Abstract
Cardiomyopathies are common disorders resulting in heart failure; the most frequent form is dilated cardiomyopathy (DCM), which is characterized by dilatation of the left or both ventricles and impaired systolic function. DCM causes considerable morbidity and mortality, and is one of the major causes of sudden cardiac death. Although about one-third of patients are reported to have a genetic form of DCM, reported mutations explain only a minority of familial DCM. Moreover, the recessive neonatal isolated form of DCM has rarely been associated with a mutation. In this study, we present the association of a mutation in the SDHA gene with recessive neonatal isolated DCM in 15 patients of two large consanguineous Bedouin families. The cardiomyopathy is presumably caused by the significant tissue-specific reduction in SDH enzymatic activity in the heart muscle, whereas substantial activity is retained in the skeletal muscle and lymphoblastoid cells. Notably, the same mutation was previously reported to cause a multisystemic failure leading to neonatal death and Leigh's syndrome. This study contributes to the molecular characterization of a severe form of neonatal cardiomyopathy and highlights extreme phenotypic variability resulting from a specific missense mutation in a nuclear gene encoding a protein of the mitochondrial respiratory chain.
Keywords: neonatal isolated cardiomyopathy, recessive inheritance, SDHA, mutation
Introduction
Cardiomyopathies are the most common disorders resulting in heart failure. Dilated cardiomyopathy (DCM; MIM 115200) is characterized by cardiac dilatation and reduced systolic function. DCM is the most frequent form of cardiomyopathy and the major cause of cardiac transplantation in children, accounting for >50% of all cardiac transplantations performed in patients between 1 and 10 years of age. Many factors may contribute to the development of this disorder, although most commonly the etiology is unknown. A heritable pattern is present in 20–30% of the cases;1 however, reported mutations explain only a minority of familial DCM.2 Although diverse modes of inheritance have been demonstrated, most familial DCM pedigrees show an autosomal dominant pattern of inheritance, usually presenting in the second or third decade of life. DCM with recessive inheritance has been described five times and the affected genes identified in four of the cases: (1) CMD3a, a fatal congenital DCM reported in one family (OMIM 300069) (Online Mendelian Inheritance in Man (OMIM) http://www.ncbi.nlm.nih.gov/OMIM)), is caused by mutations in tafazin (the function of this gene is not known). Mutations in this gene also cause Barth's syndrome (OMIM 302060). (2) CMD1X (OMIM 611615) with or without mild proximal muscle weakness, caused by mutations in the gene encoding fukutin (FKTN). Defects in this gene also cause additional types of muscular dystrophies: the Fukuyama-type congenital muscular dystrophy, limb-girdle muscular dystrophy type 2M, and the Walker–Warburg syndrome. (3) CMD2A (OMIM 611880) was reported in one family; the disease appeared in the second decade of life and is caused by a mutation in the gene encoding the cardiac structural component troponin I (TNNI3). (4). A myopathy with fatal cardiomyopathy (OMIM 611705) reported in one family is caused by mutation in the gene encoding titin (TTN). Mitochondrial dysfunction frequently affects the heart and may cause both hypertrophic cardiomyopathy and DCM.3 Nuclear encoded genes affecting mitochondrial functions are known to cause DCM. For example, a rare genetic disorder of the fatty acid β-oxidation cycle caused by mutations in both alleles of the α-subunit (HADHA) of the mitochondrial trifunctional protein may result in a severe neonatal cardiomyopathy with hypoketotic hypoglycemia and hepatic encephalopathy, often progressing to coma and neonatal death.4, 5 Another example is the recent finding of a nonsense mutation in Coenzyme Q10, a mobile lipophilic electron carrier located in the inner mitochondrial membrane. The mutation results in multisystem disease, including cardiomyopathy.6
Succinate dehydrogenase (SDH, E.C. 1.3.5.1) deficiency is a rare condition in humans, representing 2% of mitochondrial respiratory chain (RC) disorders.7 Its clinical presentation is highly variable, ranging from early-onset encephalomyopathies to tumor susceptibility in adults.8, 9 SDH catalyzes the conversion of succinate to fumarate and is a component of the mitochondrial RC (complex II) and the Krebs cycle. SDH is composed of four subunits, all encoded in the nuclear DNA – two soluble proteins, the flavoprotein (Fp, SDHA), and the Fe-S protein (SDHB) – which are anchored to the inner membrane by subunits SDHC and SDHD.8, 9 Pathogenic mutations in the SDHA gene have rarely been documented in children, and all but one case have been reported in patients with Leigh's syndrome.7, 10, 11, 12 The single case not presenting with Leigh's syndrome describes death at infancy, before any sign of the syndrome could be detected, following a respiratory infection and severe hypoglycemia.13 A late-onset neurodegenerative disease with progressive optic atrophy, ataxia, and myopathy was tentatively ascribed to a heterozygous mutation in a conserved region of the SDHA protein.14 In this report, we present the association of a mutation in the SDHA gene with the clinical manifestation and interfamilial variability of 15 patients diagnosed with DCM.
