocytes and lymphocytes, thereby inducing aberrant internalization and degradation of the receptor (Don et al., 2007). This renders the cells unresponsive to the serum lipid S1P, and inhibiting their egress from lymphoid organs. Furthermore, in addition to its effect on lymphocyte homing, this drug because of its lipophilic nature crosses the blood-brain barrier and down-regulates S1PR1 expression in astrocytes and thereby reduces astrogliosis and inflammation. This phenomenon indirectly helps in the structural restoration of the CNS parenchyma (Brinkmann, 2009). Therefore, S1P – S1PR1 signaling is an important target for controlling T cell homing in MS (Fig. 2) and accordingly, FTY720 has been found to reduce the number of lesions detected on MRI and clinical disease activity in MS patients (Kappos et al., 2006).
Destabilization of myelin
Alterations in myelin lipids play a significant role in myelin destabilization and this appear in MS normal appearing white matter prior to evidence of demyelination suggesting that they are causative events in the course of this disease. GalC, the most abundant lipid in myelin, is decreased in myelin from MS normal appearing white matter. Decrease in non-hydroxylated form of GalC play a significant role in destabilization of myelin. Further, disturbances in myelin lipids promote myelin fluidity, decreases myelin adhesion, increases membrane curvature, and promote vesiculation leading to apoptosis (Fraser et al., 1986).
Apoptosis
Oxidative stress has been proposed to play an important role in oligodendroglial death in MS (Gilgun-Sherki et al., 2004, Smith et al., 1999). Interestingly, in vivo oxidation markers have been identified in white matter tissue of MS patients, along with perturbations in glutamate homeostasis, which correlate with disease severity (Gilgun-Sherki et al., 2004, Smith et al., 1999). However, molecular mechanisms that couple oxidative stress to the loss of oligodendrocytes were poorly understood.
We have recently demonstrated the importance of NSMase–ceramide pathway in mediating oxidative stress-induced apoptosis and cell death of human primary oligodendrocytes. We have found that various oxidative stress-inducing agents, such as, superoxide radical produced by hypoxanthine and xanthine oxidase, hydrogen peroxide, aminotriazole capable of inhibiting catalase and increasing intracellular level of H2O2, or reduced glutathione-depleting diamide induce the activation of NSMase and the production of ceramide in human oligodendrocytes (Jana & Pahan, 2007). It is interesting to note that antisense knockdown of neutral, but not acidic, sphingomyelinase ablates oxidative stress-induced apoptosis and cell death in oligodendrocytes. Back–et al (Back et al., 1998) have also reported that oligodendrocytes are vulnerable to glutathione depletion caused by cystine deprivation, buthionine sulfoximine and diethylmaleate. Moreover, increases in the concentration of extracellular glutamate lead to a sustained activation of oligodendrocyte AMPA/kainate receptors resulting in the production of higher levels of ROS and apoptosis of oligodendrocytes (Benarroch, 2009). All these evidences suggest that oxidative stress and increased ceramide concentration via NSMase activation may represent an important component in the loss of oligodendrocytes in MS.
Intracellular signal transduction pathways activated by sphingolipids in MS
Role of P75 NTR
The discovery that neurotrophins bind to p75NTR and stimulate SM hydrolysis with subsequent ceramide elevation (Fig. 1) highlights the importance of ceramide in the regulation of survival and death signaling pathways in the CNS. Recent studies on the pathogenesis of MS white matter plaques have revealed upregulated p75NTR messenger RNA and protein in oligodendrocytes from MS plaques, but not in control white matter (Dowling et al., 1999). More interestingly, CNPase and p75NTR immunoreactivity co-localize with TUNEL staining indicating that the majority of dying cells were p75NTR expressing oligodendrocytes. Further, the observations that nerve growth factor (NGF) levels are elevated in the CSF of MS patients (Laudiero et al., 1992) and that MS lesions contain both apoptotic oligodendrocytes and immature oligodendrocytes with increased p75NTR expression (Ozawa et al., 1994, Dowling et al., 1999) raise the possibility that p75NTR may play a role in the pathogenesis of MS. In addition, retroviral expression of Trk A in mature oligodendrocytes prevents NGF-induced p75NTR-dependent apoptosis further reinforcing p75NTR-induced ceramide elevation in this death signaling pathway (Yoon et al., 1998).
