Abstract
The PI3K-Akt pathway is dysregulated in the majority of solid tumors. Pharmacological inhibition of Akt is a promising strategy for treating tumors resistant to growth factor receptor antagonists due to mutations in PI3K or PTEN. We have developed allosteric, isozyme-specific inhibitors of Akt activity and activation, as well as ex vivo kinase assays to measure inhibition of individual Akt isozymes in tissues. Here we describe the relationship between PK, Akt inhibition, hyperglycemia and tumor efficacy for a selective inhibitor of Akt1 and Akt2 (AKTi). In nude mice, AKTi treatment caused transient insulin resistance and reversible, dose-dependent hyperglycemia and hyperinsulinemia. Akt1 and Akt2 phosphorylation was inhibited in mouse lung with EC50 values of 1.6 and 7 μM, respectively, and with similar potency in other tissues and xenograft tumors. Weekly subcutaneous dosing of AKTi resulted in dose-dependent inhibition of LNCaP prostate cancer xenografts, an AR-dependent tumor with PTEN deletion and constitutively activated Akt. Complete tumor growth inhibition was achieved at 200 mpk, a dose that maintained inhibition of Akt1 and Akt2 of greater than 80% and 50%, respectively, for at least 12 hours in xenograft tumor and mouse lung. Hyperglycemia could be controlled by reducing Cmax, while maintaining efficacy in the LNCaP model, but not by insulin administration. AKTi treatment was well tolerated, without weight loss or gross toxicities. These studies supported the rationale for clinical development of allosteric Akt inhibitors and provide the basis for further refining of pharmacokinetic properties and dosing regimens of this class of inhibitors.
Keywords: Akt, inhibitor, isozyme-specific, pharmacodynamics, hyperglycemia
Introduction
The PI3K signaling pathway is dysregulated in the majority of human tumors (Cantley P1). Constitutive signaling can result from growth factor receptor (GFR) amplification or mutation, from activating mutations in the p110 α subunit of PI3K, from mutation or deletion of PTEN, or from Akt amplification (see refs. 2–5), (Carpten JD et al.6). The PI3K signaling cascade regulates cellular and tissue homeostasis, including cell growth and proliferation and glucose homeostasis (Cantley LC1, Cohen P7). Of particular relevance for tumorigenesis and cancer treatment strategies, the PI3K pathway transduces survival signals when the cell undergoes stress, whether intrinsic (genotypic) or extrinsic (hypoxia, cytotoxic drugs). Dysregulation of the PI3K pathway downstream of GFRs, such as EGFR, Her2 or IGF-1R, correlates with resistance to GFR antagonists in the clinic and in preclinical models. Similarly, overexpression or activation of GFR that bind to and activate PI3K, such as Her3, can cause resistance to antagonists of EGFR (Engelman, JA et al8).
Akt protein kinase, also known as protein kinase B, is an essential mediator of PI3K signaling. Constitutively activated Akt1 is transforming and Akt knock-down reduces tumor frequency and occurrence in preclinical models (Maroulakou, IG et al.9, Majumder, PK et al.10). Akt protein kinase is a small family of three highly related kinases. Despite their high homology, the three isozymes have distinct physiological functions (Yang, ZZ et al.11). The predominant phenotypes of mouse knock-outs are reduced body size and cell size for Akt1, a diabetic phenotype with type-2-like insulin resistance for Akt2 (Garofalo, RS et al.12) and reduced brain size for Akt3 (Easton, RM et al.13). Akt1+/−/Akt2null mice have an even more pronounced diabetic phenotype, while double null embryos do not survive (Peng, Xd et al.14). To what extent the differences among isozymes are the result of, such as different downstream effectors or of tissue-specific expression are not clear. Gene expression profiling shows that expression of Akt1 and Akt2 is ubiquitous, while that of Akt3 is more restricted, primarily to tissues of neuroectoderm origin. Specific isoform stimulation has been investigated in rat adipocytes and hepatocytes (Walker KD et al.15); however, no comprehensive data on Akt kinase activity in different tissues in vivo have been reported to date.
The contribution of individual Akt isozymes to human tumorigenesis remains to be defined. Akt2 and, to a lesser extent, Akt1 are amplified in human tumors at low frequency (Yuan ZQ et al.16) and mutations have been identified in Akt1 (Carpten JD et al.6). Akt3 appears to play an important role in melanomas (Robertson GP et al.17) and possibly glioblastoma, consistent with the predominant expression in cells of neuro-ectoderm origin and the mouse knock-out phenotype. Mouse tumor models indicate a role for Akt1 and Akt2 in tumor initiation and maintenance. Crosses of Akt1 knock-out mice with PTEN+/− mice (Chen ML et al.18), with v-H-ras mice (Skeen JE et al.19), or with MMTV-ErbB2 mice (Maroulakou IG et al.9) show delayed and reduced levels of tumor initiation. No corresponding crosses with two or more Akt isozyme knock-down have been reported. Because of the overlapping expression, if not function, of Akt isozymes and the embryonically lethal phenotype of Akt1/Akt2 double knock-outs, it is not clear what spectrum of Akt isozyme inhibition will result in maximal efficacy with acceptable toxicity.
Because of its central role in the PI3K pathway, Akt has been the target of intensive drug discovery efforts for several years (Hennessy BT et al.20, Collins I et al.21). The development of specific Akt inhibitors posed a challenge because of the high homology of the three Akt isozymes with each other and with members of the AGC family of protein kinases (Reuveni H et al.22). In particular, the development of specific ATP-competitive inhibitors has proven challenging (Zhu GD et al.23). To date, all reported ATP-competitive inhibitors are pan-Akt inhibitors, as expected based on the conserved active sites of the three isozymes. We have previously reported the identification of allosteric Akt inhibitors that are not ATP-competitive and depend on the pleckstrin-homology (PH) domain for binding (Lindsley CW et al.24). These allosteric inhibitors function by blocking the kinase activity of Akt in vitro and by preventing phosphorylation and activation of Akt by PDK1 and mTORC2 in cells. In contrast to ATP-competitive inhibitors, these allosteric inhibitors provide an opportunity for manipulating the isozyme profile (Lindsley CW et al.25) and for optimizing or tailoring the profile for maximal therapeutic index of different tumor types. MK-2206, a compound from this class of Akt inhibitors, has recently entered clinical development (Tolcher AW et al.26).
In this report we describe the pharmacokinetic and pharmacodynamic properties of a selective, allosteric inhibitor of Akt1 and 2 (AKTi), previously shown to be effective in xenograft tumor models with dysregulated Akt signaling (see refs 27–28). We show that as a result of the allosteric mechanism, inhibition of individual Akt isozymes and of downstream signaling can be achieved without the concomitant hyperphosphorylation of Akt seen with ATP-competitive compounds. Using multiple dosing schedules we establish the correlation between the pharmacokinetic properties of the inhibitor, the inhibition of individual Akt isozymes in multiple tissues and the impact on glucose homeostasis. We furthermore show that complete tumor growth inhibition in the LNCaP xenograft model can be achieved at well-tolerated doses associated with reversible hyperglycemia. The data presented provide novel insights into Akt signaling by correlating the pharmacokinetic and pharmacodynamic profile of an allosteric Akt inhibitor with tumor efficacy and mechanism-based impact on glucose homeostasis and supported the development of clinical candidate MK-2206.
Materials and Methods
Reagents
Antibodies were obtained from the following suppliers: pAkt T308 (cat. # 9275), Akt, β-actin (cat. # 4967), pTSC2 T1462 (cat. # 3611), pS6 protein (cat.#2212) from Cell Signaling; cleaved PARP from BD Pharmingen ; GAPDH from RDI; and total TSC2 from Santa Cruz Biotech (cat # sc-893). Immunoblot analysis was performed as previously described (Defeo-Jones D et al.29). Rabbit and sheep polyclonal antibodies for isozyme-specific immuno-precipitation of Akt1, 2 and 3 were raised against the hinge regions of Akt. Human Akt1 (amino acids 113–151), human Akt2 (amino acids 102–153), human Akt3 (amino acids 101–152), and mouse Akt2 (amino acids 102–153) were cloned into pGEX-4T. GST-fusion proteins were expressed in E. coli and purified over glutathione-agarose following manufacturer's instructions. Antibodies were affinity purified by sequential chromatography over glutathione agarose with immobilized GST-Akt or GST, and were validated for use in immunoprecipitation kinase assays. Using recombinant full-length Akt proteins with affinity tags, generated as described previously (Barnett SF et al30), each antibody was shown (i) to be strictly specific for the respective human or mouse Akt isozyme, (ii) to immuno-precipitate equal amounts of Akt isozymes as judged by Western, and (iii) not to interfere with kinase activity when Akt was preincubated with excess antibody prior to in vitro kinase assays.