Methods
Patients
The study was approved by the Soroka Medical Center Review Board. The patients' medical records were carefully reviewed, and details of their somatic growth, psychomotor development, clinical course, hospitalizations, and laboratory results were recorded. Their parents and siblings were interviewed and underwent a complete physical examination (particularly focused on the cardiac and neuromuscular systems). The patients' evaluation included: echocardiology: transthoracic two-dimensional and Doppler echocardiography performed using a System Vivid 7 echocardiograph (GE Medical Systems, Saskatchewan, Canada). Measurements of left ventricular (LV) end-diastolic dimension (LVED) and LV end-systolic dimension (LVES) were obtained in accordance with the recommendations of the American Society of Echocardiography.15 Fractional shortening (FS) was calculated as ((LVED−LVES)/LVED) × 100. Dimensions were corrected for age and body surface area according to the formula of Henry et al16: (LVED (percent predicted)=(measured LVED/predicted LVED) × 100; predicted LVED=(45.3 × body surface area (BSA)0.3)−(0.03 × Age)−7.2). LV abnormalities were classified as follows: DCM, LVED ≥117% predicted, and FS <25% in the absence of known causes of ventricular dilatation.16, 17
The method for assessing regurgitation of the mitral valve relies on measuring the jet length and diameter of the vena contracta (the narrowest area of the jet); the premise is that the greater the jet length and the wider the orifice diameter, the more severe the degree of regurgitation.18, 19, 20, 21 LV noncompaction (LVNC) diagnosis was determined according to the echocardiographic criteria described by Jenni et al:22, 23 ECG, chest X-ray, and serial echocardiography were performed every month, as well as blood lactate, pyruvate and carnitine, amino and organic acids, CPK, and troponin I. The activities of the mitochondrial RC complexes I–V were determined by spectrophotometric and polarographic methods as described previously.24, 25
Molecular analyses
DNA was prepared from peripheral blood. Whole genome search for linkage was performed using the Affymetrix (Santa Clara, CA, USA) GeneChip Human Mapping 250K Nsp or Sty Arrays containing ∼262 000 SNPs. The genotype calls were determined using Affymetrix GeneChip Genotyping Analysis Software (GTYPE) and dedicated software (KinSNP) developed in-house to automatically perform autozygosity analysis of the microarray results. VNTR analysis was performed according to the study by Parvari et al.26 RNA of lymphoblastoid cells was extracted using the EZ-RNA II kit from Biological Industries (Beit Haemeq, Israel) according to the manufacturer's instructions; cDNA was synthesized by the Reverse iT first-strand synthesis kit from ABgene (Epsom, UK) with an oligo d(T) primer. The SDHA cDNA was PCR amplified in three overlapping fragments that were directly sequenced on an AB373 apparatus, after digestion of the free PCR primers by a combination of shrimp alkaline phosphatase 0.6U and 6U Exonuclease I (both from Fermentas, Vilnius, Lithuania).
The mutation was identified on genomic DNA by direct sequencing of a PCR product using primers, forward: GTGCACACTGTTGGGCCCTT and reverse: GCCCCGTGACTCCTTCCGT, which amplify exon 13, the mutation changing the first nucleotide in this exon. The 3′ first nucleotide and two nucleotides of the forward and reverse primers, respectively, are unique to chromosome 5 and do not exist on the pseudogene SDHA gene on chromosome 3. Under the amplification conditions of 30 cycles of 94° 1′, 60° 1′, and 72° 1′, only the sequence of chromosome 5 is amplified. This was validated by BLAST analysis revealing 100% identity to chromosome 5 and the 11 nucleotides differences form the sequence of chromosome 3.