Role of Fas
Fas/CD95, a member of the TNF receptor (TNFR) superfamily, is expressed on cell surface and is responsible for transducing cell death signals when cross-linked by agonist antibodies or by FasL (Nagata & Golstein, 1995). Immunohistochemical analysis of CNS tissues from MS subjects exhibited elevated Fas expression in oligodendrocytes in chronic MS lesions compared with control subjects (D'Souza et al., 1996). In such lesions, microglia and infiltrating lymphocytes display intense immunoreactivity to FasL. More interestingly, TUNEL positive cells co-localize with FasL in MS lesions. These interesting observations indicate that Fas-FasL system is involved in observed apoptosis in MS (D'Souza et al., 1996). In addition, in EAE model, mice mutant for FasL show a significant decrease in disease onset and a remarkable reduction in demyelination (Sabelko-Downes et al., 1999a, Dittel, 2000). It has been found that adoptive transfer of FasL deficient encephalitogenic T cells to wild type (WT) mice results in partial reduction of disease severity compared with the transfer of WT encephalitogenic T cells, proving that FasL expression by T lymphocytes is important for EAE pathogenesis (Sabelko-Downes et al., 1999a, Dittel, 2000). On the other hand, mice deficient for Fas receptor are partially resistant to EAE induction by immunization with myelin antigens (Probert et al., 2000, Sabelko-Downes et al., 1999b, Dittel, 2000). Hence, encephalitogenic T cells activating Fas signal transduction are crucial for the pathogenesis of EAE. Moreover, treatment with IFN-γ increases Fas expression on oligodendrocytes to enhance their susceptibility to FasL-induced apoptosis. Mounting evidences indicate that ceramide is involved in CD95-induced cell death (Kolesnick et al., 1994, Hannun & Obeid, 2008, Spiegel & Milstien, 2003).
Modulation of MAPK cascades
Mammalian MAPKs include extracellular signal-regulated kinases (ERKs), c-jun N-terminal kinases (JNKs), and p38 subgroups. JNK/p38 and ERK2 are differentially regulated by sphingolipid products in oligodendrocytes supporting that a dynamic balance between ERK2 and JNK/p38 cascades is important in determining the cell fate. It has been shown that ceramide causes sustained activation of p38α in oligodendrocytes and this effect is inhibited by SB203580 and by p38α dominant negative mutant (Hida et al., 1999). The activation of p38α may be due to a decrease in K+ ion influx leading to caspase activation. Further, ceramide is found to inhibit K+ ion influx via a ras- and raf-1-dependent pathway in cultured oligodendrocytes (Hida et al., 1998) and this inhibition may contribute to ceramide-induced activation of p38α and perhaps an inhibition of ERK2. On the other hand, the same group has delineated that sph and S1P activate ERK2 (Hida et al., 1999). According to Bassi et al (Bassi et al., 2006) astroglial proliferation by S1P is dependent on G protein-coupled ERK pathway. Therefore, sphingolipids are important regulators of MAPK cascades.
Conclusion
The sphingolipid signaling pathway is an important regulator of cellular signaling in which two lipid second messengers (ceramide and S1P) are major players. Alterations in ceramide and S1P production contribute to autoimmune demyelination in MS. This conclusion is based on the fact that ceramide induces apoptosis of oligodendrocytes and neurons, that S1P mediates lymphocyte egress from lymphoid tissues into the circulation, and that FTY720, a blocker of S1PR1, reduces clinical disease activity in MS patients.
Neuronal and oligodendrocyte death underlies the symptoms of many human neurological disorders. Therefore, unraveling the mechanisms involved in rescuing and sustenance of neurons and oligodendrocytes within and near the lesions of patients suffering from different neurodegenerative diseases including MS is of high priority in developing effective therapies. Our findings and those of other investigators indicate that activation of the neutral sphingomyelinase–ceramide pathway via oxidative stress mechanisms plays a cardinal role in the death of oligodendrocytes. On the other hand, S1P interaction with its receptor S1PR1 is needed for the egress of autoimmune cells from lymphoid organs. Taken together, pharmacological inhibitors of neutral sphingomyelinase and/or antagonists of S1P receptor may provide new therapeutic tools to halt the neuroinflammation and demyelination in MS.
