Abstract
Although graft-versus-host (GVH) disease (GVHD) is usually associated with graft versus leukemia (GVL), GVL can occur in the absence of clinical GVHD. There is evidence to suggest that GVL and GVH are mediated by different clones of T cells. The objective of this study was to identify the two types of T cells based on their receptor sequences. To this end we used irradiated nonleukemic cells from recipients as stimulator cells in a primary mixed leukocyte reaction (MLR). The activated CD4+ donor T cells that expressed CD25 were purified by cell sorting. To prepare GVL-specific T cells, alloreactive T cells in the primary MLR were first depleted with an anti-CD25 immunotoxin. The remaining T cells had negligible alloreactivity in a secondary MLR. The allodepleted cells were then stimulated by using purified leukemia cells from the same individual as stimulator cells, and the CD25+-activated cells were purified by cell sorting. The GVL- and GVH-specific T cells were analyzed for their T cell receptor (TCR) clonality by using anchored RT-PCR of all the TCRβ locus complementarity-determining region 3 (CDR3) sequences. By comparing TCRβ CDR3 sequences from transformed bacterial colonies, we were able to demonstrate that T cells mediating GVH were different from those mediating GVL in each of the eight HLA-mismatched and one HLA-matched donor/recipient pairs. By using the appropriate TCRβ CDR3-specific primers and probes, the GVH- and GVL-specific clones were monitored in a recipient undergoing an allogeneic stem cell transplant from her HLA-matched related donor.
A major challenge in the field of allogeneic hematopoietic stem cell transplantation (HSCT) is to prevent the alloreactivity that leads to graft-versus-host (GVH) disease (GVHD) while preserving a graft-versus-leukemia (GVL) effect (1–5). After allogeneic HSCT, T cells mount an alloresponse against minor histocompatibility antigens of the recipient (in the case of HLA-matched transplants) and against minor and major histocompatibility antigens (in the case of HLA-mismatched transplants) (6, 7). GVHD is a leading cause of morbidity and mortality after HSCT (1, 2). A pronounced GVL effect of allogeneic HSCT has been well documented in animal studies and by clinical observations (4, 6–9). The most compelling evidence for a GVL response in humans is the observation of complete remissions in recipients with relapsed malignancy after HSCT who receive donor leukocyte infusions. Such remissions have been observed in ≈70–80% of recipients with chronic myeloid leukemia in chronic-phase relapse (10–14) and to a lesser extent in recipients with acute myeloid leukemia (AML), chronic lymphocytic leukemia, multiple myeloma, and low-grade lymphomas (3, 9, 10, 12). Although GVHD usually is associated with a GVL response (8, 9), GVL can occur in the absence of clinical GVHD (3, 5, 9).
In vitro data support the existence of hematopoietic lineage-restricted alloresponses within T cell receptor (TCR)β V families. It has been demonstrated that donor CD4+ T cell clones, generated against a single HLA-A locus mismatched recipient with chronic myeloid leukemia, had distinct GVL or GVH reactivity. Furthermore, clones mediating GVL and GVH had different TCRβ V specificities (15, 16). Flow cytometry and a TCRβ complementarity-determining region 3 (CDR3) length repertoire analysis (TCR spectratyping) have been used to identify clonal expansion of GVL- or GVH-specific donor lymphocytes (11, 17) and, in one study, to identify a unique TCR sequence in a GVL-specific clone (17). Spectratyping has resulted in the characterization of a limited number of TCRβ V families but not the unique TCRβ CDR3 sequences specific for every individual T cell clone. Other approaches, using HLA-2/peptide tetramers, are limited by the HLA class I phenotypes and the identification of peptides that specifically bind to particular HLA molecules (18).
