Abstract
Freezing patterns in the high alpine cushion plants Saxifraga bryoides, Saxifraga caesia, Saxifraga moschata and Silene acaulis were studied by infrared thermography at three reproductive stages (bud, anthesis, fruit development). The single reproductive shoots of a cushion froze independently in all four species at every reproductive stage. Ice formation caused lethal damage to the respective inflorescence. After ice nucleation, which occurred mainly in the stalk or the base of the reproductive shoot, ice propagated throughout that entire shoot, but not into neighboring shoots. However, anatomical ice barriers within cushions were not detected. The naturally occurring temperature gradient within the cushion appeared to interrupt ice propagation thermally. Consequently, every reproductive shoot needed an autonomous ice nucleation event to initiate freezing. Ice nucleation was not only influenced by minimum temperatures but also by the duration of exposure. At moderate subzero exposure temperatures (−4.3 to −7.7 °C) the number of frozen inflorescences increased exponentially. Due to efficient supercooling, single reproductive shoots remained unfrozen down to −17.4 °C (cooling rate 6 K h−1). Hence, the observed freezing pattern may be advantageous for frost survival of individual inflorescences and reproductive success of high alpine cushion plants, when during episodic summer frosts damage can be avoided by supercooling.
Keywords: Alpine cushion plant, Ice nucleation, Inflorescence freezing pattern, Summer frost survival, Supercooling, Thermal ice barrier
1. Introduction
In high alpine environments, plant life is mainly limited by low temperatures [1]. The alpine climate is characterized by short and cold growing seasons and stochastic weather conditions [2]. In winter, with the lowest temperatures to occur, prostrate high alpine plants such as cushion plants are usually sufficiently protected by frost hardening and snow coverage. Plants are only occasionally frost damaged at snow free sites [3]. The main threats are episodic frost events during the growing period, when plants have the lowest level of frost resistance [4]. The risk of frost damage increases with altitude, as the frequency and severity of cold spells is increased [5]. These frost events either are the consequences of cold waves [4], or caused by radiative heat loss, which can cool the plants below ambient temperature for several degrees in alpine environments [6].
Survival of summer frosts can be ensured either by freezing tolerance or by prevention of ice nucleation. While fully matured leaves of alpine woody and herbaceous plants can tolerate the formation of extracellular ice and subsequent freeze-dehydration to some extent [5], growing plant parts in the elongation phase do not tolerate freezing at all [7,8]. After cells are fully expanded and differentiated, frost resistance increases to values of the old leaves [9]. Generally, little knowledge exists about the frost resistance of reproductive tissues. Usually the reproductive organs of plants are most sensitive to frost, especially the style and ovules [3]. In a previous study, vegetative shoots of rosettes and cushion plants of several high alpine species of various growth forms were not frost injured, while at the same time the reproductive shoots got lethally damaged [4]. The inflorescences of the nival cushion plant Saxifraga bryoides appeared exceptionally vulnerable at late bud stage, during anthesis and the early fruit development [10]. During regular cold snaps at two subnival locations in the Tyrolean Alps (2650 and 2880 m a.s.l.) most inflorescences at these stages were frost damaged, but nonetheless older fruits and young buds from the second flowering cohort survived.
The freezing pattern in high alpine plants was studied by infrared thermography to some extent [11,12], but we lack information about reproductive organs. Freezing pattern in reproductive organs has been studied in fruit crops revealing protective structural (anatomical) ice barriers between the stem and the flowers that prevent ice propagation from the stem into the freezing sensitive flower organs [13,14]. In alpine plants, the freezing pattern depends markedly on the growth form [11]. In most investigated woody and herbaceous species ice propagated throughout the entire shoot once ice nucleation has occurred. In graminoids and in cushion plants such as Silene acaulis ice barriers prevent ice propagation to other leaves or shoots. In the present study, the freezing pattern in the high alpine cushion plants S. bryoides, Saxifraga caesia, Saxifraga moschata and S. acaulis were analysed at various reproductive stages (bud, anthesis, fruit development). We hypothesized, that the cushion growth form is advantageous for frost survival of the reproductive organs. Ice propagation between reproductive shoots may be prevented by an ice barrier and hence single inflorescences would survive cold spells due to supercooling.
2. Materials and methods
2.1. Plant material
Freezing patterns were studied in four high alpine cushion plants S. bryoides L., S. caesia L., S. moschata Wulfen and S. acaulis [L.] Jacq. at three reproductive stages (bud, anthesis, and fruit development). Rooted plants of the investigated species were collected in spring 2008 at two natural sites in the Tyrolean Alps: S. bryoides at a subnival site in the Tyrolean Central Alps in the forelands of the Stubai Glacier (2880 m a.s.l., 46°59′18′′N, 11°06′58′′E); S. caesia, S. moschata, and S. acaulis at an alpine site in the northern calcareous mountain range north of Innsbruck on Mt. Hafelekar (2334 m a.s.l., 47°18′45′′N, 11°23′00′′E). Individuals were potted in plastic containers (8 cm × 8 cm × 8.5 cm) filled with an alpine soil mixture. Potted plants were cultivated under natural environmental conditions in the Alpine Garden of the University of Innsbruck on Mt. Patscherkofel (1950 m a.s.l., 47°12′37′′N, 11°27′08′′E). Freezing experiments were conducted with properly rooted and well-watered plants in the laboratory throughout the summers of 2008 and mainly 2009.