Acknowledgement
This work was supported by grants from National Institutes of Health (NS39940 and NS39940-10S1).
References
- Abo-Krysha N, Rashed L. The role of iron dysregulation in the pathogenesis of multiple sclerosis: an Egyptian study. Mult Scler. 2008;14:602–608. doi: 10.1177/1352458507085550. [DOI] [PubMed] [Google Scholar]
- Alberdi E, Sanchez-Gomez MV, Marino A, Matute C. Ca(2+) influx through AMPA or kainate receptors alone is sufficient to initiate excitotoxicity in cultured oligodendrocytes. Neurobiol Dis. 2002;9:234–243. doi: 10.1006/nbdi.2001.0457. [DOI] [PubMed] [Google Scholar]
- Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R, Gard AL, Pfeiffer SE. Stimulation of oligodendrocyte differentiation in culture by growth in the presence of a monoclonal antibody to sulfated glycolipid. J Neurosci Res. 1988;21:260–267. doi: 10.1002/jnr.490210218. [DOI] [PubMed] [Google Scholar]
- Bansal R, Pfeiffer SE. Reversible inhibition of oligodendrocyte progenitor differentiation by a monoclonal antibody against surface galactolipids. Proc Natl Acad Sci U S A. 1989;86:6181–6185. doi: 10.1073/pnas.86.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50 Suppl:S91–S96. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiology of aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. author reply 49–62. [DOI] [PubMed] [Google Scholar]
- Bassi R, Anelli V, Giussani P, Tettamanti G, Viani P, Riboni L. Sphingosine-1-phosphate is released by cerebellar astrocytes in response to bFGF and induces astrocyte proliferation through Gi-protein-coupled receptors. Glia. 2006;53:621–630. doi: 10.1002/glia.20324. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. Journal of lipid research. 2003;44:2221–2233. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- Beard J. Recent evidence from human and animal studies regarding iron status and infant development. The Journal of nutrition. 2007;137:524S–530S. doi: 10.1093/jn/137.2.524S. [DOI] [PubMed] [Google Scholar]
- Beard JL, Wiesinger JA, Connor JR. Pre- and postweaning iron deficiency alters myelination in Sprague-Dawley rats. Developmental neuroscience. 2003;25:308–315. doi: 10.1159/000073507. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Oligodendrocytes: Susceptibility to injury and involvement in neurologic disease. Neurology. 2009;72:1779–1785. doi: 10.1212/WNL.0b013e3181a6b123. [DOI] [PubMed] [Google Scholar]
- Benveniste EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med. 1997;75:165–173. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- Blewett MM. Hypothesized role of galactocerebroside and NKT cells in the etiology of multiple sclerosis. Med Hypotheses. 2008;70:826–830. doi: 10.1016/j.mehy.2007.07.037. [DOI] [PubMed] [Google Scholar]
- Boggs JM, Menikh A, Rangaraj G. Trans interactions between galactosylceramide and cerebroside sulfate across apposed bilayers. Biophys J. 2000;78:874–885. doi: 10.1016/S0006-3495(00)76645-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton C, Paul C. Glutamate receptors in neuroinflammatory demyelinating disease. Mediators Inflamm. 2006;2006:93684. doi: 10.1155/MI/2006/93684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari S, Fung YK, Pahan K. Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J Neurosci. 2006;26:4930–4939. doi: 10.1523/JNEUROSCI.5480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Schonfeld E, Isharel I, Yavin E. Docosahexaenoic acid-dependent iron accumulation in oligodendroglia cells protects from hydrogen peroxide-induced damage. Journal of neurochemistry. 2008;105:1325–1335. doi: 10.1111/j.1471-4159.2008.05234.x. [DOI] [PubMed] [Google Scholar]