We have used a technique that allows us to identify all the TCRβ CDR3 sequences of antigen-stimulated T cells. To accomplish this, we first carried out a mixed leukocyte reaction (MLR) using normal cells from the recipient. The activated CD25+CD4+ cells then were sorted to purify the alloreactive T cells. To prepare the GVL-specific T cells, the activated cells in the primary MLR (in which nonleukemic cells from a recipient were used as stimulators) were treated with an anti-CD25 immunotoxin (IT) to eliminate the alloreactive T cells. The remaining allodepleted cells were used in a secondary MLR with leukemia cells from the same recipient. The CD25+ T cells were purified on the cell sorter. The unique TCRβ CDR3 sequences from the GVH- and GVL-specific T cells then were amplified by using anchored RT-PCR and sequenced. In theory, this procedure allowed us to amplify all the TCRβ CDR3 sequences from the GVH- and GVL-specific T cells without knowing which antigens these receptors recognized (19, 20). Based on the known TCRβ CDR3 sequence of an individual T cell clone we prepared and used clone-specific primers and probes to monitor the appearance of GVH- and GVL-specific clones from a recipient undergoing an allogeneic HSCT (20).
Materials and Methods
Recipient Population and Specimen Collection.
After receiving signed, informed consent, anticoagulated peripheral blood specimens were obtained from recipients and healthy donors. All studies involving these blood samples were approved by the Institutional Review Board of the University of Texas Southwestern Medical Center. Four patients had AML and two had acute lymphatic leukemia. Four donors were HLA-mismatched healthy volunteers and one sibling donor was HLA-A, -B, -C, and -DR-matched with one of the AML recipients. This recipient had refractory AML and underwent an allogeneic HSCT from her HLA-matched donor but experienced a hematological relapse 52 days after the transplant.
MLR and Selective Depletion with an Anti-CD25 IT.
Cells were incubated at 37°C in an atmosphere of 5% CO2 in a complete medium consisting of X-VIVO 15 (BioWhittaker) supplemented with 5% heat-inactivated type A+B+ human serum. Only two recipients had sufficient numbers of nonleukemic peripheral blood mononuclear cells (PBMCs) to use as stimulator cells. T cells therefore were isolated from the remaining four recipients by positive selection on anti-CD3 magnetic beads (Miltenyi Biotec, Auburn, CA), expanded by culturing with 5 μg/ml phytohemagglutinin (Sigma–Aldrich) and 100 units/ml IL-2 (R & D Systems), and used as nonleukemic stimulator cells. Donor PBMCs (5 × 107) were activated in a primary one-way MLR for 6 days with 1 × 107 irradiated (25 Gy) nonleukemic cells from the recipient. The cells were harvested, stained with FITC-labeled anti-human CD4, and phycoerythrin-labeled anti-human CD25 mAbs (Becton Dickinson/PharMingen, San Diego). Another 5 × 107 donor PBMCs were activated in a one-way MLR with 1 × 107 irradiated (25 Gy) recipient nonleukemic cells for 24 h, and then the cells were subjected to selective depletion of alloreactive donor T cells by culturing the cells for 24 h with the anti-CD25 IT RFT5-SMPT-dgRTA and 6 mM NH4Cl (21, 22, ¶). The remaining cells were restimulated in a secondary MLR for an additional 6 days by using purified leukemia cells from the same individual as stimulators. The cells then were harvested and stained with FITC-labeled anti-human CD4 and phycoerythrin-labeled anti-human CD25 mAbs. CD4+CD25+ cells from both the primary (GVH-specific) and secondary (GVL-specific) MLRs were sorted on a FACSVantage (Becton Dickinson/PharMingen).
Clonotypic Assay for the Identification, Sorting, and Quantification of Alloreactive CD4+ T Cells.