2.2. Freezing experiments
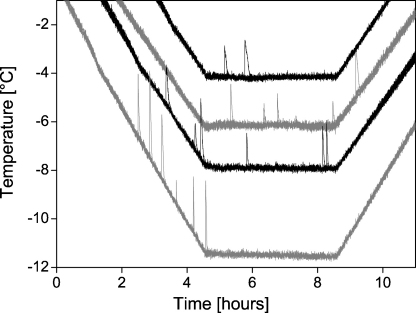
During night frosts in nature, cushion plants are exposed to a temperature gradient where the interior of the cushion remains several K warmer than their surface [15]. This warming effect has been termed thermal buffering [3]. In order to simulate the natural situation in the experiments the below ground organs were at non-freezing temperatures. For the detection of freezing patterns with the infrared camera, two experimental settings with different temperature profiles were used (A and B), a third temperature profile was applied to investigate the distribution of freezing exotherms at different exposure temperatures (C). (A) For the detection of whole plant freezing patterns and the freezing temperature range, plant temperatures were lowered in a freezing chamber at a controlled cooling rate of 6 K h−1 until all inflorescences of the cushion were frozen. When air temperature (at the height of the inflorescences) had been lowered to −15 °C, the temperature inside the cushion remained above 0 °C during the whole experiment (data not shown). Number of investigated cushions for this experimental setting at the different reproductive stages (bud/anthesis/fruit development): S. bryoides (1/2/3), S. caesia (3/1/1), S. moschata (1/3/1), and S. acaulis (0/1/2). (B) For the detection of the supercooling potential of reproductive shoots of the three Saxifrages, whole cushions were exposed to moderate subzero temperatures ranging from −4.3 °C to −7.7 °C for 15 h (cooling and thawing rate of 6 K h−1). Single cushions were investigated at following exposure temperatures and reproductive stages: S. bryoides (bud: −4.3 °C, −7.6 °C), S. caesia (anthesis: −5.9 °C; bud: −4.3 °C, −5.8 °C, −6.0 °C, −7.5 °C), and S. moschata (anthesis: −4.6 °C, −6.0 °C; fruit development: −5.9 °C, −7.7 °C). The number of inflorescence freezing events was accumulated during the exposure. The progression of inflorescence freezing was described by asymptotic exponential curves (y = a − bcx, curve fitting with Origin, OriginLab Corporation, Northampton, MA, U.S.A.). The number of frozen inflorescences at the end of the exposure, and after 1, 2, and 4 h were plotted against the exposure temperature (data of all species and phenological phases were used). Based on this data, survival curves for the different exposure times and the frost survival parameter LT50 (i.e. temperature at which 50% of the inflorescences were lethally damaged) were calculated (Logistic sigmoid curve fitting with Fig.P, Fig.P Software Corporation, Durham, NC, U.S.A.). To prevent freezing of the soil during prolonged exposure to subzero temperatures the pots were kept unfrozen in a thermally insulated box with a controlled electric heating beneath the pots (12 W heating power, realized by a power resistor and ventilation). (C) The dependence of ice nucleation temperatures and supercooling ability on the temperature profile was additionally studied by freezing exotherm measurements. Whole cushions of S. moschata inflorescences during anthesis were exposed to −4 °C, −6 °C, −8 °C and −11.5 °C for 4 h (cooling and thawing rate of 2 K h−1). In each cushion, the temperatures of six inflorescences were recorded by thermocouples.
2.3. Infrared thermography
Temperatures and freezing pattern were measured throughout the entire freezing experiments with a digital infrared camera (FLIR Systems ThermaCAM S60, FLIR Systems AB, Danderyd, Sweden). Measurements were conducted with a spatial resolution of 200 μm achieved by the use of a close-up lens (LW64/150), and a maximal time resolution of 25 images s−1. The control of the infrared camera and infrared data analysis were done with the software ThermaCAM Researcher (FLIR Systems AB, Danderyd, Sweden). The original infrared data were further analysed by performing an infrared differential thermal analysis (IDTA) as described by Hacker and Neuner [16]. IDTA is based on the subtraction of a reference image, captured just before the occurrence of freezing, from the subsequent sequence of images. The resulting sequence of differential images shows only temperature changes due to the release of heat during freezing, while background temperature fluctuations are canceled out. In the resulting IDTA images, freezing is indicated by brightening of the tissue, while unfrozen areas remain black. Temperatures were additionally measured by fine-wire thermocouples attached at the height of the inflorescences. Videos, showing the freezing patterns described in this article, are available on following website: <http://www.uibk.ac.at/botany/stressphysiology>, Navigation “Ice propagation”.