- Brinkmann V. FTY720 (fingolimod) in Multiple Sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol. 2009;158:1173–1182. doi: 10.1111/j.1476-5381.2009.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ. Microglia as liaisons between the immune and central nervous systems: functional implications for multiple sclerosis. Glia. 2002;40:218–231. doi: 10.1002/glia.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cremesti AE, Goni FM, Kolesnick R. Role of sphingomyelinase and ceramide in modulating rafts: do biophysical properties determine biologic outcome? FEBS Lett. 2002;531:47–53. doi: 10.1016/s0014-5793(02)03489-0. [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, Yao L, Oetinger M, Cort L, Blankenhorn EP, Greenstein JI. Secreted phospholipase A2 activity in experimental autoimmune encephalomyelitis and multiple sclerosis. J Neuroinflammation. 2006;3:26. doi: 10.1186/1742-2094-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza SD, Bonetti B, Balasingam V, Cashman NR, Barker PA, Troutt AB, Raine CS, Antel JP. Multiple sclerosis: Fas signaling in oligodendrocyte cell death. The Journal of experimental medicine. 1996;184:2361–2370. doi: 10.1084/jem.184.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittel BN. Evidence that Fas and FasL contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Arch Immunol Ther Exp (Warsz) 2000;48:381–388. [PubMed] [Google Scholar]
- Don AS, Martinez-Lamenca C, Webb WR, Proia RL, Roberts E, Rosen H. Essential requirement for sphingosine kinase 2 in a sphingolipid apoptosis pathway activated by FTY720 analogues. The Journal of biological chemistry. 2007;282:15833–15842. doi: 10.1074/jbc.M609124200. [DOI] [PubMed] [Google Scholar]
- Dowling P, Ming X, Raval S, Husar W, Casaccia-Bonnefil P, Chao M, Cook S, Blumberg B. Up-regulated p75NTR neurotrophin receptor on glial cells in MS plaques. Neurology. 1999;53:1676–1682. doi: 10.1212/wnl.53.8.1676. [DOI] [PubMed] [Google Scholar]
- Dringen R, Pawlowski PG, Hirrlinger J. Peroxide detoxification by brain cells. J Neurosci Res. 2005;79:157–165. doi: 10.1002/jnr.20280. [DOI] [PubMed] [Google Scholar]
- Ferriero DM. Oxidant mechanisms in neonatal hypoxia-ischemia. Developmental neuroscience. 2001;23:198–202. doi: 10.1159/000046143. [DOI] [PubMed] [Google Scholar]
- Fraser PE, Moscarello MA, Rand RP, Deber CM. Spontaneous vesicularization of myelin lipids is counteracted by myelin basic protein. Biochimica et biophysica acta. 1986;863:282–288. doi: 10.1016/0005-2736(86)90268-3. [DOI] [PubMed] [Google Scholar]
- Fujinami RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev. 2006;19:80–94. doi: 10.1128/CMR.19.1.80-94.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol. 2004;251:261–268. doi: 10.1007/s00415-004-0348-9. [DOI] [PubMed] [Google Scholar]
- Grassme H, Bock J, Kun J, Gulbins E. Clustering of CD40 ligand is required to form a functional contact with CD40. The Journal of biological chemistry. 2002a;277:30289–30299. doi: 10.1074/jbc.M200494200. [DOI] [PubMed] [Google Scholar]
- Grassme H, Jendrossek V, Bock J, Riehle A, Gulbins E. Ceramide-rich membrane rafts mediate CD40 clustering. J Immunol. 2002b;168:298–307. doi: 10.4049/jimmunol.168.1.298. [DOI] [PubMed] [Google Scholar]
- Grassme H, Schwarz H, Gulbins E. Molecular mechanisms of ceramide-mediated CD95 clustering. Biochem Biophys Res Commun. 2001;284:1016–1030. doi: 10.1006/bbrc.2001.5045. [DOI] [PubMed] [Google Scholar]
- Greenfield S, Brostoff S, Hogan E. Evidence for defective incorporation of proteins in myelin of the quaking mutant mouse. Brain research. 1977;120:507–515. doi: 10.1016/0006-8993(77)90403-6. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. Journal of neurochemistry. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Hannun YA. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Hida H, Nagano S, Takeda M, Soliven B. Regulation of mitogen-activated protein kinases by sphingolipid products in oligodendrocytes. J Neurosci. 1999;19:7458–7467. doi: 10.1523/JNEUROSCI.19-17-07458.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida H, Takeda M, Soliven B. Ceramide inhibits inwardly rectifying K+ currents via a Ras- and Raf-1-dependent pathway in cultured oligodendrocytes. J Neurosci. 1998;18:8712–8719. doi: 10.1523/JNEUROSCI.18-21-08712.