Recombinant insulin was obtained from Invitrogen. Naphthyridinone AKTi was synthesized and characterized as described (Bilodeau MT et al28). ATP-competitive Akt inhibitor A443654 (Luo Y et al31) was synthesized in-house for benchmark studies.
Cell lines
LNCaP, C33a and mouse MMT060562 cell lines were obtained from ATCC and were maintained in RPMI + 10% FBS, α-MEM with 10% FBS and MEM with 10% FBS, respectively.
Kinase assays
Recombinant enzyme assays and AKTi IC50 determinations were performed using an HTRF assay as previously described (Barnett SF et al30). Inhibition of Akt isozymes in cell culture was measured in an immunoprecipitation assay as previously described (Defeo-Jones D et al.29). Briefly, cell lysates were prepared from cells treated with inhibitor for 4 hrs. Akt isozymes were immuno-captured with isoform-specific antibodies, washed thoroughly to remove contaminating kinases and residual AKTi, and assayed in an HTRF kinase assay with peptide substrates.
Translocation assays
For Akt translocation assays C33a cells were seeded at 6,000 cells on chamber slides and treated with 5 μM AKTi for 4 hrs. Cells were fixed in 4% paraformaldehyde for 15 min at room temperature, followed by washing and blocking in 3% BSA, 0.1% Triton-X100 in Tris-buffered saline. Fixed cells were probed with rabbit anti-Akt 1 primary antibody (at 1:200) for 1 hr at room temperature, washed twice with PBS, and probed with Alexa Fluor 488-labeled rabbit secondary antibody at 1:1000 dilution (Molecular Probes).
GLUT4 translocation and surface expression was measured using HEK-IRS cells expressing c-myc-tagged GLUT4 as described (Liu F et al32). Briefly, cells were seeded into 96-well plates; serum starved overnight, then treated with either vehicle (DMSO) or 5 μM AKTi for 1 hr, followed by insulin stimulation for 30 min. Cells were fixed and stained with Europium-labeled anti-Myc antibody for quantitation of surface-expressed GLUT4.
PK/PD measurements
All PK/PD studies were conducted in Balb/c male nude mice except as indicated. For blood glucose analyses animals were tail-bled at the specified times and glucose levels were measured using a LifeScan™ glucose meter. For plasma insulin analyses whole blood was removed via cardiac puncture from animals at study termination. Blood was centrifuged at 1200 × g for 15 min, plasma was collected and insulin levels were measured following the manufacturer's protocol (Alpco Diagnostics). AKTi concentrations in blood were determined by LC-MS/MS (see Supplemental methods).
For analysis of Akt inhibition in mouse tissues and human tumor xenografts, tissues were harvested after blood collection and flash-frozen in liquid nitrogen. Tissues were homogenized at 4ºC in lysis buffer (30 mM Tris, 1% Triton X-100, 1 mM EDTA, 1 mM sodium pyrophosphate, 10 mM β-glycerol phosphate, 10 mM sodium fluoride, 0.5 mM sodium orthovanadate, 1 μM microcystin-LR, 1× protease cocktail inhibitor (Calbiochem)). Homogenates were centrifuged at 15,000 × g for 30 min at 4ºC and supernatants were collected and stored at −70ºC. Protein concentrations were determined using the Bio-Rad Dc assay (Bio-Rad Laboratories). Protein A and protein G ELISA plates (Pierce) were prepared for immuno-capture by coating with isoform-specific Akt antibodies at concentrations of 20 to 150 ng per well, using rabbit anti-hAkt1 and anti-hAkt3 antibodies for both xenograft and mouse tissues, rabbit anti-hAkt 2 antibody for xenografts, and sheep anti-mAkt2 antibody for rodent tissues. Rabbit IgG (Santa Cruz) and sheep IgG (Santa Cruz) were used for background controls. After incubation for 4 hr at 4ºC, plates were washed with buffered saline + 0.1% Tween20. Tissue homogenates were diluted (final buffer concentrations of 50 mM Hepes pH 7.5, 0.1% PEG, 100 nM EDTA, 100 nM EGTA, 2 mM β-glycerophosphate, 50 mM KCl, 150 μM ATP, 10 mM MgCl2, 5% glycerol, 1 mM DTT, protease inhibitor cocktail, 0.1% BSA) and added to antibody-coated plates at 2 to 50 μg per well. Following incubation overnight at 4ºC plates were washed and the kinase activity of captured Akt isozymes was measured in an HTRF assay as previously described (Defeo-Jones D et al.29).
Tumor efficacy studies
Balb/c male nude mice (Charles River) of 10–12 weeks of age were injected subcutaneously with 15×106 LNCaP cells in 80% matrigel (BD Biosciences) in 500-μl injection volumes. Once xenografts reached volumes of approximately 200 mm3, mice were randomized, with 10 animals per treatment group. AKTi was formulated for subcutaneous injection in 25% hydroxypropyl-beta-cyclodextrin, adjusted to pH 5.0, and was administered once weekly at doses ranging from 20 to 200 mpk per day. Animals were weighed and tumors were callipered once weekly. Tumor volumes were calculated using the equation V = 0.5 × L × W2. All studies were conducted under IACUC guidelines.
Results
Inhibition of Akt activity and activation in vitro
We have evaluated several series of allosteric Akt inhibitors for potency, cell activity and PK properties suitable for in vivo studies. A naphthyridinone with appropriate PK properties was selected for proof-of-concept studies (Figure S1, (Bilodeau MT et al28)). This compound, subsequently referred to as AKTi, is a potent and selective inhibitor of Akt, related in structure to previously described Akt antagonists (Barnett SF et al33). AKTi preferentially inhibits Akt1 and Akt2 over Akt3, with IC50 values of 3.5, 40 and 1900, respectively ((Bilodeau MT et al28), Table S1). Human and mouse isozymes are inhibited with similar potency, a prerequisite for interpretation of in vivo efficacy and tolerability data. Inhibition of kinase activity by compounds in this class is strictly dependent on the presence of the PH domain and is not competitive with ATP ((Barnett SF et al30), Table S1, and data not shown). The specificity of AKTi was evaluated against a panel of 147 unique kinases (Table S2). At an inhibitor concentration of 10 μM and ATP concentration between 10 and 100 μM, only seven kinases were inhibited by more than 50%, with inhibitory activities likely lower at physiological ATP concentrations. None of these kinases are components of the PI3K-Akt-mTOR signaling cascade. No correlation was found between kinase inhibition and either sequence homology in the kinase domain or presence of a PH domain (ROCK2 being the only PH domain-containing kinase in the counterscreen panel).
Activation of Akt involves membrane translocation and binding of second messenger PIP3, followed by phosphorylation of T308 in the activation loop by PDK1 and of S473 by mTORC2. As reported previously (Defeo-Jones et al29), allosteric Akt inhibitors not only block Akt kinase activity but also inhibit Akt activation by preventing phosphorylation of Akt on T308 and S473 in cells. To quantitate the inhibition of Akt phosphorylation by AKTi, C33a cervical carcinoma cells and MMT060562 mouse cells were treated with increasing drug concentrations, followed by immunoprecipitation of individual isozymes from cell lysates and determination of kinase activity with exogenously added peptide substrate. AKTi treatment of cells blocks activation of individual Akt isozymes with the same rank order of selectivity as seen in the enzyme assay, although with higher absolute EC50 values and increased specificity for Akt1 (Table S1).