3. Results
3.1. Freezing patterns in whole cushion plants
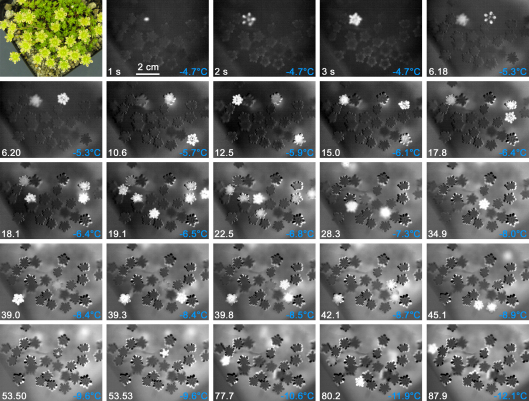
A sequence of IDTA images determined during freezing of a S. moschata cushion during anthesis illustrates, that all reproductive shoots and the vegetative shoots froze independently from each other (Fig. 1). Freezing is indicated in the IDTA image by brightening, which diminished after several minutes when most water has frozen. In every reproductive shoot, autonomous ice nucleation is required to initiate the freezing process. Ice propagation between single reproductive or vegetative shoots was not observed. Consequently, freezing of all reproductive shoots of the cushion occurred in a broad temperature range from −4.7 °C to −12.1 °C and was finished only after 90 min (at a cooling rate of 6 K h−1). All inflorescences were lethally damaged, but 40% of the leaf bearing bases of the reproductive shoots survived this freezing treatment (data not shown, minimum temperature was −15 °C).
Fig. 1.
The sequence of IDTA images of a flowering S. moschata cushion recorded during controlled freezing shows that all inflorescences froze independent from each other. A brightening of the inflorescences or vegetative shoots (bright spots out of focus) indicates freezing. When most water in the respective organs was frozen and no more heat release was detectable, plant tissues became black again. Freezing of all inflorescences occurred in a wide temperature range from −4.7 °C to −12.1 °C within 90 min. White numbers indicate the time in minutes after the first freezing event, if not other specified.
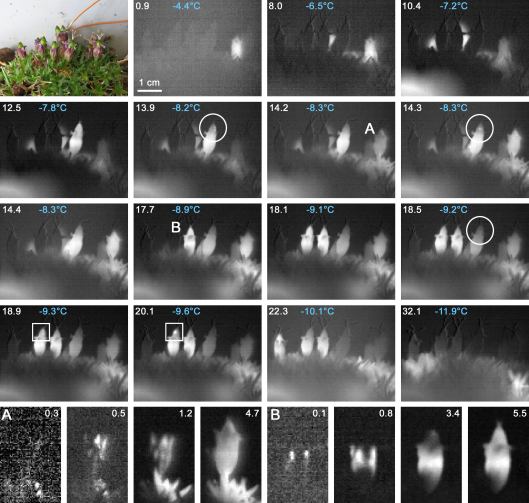
The same freezing pattern as found in S. moschata during anthesis was detected in a S. acaulis cushion during fruit development (Fig. 2). The single fruits and vegetative shoots froze independently from each other and each inflorescence required an autonomous ice nucleation event. Ice propagation between frozen and unfrozen shoots could not be detected. All fruits of the cushion froze in a wide temperature range from −4.4 °C to −11.9 °C. Ice nucleation either occurred in the leaf bearing base of the reproductive shoot (Fig. 2, detail A), or most times (six out of seven fruits) in the calyx outside the fruit (Fig. 2, detail B). After the main freezing exotherm additional freezing events could be observed inside two fruits (Fig. 2, freezing sites are marked with white circles and squares respectively).
Fig. 2.
The sequence of IDTA images of a fruiting S. acaulis cushion during controlled freezing shows that all single fruits and vegetative shoots froze independently from each other. Each shoot required autonomous ice nucleation to initiate the freezing process. Ice was either nucleated in the leaf bearing base of the reproductive shoot (A), from where ice propagated into the fruit, or more often in the calyx (B). Inside two fruits, additional freezing events were detectable after the main freezing exotherm. White numbers indicate the time in minutes after the first freezing event; subfigures (A and B) white numbers indicate time in seconds after ice nucleation.
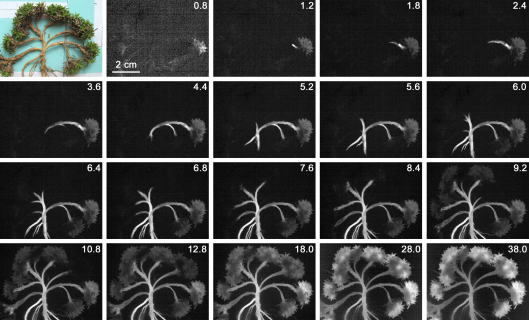
The freezing patterns in S. moschata during anthesis and S. acaulis during fruit development were in principal the same in all four investigated cushion plants at all reproductive stages. For the determination, whether anatomical ice barriers are existent in cushions, the cushion structure of a S. acaulis cushion was completely loosened. Then the vegetative shoots were shifted apart in order to prevent thermal buffering and hence to produce a homogenous temperature in all plant parts during cooling. The sequence of IDTA images shows unhindered ice propagation throughout the whole shoot within 13 s, subsequent to ice nucleation at −3.7 °C occurring in one of the vegetative shoots (Fig. 3).