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, O'Connor T, Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells. 2003;8:379–391. doi: 10.1046/j.1365-2443.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- Jahng AW, Maricic I, Pedersen B, Burdin N, Naidenko O, Kronenberg M, Koezuka Y, Kumar V. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. The Journal of experimental medicine. 2001;194:1789–1799. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Pahan K. Oxidative stress kills human primary oligodendrocytes via neutral sphingomyelinase: implications for multiple sclerosis. J Neuroimmune Pharmacol. 2007;2:184–193. doi: 10.1007/s11481-007-9066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana M, Pahan K. Redox regulation of cytokine-mediated inhibition of myelin gene expression in human primary oligodendrocytes. Free Radic Biol Med. 2005;39:823–831. doi: 10.1016/j.freeradbiomed.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayadev S, Hayter HL, Andrieu N, Gamard CJ, Liu B, Balu R, Hayakawa M, Ito F, Hannun YA. Phospholipase A2 is necessary for tumor necrosis factor alpha-induced ceramide generation in L929 cells. The Journal of biological chemistry. 1997;272:17196–17203. doi: 10.1074/jbc.272.27.17196. [DOI] [PubMed] [Google Scholar]
- Juurlink BH, Thorburne SK, Hertz L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia. 1998;22:371–378. doi: 10.1002/(sici)1098-1136(199804)22:4<371::aid-glia6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kanter JL, Narayana S, Ho PP, Catz I, Warren KG, Sobel RA, Steinman L, Robinson WH. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat Med. 2006;12:138–143. doi: 10.1038/nm1344. [DOI] [PubMed] [Google Scholar]
- Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- Kasai N, Pachner AR, Yu RK. Anti-glycolipid antibodies and their immune complexes in multiple sclerosis. J Neurol Sci. 1986;75:33–42. doi: 10.1016/0022-510x(86)90048-1. [DOI] [PubMed] [Google Scholar]
- Kolesnick R, Golde DW. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994;77:325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Kolesnick RN, Haimovitz-Friedman A, Fuks Z. The sphingomyelin signal transduction pathway mediates apoptosis for tumor necrosis factor, Fas, and ionizing radiation. Biochem Cell Biol. 1994;72:471–474. doi: 10.1139/o94-063. [DOI] [PubMed] [Google Scholar]
- Kunz M, Ibrahim SM. Cytokines and cytokine profiles in human autoimmune diseases and animal models of autoimmunity. Mediators Inflamm. 2009;2009:979258. doi: 10.1155/2009/979258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudiero LB, Aloe L, Levi-Montalcini R, Buttinelli C, Schilter D, Gillessen S, Otten U. Multiple sclerosis patients express increased levels of beta-nerve growth factor in cerebrospinal fluid. Neurosci Lett. 1992;147:9–12. doi: 10.1016/0304-3940(92)90762-v. [DOI] [PubMed] [Google Scholar]
- LeVine SM. Oligodendrocytes and myelin sheaths in normal, quaking and shiverer brains are enriched in iron. Journal of neuroscience research. 1991;29:413–419. doi: 10.1002/jnr.490290317. [DOI] [PubMed] [Google Scholar]
- Marbois BN, Faull KF, Fluharty AL, Raval-Fernandes S, Rome LH. Analysis of sulfatide from rat cerebellum and multiple sclerosis white matter by negative ion electrospray mass spectrometry. Biochimica et biophysica acta. 2000;1484:59–70. doi: 10.1016/s1388-1981(99)00201-2. [DOI] [PubMed] [Google Scholar]
- Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- Marusic S, Leach MW, Pelker JW, Azoitei ML, Uozumi N, Cui J, Shen MW, DeClercq CM, Miyashiro JS, Carito BA, Thakker P, Simmons DL, Leonard JP, Shimizu T, Clark JD. Cytosolic phospholipase A2 alpha-deficient mice are resistant to experimental autoimmune encephalomyelitis. The Journal of experimental medicine. 2005;202:841–851. doi: 10.1084/jem.20050665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusic S, Thakker P, Pelker JW, Stedman NL, Lee KL, McKew JC, Han L, Xu X, Wolf SF, Borey AJ, Cui J, Shen MW, Donahue F, Hassan-Zahraee M, Leach MW, Shimizu T, Clark JD. Blockade of cytosolic phospholipase A2 alpha prevents experimental autoimmune encephalomyelitis and diminishes development of Th1 and Th17 responses. J Neuroimmunol. 2008;204:29–37. doi: 10.1016/j.jneuroim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Matei I, Matei L. Cytokine patterns and pathogenicity in autoimmune diseases. Rom J Intern Med. 2002;40:27–41. [PubMed] [Google Scholar]