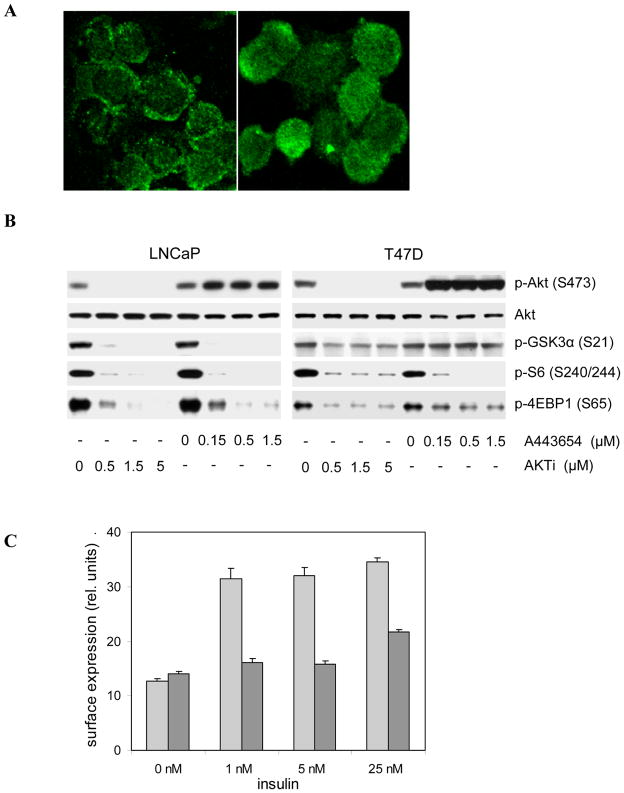
The mechanism of action of PH domain-dependent allosteric inhibitors is not entirely understood (Collins I et al21& Calleja V et al34). However, the enzyme and cell culture data are consistent with a model in which inhibitor binding induces a closed conformation that occludes binding sites for activating kinases PDK1 and mTORC2, and Akt substrates, and disrupts membrane localization (Figure S2). As shown in immunofluorescence studies with C33a cells, Akt localizes primarily to the plasma membrane as a consequence of high PIP3 levels in this PTEN-deficient cell line. AKTi treatment of C33a cells results in redistribution of Akt1 from the membrane to the cytoplasm (Figure 1A). In all tested cell lines, AKTi treatment results in a shift towards unphosphorylated Akt (Figure 1B; (She QB et al27). The inability of PDK1 and mTORC2 to phosphorylate Akt is likely a direct consequence of this relocalization. ATP-competitive Akt inhibitors are known to cause hyper-phosphorylation of Akt as a result of negative regulatory feed-back loops involving mTOR and p70 S6 kinase ( O'Reilly KE et al35 & Han EKH 36). The cytoplasmic relocalization of Akt following treatment with allosteric inhibitors prevents such feed-back phosphorylation. As shown in Figure 1B, allosteric inhibitors and ATP-competitive inhibitors, such as A-443654 (Luo Y et al31), are equally effective at blocking phosphorylation of S6 protein and 4EBP-1 in LNCaP and T47D cells; however, AKTi treatment does not induce hyper-phosphorylation of Akt.
Figure 1. In vitro mechanism of Akt inhibition by AKTi.
(A) AKTi treatment inhibits the translocation of endogenous Akt1 in vitro. C33a cells were treated with 5 μM AKTi (right panel) or vehicle (left panel) for 3 hrs. Akt1 was detected with an isozyme-specific antibody and visualized with an Alexa Fluor 488 secondary antibody. (B) Inhibition of Akt phosphorylation by allosteric inhibitor, AKTi and feed-back hyper-phosphorylation by ATP-competitive inhibitor, A443654. Western blot analyses of LNCaP and T47D cells treated with vehicle or the indicated concentrations of AKTi and A443654 for 3 hrs. (C) Inhibition of GLUT4 translocation by AKTi. HEK cells expressing c-myc-tagged GLUT4 were incubated with 5 μM AKTi (dark bars) or vehicle (light bars) for 1 hour, followed by stimulation with the indicated concentrations of insulin for 30 min. Surface expression of GLUT4 was measured with a Europium-labeled anti-Myc antibody. Representative data from one of two independent experiments are shown. Error bars represent standard deviations of triplicate values. P-values were ≤ 0.005 for AKTi vs vehicle control for all insulin-stimulated conditions (one-tailed unpaired t-test).
In addition to down-regulating translational activity via mTOR, Akt inhibition affects regulators of glucose metabolism and survival signaling. Insulin receptor activation has been shown to cause Akt2-dependent translocation of glucose transporter GLUT4 to the plasma membrane (Miinea CP et al37). AKTi treatment prevents the translocation and cell surface presentation of GLUT4 in HEK-IRS1 cells (Figure 1C). In C33a cells, transcription factor FKHR is retained in the cytoplasm as a consequence of constitutive phosphorylation by Akt and binding to 14-3-3 proteins. AKTi treatment of C33a cells expressing a GFP-FKHR fusion protein results in nearly complete nuclear translocation of FKHR (Supplemental Figure S3).
Correlation between PK and Akt inhibition in vivo
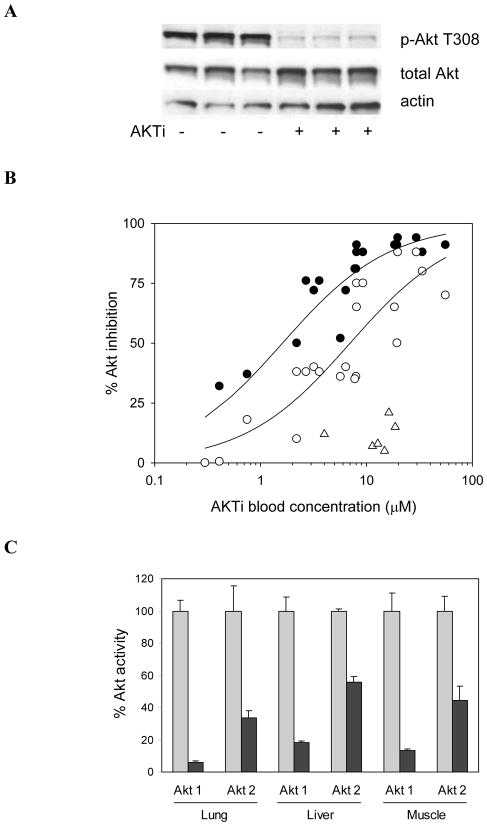
To evaluate the pharmacodynamic effect of allosteric Akt inhibitors in vivo, we analyzed the inhibition of Akt isozymes in multiple tissues in nude mice. Subcutaneous administration of AKTi resulted in peak plasma levels between 1 and 3 hours, depending on dose. A single dose of 50 mpk was sufficient to cause nearly complete inhibition of Akt phosphorylation (pAkt T308) in lungs of female mice harvested 3 hours after dosing (Figure 2A). To quantify the inhibition of individual Akt isozymes in mouse tissues, we immunoprecipitated Akt from tissue lysates with isozyme-specific antibodies and measured kinase activities ex vivo. From several studies in female and male mice treated with varying doses of AKTi we determined EC50 values for inhibition of Akt1, Akt2 and Akt3 in lung of 1.6±0.5 μM, 7±5 μM, and >30 μM, respectively (Figure 2B). The rank order of potency against individual mouse isozymes in vivo is consistent with the in vitro data (Table S1), though the ratio of EC50 values for Akt1 and Akt2 is approximately 8-fold smaller in vivo than in cell culture. The in vivo EC50 values are also substantially higher than those measured in cell culture, a shift in potency at least partly due to the low free fraction of AKTi in mouse blood (3%). Similar levels of inhibition were seen in lung, liver and muscle of treated animals for each isozyme (Figure 2C). Of note, the absolute kinase activities of individual Akt isozymes vary significantly between tissues (Table 1, basal activity). Akt1 and Akt3 are the most active isozymes in lung. Akt2 activity dominates in muscle and, to a lesser extent, in liver, consistent with the major role of Akt2 in regulating glucose metabolism.
Figure 2. Correlations between PK and pharmacodynamic effects in mouse tissues.
