Abstract
As shown by genome-wide association studies single-nucleotide polymorphisms (SNPs) within intron 1 of the FTO gene are associated with the body mass index and type II diabetes, although the functional significance of these SNPs has remained unclear. Using primer extension assays, we have determined the ratio of allelic FTO transcript levels in unspliced heterogeneous nuclear RNA preparations from blood of individuals heterozygous for SNP rs9939609. Allelic expression ratios of the neighboring RPGRIP1L gene were investigated in individuals who were heterozygous for SNP rs4784319 and heterozygous or homozygous for rs9939609. In each of five individuals, the FTO transcripts containing the A (risk) allele of rs9939609 were more abundant than those with T allele (mean 1.38; 95% confidence interval 1.31–1.44). Similar results were obtained in a fibroblast sample. We also observed skewed allelic expression of the RPGRIP1L gene in blood, but skewing was independent of the FTO genotype. Our data suggest that increased expression of FTO is associated with increased body mass.
Keywords: allelic expression, FTO, SNP
Introduction
Recent genome-wide association studies have revealed a strong association between a block of single-nucleotide polymorphisms (SNPs) in the fat mass and obesity-associated (FTO) gene, body mass index (BMI) and other obesity-related traits in children and adults of different populations.1, 2 The obesity-associated SNPs are located in intron 1 of the FTO gene, which contains nine exons (Figure 1). Using bioinformatics analysis, Gerken et al3 have proposed that the FTO protein is an Fe(II) and 2-oxoglutarate-dependent oxygenase.3 Loss-of-function mutation in FTO causes severe growth retardation and multiple malformations in homozygotes,4 whereas loss of one functional copy of this gene seems to be compatible with both lean and obese phenotype.5 Leanness, postnatal growth retardation and a higher metabolic rate were observed in Fto knockout mice7 and in mice with a missense mutation in exon 6.8
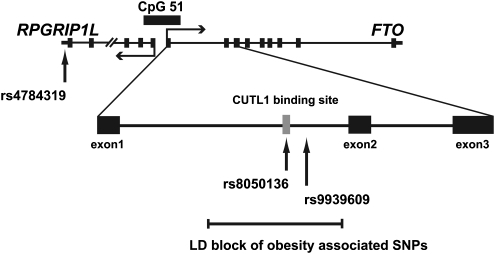
Figure 1.
Physical map of the FTO and RPGRIP1L loci. The FTO and RPGRIP1L genes are located on the long arm of chromosome 16, share a CpG island with 51 CpG dinucleotides and are transcribed in opposite directions. The obesity-associated SNPs are in strong LD and located within intron 1 of the FTO gene. The variants used in this study as well as the CUTL1-binding site identified by Stratigopoulos et al9 are indicated.
The FTO gene shares a CpG island with the adjacent RPGRIP1L gene, which is transcribed in the opposite direction, suggesting that the two genes are coregulated. The RPGRIP1L protein is located in the cilia and centrosomes.6 Loss-of-function mutations in RPGRIP1L cause Joubert syndrome type 7 or Meckel syndrome type 5. It can be noted that FTO and RPGRIP1L genes are ubiquitously expressed and show similarity of expression profile both in fetal and adult tissues (data not shown).
As the obesity-associated SNPs in the FTO gene are intronic, their functional significance is unclear. It is possible that one of the SNPs is located in a regulatory sequence and that the risk allele increases or decreases the transcription rate, but strong linkage disequilibrium (LD) of these SNPs makes it difficult to identify the functionally relevant SNP. In 2008, Stratigopoulos et al9 reported that the risk allele (A) of SNP rs8050136 preferentially bound to the transcription factor CUTL1 in human fibroblast DNA and that an siRNA knockdown of CUTL1 by 70% decreased FTO and RPGRIP1L expression by 90 and 65%, respectively. However, these findings are not consistent with their model in which FTO and/or RPGRIP1L mediate suppressive effects on energy intake. To fit the physiological model, CUTL1 should preferentially bind to the non-risk allele of rs8050136. Furthermore, several studies have failed to reveal any influence of the FTO genotype on total mRNA level of FTO or RPGRIP1L.10, 11, 12 Recently, an association between intronic variation of the FTO gene and transcript levels of the retinoblastoma-like 2 (RBL2) gene, which maps 270 kb upstream of FTO, was reported.13 One problem in interpreting the previous findings is that the CCAAT-displacement activity of CUTL1 was implicated in the transcriptional repression of several genes, whereas some CUTL1 isoforms were found to participate in the transcriptional activation.14 Another problem is that studies on cis-regulatory effects on gene transcription in humans are hampered by the fact that the tested individuals unavoidably differ in genetic background, age, life events and environment. To detect subtle differences in transcript levels, very large numbers of individuals would have to be tested. These problems can be circumvented by determining the ratio of allelic transcript levels in heterozygous individuals, in whom each allele serves as an internal control for the other.15, 16, 17, 18 For this approach, only few subjects are needed.