Fig. 3.
In a vegetative S. acaulis cushion shoots have been put apart to prevent thermal buffering. The sequence of IDTA images shows unhindered ice propagation throughout the whole shoot system via the connecting stems after ice has nucleated in one vegetative shoot. Anatomical ice barriers apparently did not exist. Numbers indicate the time in seconds after initial ice nucleation.
3.2. Sites of ice nucleation
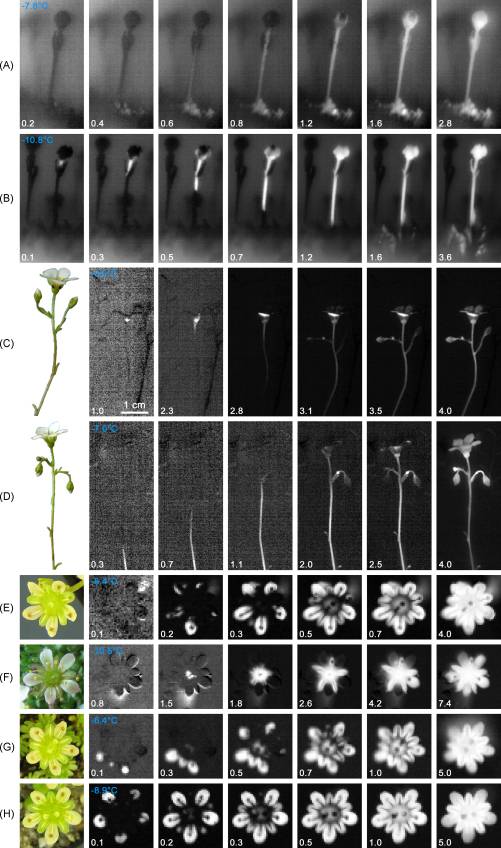
Sites of ice nucleation and ice propagation patterns within reproductive shoots are shown for S. moschata and S. caesia (Fig. 4). Ice nucleation occurred mainly in the stalk (Fig. 4B) or in single flowers (Fig. 4C), in the latter in the ovary or the calyx. Less frequent was ice nucleation in the basis of the reproductive shoot (Fig. 4A and D). Subsequent to ice nucleation, ice propagated throughout the entire inflorescence including all branchings independent of the ice nucleation site. Ice nucleation in the stalk and the leaf-bearing basis of the reproductive shoot caused immediate ice propagation, while after ice nucleation in the flowers, ice was restricted for a short time at the nucleation site. However, when ice encountered the vascular tissue, ice spread without hindrance throughout the complete reproductive shoot. The rate of ice propagation in the inflorescence stalk was significantly higher at lower temperatures (Fig. 4C: 4.0 cm s−1 at −8.9 °C; Fig. 4D: 3.0 cm s−1 at −7.0 °C; 1.6 cm s−1 at −3.4 °C). When the freezing patterns were observed in top view of the flowers, the site of ice nucleation was difficult to determine, if at all (Fig. 4E–H). Ice nucleation beneath the flower was clearly detectable when the inflorescence stalk was bent. From the symmetric freezing patterns of the petals and sepals as in Fig. 4H, ice nucleation in the leaf bearing base of the reproductive shoot or in the stalk can be assumed.
Fig. 4.
Freezing patterns in reproductive shoots of S. moschata (A and B, buds in lateral view; E–H, flowers in top view) and S. caesia (C and D, flowers in lateral view) were visualized by sequences of IDTA images during freezing. In the lateral view, two main ice nucleation sites could be clearly differentiated: Ice nucleation in the leaf bearing base of the reproductive shoot (A and D) and in the inflorescence (either in the stalk (B) or in the flower itself (C)). Subsequent to nucleation, ice propagated throughout the reproductive shoot either immediately (A, B and D) or was retarded for a second after ice nucleation in the flower (C). Ice nucleation in four S. moschata flowers observed in top view occurred mainly beneath the flower. Freezing of the stalk can be either clearly seen (E or F) or the blossom hides the view (G and H). Numbers indicate the time in seconds after ice nucleation. Scale: (A and B) height of inflorescences is about 2 cm; (E–H) diameter of flowers is about 1 cm.
Cushion structure appeared to influence ice nucleation sites. In a compact cushion of S. caesia bearing 45 reproductive shoots ice nucleation occurred mainly in the stalks (53%), in the flower bud itself (24%), in leaves of the stalk (16%), and more rarely in the leaf-bearing base of the reproductive shoots (7%). In cushions without a compact structure, i.e. with considerable air spaces between shoots, the chance for ice nucleation in the leaf-bearing base of the reproductive shoots was increased (data not shown).