(A) Inhibition of Akt phosphorylation in lungs of female nude mice treated with 50 mpk AKTi for 3 hrs. The Western blot of lung lysates from three treated and vehicle control animals is representative of multiple independent experiments. (B) PK/PD correlation for Akt activity in lungs of nude mice treated with AKTi. Animals were administered single subcutaneous doses of AKTi, ranging from 25 to 200 mpk, and were sacrificed for determination of lung PD and AKTi blood levels within 2 to 3 hours after dosing. Akt isozyme activity was determined by IP-kinase assay of lung lysates and data were combined from several separate experiments including male and female mice. Closed circles, Akt1; open circles, Akt2; open triangles, Akt3. (C) Inhibition of Akt1 and Akt2 activity in lung, liver and muscle of male nude mice. Animals were administered a single subcutaneous dose of 100 mpk AKTi. Organs were harvested after 2 hours and Akt isozyme activity was measured by IP-kinase assay in homogenized tissues of treated (black bars) and control animals (gray bars) and was normalized against the corresponding control group. Values and error bars represent averages and standard deviation from 3 animals per group, respectively. P-values were 0.005 for all comparisons of vehicle vs treated groups (one-tailed unpaired t-test).
Table 1.
Stimulation of Akt isozyme activities by exogenous insulin in multiple tissues
| Insulin dose (μg)* | 0 | 1.0 | 5.0 | |
| Insulin, plasma (ng/ml)† | 0.7 ± 0.3 | 14 ± 5.2 | 77 ± 21 | |
| Akt kinase activity | % basal‡ | fold increase§ | ||
| Lung | Akt1 | 100 | 1.1 ± 0.2 | 1.3 ± 0.3 |
| Akt2 | 46 ± 12 | 1.1 ± 0.4 | 1.3 ± 0.3 | |
| Akt3 | 139 ± 36 | 1.2 ± 0.3 | 1.4 ± 0.3 | |
| Liver | Akt1 | 14 ± 5.9 | 5.7 ± 2.0 | 9.2 ± 3.3 |
| Akt2 | 31 ± 9.7 | 1.9 ± 0.7 | 2.5 ± 0.9 | |
| Akt3 | 11 ± 3.0 | 1.4 ± 0.6 | 1.6 ± 0.2 | |
| Muscle | Akt1 | 26 ± 6.1 | 2.4 ± 0.9 | 3.5 ± 0.5 |
| Akt2 | 315 ± 98 | 1.7 ± 0.7 | 2.4 ± 0.6 | |
| Akt3 | 10 ± 3.7 | 1.6 ± 0.8 | 1.9 ± 0.8 | |
Insulin dose per mouse, injected intraperitoneally
Insulin plasma levels 30 min after injection (average and standard deviation for 3 mice per group)
Percent Akt isozyme activity in vehicle-treated animals; normalized to Akt1 activity in lung lysate (mice sacrificed at 30 min; 3 animals per group; triplicate kinase assays per tissue lysate)
Fold increase in kinase activity in insulin-treated over vehicle-treated animals, measured 30 min after insulin injection
In vivo correlation of PK, Akt inhibition and glucose homeostasis
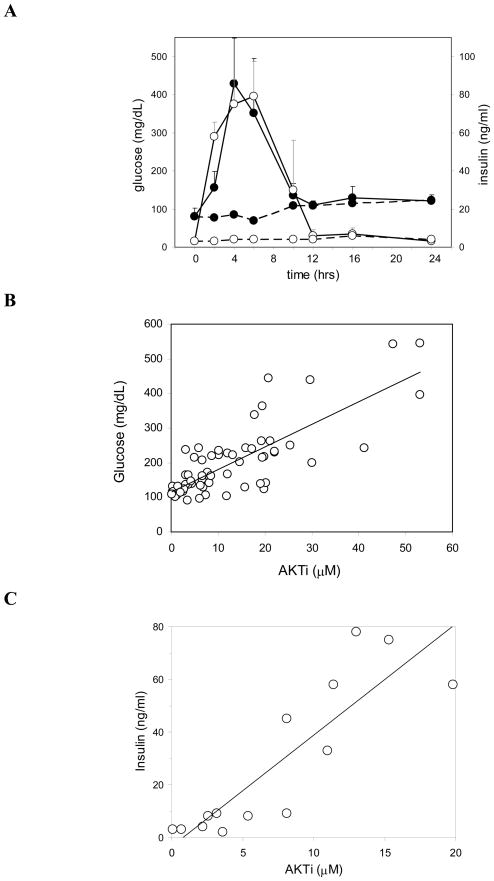
The PI3K-Akt pathway is a key regulator of glucose homeostasis and its inhibition is well known to cause hyperglycemia (Cohen P et al7). Analysis of glucose and insulin levels after single or multiple doses of AKTi showed dose-dependent, rapid and transient elevations of both serum glucose and insulin. Figure 3A shows a time course of blood glucose and insulin in female nude mice after a cumulative 150-mpk dose of AKTi. Hyperinsulinemia is pronounced, with a 40-fold increase over vehicle control animals (2 to 5 ng/ml), but fully reversible. Glucose levels reached 400 to 500 mg/dL, approximately a 5-fold increase over those in control animals. The dose-response relationship between pharmacokinetics (PK) and glucose and insulin in blood is shown in Figure 3, panels B and C, with data combined from several independent experiments. Glucose and insulin levels appear to be very sensitive to inhibition of Akt2, the isozyme believed to be primarily responsible for regulation of glucose homeostasis. As shown in Figure 3B, at blood concentrations of 10 μM AKTi (a level that results in approximately 50% inhibition of Akt2 in all tested tissues, see Figure 2C) blood glucose levels nearly doubled compared to vehicle controls (normal range of 80 to 120 mg/dL).
Figure 3. PK/glucose/insulin correlations.
(A) Glucose and insulin time course in female nude mice treated with 150 mpk AKTi. Compound or vehicle was administered subcutaneously as three consecutive doses of 50 mpk each at time 0, 3 and 6 hrs. Blood was collected from treated animals (solid lines) and vehicle controls (dashed lines) at the indicated time points for determination of blood glucose (filled circles) and serum insulin (open circles) levels. Values represent averages for three animals per group, with error bars denoting standard deviations for glucose (black) and insulin (grey) of AKTi treated groups. Data are representative for one of two similar experiments. (B) PK-glucose correlation and (C) PK-insulin correlation in male nude mice bearing LNCaP tumors. Glucose, insulin and blood PK measurements were taken between 1 and 24 hrs after subcutaneous administration of AKTi, at doses ranging from 20 to 200 mpk. Data were compiled from several independent experiments, with each data point representing an average from 3 animals.
Pharmaceutical Akt inhibition is predicted to induce transient insulin resistance. The finding of concomitant hyperglycemia and hyperinsulinemia is consistent with this expectation and raises the question, as to what extent hyperinsulinemia and saturation of insulin receptor signaling might counteract pathway inhibition by AKTi, particularly in insulin-responsive tissues. To better understand the effect of hyperinsulinemia on Akt activity in different tissues, we injected mice intraperitoneally with either 1 μg or 5 μg of insulin in the absence of AKTi treatment. Plasma insulin levels, measured 30 min after injection, increased by about 20 and 100-fold, respectively (Table 1). In lung, exogenous insulin had a minor impact on the activity level of all three Akt isozymes, causing a maximal increase of 40% for Akt3. Since insulin levels stayed below 100 ng/ml in all efficacy studies with AKTi, hyperinsulinemia has a minimal impact on pharmacodynamic measurements in lung in such studies. Akt activation in muscle was more significant, with Akt1 being most sensitive to insulin, followed by Akt2 and Akt3. As expected, the strongest activation of Akt was seen in liver, a highly insulin-responsive tissue. Akt1 showed the largest dynamic range in this tissue, with the lowest basal activity level and a nearly 10-fold induction. Based on studies with exogenous insulin, the potential of hyperinsulinemia to counteract pathway inhibition by Akt inhibitors thus appears greatest in liver, followed by muscle, and may explain the slightly less effective inhibition of Akt in these tissues (Figure 2C and data not shown). In the presence of Akt inhibitors, further sensitization to insulin may result from activation of mTOR-dependent feed-back loops and upregulation of receptors and signaling intermediates, a mechanism the current studies do not address.