Materials and methods
Study cohort
The study was approved by the ethics committee of the University Hospital Essen. Blood samples and skin biopsies from normal weight individuals (BMI 18.5–25) were obtained after informed consent was given.
Genotyping
Subjects were genotyped by sequence analysis of genomic DNA (gDNA) extracted from whole blood with EZ1 DNA Blood Kit (Qiagen, Hilden, Germany). Sequence reactions were carried out with Big Dye Terminators (BigDye Terminator v1.1 Cycle Sequencing Kit, Applied Biosystems, Foster City, CA, USA). Reaction products were analyzed with an ABI 3100 Genetic Analyzer and Sequencing Analysis software (Applied Biosystems).
Preparation of hnRNA and total RNA
For heterogeneous nuclear RNA (hnRNA) extraction, lymphocytes were isolated with Ficoll-Paque PLUS (GE Healthcare, Waukesha, WI, USA) from fresh blood collected in EDTA tubes. Skin fibroblasts were cultured in AmnioMAX+M-C100 medium containing 20% AmnioMAX Supplement (Gibco, Invitrogen, San Diego, CA, USA) at 37°C in a humidified atmosphere containing 5% CO2. Both lymphocytes and fibroblasts were subjected to hnRNA extraction with the Cytoplasmic & Nuclear RNA Purification Kit (Norgen Biotek Corporation, Thorold, ON, Canada). DNase treatment was carried out in solution followed by cleaning up on spin columns (Qiagen, Hilden, Germany). To minimize loss of hnRNA, all steps were carried out as quickly as possible. Isolated hnRNA was dispensed in several aliquots and frozen in liquid nitrogen. For testing SNP rs4784319, we used total RNA extracted from blood with the PAXgene blood RNA Kit (PreAnalytiX, Hombrechtikon, Switzerland).
Allelic expression assays
Reverse transcription and primer extension assays were performed with kits from Applied Biosystems. Total RNA from blood was reverse transcribed with random hexamers, whereas cDNA from hnRNA was primed with sequence-specific primers FTO R1 or FTO R2 (all primer sequences and annealing temperatures are given in the Supplementary Table S1). For amplification, the GoTaq DNA Polymerase Kit (Promega, Madison, WI, USA) was used. FTO genomic DNA and cDNA were amplified with primers FTO F1 and FTO R1. RPGRIP1L genomic DNA was amplified with primers gRP F and gRP R, cDNA was amplified with cRP F and cRP R. SNPs in the primer-binding sites were excluded by sequencing across these regions in all individuals. Samples were heated at 95°C for 2 min, followed by 35 cycles at 95°C for 30 s, 55°C (FTO gDNA and cDNA), 60°C (RPGRIP1L gDNA) and 62°C (RPGRIP1L cDNA) for 30 s, and at 72°C for 40 s, finally at 72°C for 5 min. Using equal amount of amplicons from cDNA and genomic DNA, primer extension assays were carried out with Snapshot R primers and ABI Prism SNaPshot Kit. Reaction conditions were as follows: 96°C for 3 min, 25 cycles at 96°C for 10 s, 53°C (FTO, rs9939609) and 41°C (RPGRIP1L, rs4784319) for 5 s, and at 60°C for 30 s. The reaction products were analyzed by gel capillary electrophoresis on ABI 3700 DNA Analyzer and the electropherograms were analyzed with the Gene Mapper 4.0 software (Applied Biosystems). Allelic DNA ratios were used to normalize the cDNA ratios. Means and confidence intervals were calculated with JMP7 (SAS, Cary, NC, USA).
Results
To measure the allelic ratios of FTO and RPGRIP1L transcripts, we developed fluorescence-tagged single-nucleotide primer extension assays. This approach has been successfully used by us and others for detecting skewed and non-skewed allelic expression of many genes (see for example Serre et al18 and Kanber et al19). For FTO, we used unspliced hnRNA because (i) all obesity-associated SNPs are of intronic location and (ii) no expressed polymorphism in LD with these SNPs is known. We chose the clinically associated SNP rs9939609 within intron 1 of FTO as a marker (Figure 1), because it was tested in many independent studies of large Caucasian populations. SNP rs9939609 is in complete LD with SNP rs8050136, and the A (risk) allele of rs9939609 is associated with the A allele of rs8050136 (data not shown). For RPGRIP1L, we used an expressed SNP located in the 3′ untranslated region (rs4784319) (Figure 1). As SNPs in this gene are not in LD with the obesity-associated FTO SNPs and there is no easy way of establishing phase in double heterozygotes, we decided to determine the allelic expression pattern of the RPGRIP1L gene in RPGRIP1L heterozygous individuals with different FTO genotypes. If the obesity-associated FTO SNPs affected RPGRIP1L expression, we should observe allelic expression imbalance of RPGRIP1L in FTO heterozygotes, but not in FTO homozygotes.