Results
Patients
In all, 15 Bedouin patients of one tribe who presented with cardiomyopathy, between the ages of 32 weeks in utero to 10 years of age, were enrolled. The laboratory indices were normal except for mildly increased lactate of 3.7 mmol/l. The electrocardiogram of all patients was sinus rhythm with LV hypertrophy and normal QTC interval. Cardiomyopathies were categorized according to World Health Organization cardiomyopathy classification.27 At presentation, all patients had normal growth (30th–50th percentile) and a normal neuromuscular examination, including muscle bulk and strength, reflexes, and gait. The psychomotor development was appropriate for age. During follow-up visits, the neuromuscular examinations remained normal and none of the patients had seizures. In two patients, a brain MRI was performed without any evidence of focal lesions in the basal ganglia or grey matter or cortex or brainstem, ruling out Leigh's syndrome. Eight infants were diagnosed with LVNC. The patients' characteristics are presented in Table 1. RC enzymes were assessed in the skeletal muscle of two patients and in the cardiac muscle of two patients from biopsy samples collected immediately post mortem. The results showed reduced activity of complex II in the muscle (50–60% residual activity) and marked reduction in activity in the cardiac muscle (15–18% residual activity) (Table 2). Partially decreased activity of complex I was found in patient A5. The possibility that this decrease is caused by a defect in iron–sulfur metabolism was excluded by the normal activity of aconitase measured in the muscle mitochondria (results not shown).
Table 1. Clinical data of all patients.
| Case | Age at onset (months) | Age at death (months) | Age alive (years) | Sex | Primary clinical features and follow-up (f/u) | Echo data | LV noncompaction (LVNC) | LV function FS% |
|---|---|---|---|---|---|---|---|---|
| A6 | 2 | 2 | F | Respiratory distress, CHF, cardiogenic shock | LV dilatation LVEDD – 39 mm Noncontracting LV | Noncompaction LV | <10 | |
| A7 | 8 | 14 months, psychomotor development adequate to age | M | Cardiomegaly in X-ray asymptomatic f/u – asymptomatic | LV dilatation LVEDD – 36 mm Mild LVH, mild MVI Mild LV dysfunction | 25 | ||
| A8 | 4 | 2 years, adequate psychomotor development to age | F | Respiratory distress f/u – frequently hospitalized due to CHF | LV dilatation LVEDD – 44 mm LV dysfunction Moderate LVH | 15–17 | ||
| A9 | 2 | 11 | M | Respiratory distress f/u – frequently hospitalized due to CHF | LV dilation LVEDD – 44 mm LV dysfunction Moderate MVI Moderate LVH | 12–15 | ||
| A10 | 3 | 5 | F | Respiratory distress, CHF | LV dilation LVEDD – 42 mm Noncontracting LV | 11–13 | ||
| A11 | 2 | 2 | F | Respiratory distress, CHF | LV dilation LVEDD – 37 mm Noncontracting LV | Noncompaction LV | <10 | |
| A12 | 5 | 7 years, normal school performance | M | Mild respiratory distress f/u – exercise intolerance | LV dilation LVEDD – 46 mm LV dysfunction Mild MVI Mild LV hyperthropy | 23–25 | ||
| A13 | 6 | 6 | M | Respiratory distress, CHF cardiogenic shock | LV dilatation LVEDD – 42 mm Noncontracting LV | Noncompaction LV | <10 | |
| B1 | 33 weeks of gestation | 2 | M | Respiratory distress, CHF cardiogenic shock | LV dilatation LVEDD – 36 mm Noncontraction LV | Noncompaction LV | <10 | |
| B2 | 1 | 8 years, normal school performance | F | Respiratory distress frequently hospitalized at 1 year of age, f/u – exercise intolerance | LV dilatation LVEDD – 50 mm LV dysfunction Mild MVI, mild LVH | 22–23 | ||
| B3 | 3 | 8 | F | Respiratory distress CHF | LV dilatation LVEDD – 44 mm Severe LV dysfunction | Noncompaction LV | 13 | |
| B4 | 32 weeks of gestation | 1 | F | Respiratory distress, CHF, sudden death at home | LV dilation LVEDD – 33 mm Noncontracting LV | Noncompaction LV | <10 | |
| B5 | 32 weeks of gestation | 14 months, walked at age 12 months | M | Respiratory distress f/u – frequently hospitalized due to CHF | LV dilatation LVEDD – 43 mm Mild MVI Moderate to severe LV dysfunction | Noncompaction LV | 12–18 | |
| C1 | 4 | 4 | M | Respiratory distress CHF, cardiogenic shock | LV dilatation LVEDD – 43 mm Noncontraction LV | <10 | ||
| D1 | 8 | 11 years, normal school performance | M | At presentation – respiratory distress f/u – exercise intolerance | LV dilatation LVEDD – 48 mm Mild LVH, mild MVI Mild LV dysfunction | 24–26 |
Abbreviations: CHF, congestive heart failure; FS, fractional shortening; LV, left ventricle; LVEDD, left ventricle end-diastolic diameter; LVH, left ventricle hypertrophy; MVI, mitral valve insufficiency.