3.3. Ice nucleation temperatures
When the temperatures were lowered at a controlled cooling rate of 6 K h−1 down to a minimum temperature with all inflorescences frozen, ice nucleation in reproductive shoots occurred in a wide range of temperatures (Table 1). First ice nucleation events were detected around −4 °C in all species and reproductive stages, while minimal ice nucleation temperatures varied between −7.6 °C in S. bryoides flowers and −17.4 °C in S. acaulis flowers. Hence, the resulting range of ice nucleation temperatures was between 3.8 °C in S. moschata flowers and 13.7 °C in S. acaulis flowers.
Table 1.
Maximum, mean and minimum ice nucleation temperatures and their total range measured in inflorescences of the four investigated cushion plants S. bryoides, S. caesia, S. moschata and S. acaulis at the three reproductive stages.
| Species | Phase | No. of cushions | No. of inflorescences | Maximum (°C) | Mean (°C) | Minimum (°C) | Range (K) |
|---|---|---|---|---|---|---|---|
| S. bryoides | Bud | 1 | 42 | −3.7 | −6.7 | −10.3 | 6.6 |
| S. bryoides | Flower | 1 | 21 | −3.6 | −5.3 | −7.6 | 4.0 |
| S. caesia | Bud | 2 | 93 | −4.7 | −9.2 | −13.8 | 9.1 |
| S. caesia | Flower | 1 | 7 | −2.8 | −6.3 | −8.9 | 6.1 |
| S. moschata | Bud | 1 | 9 | −7.8 | −10.3 | −11.6 | 3.8 |
| S. moschata | Flower | 3 | 54 | −3.8 | −8.1 | −12.8 | 9.0 |
| S. moschata | Fruit | 1 | 18 | −4.2 | −8.0 | −11.4 | 7.2 |
| Silene acaulis | Bud | 2 | 45 | −3.7 | −11.0 | −17.4 | 13.7 |
| Silene acaulis | Fruit | 1 | 7 | −4.4 | −8.0 | −10.3 | 5.9 |
The ice nucleation temperatures and the duration of supercooling were markedly affected by the cooling profile itself, which is representatively shown for S. moschata flowers (Fig. 5). At the lowest exposure temperature of −11.5 °C, all freezing exotherms occurred during the cooling phase and were at mean significantly lower than in the more moderate freezing temperature scenarios. At the exposure temperature of −8 °C, freezing exotherms occurred not only during cooling, but also during the exposure phase. This was more pronounced at the exposure temperature of −6 °C, when freezing exotherms occurred during the whole exposure phase and even during thawing. At the highest exposure temperatures of −4 °C and −6 °C, several flowers (four and one flower respectively) remained supercooled during the entire freezing treatment and no freezing exotherms were detected.
Fig. 5.
Ice nucleation temperature and the duration of supercooling of six single flowers of four S. moschata cushions depended markedly on the severity of the freezing treatment. At an exposure temperature of −11.5 °C, all freezing exotherms occurred during cooling, while at higher exposure temperatures, the freezing exotherms appeared mainly during the exposure and even thawing phase. At the exposure temperature of −4 °C and −6 °C, some flowers (four and one flower respectively) remained supercooled during the entire freezing treatment and no exotherms were detected.
3.4. Survival rates of inflorescences at higher subzero temperatures
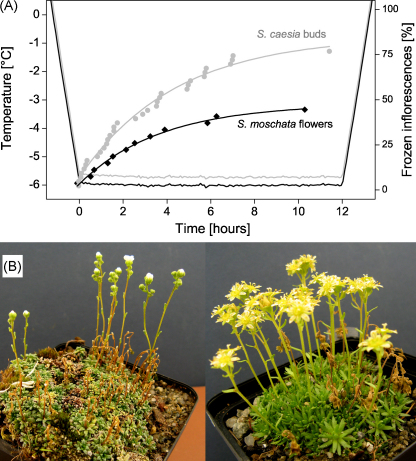
The cooling profile markedly influenced the ice nucleation pattern, as described in the previous section. Especially at higher temperatures prolonged supercooling of inflorescences occurred. A cushion of S. caesia during bud phase and S. moschata during anthesis was exposed for 12 h at minimum temperatures of −5.8 °C and −6.0 °C respectively with controlled cooling and thawing rates of 6 K h−1 (Fig. 6A). First inflorescences froze, when the target temperature had been reached, but freezing of inflorescence was observed during the entire exposure in both species. Ice nucleation occurred most frequent at the beginning of the exposure but decreased with the duration of the exposure. The accumulated number of freezing events was expressed as asymptotic exponential function, which describes the freezing progression adequately (Fig. 6A). Freezing occurred in 77% of S. caesia flower buds and in 44% of open flowers in S. moschata causing full frost damage to the reproductive organs (Fig. 6B). Inflorescences without detectable freezing during the whole experiment survived and developed further.
Fig. 6.
(A) A cushion of S. caesia at bud stage and S. moschata during anthesis have been exposed to −5.8 °C and −6.0 °C for 12 h (cooling and thawing rate 6 K h−1). First inflorescences froze when the temperatures have reached theirs minimums. But freezing occurred during the whole exposure phase, accumulated number of inflorescence freezing events are indicated by gray circles for S. caesia and black diamonds for S. moschata respectively. The progression of inflorescence freezing is additionally depicted by fitted asymptotic exponential curves. (B) At the end of the treatment, 77% of S. caesia buds and 44% of S. moschata flowers were frozen and thereby lethally damaged, while unfrozen inflorescences survived the freezing treatment.