Correlation between pathway inhibition and efficacy in LNCaP xenograft model
We have previously demonstrated that AKTi is effective as a single agent in several xenograft tumor models (She QB et al27 & Bilodeau et al28) when dosed daily or every other day. For the studies presented below we selected a xenograft model that would allow less frequent dosing, in keeping with the intermittent dosing expected to be used with chemotherapeutic combinations in the clinic. LNCaP prostate cancer cells lack PTEN, have elevated levels of phosphorylated Akt and are sensitive to combinations of allosteric Akt inhibitors and multiple cytotoxic agents (Defeo-Jones et al29). Exploratory efficacy studies with weekly dosing in the LNCaP xenograft model showed surprising single-agent activity at 100 mpk AKTi (Supplemental Figure S4). To approximate the pharmacokinetic profile of a prolonged single-day infusion we chose to administer the compound subcutaneously, split into four equal doses and given three hours apart. This dosing regimen resulted in blood levels with peak-to-trough variation of less than 10-fold for nearly 12 hours and was well tolerated, without any signs of gross toxicity. Animals in all treatment and vehicle groups did lose weight, the result of the cachexic nature of large LNCaP tumors (Figure S4, A and B). Pharmacodynamic analyses in lungs and tumors of animals sacrificed two hours after the last dose showed similar levels of Akt1 and Akt2 inhibition in both tissues, reaching >90% and ~ 50% inhibition of Akt1 and Akt2 in LNCaP tumors, respectively.
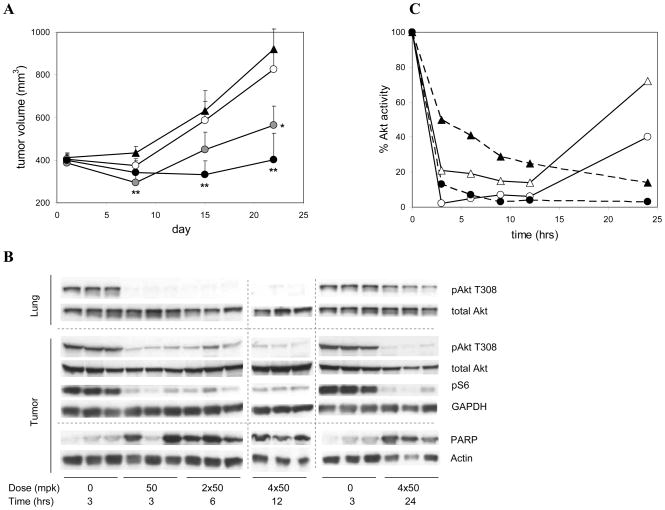
Cell culture studies with LNCaP cells had shown that induction of apoptosis by AKTi was time-dependent, requiring at least 12 hours of Akt inhibition for maximal stimulation of Trail ligand-mediated apoptosis (Defeo-Jones et al29) and data not shown). To test whether tumor efficacy and apoptosis induction in vivo also required a minimal duration of Akt inhibition, we varied the length of target exposure by varying the numbers of sequential doses of AKTi. LNCaP tumor-bearing animals were administered either one, two or four subcutaneous 50-mpk doses of AKTi, spaced at 3-hour intervals, on days 1, 8, 15 and 22. The 4× 50-mpk dosing regimen (200 mpk per day; once weekly) resulted in nearly complete tumor growth inhibition, with a trend toward regression at the earlier time points (Figure 4A). The animals in this group showed no signs of gross toxicity and maintained their body weight (−0.2±2.5 gm from day 1 to 22), while the control group lost significant body weight (−3.2±2.3 gm). The 2× 50-mpk dosing regimen was less efficacious but still resulted in significant tumor growth inhibition of > 70% at day 22. To monitor pathway inhibition and apoptosis in tissues, three animals in each treatment group were sacrificed 3 hours after the last dose on day one. Apoptosis induction in tumor, as measured by PARP cleavage, was nearly maximal already after a single 50-mpk dose (Figure 4B), suggesting that the increased efficacy of the multi-dose regimens was not due to a threshold for apoptosis triggering but to cumulative cytotoxic and/or cytostatic effects of Akt inhibition.
Figure 4. Correlation between tumor efficacy and duration of Akt inhibition in LNCaP xenograft tumors.
(A) Time course of LNCaP tumor growth in male nude mice treated with one, two, or four doses of 50 mpk AKTi per day, repeated once weekly for three weeks. Individual doses were administrated subcutaneously at three-hour intervals. Vehicle controls (triangles) and compound-treated groups (circles), with dosing regimens as follows: 1× 50 mpk (open circles), 2 × 50 mpk (grey circles), or 4 × 50 mpk (closed circles). Values and error bars are averages and SEM for 11 animals per group. Asterisks denote p-values of < 0.05 (*) and < 0.01 (**) versus vehicle group (one-tailed unpaired t-test). (B) Western blots of lung and tumor lysates probed for pAkt (T308), total Akt, pS6 protein, and cleaved PARP as indicated. Animals were dosed with one, two, or four doses of 50 mpk AKTi and were sacrificed 3 or 15 hours after the last dose. Results from three individual animals each are shown per time point. Vertically aligned bands are from single lysates. Dotted lines delineate blots from separate gels. GAPDH and actin controls shown for equal loading. Results shown are from a single experiment and are consistent with results from several experiments conducted under different but overlapping sets of conditions. (C) Time course of Akt1 and Akt2 inhibition in lung and tumor. Animals were dosed with 4 × 50 mpk AKTi at 3 hour intervals and sacrificed at the indicated time points. Activity of Akt1 (circles) and Akt2 (triangles) in tumor (closed symbols) and lung (open symbols) was measured by IP-kinase assay in homogenized tissues and normalized to vehicle-treated controls. Data represents averages of three animals per group.
Akt inhibition in lung, as measured by Western blotting of pAkt T308 levels, was already maximal after a single 50-mpk dose and remained suppressed through the subsequent 3 doses, but had nearly returned to base line at 24 hours (15 hours after the last dose; Figure 4B). In contrast, Akt inhibition in tumor was maintained for at least 15 hours after the last dose, as was inhibition of phosphorylation of downstream effector S6 protein (Figure 4B). A more detailed analysis of individual Akt isozymes in tissue lysates showed delayed onset of Akt1 and Akt2 inhibition in tumor but not lung, and confirmed the long-lasting inhibition in tumor (Figure 4C). Analyses of AKTi levels in lung and tumor tissues over time showed delayed drug clearance in tumor, but not lung, when compared to blood levels (data not shown), consistent with the prolonged pharmacodynamic effect in tumor.
PK profile determines hyperglycemia but not tumor efficacy
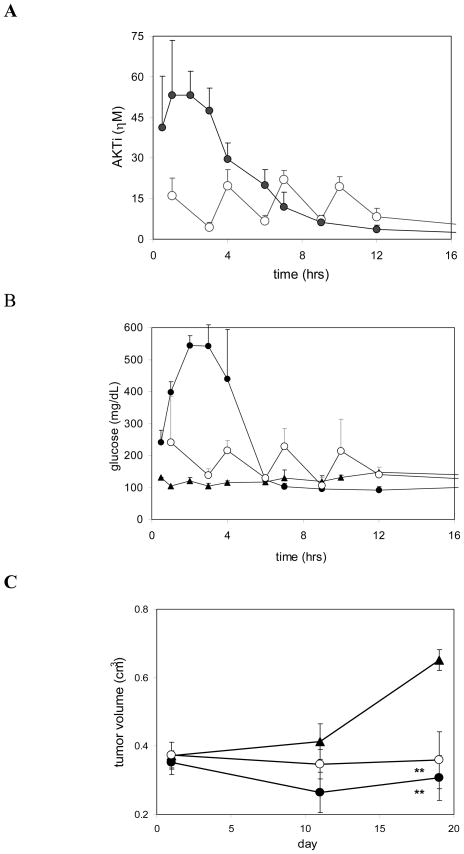
The above study, together with additional LNCaP tumor studies with weekly dosing regimens (not shown), demonstrated that complete tumor growth inhibition could be achieved when Akt signaling in the tumor was inhibited for at least 15 hours, with continuous inhibition of the individual isozymes of greater than 80% for Akt 1 and 50% for Akt2. These studies did not address the questions, however, if efficacy was driven by AUC or Cmax,, or if hyperglycemia would be tightly linked with efficacy To test whether a single subcutaneous bolus dose could be superior to a multi-dose regimen and to explore the feasibility of short IV infusion in the clinic, we compared efficacy and impact on glucose metabolism of a 200-mpk dose with a 4 × 50-mpk fractionated dose, both given once weekly for 3 weeks. The two dosing regimens resulted in distinct PK profiles (Figure 5A), with the expected higher Cmax (53 vs 22 μM) and higher exposure during the first 6 hours for the 200-mpk bolus dose, but with almost identical overall exposure (AUC(0–24) 230 vs 213 μM*hr). Blood glucose levels closely tracked with the variations in AKTi levels, both temporally and proportionate with drug concentrations (Figure 5B). Consequently, the 200-mpk bolus dose triggered pronounced, but transient hyperglycemia, while the 4× 50-mpk protocol resulted in more moderate glucose excursions. In contrast to blood glucose, tumor efficacy showed less dependence on the dosing protocol (Figure 5C). The bolus dose resulted in more pronounced regression of the LNCaP tumors (10% vs 5% at the end of week 3); however the efficacy difference between the two dosing protocols did not reach statistical significance. Akt1 and Akt2 inhibition in tumors at the 12-hour time point was comparable in the two treatment groups, with 89% and 40% inhibition for the 200-mpk dose and 94% and 57% for the 4× 50-mpk dose, respectively (not shown).