LVEDD, LVH, and FS were calculated and severity was assessed according to the studies by Henry et al16, Richardson et al17, Sahn18, 19, 20, and Silverman and McElhinney21. MVI assessment and severity were assessed according to the studies by Sahn18, 19, 20 and Silverman and McElhinney21. Noncompaction LV was determined according to the studies by Jenni et al 22, 23.
Table 2. Activities of the mitochondrial respiratory chain complexes in the muscle and myocardium.
| Sample | ||||||
|---|---|---|---|---|---|---|
| Muscle mitochondria | Myocardium homogenate | |||||
| Assay | Control muscle mitochondria n=50 | B3 | A5 | Control myocardium n=3 | A8 | A5 |
| Citrate synthasea | 1990±370 | 1690 | 1800 | 251±164 | 454 | 233 |
| NADH-CoQ reductasea (complex I) | 241±92 | 149 (74%) | 110 (50%) | |||
| NADH-cytochrome c reductasea (complex I+III) | 586±234 | 413 (84%) | 335 (73%) | |||
| Succinate – cytochrome c reductasea (complex II+III) | 312±118 | 133 (51%) | 115 (64%) | 27±13 | 10 (21%) | 2 (8%) |
| Succinate-CoQ reductasea (complex II) | 72±26 | 30 (50%) | 39 (60%) | |||
| Succinate dehydrogenasea (complex II) | 260±86 | 118 (54%) | 131 (56%) | 48±25 | 13 (15%) | 8 (18%) |
| Ubiquinol-cytochrome c reductasea (complex III) | 4270±970 | 3800 (106%) | 3000 (99%) | |||
| Cytochrome c oxidasea (complex IV) | 1155±420 | 1135 (117%) | 1128 (108%) | 197±118 | 354 (100%) | 147 (81%) |
| Mg++ATPase (complex V)a | 536±193 | 828 (184%) | 675 (111%) | |||
| Pyruvate+malate oxidationb | 67±32 | 59 (105%) | ||||
| Succinate oxidationb | 94±36 | 20 (26%) | ||||
The assignment of patients is according to Figure 1.
Activities of the five mitochondrial respiratory chain complexes were measured spectrophotometrically in nmol/min per mg.
Activities of the five mitochondrial respiratory chain complexes were measured by oxygen consumption in nat O/min per mg protein.
Values in brackets present activities as a percentage of the control mean, normalized for citrate synthase activity.