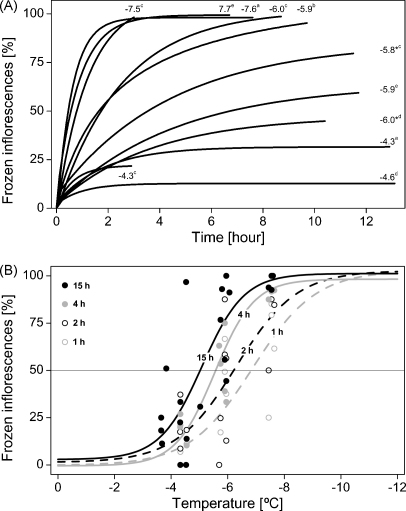
Freezing progression and frost damage rates of the inflorescences of the three Saxifrages at the three reproductive stages (bud, anthesis, and fruit development) were surveyed at exposure temperatures ranging from −4.3 °C to −7.7 °C and an exposure time of 15 h (Fig. 7). At high exposure temperatures of around −4.5 °C, freezing was detected only in 14–33% of all inflorescences, while at exposure temperatures of around −7.5 °C more than 90% inflorescences were already frozen within a few hours (Fig. 7A). At an exposure temperature of −6 °C, the biggest variation could be observed (44–94% frozen inflorescences). At high exposure temperatures, the maximum frost damage was reached after a few hours indicated by flattening of the fitted curve, while in the intermediate temperatures an increase was detectable during the entire exposure time. From these curves, the number of frozen inflorescences was determined after 1, 2, 4, and 15 h and plotted against the exposure temperature (Fig. 7B). Frost survival rates of inflorescences were markedly affected by the duration of exposure with high LT50 values at longer duration and vice versa −5.1 °C (15 h), −5.5 °C (4 h), −6.3 °C (2 h), and −6.9 °C (1 h).
Fig. 7.
(A) The occurrence of freezing events of reproductive shoots of the three Saxifrages at the three investigated reproductive stages were monitored at different exposure temperatures for 15 h (* 12 h exposure time; superscripted letters indicate species and reproductive stage: (a) S. bryoides bud, (b) S. caesia anthesis, (c) S. caesia bud, (d) S. moschata anthesis, (e) S. moschata fruit development). The progression of inflorescence freezing is depicted by asymptotic exponential curves (see Fig. 6A; single data points not shown). (B) From these curves, the number of frozen inflorescences was determined after 1, 2, 4, and 15 h and plotted against the exposure temperature. Frost survival of inflorescences increased markedly at shorter durations of exposure, as indicated by LT50 values: −5.1 °C (15 h); −5.5 °C (4 h); −6.3 °C (2 h); and −6.9 °C (1 h).
4. Discussion
While vegetative shoots and leaves of high alpine cushion plants are known to survive extracellular freezing in summer [5], the reproductive organs of the investigated species were not ice tolerant. Only supercooling prevented frost damage of the reproductive organs. The reproductive shoots of the investigated cushion plants S. bryoides, S. caesia, S. moschata and S. acaulis showed unique freezing patterns at all reproductive stages (bud, anthesis, fruit development). Each single reproductive shoot froze independent from each other and needed an autonomous ice nucleation event, which corroborated the findings of separate freezing events in vegetative shoots of a cushion of S. acaulis [11]. The detected ice barrier is not a structural one, which could be demonstrated for a loosened S. acaulis cushion. Ice propagated unhindered throughout the whole cushion when the structural thermal buffering of the cushion [3] was inhibited. Ice propagation between shoots was thermally blocked. This can be expected to be close to the situation at a natural growing site, where the interior of cushion plants has been shown to remain several centigrade's warmer than the cushion surface [15]. Structural ice barriers are present in graminoids due to the specific organization of the vascular system [11,12,17,18] and in overwintering flower buds of several Rhododendron species [19,20], Vitis vinifera [21] and peach [22]. The additional freezing events detected inside of S. acaulis fruits after the main freezing exotherm, originate very likely from freezing of single seeds. During maturation drying, the seeds will become separated from the carpel whereby an ice barrier may develop. In alpine plant species, the whole maturation phase is known to be rather insensitive to low temperatures (S. moschata [23], Gentianella germanica [24]). A decoupling of the seeds from the reproductive shoot as a freezing unit appears advantageous for seed survival.
