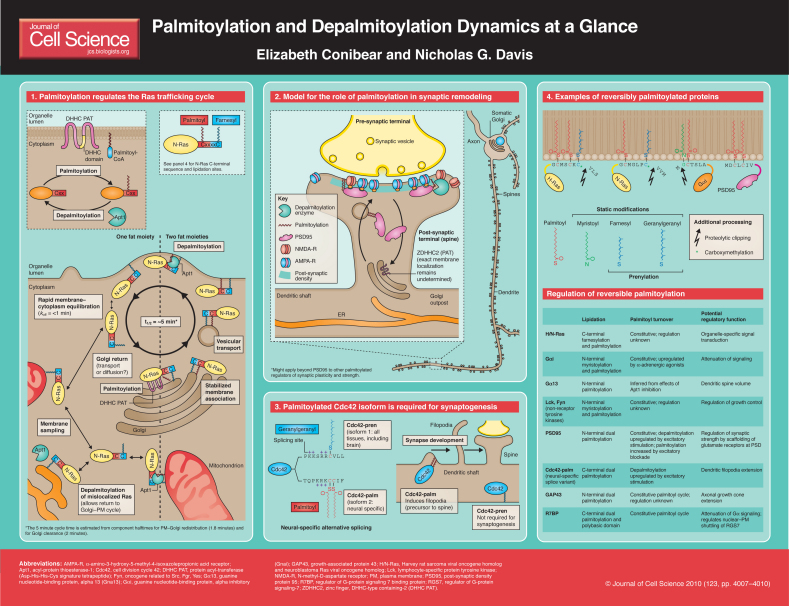
Protein palmitoylation, the thioester linkage of fatty acyl moieties (typically, saturated 16C palmitate) to cysteine, is a lipid modification that serves both to tether proteins to membranes and to direct their localization to membrane microdomains. Unlike the two other types of lipid modification that also tether proteins to cytosolic membrane surfaces, namely prenylation and myristoylation, which remain attached to the protein throughout its lifetime, a distinguishing feature of palmitoylation is its reversibility. This ‘At a Glance’ poster article focuses on this one aspect of palmitoylation – the dynamic regulation of membrane association of proteins through the regulated addition and removal of palmitoyl modifications. Other aspects of protein palmitoylation are covered in a number of recent reviews (Fukata et al., 2004; Nadolski and Linder, 2007; Planey and Zacharias, 2009; Zeidman et al., 2009).
The Ras proto-oncogene family members H-Ras and N-Ras (hereafter referred to as H/N-Ras) provide the best example of proteins whose localization is dynamically regulated by reversible palmitoylation (see Poster, panel 1). Palmitoylation at the Golgi stabilizes the association of H/N-Ras with membranes, thereby facilitating its vesicular trafficking to the plasma membrane (PM). There, depalmitoylation releases H/N-Ras into the cytoplasm, allowing its return to the Golgi for another round of palmitoylation. Because the Golgi and the PM pools of H/N-Ras activate different downstream signaling cascades (Fehrenbacher et al., 2009), this regulation of localization also regulates signaling.
Similar palmitoylation-driven protein cycling between Golgi and PM has been documented for several other signaling regulators, including the heterotrimeric G-protein subunit Giα and the non-receptor tyrosine kinase Fyn (Rocks et al., 2010; Rocks et al., 2005) (see Poster, panel 4). Nonetheless, it remains unclear how widely the Ras paradigm applies. Below, we focus on the Ras cycle as an example of how palmitoylation and depalmitoylation act to dynamically regulate protein localization. We
then describe recent findings suggesting that dynamic changes in palmitoylation at the synapse influence the local control of receptor flow to and from synaptic membranes, and might play a major role in synaptic plasticity.
Lipid tethers – one is not enough
The dynamic Ras cycle is driven in large part by the striking differences in membrane avidity afforded by one versus two lipid tethers (Shahinian and Silvius, 1995). A single lipid tether provides hydrophobicity for a strong but kinetically unstable association with membranes, with measurable membrane off-rates of less than 1 minute. Consequently, a protein with just a single lipid tether ‘jitters’ on and off the bilayer, and is able to sample multiple endomembrane systems within the cell [e.g. endoplasmic reticulum (ER), mitochondria; see Poster, panel 1]. The addition of a second tether increases membrane off-rates to many hours, which greatly stabilizes the association of the protein with the membrane and enables its productive transport by vesicular trafficking.