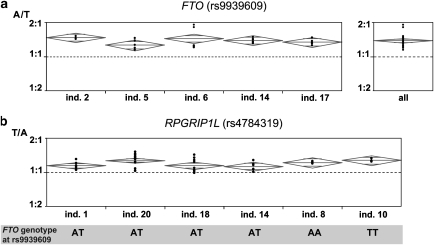
For determining allelic FTO transcript levels, we investigated hnRNA from blood of five individuals heterozygous for rs9939609. All assays were performed in sextuplicate. There was no evidence for DNA contamination of the hnRNA samples (Supplementary Figure 1). As shown in Figure 2a, there was very little inter-assay variation of allelic ratios. In each of the five individuals, relative transcript levels were skewed to similar degrees in favor of the A (risk) allele (mean 1.38; 95% confidence interval 1.31–1.44). In skin fibroblasts from individual 18, we observed a ratio of 1.31 (95% confidence interval 1.23–1.39, data not shown).
Figure 2.
Allelic expression studies. (a) Allelic expression of the FTO gene in five individuals. The ratio of the two alleles of SNP rs9939609 is skewed in favor of the A (risk) allele in all individuals investigated. Results are displayed as diamonds, in which the horizontal line represents the mean value and the top and bottom of the diamonds represent the 95% confidence interval. (b) Allelic expression of the RPGRIP1L gene in six individuals with different FTO genotypes. In each individual, the ratio of the two alleles of SNP rs4784319 is skewed in favor of the T allele, independently of the FTO genotype.
To investigate the effect of the obesity-associated FTO SNPs on allelic RPGRIP1L transcript levels, we tested blood RNA of six individuals who were heterozygous for the RPGRIP1L SNP rs4784319. Of these, four were heterozygous for the FTO SNP rs9939609, one was homozygous for the A (risk) allele of this SNP, and one was homozygous for the T allele. As shown in Figure 2b, we observed similar degrees of skewing in favor of the T allele in all six individuals. These results suggest that RPGRIP1L gene expression is not affected by the FTO genotype, but by cis-regulatory variation, which is in LD with rs4784319.
Discussion
Using single-nucleotide primer extension assays we have determined allelic expression ratios of the FTO and RPGRIP1L genes. We demonstrate for the first time (i) that the obesity-associated FTO SNPs affect FTO and not RPGRIP1L expression and (ii) that the primary FTO transcript made from the risk allele is more abundant than the transcript made from the non-risk allele, at least in blood cells and skin fibroblasts. The observed skewing cannot be due to the presence of genomic DNA in our hnRNA preparations, because (i) there was no evidence for DNA contamination (Supplementary Figure 1) and (ii) allelic PCR products from genomic DNA would be present in equal amounts.
Our observation that allelic expression imbalance of the RPGRIP1L gene is not dependent on the FTO genotype is consistent with previous studies reporting no association between RPRGIP1L variation and obesity.20 The degree of skewing of allelic FTO expression is remarkably similar in our subjects, suggesting that most of the variation in FTO expression is due to cis-regulatory variation in intron 1 of this gene. As the determination of allelic expression with intronic SNPs gives very similar estimates with those obtained with exonic SNPs,18 we can assume that allelic mRNA levels are also skewed. Higher mRNA levels might translate into higher protein levels, but, of course, we have no proof for this assumption.
One limitation of our study is the fact that we could analyze easily accessible cell types only. Blood cells and fibroblasts are most probably not involved in body weight regulation, but we do not know the cell type through which FTO exerts its effect, and this cell type may not be accessible. On the other hand, FTO is ubiquitously expressed. Therefore, it not unreasonable to assume that the allelic expression ratios found in blood cells and fibroblasts are similar to those in many other cells.
On the basis of our results, we propose that increased rather than decreased expression of FTO is causally evolved in increased BMI measures and obesity-associated traits. This interpretation is consistent with the mouse models, in which decreased levels of Fto cause leanness.7, 8 Increased expression of the risk allele as shown here is compatible with a role of CUTL1 in activating FTO, as originally suggested by Stratigopoulos et al,9 although other transcriptional activators may have a role. It is tempting to speculate that subtle reduction of FTO expression by pharmacological intervention may contribute to the prevention of obesity.