Rapid, unbiased identification and quantification of specific T cell clones were performed on sorted populations of activated T cells by RT-PCR and quantitative clonotypic PCR of TCRβ CDR3 sequences as described (19). Briefly, mRNA was extracted from the sorted antigen-activated T cells. Template switch-anchored PCR was used to amplify the V(D)J-region sequences. The sequences were used to transform bacteria, and for each PCR product, 50–60 colonies were selected and sequenced to obtain TCRβ CDR3 sequences corresponding to all sorted T cells that had been activated in the MLR (19). Clones were defined by the presence of at least two identical DNA sequences of the TCRβ CDR3. In the case of the HLA-identical recipient/donor pair, the primers and probes specific for each CD4+ clone were prepared by using Applied Biosystems software program PRIMER EXPRESS 1.5, and the real-time quantitative PCR was performed on posttransplant samples by using an Applied Biosystems PRISM 7700 real-time thermal cycler.
Statistics.
All data are expressed as means ± SD. Statistical analysis of all processed data were performed with Microsoft EXCEL software. Comparisons were made by using a two-tailed Student's t test.
Results
Selective Depletion of Alloreactive T Cells Preserves the Antileukemic Activity of the Treated Cells.
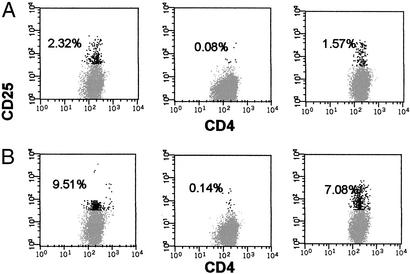
One HLA-matched and eight HLA-mismatched MLRs were performed by using donor PBMCs as responders and irradiated PBMCs or irradiated expanded T cells as stimulators. Smaller numbers of responders were activated in a primary MLR from the HLA-matched pair (2.32% CD4+CD25+ cells) in comparison to HLA-mismatched pairs (10.01 ± 2.98% CD4+CD25+ cells). Alloreactive cells were selectively depleted with an anti-CD25 IT. The remaining allodepleted cells contained 0.15 ± 0.05% CD4+CD25+ cells. Secondary MLRs then were performed by using irradiated leukemic cells as stimulators. There was good preservation of antileukemic reactivity in both HLA-matched (1.57% CD4+CD25+ cells) and HLA-mismatched pairs (6.09 ± 1.65% CD4+CD25+ cells). The GVL responses were similar regardless of whether the AML or acute lymphatic leukemia cells were used as stimulators. Activated T cells were better stimulators than PBMCs in HLA-mismatched pairs (P < 0.01). Fig. 1 shows the flow-cytometric results of the HLA-matched and one of the HLA-mismatched pairs. Table 1 summarizes the results of the MLRs. As can be seen in Table 1, when the donor and recipient were HLA-matched, the percentages of both alloreactive and leukemia-reactive cells were lower, reflecting the fact that there were fewer mismatched antigens.
Figure 1.
Stimulation of alloreactive and leukemia-reactive CD4+ T cells. Flow-cytometric analysis of activated CD4+CD25+ T cells in the primary MLR using nonleukemic cells as stimulators (Left), after the anti-CD25 IT treatment (Center), and in a secondary MLR using leukemic cells as stimulators after selective depletion of alloreactive cells with the anti-CD25 IT (Right). The percentages of activated cells (black dots) are shown. (A) HLA-matched pair. (B) HLA-mismatched pair.
Table 1.
Characteristics of alloreactive and leukemia-reactive CD4+CD25+ T cells
| Pairs studied | Donor no./HLA match | Recipient no./alloreactive stimulators | Alloreactive CD4+CD25+ cells, % | Alloreactive CD4+CD25+ cells after IT treatment, % | Recipient no./leukemic stimulators | Leukemia-reactive CD4+CD25+ cells, % |
|---|---|---|---|---|---|---|
| 1 | 1/Yes | 1/PBMC | 2.32 | 0.08 | 1/AML | 1.57 |
| 2 | 2/No | 2/PBMC | 9.51 | 0.14 | 2/AML | 7.08 |
| 3 | 3/No | 2/PBMC | 6.39 | 0.11 | 2/AML | 4.70 |
| 4 | 4/No | 3/Act. T | 15.26 | 0.22 | 3/AML | 8.12 |
| 5 | 5/No | 4/Act. T | 12.16 | 0.16 | 4/AML | 7.80 |
| 6 | 2/No | 5/Act. T | 7.66 | 0.18 | 5/ALL | 3.87 |
| 7 | 3/No | 5/Act. T | 9.88 | 0.20 | 5/ALL | 6.81 |
| 8 | 4/No | 6/Act. T | 7.30 | 0.12 | 6/ALL | 4.15 |
| 9 | 5/No | 6/Act. T | 11.91 | 0.18 | 6/ALL | 6.15 |
Act. T, expanded activated T cells; ALL, acute lymphatic leukemia.