- McCord JM, Edeas MA. SOD, oxidative stress and human pathologies: a brief history and a future vision. Biomed Pharmacother. 2005;59:139–142. doi: 10.1016/j.biopha.2005.03.005. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Menon KK, Piddlesden SJ, Bernard CC. Demyelinating antibodies to myelin oligodendrocyte glycoprotein and galactocerebroside induce degradation of myelin basic protein in isolated human myelin. J Neurochem. 1997;69:214–222. doi: 10.1046/j.1471-4159.1997.69010214.x. [DOI] [PubMed] [Google Scholar]
- Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Moscatelli EA, Isaacson E. Gas liquid chromatographic analysis of sphingosine bases in sphingolipids of human normal and multiple sclerosis cerebral white matter. Lipids. 1969;4:550–555. doi: 10.1007/BF02531040. [DOI] [PubMed] [Google Scholar]
- Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Newcombe J, Uddin A, Dove R, Patel B, Turski L, Nishizawa Y, Smith T. Glutamate receptor expression in multiple sclerosis lesions. Brain Pathol. 2008;18:52–61. doi: 10.1111/j.1750-3639.2007.00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohler B, Graf K, Bragg R, Lemons T, Coe R, Genain C, Israelachvili J, Husted C. Role of lipid interactions in autoimmune demyelination. Biochimica et biophysica acta. 2004;1688:10–17. doi: 10.1016/j.bbadis.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Suchanek G, Breitschopf H, Bruck W, Budka H, Jellinger K, Lassmann H. Patterns of oligodendroglia pathology in multiple sclerosis. Brain. 1994;117(Pt 6):1311–1322. doi: 10.1093/brain/117.6.1311. [DOI] [PubMed] [Google Scholar]
- Pahan K, Sheikh FG, Khan M, Namboodiri AM, Singh I. Sphingomyelinase and ceramide stimulate the expression of inducible nitric-oxide synthase in rat primary astrocytes. The Journal of biological chemistry. 1998;273:2591–2600. doi: 10.1074/jbc.273.5.2591. [DOI] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal Bansal. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Plo I, Ghandour S, Feutz AC, Clanet M, Laurent G, Bettaieb A. Involvement of de novo ceramide biosynthesis in lymphotoxin-induced oligodendrocyte death. Neuroreport. 1999;10:2373–2376. doi: 10.1097/00001756-199908020-00028. [DOI] [PubMed] [Google Scholar]
- Podbielska M, Hogan EL. Molecular and immunogenic features of myelin lipids: incitants or modulators of multiple sclerosis? Mult Scler. 2009;15:1011–1029. doi: 10.1177/1352458509106708. [DOI] [PubMed] [Google Scholar]
- Probert L, Eugster HP, Akassoglou K, Bauer J, Frei K, Lassmann H, Fontana A. TNFR1 signalling is critical for the development of demyelination and the limitation of T-cell responses during immune-mediated CNS disease. Brain. 2000;123(Pt 10):2005–2019. doi: 10.1093/brain/123.10.2005. [DOI] [PubMed] [Google Scholar]
- Raine CS, Johnson AB, Marcus DM, Suzuki A, Bornstein MB. Demyelination in vitro. Absorption studies demonstrate that galactocerebroside is a major target. J Neurol Sci. 1981;52:117–131. doi: 10.1016/0022-510x(81)90140-4. [DOI] [PubMed] [Google Scholar]
- Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- Sabelko-Downes KA, Cross AH, Russell JH. Dual role for Fas ligand in the initiation of and recovery from experimental allergic encephalomyelitis. The Journal of experimental medicine. 1999a;189:1195–1205. doi: 10.1084/jem.189.8.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabelko-Downes KA, Russell JH, Cross AH. Role of Fas--FasL interactions in the pathogenesis and regulation of autoimmune demyelinating disease. J Neuroimmunol. 1999b;100:42–52. doi: 10.1016/s0165-5728(99)00191-5. [DOI] [PubMed] [Google Scholar]