Identification of the mutation on the SDHA gene
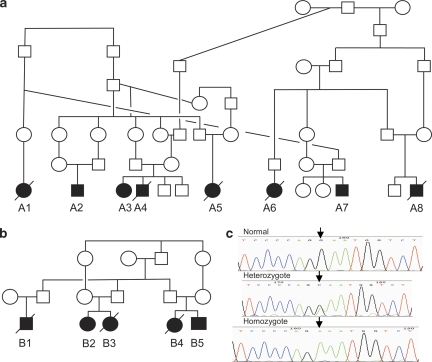
The families present a recessive pattern of inheritance. All patients belong to the same tribe and share a family name; 13 could be traced to two large families (Figures 1a and b); for 2 additional patients, family relations could not be established. As this disease is very rare and the families are consanguineous, we predicted that it is caused by homozygosity of the mutation inherited from a common founder. A whole genome search for linkage was performed on three of the patients of family B (B2, B4, and B5, see Figure 1) using the Affymetrix GeneChip Arrays containing ∼262 000 SNPs. Two chromosomal segments >4.0 cM were shared by the three patients analyzed. One of these segments (4.8 cM) was excluded by analysis of a VNTR in the interval as it showed heterozygosity in the patients. The other segment (5.6 cM) included the SDHA gene. Direct sequencing of this gene was carried out on cDNA derived from an established lymphoblastoid cell line of patient B5. The homozygous transition, c.1664G to A, was observed in the Fp cDNA resulting in the change of glycine at position 555 to glutamic acid (G555E). To confirm this finding, we further identified the mutation in genomic DNA by direct sequencing of a PCR product using amplification conditions that enable the amplification of the SDHA gene on chromosome 5, but not the SDHA pseudogene on chromosome 3 (Figure 1c). All patients and available family members (in total 34 individuals) were evaluated: all patients are homozygous for the G555E allele, all healthy siblings are either heterozygous or homozygous for the normal allele; all available parents of affected patients were heterozygous for the mutation with one exception. To our surprise, the father of patient A2 was homozygous for the mutation. He reported that three of his siblings had died at a young age due to cardiovascular failure; hence, none were available for verification of this mutation. He was clinically assessed and his medical records were pursued because of this finding, revealing no visits to the clinic in his childhood and no previous hospitalizations. His physical assessment was negative for any symptoms or other suspicious factors. In addition, his electrocardiogram and the echo study exhibited normal LV function and dimension. He was not amenable to a stress test, but notably, his occupation demands heavy physical work. To further elucidate this finding, we verified whether his SDHA gene is identical to that of the patients' gene by comparing their haplotypes. The analyses of three adjacent polymorphic microsatellite markers on both sides of the SDHA gene: D5S2488, D5S392, and a dinucleotide polymorphic repeat on the sequence acc. no. AC021087 and the SNP rs13070 adjacent to the mutation determined that the father is homozygous for the chromosome locus harboring the G555E mutation, similar to the patients (not shown). G555E was previously reported twice as a disease-causing mutation: in a patient with a lethal infantile presentation13 and in a patient with a relatively mild Leigh's syndrome.12
Figure 1.
Pedigrees of two families from the same tribe and the identification of the c.G1664A (G555E) mutation on genomic DNA. (a, b) Families. (c) Partial sequence chromatogram presenting the c.G1664A change causing G555E.
Both previous studies reporting G555E as the disease-causing mutation demonstrated a defect in the assembly of mitochondrial complex II.12, 13 Accordingly, we hypothesized that differences in amino acids of the other subunits of complex II, proposed to contact SDHA (SDHB and SDHD), may enable assembly of this complex in the father, thus explaining his normal heart evaluation. To test this hypothesis, we sequenced exons of the SDHB and SDHD genes from the father, his affected son (patient A2), and two severely affected patients (A5 and A8) but found no differences in the sequence in any of them. Thus, the possibility of a difference in complex assembly due to variations of amino acids of the subunits as the cause of the variability in phenotype was excluded. Recently, a candidate modifier gene, the first SDH assembly factor (SDHAF1), found to be mutated in infantile leukoencephalopathy with defective SDH activity was described by Ghezzi et al.28 This factor is highly expressed in the brain and heart; thus, we tested whether this gene differs between the unaffected homozygous father and affected patients by sequencing the protein coding region of the gene. We found no sequence differences between the father, his unaffected son, a severely affected patient (B4), and two less affected patients (A3 and B5). Finally, we verified the enzymatic activity of complex II in lymphoblastoid cells established from the father in comparison with his patient son, a mild patient (B5), the heterozygous mother, and four controls. The enzymatic activity of the father's complex was decreased by 42%, being more similar to that of the patients compared with the heterozygous mother and controls (Table 3). Thus, presently, we lack an explanation for the normal phenotype of the father.
Table 3. Activities of the mitochondrial respiratory chain in lymphoblastoid cells.
| Lymphoblastoid homogenate | |||||
|---|---|---|---|---|---|
| Assay | Controls n=4 | Patient B5 | Patient A7 | Father of Patient A2 | Mother of Patient A2 |
| Citrate synthasea | 64±9 | 72 | 50 | 76 | 57 |
| Succinate – cytochrome c reductasea (complex II+III) | 20±5 | 10 (45%) | 12 (77%) | 16 (67%) | 16 (90%) |
| Succinate dehydrogenasea (complex II) | 27±5 | 19 (63%) | 13 (62%) | 19 (60%) | 28 (117%) |
| Cytochrome c oxidasea (complex IV) | 86±6 | 105 (109%) | 69 (103%) | 79 (77%) | 71 (92%) |
Activities of mitochondrial respiratory chain complexes II–IV were measured spectrophotometrically. Values in brackets present activities as a percentage of the control mean, normalized for citrate synthase activity.
nmol/min per mg.