Acknowledgments
We thank all individuals for donating blood and skin biopsies, physicians for drawing blood, Regina Kubica for technical assistance with fibroblast cultures and the Bundesministerium für Bildung und Forschung (NGFN plus 01GS0820) for financial support.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
WEB RESOURCES
The URLs used for this study are as follows: UCSC Human Genome Browser Gateway at http://genome.ucsc.edu/NCBI Single Nucleotide Polymorphism at http://www.ncbi.nlm.nih.gov/projects/SNP/HapMap Genome Browser at http://hapmap.ncbi.nlm.nih.gov/cgi-perl/gbrowse/hapmap3r2_B36/.
Supplementary Material
References
- Frayling TM, Timpson NJ, Weedon NM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–892. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina C, Meyre D, Gallina S, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- Gerken T, Girard ChA, Tung YL, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–1474. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissel S, Reish O, Proulx K, et al. Loss-of-Function mutation in the dioxygenase-encoding FTO gene causes severe growth retardation and multiple malformations. Am J Hum Genet. 2009;85:106–111. doi: 10.1016/j.ajhg.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyre D, Proulx K, Kawagoe-Takaki H, et al. Prevalence of loss of function FTO mutations in lean and obese individuals. Diabetes. 2010;59:311–318. doi: 10.2337/db09-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delous M, Lekbir Baala L, Salomon R, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- Fischer J, Koch L, Emmerling Ch, et al. Inactivation of the Fto gene protects from obesity. Nature. 2009;458:894–898. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- Church C, Lee S, Bagg EAL, et al. A mouse model for the metabolic effects of the human fat mass and obesity associated FTO gene. PLoS Genet. 2009;5:e1000599. doi: 10.1371/journal.pgen.1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratigopoulos G, Padilla SL, LeDuc CA, et al. Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol. 2008;294:1185–1196. doi: 10.1152/ajpregu.00839.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöting N, Schleinitz D, Ruschke K, et al. Inverse relationship between obesity and FTO gene expression in visceral adipose tissue in humans. Diabetologia. 2008;51:641–647. doi: 10.1007/s00125-008-0928-9. [DOI] [PubMed] [Google Scholar]
- Wåhlén K, Sjölin E, Hoffstedt J. The common rs9939609 gene variant of the fat mass- and obesity-associated gene FTO is related to fat cell lypolisis. J Lipid Res. 2008;49:607–611. doi: 10.1194/jlr.M700448-JLR200. [DOI] [PubMed] [Google Scholar]
- Louise GG, Nilsson E, Ling C, et al. Regulation and function of FTO mRNA expression in human skeletal muscle and subcutaneous adipose tissue. Diabetes. 2009;58:2402–2408. doi: 10.2337/db09-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowett BMJ, Curran JE, Johnson MP, et al. Genetic variation at the FTO locus influences RBL2 gene expression. Diabetes. 2010;59:726–732. doi: 10.2337/db09-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansregret L, Nepveu A. The multiple roles of CUX1: insights from mouse models and cell-based assays. Gene. 2008;412:84–94. doi: 10.1016/j.gene.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Yan H, Yuan W, Velculescu VE, Vogelstein B, Kinzler KW. Allelic variation in human gene expression. Science. 2002;297:1143. doi: 10.1126/science.1072545. [DOI] [PubMed] [Google Scholar]
- Lo HS, Wang Z, Hu Y, et al. Allelic variation in gene expression is common in the human genome. Genome Res. 2003;13:1855–1862. doi: 10.1101/gr.1006603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastinen T, Sladek R, Gurd S, et al. A survey of genetic and epigenetic variation affecting human gene expression. Physiol Genomics. 2004;16:184–193. doi: 10.1152/physiolgenomics.00163.2003. [DOI] [PubMed] [Google Scholar]
- Serre D, Gurd S, Ge B, et al. Differential allelic expression in the human genome: a robust approach to identify genetic and epigenetic cis-acting mechanisms regulating gene expression. PLoS Genet. 2008;4:e1000006. doi: 10.1371/journal.pgen.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanber D, Berulava T, Ammerpohl O, et al. The human retinoblastoma gene is impinted. PLoS Genet. 2009;5:e1000790. doi: 10.1371/journal.pgen.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson JA, Danielsson P, Svensson V, et al. Major gender difference in association of FTO gene variant among several obese children with obesity and obesity related phenotypes. Biochem Biophys Res Commun. 2008;368:476–482. doi: 10.1016/j.bbrc.2008.01.087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.