Soon after their biosynthesis, but before palmitoylation, H/N-Ras are first farnesylated on their consensus C-terminal CaaX motif by the soluble cytoplasmic farnesyl-transferase. The attached farnesyl moiety allows H/N-Ras to transiently interact with the membrane, facilitating subsequent membrane-based processing – that is, the proteolytic removal of the C-terminal aaX tripeptide, followed by carboxy-methylation on the new C terminus and, finally, palmitoylation of proximal cysteines (see Poster, panel 4). As with palmitoylation, prenylation also involves attachment of prenyl moieties (either the 15C farnesyl or the 20C geranylgeranyl) to cysteine, but via a thioether, as opposed to thioester, linkage. Prenylation is not reversible. Thus, in the Ras cycle, farnesylation remains in place throughout and the cycle is driven solely by the changing palmitoylation status. The palmitate addition stabilizes the association of H/N-Ras with the membrane, facilitating its vesicular transport from the Golgi to the PM, whereas palmitate removal allows H/N-Ras release from the PM and return to the Golgi (see Poster, panel 1).
Giα and Fyn, which also undergo palmitoylation-driven Golgi–PM cycling, are lipidated at their N termini by both myristoylation and palmitoylation (see Poster, panel 4). Like prenylation, myristoylation is a static modification, whereas palmitoylation is reversible and dynamic, facilitating Golgi–PM cycling similar to that seen for H/N-Ras (Rocks et al., 2010; Rocks et al., 2005). Other proteins showing palmitoyl cycling include the two neuronal proteins PSD95, which functions to scaffold glutamate receptors at post-synaptic membranes, and GAP43, which functions in axonal growth cone extension. These two proteins differ from the other examples discussed above (i.e. H/N-Ras, Giα and Fyn) in that they are neither prenylated nor myristoylated, but instead rely solely on palmitoylation at multiple cysteines for their membrane tethering (see Poster, panel 4) (Fukata and Fukata, 2010).
Palmitoylation enzymes – the DHHC PATs
Palmitoylation occurs in a wide variety of sequence contexts, within both soluble and transmembrane proteins, thus complicating the identification of consensus sequences. This large substrate diversity is accommodated by a large array of palmitoylation enzymes, the recently identified family of DHHC protein acyl-transferases (PATs) (Fukata et al., 2004; Huang et al., 2004; Lobo et al., 2002; Roth et al., 2002; Roth et al., 2006). Mammals express 23 distinct DHHC PATs, which together mediate the bulk of protein palmitoylation within the cell. Unlike the prenyl- and myristoyl-transferases, which are soluble cytoplasmic enzymes, the DHHC PATs are multi-pass transmembrane proteins with four to six predicted transmembrane domains (TMDs). Their defining sequence element – the 50-residue, zinc-finger-like DHHC cysteine-rich domain, which is thought to comprise the core of the active site – is displayed within a cytosolic loop domain (see Poster, panel 1). The DHHC PATs localize mainly to endomembrane compartments (ER, Golgi, endosomes), but are also found at the PM (Ohno et al., 2006).
Depalmitoylation enzymes – APT1
Only one enzyme has so far been definitively shown to mediate protein depalmitoylation in vivo – acyl-protein thioesterase-1 (APT1). APT1 was initially identified by its in vitro depalmitoylation activity towards the two palmitoylated substrates Giα and H-Ras (Duncan and Gilman, 1998). Later work expanded the list of in vitro APT1 substrates; however, compelling support for an in vivo role for APT1 in depalmitoylation was lacking until fairly recently. First, an investigation of neuronal micro RNAs (miRNAs) found that control of hippocampal dendritic spine morphogenesis is mediated by miRNA-138 downregulation of mRNA encoding APT1, with APT1 being shown, in turn, to act upon palmitoylated Gα13 (Siegel et al., 2009). Subsequently, Dekker et al. developed a new APT1-specific inhibitor, termed palmostatin-B, which they used to interrupt the Ras acylation cycle, thereby demonstrating that APT1 mediates in vivo release of H/N-Ras from the PM (Dekker et al., 2010). Finally, Rocks et al. demonstrated depalmitoylation activity to be widely distributed throughout the cell, which they suggest provides a means for redirecting palmitoylated proteins that might be mislocalized to different cellular compartments back into the Golgi–PM cycling loop (Rocks et al., 2010). Interestingly, a recent proteomic analysis of cellular palmitoylation suggests that APT1 itself is likely to be palmitoylated, perhaps providing a means for it to access its membrane-localized substrates (Yang et al., 2010).