Discussion
We have described the cardiac features of an autosomal-recessive DCM associated with mutation G555E in the SDHA gene. This condition, unfortunately, is marked by high mortality, with two-thirds succumbing to cardiac failure. However, one case showed that homozygosity for the mutation shows nonpenetrance for the cardiomyopathy in spite of a reduction in the SDH mitochondrial activity in lymphoblast cells comparable with the reduction observed in other patients. Overall, the rate of death due to cardiac complications in our patients is higher than that reported for DCM in general,29 but similar to that reported for cardiomyopathy associated with mitochondrial disease, in which cardiac function deteriorates rapidly regardless of the associated RC defect.30
The mutation G555E in the SDHA gene was reported twice before: in a patient who died at 5.5 months from respiratory difficulties and severe hypoglycemia, also presenting with severe hypotonia, hepatosplenomegaly, and cardiac dysrhythmia with cardiomegaly.13 The second patient presented with a relatively mild Leigh's syndrome at 22 months.12 Our patients exhibiting isolated cardiomyopathy differ markedly from the other reported cases. Notably, distinct clinical phenotypes of encephalomyopathy in one pedigree and fatal hypertrophic cardiomyopathy in another were reported to associate with the same mutation as the cause of mitochondrial elongation factor EFT.31 Aiming to elucidate the different clinical presentations of the G555E mutation, Pagnamenta et al12 compared enzymatic activity in the muscle and fibroblasts of the two patients and levels of the holo-complex II in both patients' fibroblasts, and searched for additional mutations in SDHB but found none. Our findings of no changes in SDHB and no correlation between the enzymatic activity of SDH complex in lymphoblastoid cells, the only available tissue, and clinical presentation in the unaffected homozygous father, are in agreement with this study.
The possibility that G555E is not the mutation, or only partially, associated with the disease is unlikely based on the two previous reports that it abrogates the stability of mitochondrial complex II, reduces the enzymatic activity of the complex in the muscle and fibroblasts by half, and was not found in 186 control chromosomes.12, 13 Furthermore, it was recognized that inherited deficiencies of SDH associated with SDHA mutations are associated with relatively high residual activities, 25±50% of control mean values.7, 10, 32, 33 In comparison, <5% residual activity is frequently measured in patients with severe defect of complex IV or I. However, patients with such SDH defects present typical Leigh's syndrome and thus do not clinically differ from patients with other RC complex defects.34 Finally, the only genomic region presenting linkage to the disease in family B was the SDHA locus, excluding the possibility of another mutation causing disease in this family.
The mutation causes a severe reduction in complex II enzymatic activity in the heart and only a mild reduction in the skeletal muscle and lymphoblastoid cells. The enzymatic activity in our patients' skeletal muscle and lymphoblastoid cells is comparable with the measurements in the skeletal muscle and fibroblasts in the two previous reports of this mutation12, 13 and in accord with previous reports presenting a partial reduction (50%) in SDH and II+III activity in the muscle as a significant finding in clinical presentations of mutations in complex II.10, 14, 33 The specific reduction in enzymatic activity in the heart compared with the other tissues tested is not easy to explain as all components of the SDH holoenzyme are encoded by the nucleus. Variable tissue-specific expression of SDH deficiency was previously reported in one patient with isolated heart involvement, affecting the heart but not the muscle, liver leucocytes, or fibroblasts, but the mutation remained unidentified.35 Variable expression of SDH deficiency was reported in late-onset optic atrophy with 50% reduction in the muscle and platelets but not in fibroblasts and lymphoblastoids.33
In conclusion, we present the association of G555E in the SDHA gene with severe neonatal isolated DCM. At present, we can only speculate that modifier genes contribute to the reported phenotypic variability of this mutation. A candidate modifier gene, the SDH assembly factor SDHAF1,28 does not appear to contribute to the intra-familial variability.