Ice nucleation occurred mainly in the stalk or the leaf-bearing base of the reproductive shoot but was also detected in the ovary and the calyx. Due to the smallness of the investigated organs, an exact localisation of the nucleation site was not possible. Intrinsic ice nucleation can be assumed, as the investigated plant samples were lacking surface water, which plays an important role in extrinsic ice nucleation [25]. In biological systems, nucleation is always heterogeneous and takes place on or around foreign particles, because the internal water is inevitable in contact with other surfaces and large molecules [26,27]. It can be expected, that ice nucleation occur in apoplastic water, as it has a higher equilibrium freezing temperature than cytoplasmic or vacuolar water [28]. In most cases, ice propagation in the stalk throughout the reproductive shoot started immediately after ice nucleation, and was associated with the vascular tissue. This corroborates findings for other alpine species of various growth forms [11] and woody species of different taxa and provenance [16,28]. In these cases, the xylem vessels itself appear to be the most probable sites of nucleation, as the likelihood of ice nucleation increases with the volume of water [26,29]. Ice nucleation in the ovary or the calyx occurred in some cases apparently not in the vessels, as ice propagation in the stalk did not start immediately, but was retarded for some seconds, which is also reported for leaves of Senecio incanus [12]. When cold spells in summer are accompanied by precipitation, ice nucleators, present at the plant surface, will become active in aqueous solutions [30]. Hence, extrinsic ice nucleation could be favoured under such conditions and critically reduce the supercooling ability of the reproductive shoots.
The initial rapid spread of ice following ice nucleation was found to occur in the vascular system [3,11,16,31]. Ice propagation rates in the inflorescence increased significantly with decreasing ice nucleation temperatures which corroborates earlier findings [11,16]. High density and compactness of the cushion favoured ice nucleation in the inflorescences due to a higher temperature gradient between the inflorescence tip and its base. In contrast, in loosened cushions ice nucleation occurred equally in the inflorescences and the leaf bearing base of the reproductive shoots due to a reduced temperature gradient. At natural sites under radiative cooling conditions, the temperature gradients could be even more pronounced than under convective cooling used in the freezing experiments, which may result in a higher probability for ice nucleation in the inflorescences itself.
As the reproductive organs of the investigated high alpine cushion plants were not ice tolerant, survival depended on efficient supercooling. Ability to maintain the supercooled state was not only increased under moderate freezing temperature but also markedly by shorter exposure times. In nature, a moderate night frost would leave a calculable probability of frost survival due to remaining supercooled reproductive shoots. A similar supercooling survival mechanism has been found in graminoids [11] that are the most frost resistant high alpine plant species in summer [5]. Particularly under the radiative freezing scenarios typical for high alpine environments during spring and summer, subzero temperatures are often lasting only for a short time period. Prevention of ice nucleation could enable an escape from freezing injury. This observed freezing and ice nucleation patterns in the reproductive shoots of high alpine cushion plants may be crucial for their reproductive success.
Acknowledgements
This work was funded by a grant from the Austrian Science Fund FWF (project 20010 to J. Wagner: “Reproduction of mountain plants under temperature stress”). We further wish to thank the Patscherkofelbahnen Ges.m.b.H. and Wintersport Tirol AG for free transportation to the research sites at Mt. Patscherkofel and Stubai Glacier respectively.
References
- 1.Körner C. Functional Plant Ecology of High Mountains Ecosystems. second ed. Springer; Heidelberg: 2003. Alpine plant life. [Google Scholar]
- 2.Billings W. Adaptations and origins of alpine plants. Arct. Alp. Res. 1974;6:129–142. [Google Scholar]
- 3.Sakai A., Larcher W. Springer Verlag; Berlin: 1987. Frost Survival of plants. Responses and Adaptation to Freezing Stress. [Google Scholar]
- 4.Larcher W., Kainmüller C., Wagner J. Survival types of high mountain plants under extreme temperatures. Flora. 2010;205:3–18. [Google Scholar]
- 5.Taschler D., Neuner G. Summer frost resistance and freezing patterns measured in situ in leaves of major alpine plant growth forms in relation to their upper distribution boundary. Plant Cell Environ. 2004;27:737–746. [Google Scholar]
- 6.Jordan D., Smith W. Energy balance analysis of nighttime leaf temperatures and frost formation in a subalpine environment. Agric. For. Meteorol. 1994;71:359–372. [Google Scholar]
- 7.Taschler D., Beikircher B., Neuner G. Frost resistance and ice nucleation in leaves of five woody timberline species measured in situ during shoot expansion. Tree Physiol. 2004;24:331–337. doi: 10.1093/treephys/24.3.331. [DOI] [PubMed] [Google Scholar]