In addition to APT1, mammals express two other APT1 homologs: APT2 (66% identify to APT1) and APTL1 (33% identity). More distant APT1 relatives are found within the large α/β-hydrolase family, which includes proteases, lipases and other proteins with poorly defined function. It will be interesting to see whether other depalmitoylation enzymes emerge from among these related proteins.
Dynamic palmitoylation at the synapse
Palmitoylation might also prove to be an important regulator of synaptic plasticity, a process whereby altered synaptic activity results in changes in synaptic size, strength, morphology and protein content. These changes are exerted locally, with the protein content of individual synapses being altered both through local translation of dendritic mRNAs and through local regulation of membrane trafficking. Dendrites contain satellite elements of the neuronal endomembrane system (e.g. ER and Golgi outposts; see Poster, panel 2), which might participate in this localized trafficking at synapse level (Hanus and Ehlers, 2008).
Many synaptic proteins are palmitoylated, suggesting that palmitoylation and depalmitoylation play a major role in regulating protein traffic to and from synaptic membranes (Fukata and Fukata, 2010; Huang and El-Husseini, 2005; Kang et al., 2008). Examples of pre-synaptic palmitoylated proteins include the SNAREs and synaptotagmins, which regulate the synaptic vesicle fusion events that release neurotransmitter into the synaptic cleft. The collection of post-synaptic proteins that are known to be palmitoylated includes the receptors that receive and process the trans-synaptic signals (e.g. the transmembrane subunits of the AMPA- and NMDA-type ionotropic glutamate receptors), the downstream effector proteins (e.g. H/N-Ras and many other small GTPases), as well as the scaffolding proteins (e.g. PSD95, AKAP79 and SAP97) that coordinate the elements of this signaling apparatus at post-synaptic membranes.
A well-studied example of how palmitoylation and depalmitoylation might regulate synaptic plasticity is provided by PSD95, which scaffolds key signaling proteins, such as the AMPA- and NMDA-type glutamate receptors at the post-synaptic PM. Synapses that strengthen and enlarge retain more PSD95 and hence more glutamate receptors. PSD95 is N-terminally palmitoylated (see Poster, panel 4) and this palmitoylation is required for its membrane scaffolding function. PSD95 palmitoylation shows a constitutive rate of turnover that is enhanced by excitatory stimulation of the synapse (e.g. glutamate). Strong excitatory stimulation accelerates PSD95 depalmitoylation and reduces the amount of PSD95 at the post-synaptic membrane, thereby reducing synaptic strength (El-Husseini Ael et al., 2002). Lowering synaptic activity has the opposite consequence: PSD95 palmitoylation and localization to the post-synaptic PM are increased (Noritake et al., 2009). The increased PSD95 palmitoylation appears to be driven by local accumulation of the PSD95-specific PAT, ZDHHC2, which translocates into the dendritic spine upon synaptic activity blockade (Noritake et al., 2009). Thus, the palmitoylation-mediated regulation of PSD95 function might involve a Golgi–PM trafficking cycle similar to that of H/N-Ras, but, in this case, with PSD95 cycling between the post-synaptic density and local Golgi outposts (see Poster, panel 2). Although thus far little is known about Golgi outpost function, this localized membrane system might provide an efficient alternative to shuttling proteins back and forth between the synapse and the somatic Golgi compartment located in the neuronal cell body (see Poster, panel 2).
Another striking example of neuronal palmitoyl regulation is provided by the two splice variants of Cdc42. Cdc42 is a small GTPase that, together with its kindred Rho proteins (e.g. Rac1, RhoA), directs cellular and neuronal morphogenesis. The canonical form, Cdc42-pren, tethers to membranes through geranyl-geranylation of a C-terminal CaaX motif (see Poster, panel 3). However, brain-specific alternative splicing alters the ten C-terminal amino acids, thus exchanging the prenylation motif for a sequence that is instead palmitoylated, yielding a second isoform, Cdc42-palm. Although the prenylated and palmitoylated isoforms are both expressed in the developing neuron, Cdc42-palm is uniquely required for the extension of the dendritic filopodia, which develop into the dendritic spines, the post-synaptic half of the synapse (Kang et al., 2008) (see Poster, panel 3). Thus, this switch from stable prenylation to reversible palmitoylation has a large effect on synaptogenesis.