Figure 5. Correlation of PK and glucose levels.
AKTi concentrations (A) and glucose levels (B) in blood collected from treated animals and controls animals. LNCaP tumor bearing male nude mice were dosed once weekly with 200 mpk AKTi, administered either via a single subcutaneous injection or as four sequential 50-mpk injections at 3-hour intervals. Drug and glucose levels were measured during the third weekly dosing cycle. 200-mpk dose (filled circles); 4× 50-mpk dose (open circles); vehicle (diamonds). Values and error bars represent averages and standard deviations of at least 3 animals per group. (C) Time course of LNCaP tumor growth. Symbols as shown above. Values and error bars represent averages and SEM of 12 animals per group. Asterisks denote p-values of < 0.01 (**) versus the vehicle group (one-tailed unpaired t-test). Results shown are from a single experiment and are consistent with results from a total of six independent PK-efficacy studies conducted under different but overlapping sets of conditions.
Similar correlations between PK, PD and efficacy were observed in a total of six independent LNCaP tumor studies conducted with variations in dose fractionation, total doses per cycle and total number of treatment cycles (Figure 4A, supplemental Figure S4 and data not shown). In some studies with female mice, similar treatment regimens with body-weight adjusted doses triggered larger glucose excursions than seen in the male mouse / LNCaP model despite comparable PK (Fig. 3A and data not shown). Whether the apparent increase in drug sensitivity may be the result of gender-specific differences in drug biodistribution or physiological response remains to be determined.
In the LNCaP model hyperglycemia can be minimized via control of AKTi pharmacokinetics with little penalty to efficacy. However, because this approach may not be compatible with maximal efficacy for other tumors we explored two complementary approaches to glucose control. In wild-type and diabetic mice hyperglycemia during oral glucose tolerance tests can be blunted by fasting or by administration of exogenous insulin. Fasting of mice overnight was partly effective at controlling hyperglycemia caused by a 100-mpk dose of AKTi, lowering both Cmax and AUC approximately 2-fold (Supplemental Figure S5). In contrast, exogenous insulin administration was not expected to significantly control glucose levels in the setting of AKTi-induced, severe insulin resistance. Indeed, intraperitoneal administration of 40 μg of insulin resulted in plasma insulin levels 10 to 50-fold higher than those induced by AKTi treatment alone but had only a minor effect on glucose levels in animals treated with 100 mpk AKTi, while causing hypoglycemia in vehicle-treated animals as expected (Supplemental Figure S5 and data not shown). Together these data suggest that control of hyperglycemia during drug-induced insulin resistance, if needed in the clinic, may be more easily achieved via appropriate dosing or formulation of Akt inhibitors than via pharmacological intervention.
Discussion
Dysregulation and activation of the PI3K-Akt signaling pathway is common in a large fraction of most human tumor types (Cantley LC et al1). Over expression and mutations of growth factor receptors (GFR) contribute to PI3K-Akt activation in many tumor subtypes and have long been recognized as causative, early events in tumorigenesis (Vivanco I et al2). Antibodies and small molecule inhibitors targeting GFRs EGFR (Erbitux, Iressa, Tarceva) and HER2 (Herceptin) are successful in the clinic and highly effective in a subset of patients, and antagonists of several other GFRs are in clinical development. Retrospective analyses of refractory tumors have been correlated with activity of redundant GFRs, mutations in the targeted GFR and failure to inhibit Akt signaling (Hynes NE et al5). The high frequency of mutations in signaling components downstream of GFR, but upstream of Akt, such as activating mutations in the p110α subunit of PI3K and mutation or deletion of tumor suppressor PTEN (Vivanco I et al2 & Karakas B et al4) suggested that tumors refractory to GFR antagonists would remain sensitive to inhibitors of PI3K-Akt signaling.
Several Akt development programs have been reported (Collins I et al21 & Lindsley CW et al25) (see refs 38–40). Compounds in clinical development include Triciribine and Perifosine (Phase 2/3), two compounds that inhibit Akt phosphorylation via incompletely understood mechanisms, ATP-competitive inhibitors, and MK-2206 (Tolcher AW et al26), an allosteric inhibitor of the class described in this report.
The development of allosteric and specific inhibitors of Akt (Lindsley CW et al24 & Bilodeau MT et al28) for the first time provided the opportunity to address the dependence of tumors on PI3K-Akt signaling via pharmaceutical intervention. Due to the allosteric mechanism of action, the enzyme-inhibitor complex is not only catalytically inactive but is also not susceptible to feedback phosphorylation by PDK1 or mTORC2. As a result, AKTi treatment of cells does not trigger hyperphosphorylation of Akt but instead leads to rapid accumulation of unphosphorylated Akt and its relocalization from membrane to cytosol. This is a fundamental difference from ATP-competitive Akt inhibitors and mTOR inhibitors (see refs 35,36,39,41) which result in accumulation of the phosphorylated, membrane-bound form of Akt. Whether this will have therapeutic implications remains to be seen. However, the inhibition of Akt phosphorylation in response to these allosteric inhibitors provides a valuable pharmacodynamic marker of their efficacy. In addition, it provides a unique opportunity for quantitating the inhibition of the individual Akt isoforms in vivo despite the lack of isozyme-specific downstream markers.
The isozyme-specificity of AKTi in enzyme assays is maintained in cell culture and in vivo, though the absolute activity ratios vary. In vivo, AKTi is specific for Akt1 and Akt2, with no significant Akt3 inhibition observed at concentrations achieved in efficacy studies. To study the pharmacodynamics of AKTi, we measured the kinase activity of individual Akt isozymes in mouse lung by isozyme-specific immunoprecipitation from tissue lysates, followed by in vitro kinase assays. Subcutaneous administration of single rising doses of AKTi to nude mice allowed us to establish a firm PK/PD correlation for Akt1 and Akt2 in lung. Similar levels of Akt inhibition were seen in liver and muscle.