Acknowledgments
We thank the families for their collaboration. We thank Professor Orly Elpeleg (Hadassah-Hebrew University Medical Center, Jerusalem) for very fruitful discussions and suggestions. The study was supported by a grant from the Israeli Ministry of Health and a grant from the Israeli Ministry of Science, Culture and Sport to RP. VS is an investigator of the Howard Hughes Medical Institute.
The authors declare no conflict of interest.
References
- Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- Kärkkäinen S, Peuhkurinen K. Genetics of dilated cardiomyopathy. Ann Med. 2007;39:91–107. doi: 10.1080/07853890601145821. [DOI] [PubMed] [Google Scholar]
- Di Donato S. Disorders related to mitochondrial membranes: pathology of the respiratory chain and neurodegeneration. J Inherit Metab Dis. 2000;23:247–263. doi: 10.1023/a:1005684029429. [DOI] [PubMed] [Google Scholar]
- Ibdah JA, Bennett MJ, Rinaldo P, et al. A fetal fatty-acid oxidation disorder as a cause of liver disease in pregnant women. N Engl J Med. 1999;340:1723–1731. doi: 10.1056/NEJM199906033402204. [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter U, Eeds A, Yue Z, Haines J, Strauss AW, Summar M. Uniparental disomy of chromosome 2 resulting in lethal trifunctional protein deficiency due to homozygous alpha-subunit mutations. Hum Mutat. 2002;20:447–451. doi: 10.1002/humu.10142. [DOI] [PubMed] [Google Scholar]
- Duncan AJ, Bitner-Glindzicz M, Meunier B, et al. A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q10 deficiency: a potentially treatable form of mitochondrial disease. Am J Hum Genet. 2009;84:558–566. doi: 10.1016/j.ajhg.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfait B, Chreyien D, Rotig A, Marsac C, Munich A, Rustin P. Compound heterozygous mutations in the flavoprotein gene of the respiratory chain complex II in a patient with Leigh syndrome. Hum Genet. 2000;106:236–243. doi: 10.1007/s004390051033. [DOI] [PubMed] [Google Scholar]
- Rustin P, Munnich A, Rotig A. Succinate dehydrogenase and human diseases: new insights into a well-known enzyme. Eur J Hum Genet. 2002;10:289–291. doi: 10.1038/sj.ejhg.5200793. [DOI] [PubMed] [Google Scholar]
- Rustin P, Rotig A. Inborn errors of complex II–unusual human mitochondrial diseases. Biochim Biophys Acta. 2002;1553:117–122. doi: 10.1016/s0005-2728(01)00228-6. [DOI] [PubMed] [Google Scholar]
- Bourgeron T, Rustin P, Chretien D, et al. Mutation of a nuclear succinate dehydrogenase gene results in mitochondrial respiratory chain deficiency. Nat Genet. 1995;11:144–149. doi: 10.1038/ng1095-144. [DOI] [PubMed] [Google Scholar]
- Horváth R, Abicht A, Holinski-Feder E, et al. Leigh syndrome caused by mutations in the flavoprotein (Fp) subunit of succinate dehydrogenase (SDHA) J Neurol Neurosurg Psychiatry. 2006;77:74–76. doi: 10.1136/jnnp.2005.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnamenta AT, Hargreaves IP, Duncan AJ, et al. Phenotypic variability of mitochondrial disease caused by a nuclear mutation in complex II. Mol Genet Metab. 2006;89:214–221. doi: 10.1016/j.ymgme.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Van Coster R, Seneca S, Smet J, et al. Homozygous Gly555Glu mutation in the nuclear-encoded 70 kDa Flavoprotein gene causes instability of the respiratory chain complex II. Am J Med Genet. 2003;120A:13–18. doi: 10.1002/ajmg.a.10202. [DOI] [PubMed] [Google Scholar]
- Birch-Machin MA, Taylor RW, Cochran B, Ackrell BA, Turnbull DM. Late-onset optic atrophy, ataxia, and myopathy associated with a mutation of a complex II gene. Ann Neurol. 2000;48:330–335. [PubMed] [Google Scholar]
- Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiography. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- Henry WL, Gardin JM, Ware JH. Echocardiographic measurements in normal subjects from infancy to old age. Circulation. 1980;62:1054–1061. doi: 10.1161/01.cir.62.5.1054. [DOI] [PubMed] [Google Scholar]
- Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- Sahn DL. Applications of color flow mapping in pediatric cardiology. Cardiol Clin. 1989;7:255–264. [PubMed] [Google Scholar]
- Sahn DJ. Instrumentation and physical factors related to visualization of stenotic and regurgitant jets by Doppler color flow mapping. J Am Coll Cardiol. 1988;12:1354–1365. doi: 10.1016/0735-1097(88)92621-6. [DOI] [PubMed] [Google Scholar]
- Sahn DJ. Real-time two-dimensional Doppler echocardiographic flow mapping. Circulation. 1985;71:849–853. doi: 10.1161/01.cir.71.5.849. [DOI] [PubMed] [Google Scholar]
- Silverman NH, McElhinney DB. Atrioventricular valve dysfunction: evaluation by doppler and cross-sectional ultrasound. Ann Thorac Surg. 1998;66:653–658. doi: 10.1016/s0003-4975(98)00583-9. [DOI] [PubMed] [Google Scholar]
- Jenni R, Oechslin EN, van der Loo B.Isolated ventricular non-compaction of the myocardium in adults Heart 20079311–15.Published online first: 2 May 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart. 2001;86:666–671. doi: 10.1136/heart.86.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saada A, Bar-Meir M, Belaiche C, Miller C, Elpeleg O. Evaluation of enzymatic assays and compounds affecting ATP production in mitochondrial respiratory chain complex I deficiency. Anal Biochem. 2004;335:66–72. doi: 10.1016/j.ab.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Reisch AS, Elpeleg O. Biochemical assays for mitochondrial activity: assays of TCA cycle enzymes and PDHc. Methods Cell Biol. 2007;80:199–222. doi: 10.1016/S0091-679X(06)80010-5. [DOI] [PubMed] [Google Scholar]
- Parvari R, Hershkovitz E, Kanis A, et al. Homozygosity and linkage-disequilibrium mapping of the syndrome of congenital hypoparathyroidism, growth and mental retardation, and dysmorphism to a 1-cM interval on chromosome 1q42-43. Am J Hum Genet. 1998;63:163–169. doi: 10.1086/301915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- Ghezzi D, Goffrini P, Uziel G, et al. SDHAF1, encoding a LYR complex-II specific assembly factor, is mutated in SDH-defective infantile leukoencephalopathy. Nat Genet. 2009;40:654–656. doi: 10.1038/ng.378. [DOI] [PubMed] [Google Scholar]
- Towbin JA, Bowles NE. Arrhythmogenic inherited heart muscle disease in children. J Electrocardiol. 2001;43 (Suppl:151–165. doi: 10.1054/jelc.2001.28859. [DOI] [PubMed] [Google Scholar]
- Scaglia F, Towbin JA, Craigen WJ, et al. Clinical spectrum, morbidity, and mortality in 113 pediatric patients with mitochondrial disease. Pediatrics. 2004;114:925–931. doi: 10.1542/peds.2004-0718. [DOI] [PubMed] [Google Scholar]
- Smeitink JAM, Elpeleg O, Antonick H, et al. Distinct clinical phenotypes associated with a mutation in the mitochondrial translation elongation factor EFTs. Am J Hum Genet. 2007;79:869–877. doi: 10.1086/508434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustin P, Munnich A, Rötig A. Succinate dehydrogenase and human diseases: new insights into a well-known enzyme. Eur J Hum Genet. 2002;10:289–291. doi: 10.1038/sj.ejhg.5200793. [DOI] [PubMed] [Google Scholar]
- Taylor RW, Birch-Machin MA, Schaefer J, et al. Deficiency of complex II of the mitochondrial respiratory chain in late-onset optic atrophy and ataxia. Ann Neurol. 1996;39:224–232. doi: 10.1002/ana.410390212. [DOI] [PubMed] [Google Scholar]
- Munnich A, Rustin P. Clinical spectrum and diagnosis of mitochondrial disorders. Am J Med Genet. 2001;106:4–17. doi: 10.1002/ajmg.1391. [DOI] [PubMed] [Google Scholar]
- Rustin P, Lebidois J, Chretien D, et al. The investigation of respiratory chain disorders in heart using endomyocardial biopsies. J Inherit Metab Dis. 1993;16:541–544. doi: 10.1007/BF00711676. [DOI] [PubMed] [Google Scholar]