In addition to the soluble, hydrophilic proteins discussed above, for which palmitoylation and depalmitoylation serve to regulate membrane association, palmitoylation also has been documented for a large number of transmembrane proteins. For these proteins, which are embedded within the membrane bilayer through their hydrophobic transmembrane domains, palmitoylation might influence localization by directing lateral in-bilayer segregation into membrane microdomains. Alternatively, palmitoylation could change the tilt of transmembrane segments within the bilayer, thereby altering conformation. Are palmitoylated transmembrane proteins also regulated by reversible palmitoylation? Palmitoylation of the transmembrane subunits of post-synaptic AMPA- and NMDA-type ionotropic glutamate receptors has been suggested to regulate their trafficking between the post-synaptic PM and endomembrane compartments (Fukata and Fukata, 2010; Hayashi et al., 2009). It remains unclear, however, whether the palmitoylation of these multisubunit ligand-regulated ion channels is, in fact, reversible. Furthermore, for the nicotinic acetylcholine receptor, another neuronal ionotropic receptor, palmitoylation appears to be required at the level of the ER for the assembly of this multisubunit channel and its subsequent delivery to the PM (Drisdel et al., 2004). Indeed, a more general role for palmitoylation in ER export and quality control is suggested by the failed ER export of two other transmembrane proteins, yeast chitin synthase Chs3 and the mammalian Kv1.5 potassium channel, that accompanies inhibition of their palmitoylation (Lam et al., 2006; Zhang et al., 2007).
Outstanding questions
Although work during the past decade has resulted in major advances regarding both the enzymology and cell biology of protein palmitoylation and depalmitoylation, reversible palmitoylation has so far been documented for a surprisingly small number of proteins. Many central questions remain. For example, does the dynamic regulation of palmitoylation apply widely or just to a limited subset of highly regulated signaling proteins, such as H/N-Ras, Gα and PSD95? Are there other acyl-protein thioesterases beyond APT1? What factors regulate APT access to substrates? Finally, is palmitoyl cycling regulated at the level of the PAT or at the level of the APT, or both? With so many questions remaining, the next decade should be as fertile for discovery as the last.
Acknowledgments
We acknowledge financial support from the National Institutes of Health (GM65525 to N.G.D.) and from Canadian Institutes of Health Research (81156 to E.C.). Also, we thank Bill Green (University of Chicago) and David Lin (University of British Columbia) for their helpful comments. Deposited in PMC for release after 12 months.
References
- Dekker F. J., Rocks O., Vartak N., Menninger S., Hedberg C., Balamurugan R., Wetzel S., Renner S., Gerauer M., Scholermann B., et al. (2010). Small-molecule inhibition of APT1 affects Ras localization and signaling. Nat. Chem. Biol. 6, 449-456 [DOI] [PubMed] [Google Scholar]
- Drisdel R. C., Manzana E., Green W. N. (2004). The role of palmitoylation in functional expression of nicotinic alpha7 receptors. J. Neurosci. 24, 10502-10510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. A., Gilman A. G. (1998). A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21(RAS). J. Biol. Chem. 273, 15830-15837 [DOI] [PubMed] [Google Scholar]
- El-Husseini Ael D., Schnell E., Dakoji S., Sweeney N., Zhou Q., Prange O., Gauthier-Campbell C., Aguilera-Moreno A., Nicoll R. A., Bredt D. S. (2002). Synaptic strength regulated by palmitate cycling on PSD-95. Cell 108, 849-863 [DOI] [PubMed] [Google Scholar]
- Fehrenbacher N., Bar-Sagi D., Philips M. (2009). Ras/MAPK signaling from endomembranes. Mol. Oncol. 3, 297-307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M., Fukata Y., Adesnik H., Nicoll R. A., Bredt D. S. (2004). Identification of PSD-95 palmitoylating enzymes. Neuron 44, 987-996 [DOI] [PubMed] [Google Scholar]
- Fukata Y., Fukata M. (2010). Protein palmitoylation in neuronal development and synaptic plasticity. Nat. Rev. Neurosci. 11, 161-175 [DOI] [PubMed] [Google Scholar]