Akt2 and, to a lesser extent, Akt1 are known to play essential roles in insulin signaling and glucose homeostasis. Akt2 knock-out mice are glucose intolerant, show an insulin-resistance phenotype and develop type 2 diabetes with islet cell failure with age (Garofalo RS et al12). Similarly, Akt inhibitors have previously been shown to cause hyperglycemia in mice (see refs 31,40,42). As expected, AKTi treatment caused a significant but transient increase in glucose and insulin. At the highest achievable drug concentrations blood glucose increased as much as 7-fold over control. Hyperinsulinemia in response to AKTi treatment was even more pronounced, with greater than 100-fold increases over control after a 200-mpk dose. The exact cause-effect relationship between glucose and insulin during AKTi treatment remains to be elucidated. In insulin-responsive tissues, Akt2, with a possible contribution by Akt1, is an essential downstream effector of insulin receptor signaling. Akt2 activation has both acute and delayed effects on glucose uptake, for instance via phosphorylation of AS160, a Rab-GTPase activator required for translocation of the GLUT4 glucose transporter to the plasma membrane (Sano H et al43), and via transcriptional upregulation of GLUT1 expression. The inhibition of membrane translocation of GLUT4 observed in cell culture and the rapid inhibition of Akt in vivo suggest that AKTi-induced insulin resistance will rapidly lower glucose uptake in insulin-responsive tissues such as liver and adipose tissues. A concomitant rise in blood glucose, combined with the pharmacologically maintained insulin resistance, may then be responsible for the observed pronounced hyperinsulinemic response. However, several other observations suggest a more complex interplay between blood glucose and insulin. For one, insulin levels were much more sensitive to low AKTi concentrations than were glucose levels, often raising several fold in the absence of any measurable changes in glucose. This apparently glucose-independent insulin response suggested the possibility of a direct impact of Akt inhibition on pancreatic islet cells. Ex vivo treatment of isolated mouse islet cells with 5 μM AKTi had no effect on GLP-1 sensitive insulin secretion, however (data not shown). Time course studies of glucose and insulin dynamics could not establish a causal relationship between the two analytes but may have lacked the necessary resolution.
Secondly, AKTi treatment triggered significant hyperglycemia even in animals maintained in a fasted state, suggesting that a combination of gluconeogenesis and/or glycogenolysis was more likely responsible for the rising blood glucose. While simultaneous hyperinsulinemia and glucose generation would seem paradoxical, AKTi-mediated insulin resistance may well completely abrogate insulin action. As demonstrated by exogenous insulin administration, insulin receptor signaling cannot overcome the downstream blockade by AKTi. Finally, we found that prolonged Akt inhibition in mice can result in elevated levels of serum fatty acids (data not shown), indicative of an endocrine response to hypoglycemia, despite the manifest hyperglycemia. Taken together, these data suggest an alternative, or additional, mechanism underlying the dysregulated glucose homeostasis. Pharmacologically induced insulin resistance may generate intracellular glucose or energy deficits which, interpreted as a signal for low blood sugar, would trigger pancreatic insulin secretion and glucose generation by liver and adipose tissues.
Tumor efficacy studies with AKTi were conducted in the LNCaP prostate tumor xenograft model. To model intermittent clinical dosing regimens, such as those used with cytotoxic combination therapies, we limited the dosing frequency to once weekly but allowed multiple subcutaneous administrations per day to approximate target exposure expected from prolonged infusion or oral administration. Weekly dosing of AKTi as a single agent was unexpectedly efficacious in this model. LNCaP cells do not express detectable levels of Akt3 and may therefore be intrinsically more sensitive to the Akt1/2-selective inhibitor used here. However, we have demonstrated tumor efficacy with this AKTi in monotherapy in several breast cancer xenograft models (She QB et al27) as well as in ovarian A2780 xenografts that express all three isozymes (Bilodeau MT et al28). Thus the correlations described here should apply conceptually, if not quantitatively, to other models. Furthermore, Akt3 may not play as significant a role in tumorigenesis as Akt1 and 2, as suggested by the relative lack of clinical correlations for Akt3, the inability of the Akt3 knock-out, but not Akt1, to suppress tumorigenesis in the Neu mouse model (Maroulakou IG et al9), and the lack of detectable Akt3 expression in nearly half of the human tumor cell lines we evaluated for Akt isozyme expression by immunoblotting (data not shown).
The combined data from multiple studies with weekly dosing protocols in the LNCaP model show dose-dependent tumor growth inhibition, with complete growth inhibition reached at exposures that maintain 80% Akt1 and 50% Akt2 inhibition for at least 12 hours. These studies focused on the pharmacokinetics and pharmacodynamic effects therefore, we did not determine the maximum tolerated dose for this compound. The highest dose tested (200 mpk, once weekly) resulted in only transient minor tumor regressions. Higher doses or more frequent dosing may well improve efficacy further in this model. The interpretation of the relationship between pathway inhibition and efficacy is complicated by the delayed onset and the prolonged inhibition of Akt in tumor relative to lung. Based on limited pharmacokinetic analyses of AKTi and related compounds in lung and tumor tissues, the different pharmacodynamic profile in tumor appears to track with delayed drug clearance from tumor (not shown). The requirement for at least 12 hours of Akt inhibition, to achieve maximal efficacy at a given level of inhibition, does not seem to be due to a time-lag for apoptosis induction. Near-maximal PARP cleavage is detected in tumors of treated animals as soon as three hours after a single subcutaneous dose of AKTi and is maintained through the end of the study. In addition to apoptosis, cell cycle arrest is another possible outcome that may contribute to the efficacy of AKTi treatment. Cell cycle arrest in G1 is a prominent outcome of Akt inhibition in several breast cancer cell lines (She QB et al27) and prolonged Akt inhibition may well result in irreversible arrest in vivo.
The Akt-PI3K pathway transduces survival signals, impacts cell proliferation and regulates energy metabolism. The extent to which these mechanisms will contribute to the efficacy of Akt inhibitors in the clinic is unknown and may vary with tumor type, dosing regimen and drug combination. The mechanism-based impact on glucose metabolism and the potential for hyperglycemia to become a dose-limiting toxicity in the clinic have been longstanding concerns in the field. However, inhibition of glucose uptake may also prove to be a particularly effective approach against hypoxic and energy-starved tumors. Irrespective of whether glucose control will be necessary or desirable in a given clinical setting, the concerns about dose-limiting hyperglycemia and long-term impact on energy metabolism are ameliorated by three findings from the current proof-of-concept studies in nude mice: First, hyperglycemia and hyperinsulinemia are fully reversible, even after three weekly cycles. Secondly, intermittent as well as continuous treatment with this Akt1/2 inhibitor have minimal impact on body weight, with no signs of gross toxicity even at doses that cause severe hyperglycemia (this study and (She QB et al27)). The excellent tolerability and efficacy profile of the allosteric inhibitor described here may be due, in part, to its preferential inhibition of Akt1 over Akt2, the main mediator of insulin signaling. In this context it is also important to note that the particular compound used here is equipotent against human and mouse enzymes in vitro and, within the experimental limitations imposed by different serum and tumor clearance, inhibits Akt in mouse lung and human xenograft with equivalent potency. Lastly, glucose levels in mice correlate well with drug levels, allowing control of hyperglycemia via appropriate dosing regimens if needed. At least in the LNCaP xenograft model, peak drug exposure and hyperglycemia could be lowered without significant reduction in efficacy of a given dose. In some studies with female mice drug treatment triggered larger glucose increases than observed in male mice. The underlying cause for this possible gender-specific difference in drug sensitivity and the consequence, if any, for glucose control in tumor models in female mice, remains to be determined.
Control of acute hyperglycemia may be possible by fasting or pharmacological intervention. Some hypoglycemic agents have shown promise in preclinical studies with Akt pathway inhibitors (Meuillet EJ et al 42). On the other hand, insulin infusion, the standard treatment for acute hyperglycemia in the clinic, is unlikely to be effective in the context of Akt inhibition. Considering the pronounced insulin resistance phenotype induced by Akt inhibition, risk management during the initial clinical development will require exclusion of diabetic patients. Hyperglycemia may not become the dose-limiting toxicity for Akt inhibitors in the clinic (Tolcher AW et al26); whether it will become a predictor of response for Akt inhibitors remains an open and intriguing question.