- 8.Hacker J., Neuner G. Photosynthetic capacity and PSII efficiency of the evergreen alpine cushion plant Saxifraga paniculata during winter at different altitudes. Arct. Antarct. Alp. Res. 2006;38:198–205. [Google Scholar]
- 9.Neuner G., Beikircher B. Critically reduced frost resistance of Picea abies during sprouting could be linked to cytological changes. Protoplasma. 2010;243:145–152. doi: 10.1007/s00709-009-0052-9. [DOI] [PubMed] [Google Scholar]
- 10.Ladinig U., Wagner J. Dynamics of flower development and vegetative shoot growth in the high mountain plant Saxifraga bryoides L. Flora. 2009;204:63–73. [Google Scholar]
- 11.Hacker J., Neuner G. Ice propagation in dehardened alpine plant species studied by infrared differential thermal analysis (IDTA) Arct. Antarct. Alp. Res. 2008;40:660–670. [Google Scholar]
- 12.Hacker J., Spindelböck J., Neuner G. Mesophyll freezing and effects of freeze dehydration visualized by simultaneous measurement of IDTA and differential imaging chlorophyll fluorescence. Plant Cell Environ. 2008;31:1725–1733. doi: 10.1111/j.1365-3040.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 13.Carter J., Brennan R., Wisniewski M. Patterns of ice formation and movement in blackcurrant. Hortscience. 2001;36:855–859. [Google Scholar]
- 14.Workmaster B., Palta J., Wisniewski M. Ice nucleation and propagation in cranberry uprights and fruit using infrared video thermography. J. Am. Soc. Hortic. Sci. 1999;124:619–625. [Google Scholar]
- 15.Ruthsatz B. Las plantas es cojin de los semi-desiertos andinos del Nordeste Argentino. Darwinia. 1978;21:492–539. [Google Scholar]
- 16.Hacker J., Neuner G. Ice propagation in plants visualized at the tissue level by infrared differential thermal analysis (IDTA) Tree Physiol. 2007;27:1661–1670. doi: 10.1093/treephys/27.12.1661. [DOI] [PubMed] [Google Scholar]
- 17.Pearce R.S., Fuller M.P. Freezing of barley studied by infrared video thermography. Plant Physiol. 2001;125:227–240. doi: 10.1104/pp.125.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stier J.C., Filiault D.L., Wisniewski M., Palta J.P. Visualization of freezing progression in turfgrasses using infrared video thermography. Crop Sci. 2003;43:415–420. [Google Scholar]
- 19.George M.F., Burke M.J., Weiser C.J. Supercooling in overwintering Azalea flower buds. Plant Physiol. 1974;54:29–35. doi: 10.1104/pp.54.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishikawa M., Sakai A. Freezing avoidance mechanisms by supercooling in some Rhododendron flower buds with reference to water relations. Plant Cell Physiol. 1981;22:953–967. [Google Scholar]
- 21.Jones K.S., McKersie B.D., Paroschy J. Prevention of ice propagation by permeability barriers in bud axes of Vitis vinifera. Can. J. Bot: Rev. Can. Bot. 2000;78:3–9. [Google Scholar]
- 22.Quamme H. Mechanism of supercooling in overwintering peach flower buds. J. Am. Soc. Hortic. Sci. 1978;103:57–61. [Google Scholar]
- 23.Ladinig U., Wagner J. Sexual reproduction of the high mountain plant Saxifraga moschata Wulfen at varying lengths of the growing season. Flora. 2005;200:502–515. [Google Scholar]
- 24.Wagner J., Mitterhofer E. Phenology, seed development, and reproductive success of an alpine population of Gentianella germanica in climatically varying years. Bot. Acta. 1998;111:159–166. [Google Scholar]
- 25.Wisniewski M., Gusta L., Fuller M., Karlson D. Ice nucleation, propagation and deep supercooling: the lost tribes of freezing studies. In: Gusta L., Wisniewski M., Tanino K., editors. Plant Cold Hardiness: From the Laboratory to the Field. CABI; Oxfordshire, UK: 2009. pp. 1–11. [Google Scholar]
- 26.Vali G. Principles of ice nucleation. In: Lee R.E. Jr., Warren G.J., Gusta L.V., editors. Biological Ice Nucleation and its Application. American Phytopathological Society; St. Paul, Minnesota: 1995. pp. 1–28. [Google Scholar]
- 27.Wilson P.W., Heneghan A.F., Haymet A.D.J. Ice nucleation in nature: supercooling point (Scp) measurements and the role of heterogeneous nucleation. Cryobiology. 2003;46:88–98. doi: 10.1016/s0011-2240(02)00182-7. [DOI] [PubMed] [Google Scholar]
- 28.Ball M.C., Wolfe J., Canny M., Hofmann M., Nicotra A.B., Hughes D. Space and time dependence of temperature and freezing in evergreen leaves. Funct. Plant Biol. 2002;29:1259–1272. doi: 10.1071/FP02037. [DOI] [PubMed] [Google Scholar]
- 29.Ashworth E.N., Davis G.A., Anderson J.A. Factors affecting ice nucleation in plant tissues. Plant Physiol. 1985;79:1033–1037. doi: 10.1104/pp.79.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wisniewski M., Fuller M., Glenn D., Gusta L., Duman J., Griffith M. Extrinsic ice nucleation in plants: what are the factors involved and can they be manipulated? In: Li P., Palva E., editors. Plant Cold Hardiness. Gene Regulation and Genetic Engineering. Kluwer Academic Publishers; New York: 2002. pp. 223–236. [Google Scholar]
- 31.Ball M.C., Canny M.J., Huang C.X., Heady R.D. Structural changes in acclimated and unacclimated leaves during freezing and thawing. Funct. Plant Biol. 2004;31:29–40. doi: 10.1071/FP03164. [DOI] [PubMed] [Google Scholar]