- Hanus C., Ehlers M. D. (2008). Secretory outposts for the local processing of membrane cargo in neuronal dendrites. Traffic 9, 1437-1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Thomas G. M., Huganir R. L. (2009). Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron 64, 213-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K., El-Husseini A. (2005). Modulation of neuronal protein trafficking and function by palmitoylation. Curr. Opin. Neurobiol. 15, 527-535 [DOI] [PubMed] [Google Scholar]
- Huang K., Yanai A., Kang R., Arstikaitis P., Singaraja R. R., Metzler M., Mullard A., Haigh B., Gauthier-Campbell C., Gutekunst C. A., et al. (2004). Huntingtin-interacting protein HIP14 is a palmitoyl transferase involved in palmitoylation and trafficking of multiple neuronal proteins. Neuron 44, 977-986 [DOI] [PubMed] [Google Scholar]
- Kang R., Wan J., Arstikaitis P., Takahashi H., Huang K., Bailey A. O., Thompson J. X., Roth A. F., Drisdel R. C., Mastro R., et al. (2008). Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature 456, 904-909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam K. K., Davey M., Sun B., Roth A. F., Davis N. G., Conibear E. (2006). Palmitoylation by the DHHC protein Pfa4 regulates the ER exit of Chs3. J. Cell Biol. 174, 19-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo S., Greentree W. K., Linder M. E., Deschenes R. J. (2002). Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 277, 41268-41273 [DOI] [PubMed] [Google Scholar]
- Nadolski M. J., Linder M. E. (2007). Protein lipidation. FEBS J. 274, 5202-5210 [DOI] [PubMed] [Google Scholar]
- Noritake J., Fukata Y., Iwanaga T., Hosomi N., Tsutsumi R., Matsuda N., Tani H., Iwanari H., Mochizuki Y., Kodama T., et al. (2009). Mobile DHHC palmitoylating enzyme mediates activity-sensitive synaptic targeting of PSD-95. J. Cell Biol. 186, 147-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y., Kihara A., Sano T., Igarashi Y. (2006). Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim. Biophys. Acta 1761, 474-483 [DOI] [PubMed] [Google Scholar]
- Planey S. L., Zacharias D. A. (2009). Palmitoyl acyltransferases, their substrates, and novel assays to connect them (Review). Mol. Membr. Biol. 26, 14-31 [DOI] [PubMed] [Google Scholar]
- Rocks O., Peyker A., Kahms M., Verveer P. J., Koerner C., Lumbierres M., Kuhlmann J., Waldmann H., Wittinghofer A., Bastiaens P. I. (2005). An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science 307, 1746-1752 [DOI] [PubMed] [Google Scholar]
- Rocks O., Gerauer M., Vartak N., Koch S., Huang Z. P., Pechlivanis M., Kuhlmann J., Brunsveld L., Chandra A., Ellinger B., et al. (2010). The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell 141, 458-471 [DOI] [PubMed] [Google Scholar]
- Roth A. F., Feng Y., Chen L., Davis N. G. (2002). The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J. Cell Biol. 159, 23-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A. F., Wan J., Bailey A. O., Sun B., Kuchar J. A., Green W. N., Phinney B. S., Yates J. R., 3rd, Davis N. G. (2006). Global analysis of protein palmitoylation in yeast. Cell 125, 1003-1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahinian S., Silvius J. R. (1995). Doubly-lipid-modified protein sequence motifs exhibit long-lived anchorage to lipid bilayer membranes. Biochemistry 34, 3813-3822 [DOI] [PubMed] [Google Scholar]
- Siegel G., Obernosterer G., Fiore R., Oehmen M., Bicker S., Christensen M., Khudayberdiev S., Leuschner P. F., Busch C. J., Kane C., et al. (2009). A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat. Cell Biol. 11, 705-716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Di Vizio D., Kirchner M., Steen H., Freeman M. R. (2010). Proteome scale characterization of human S-acylated proteins in lipid raft-enriched and non-raft membranes. Mol. Cell Proteomics 9, 54-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman R., Jackson C. S., Magee A. I. (2009). Protein acyl thioesterases (Review). Mol. Membr. Biol. 26, 32-41 [DOI] [PubMed] [Google Scholar]
- Zhang L., Foster K., Li Q., Martens J. R. (2007). S-acylation regulates Kv1.5 channel surface expression. Am. J. Physiol. Cell Physiol. 293, C152-C161 [DOI] [PubMed] [Google Scholar]