Supplementary Material
Abbreviations List
- PI3K
phosphatidylinositol-3'-kinase
- PIP3
phosphatidylinositol-3,4,5-trisphosphate
- GFR
growth factor receptor
- PK
pharmacokinetics
- PD
pharmacodynamics
- GFP
green fluorescent protein
- FKHR
forkhead transcription factor
Reference List
- 1.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 2.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–01. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Simpson L, Takahashi M, Miliaresis C, Myers MP, Tonks N, et al. The PTEN/MMAC1 tumor suppressor induces cell death that is rescued by the AKT/protein kinase B oncogene. Cancer Res. 1998;58:5667–72. [PubMed] [Google Scholar]
- 4.Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 2006;94:455–9. doi: 10.1038/sj.bjc.6602970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 6.Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–44. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 7.Cohen P. The twentieth century struggle to decipher insulin signalling. Nat Rev Mol Cell Biol. 2006;7:867–73. doi: 10.1038/nrm2043. [DOI] [PubMed] [Google Scholar]
- 8.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 9.Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 2007;67:167–77. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- 10.Majumder PK, Yeh JJ, George DJ, Febbo PG, Kum J, Xue Q, et al. Prostate intraepithelial neoplasia induced by prostate restricted Akt activation: the MPAKT model. Proc Natl Acad Sci U S A. 2003;100:7841–46. doi: 10.1073/pnas.1232229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang ZZ, Tschopp O, Baudry A, Dummler B, Hynx D, Hemmings BA. Physiological functions of protein kinase B/Akt. Biochem Soc Trans. 2004;32:350–4. doi: 10.1042/bst0320350. [DOI] [PubMed] [Google Scholar]
- 12.Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197–08. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, et al. Role for Akt3/protein kinase B gamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–78. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng Xd, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–65. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker KS, Deak M, Paterson A, Hudson K, Cohen P, Alessi DR. Activation of protein kinase B beta and gamma isoforms by insulin in vivo and by 3-phosphoinositide-dependent protein kinase-1 in vitro: comparison with protein kinase B alpha. Biochem J. 1998;331(Pt 1) doi: 10.1042/bj3310299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan ZQ, Sun M, Feldman RI, Wang G, Ma X, Jiang C, et al. Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene. 2000;19:2324–30. doi: 10.1038/sj.onc.1203598. [DOI] [PubMed] [Google Scholar]
- 17.Robertson GP. Functional and therapeutic significance of Akt deregulation in malignant melanoma. Cancer Metastasis Rev. 2005;24:273–85. doi: 10.1007/s10555-005-1577-9. [DOI] [PubMed] [Google Scholar]
- 18.Chen ML, Xu PZ, Peng Xd, Chen WS, Guzman G, Yang X, et al. The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/− mice. Genes Dev. 2006;20:1569–74. doi: 10.1101/gad.1395006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skeen JE, Bhaskar PT, Chen CC, Chen WS, Peng Xd, Nogueira V, et al. Akt deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Cancer Cell. 2006;10:269–80. doi: 10.1016/j.ccr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–04. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 21.Collins I. Targeted small-molecule inhibitors of protein kinase B as anticancer agents. Anticancer Agents Med Chem. 2009:9. doi: 10.2174/187152009787047734. [DOI] [PubMed] [Google Scholar]
- 22.Reuveni H, Livnah N, Geiger T, Klein S, Ohne O, Cohen I, et al. Toward a PKB inhibitor: modification of a selective PKA inhibitor by rational design. Biochemistry. 2002;41:10304–14. doi: 10.1021/bi0202530. [DOI] [PubMed] [Google Scholar]
- 23.Zhu GD, Gandhi VB, Gong J, Luo Y, Liu X, Shi Y, et al. Discovery and SAR of oxindole-pyridine-based protein kinase B/Akt inhibitors for treating cancers. Bioorg Med Chem Lett. 2006;16:3424–29. doi: 10.1016/j.bmcl.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Lindsley CW, Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, et al. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett. 2005;15:761–4. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Lindsley CW, Barnett SF, Yaroschak M, Bilodeau MT, Layton ME. Recent progress in the development of ATP-competitive and allosteric Akt kinase inhibitors. Curr Top Med Chem. 2007;7:1349–63. doi: 10.2174/156802607781696864. [DOI] [PubMed] [Google Scholar]
- 26.Tolcher AW, Yap TA, Fearen I, Taylor A, Carpenter C, Brunetto AT, et al. A phase I study of MK-2206, an oral potent allosteric Akt inhibitor, in patients with advanced solid tumor. J Clin Oncol. 2009;27 (15s):3503. [Google Scholar]
- 27.She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. Plos One. 2008;3(8):e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bilodeau MT, Balitza AE, Hoffman JM, Manley PJ, Barnett SF, Defeo-Jones D, et al. Allosteric inhibitors of Akt1 and Akt2: a naphthyridinone with efficacy in an A2780 tumor xenograft model. Bioorg Med Chem Lett. 2008;18:3178–82. doi: 10.1016/j.bmcl.2008.04.074. [DOI] [PubMed] [Google Scholar]
- 29.DeFeo-Jones D, Barnett SF, Fu S, Hancock PJ, Haskell KM, Leander KR, et al. Tumor cell sensitization to apoptotic stimuli by selective inhibition of specific Akt/PKB family members. Mol Cancer Ther. 2005;4:271–9. [PubMed] [Google Scholar]
- 30.Barnett SF, DeFeo-Jones D, Fu S, Hancock PJ, Haskell KM, Jones RE, et al. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem J. 2005;385:399–08. doi: 10.1042/BJ20041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo Y, Shoemaker AR, Liu X, Woods KW, Thomas SA, Jong Rd, et al. Potent and selective inhibitors of Akt kinases slow the progress of tumors in vivo. Mol Cancer Ther. 2005;4:977–86. doi: 10.1158/1535-7163.MCT-05-0005. [DOI] [PubMed] [Google Scholar]
- 32.Liu F, Dallas-Yang Q, Castriota G, Fischer P, Santini F, Ferrer M, et al. Development of a novel GLUT4 translocation assay for identifying potential novel therapeutic targets for insulin sensitization. Biochem J. 2009;418:413–420. doi: 10.1042/BJ20082051. [DOI] [PubMed] [Google Scholar]
- 33.Barnett SF, Bilodeau MT, Lindsley CW. The Akt/PKB family of protein kinases: a review of small molecule inhibitors and progress towards target validation. Curr Top Med Chem. 2005;5:109–25. doi: 10.2174/1568026053507714. [DOI] [PubMed] [Google Scholar]
- 34.Calleja V, Laguerre M, Parker PJ, Larijani B. Role of a novel PH-kinase domain interface in PKB/Akt regulation: structural mechanism for allosteric inhibition. Plos Biol. 2009:7. doi: 10.1371/journal.pbio.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han EKH, Leverson JD, McGonigal T, Shah OJ, Woods KW, Hunter T, et al. Akt inhibitor A-443654 induces rapid Akt Ser-473 phosphorylation independent of mTORC1 inhibition. Oncogene. 2007;26:5655–61. doi: 10.1038/sj.onc.1210343. [DOI] [PubMed] [Google Scholar]
- 37.Miinea CP, Sano H, Kane S, Sano E, Fukuda M, Peranen J, et al. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J. 2005;391:87–93. doi: 10.1042/BJ20050887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q. Recent progress in the discovery of Akt inhibitors as anticancer agents. Expert Opinion on Therapeutic Patents. 2007;17:1077–130. [Google Scholar]
- 39.Rhodes N, Heerding DA, Duckett DR, Eberwein DJ, Knick VB, Lansing TJ, et al. Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res. 2008;68(7):2366–74. doi: 10.1158/0008-5472.CAN-07-5783. [DOI] [PubMed] [Google Scholar]
- 40.Crouthamel MC, Kahana JA, Korenchuk S, Zhang SY, Sundaresan G, Eberwein DJ, et al. Mechanism and management of AKT inhibitor-induced hyperglycemia. Clin Cancer Res. 2009;15:217–225. doi: 10.1158/1078-0432.CCR-08-1253. [DOI] [PubMed] [Google Scholar]
- 41.Tamburini J, Chapuis N, Bardet V, Park S, Sujobert P, Lise Willems, et al. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood. 2008;111:379–82. doi: 10.1182/blood-2007-03-080796. [DOI] [PubMed] [Google Scholar]
- 42.Meuillet EJ, Ihle N, Baker AF, Gard JM, Stamper C, Williams R, et al. In vivo molecular pharmacology and antitumor activity of the targeted Akt inhibitor PX-316. Oncol Res. 2004;14:513–27. doi: 10.3727/0965040042380487. [DOI] [PubMed] [Google Scholar]
- 43.Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.