- Schonfeld E, Yasharel I, Yavin E, Brand A. Docosahexaenoic acid enhances iron uptake by modulating iron transporters and accelerates apoptotic death in PC12 cells. Neurochemical research. 2007;32:1673–1684. doi: 10.1007/s11064-007-9378-x. [DOI] [PubMed] [Google Scholar]
- Schubert D, Piasecki D. Oxidative glutamate toxicity can be a component of the excitotoxicity cascade. J Neurosci. 2001;21:7455–7462. doi: 10.1523/JNEUROSCI.21-19-07455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segui B, Cuvillier O, Adam-Klages S, Garcia V, Malagarie-Cazenave S, Leveque S, Caspar-Bauguil S, Coudert J, Salvayre R, Kronke M, Levade T. Involvement of FAN in TNF-induced apoptosis. J Clin Invest. 2001;108:143–151. doi: 10.1172/JCI11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I, Pahan K, Khan M, Singh AK. Cytokine-mediated induction of ceramide production is redox-sensitive. Implications to proinflammatory cytokine-mediated apoptosis in demyelinating diseases. The Journal of biological chemistry. 1998;273:20354–20362. doi: 10.1074/jbc.273.32.20354. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Stoffel W, Bosio A. Myelin glycolipids and their functions. Current opinion in neurobiology. 1997;7:654–661. doi: 10.1016/s0959-4388(97)80085-2. [DOI] [PubMed] [Google Scholar]
- Tani M, Glabinski AR, Tuohy VK, Stoler MH, Estes ML, Ransohoff RM. In situ hybridization analysis of glial fibrillary acidic protein mRNA reveals evidence of biphasic astrocyte activation during acute experimental autoimmune encephalomyelitis. Am J Pathol. 1996;148:889–896. [PMC free article] [PubMed] [Google Scholar]
- Tani M, Hannun YA. Neutral sphingomyelinase 2 is palmitoylated on multiple cysteine residues. Role of palmitoylation in subcellular localization. The Journal of biological chemistry. 2007;282:10047–10056. doi: 10.1074/jbc.M611249200. [DOI] [PubMed] [Google Scholar]
- Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annual review of neuroscience. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- Van der Goes A, Wouters D, Van Der Pol SM, Huizinga R, Ronken E, Adamson P, Greenwood J, Dijkstra CD, De Vries HE. Reactive oxygen species enhance the migration of monocytes across the blood-brain barrier in vitro. FASEB J. 2001;15:1852–1854. doi: 10.1096/fj.00-0881fje. [DOI] [PubMed] [Google Scholar]
- van Meeteren ME, Teunissen CE, Dijkstra CD, van Tol EA. Antioxidants and polyunsaturated fatty acids in multiple sclerosis. Eur J Clin Nutr. 2005;59:1347–1361. doi: 10.1038/sj.ejcn.1602255. [DOI] [PubMed] [Google Scholar]
- Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- Wheeler D, Bandaru VV, Calabresi PA, Nath A, Haughey NJ. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain. 2008;131:3092–3102. doi: 10.1093/brain/awn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YP, Mizugishi K, Bektas M, Sandhoff R, Proia RL. Sphingosine kinase 1/S1P receptor signaling axis controls glial proliferation in mice with Sandhoff disease. Hum Mol Genet. 2008;17:2257–2264. doi: 10.1093/hmg/ddn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SO, Casaccia-Bonnefil P, Carter B, Chao MV. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamvil SS, Steinman L. Diverse targets for intervention during inflammatory and neurodegenerative phases of multiple sclerosis. Neuron. 2003;38:685–688. doi: 10.1016/s0896-6273(03)00326-x. [DOI] [PubMed] [Google Scholar]
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nature reviews. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zhang X, Haaf M, Todorich B, Grosstephan E, Schieremberg H, Surguladze N, Connor JR. Cytokine toxicity to oligodendrocyte precursors is mediated by iron. Glia. 2005;52:199–208. doi: 10.1002/glia.20235. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kolesnick R. Signaling through the sphingomyelin pathway. Endocrinology. 1995;136:4157–4160. doi: 10.1210/endo.136.10.7664631. [DOI] [PubMed] [Google Scholar]