Clonotypic Analysis with Alloreactive and Leukemia-Reactive Donor CD4+ T Cells.
Activated donor CD4+CD25+ cells were positively sorted from both the primary and secondary MLRs, and clonotypic assays were performed as described in Materials and Methods. The results from all evaluable TCRβ CDR3 sequences are summarized in Table 2. As shown, in the HLA-matched pair there were oligoclonal populations of both alloreactive and leukemia-reactive CD4+ T cells, which indicates that in HLA-matched pairs very few T cell clones are activated. The presence of other individual TCRβ CDR3 sequences may represent minor clones or immunoregulatory CD4+CD25+ cells. One of the clones was present in both the alloreactive and leukemia-reactive sorted cells. This clone must have escaped allodepletion because of low expression of CD25, suggesting that it was activated poorly or slowly in the alloresponse and that it emerged during the secondary MLR against the leukemia cells. This clone may recognize a common minor antigen on both the normal and leukemic cells.
Table 2.
Amino acid sequences of TCRβ CDR3 sequences from individual CD4+ T cell clones identified using the clonotypic assay
| Donor/Pt. pair no. | CDR3 sequence of dominant alloreactive clones
|
CDR3 sequence of dominant leukemia-reactive clones
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total no. of sequences | Total no. of clones | Sequence frequency of alloreactive clones, % | TCRβV | TCRβD | TCRβJ | Total no. of sequences | Total no. of clones | Sequence frequency of leukemic clones, % | TCRβV | TCRβD | TCRβJ | |
| 1 | 55 | 36 | 12 (22) | QGDSAMYLCASS* | PE | AFFGQGTRLTVV | 53 | 26 | 24 (45) | PEDSSFYICSA | SGAD | SGANYLTFGAGSRLTVL |
| 6 (11) | LGDSALYLCASS | DGTGD | EQFFGPGTRLTVL | 4 (8) | LGDSALYLCASS | GTSAS | SYNEQFFGPGTRLTVL | |||||
| 3 (6) | LGDSALYLCASS | GTSAS | SYNEQFFGPGTRLTVL | 2 (4) | PSQTSVYFCASS | PGTG | EQFFGPGTRLTVL | |||||
| 2 (4) | LEDSAMYFCAS | RAPAT | YNSPLHFGNGTRLTVT | |||||||||
| 2 | 51 | 51 | 1 (2) | Polyclonal† | 46 | 36 | 6 (13) | RGDSAAYFCASS | RRTGSG | NEQFFGPGTRLTVL | ||
| 4 (9) | LEDSAMYFCASS | REAF | YGYTFGSGTRLTVV | |||||||||
| 2 (4) | QEDSAVYLCASSL | AGTGS | SGANVLTFGAGSRLTVL | |||||||||
| 2 (4) | PNQTSLYFCASSL | QGVP | EAFFGQGTRLTVV | |||||||||
| 3 | 47 | 47 | 1 (2) | Polyclonal | 49 | 28 | 10 (20) | TNQTSMYLCASI | TRRAG | QYFGPGTRLTVT | ||
| 8 (16) | LGDSALYLCASS | FPGTSG | YNEQFFGPGTRLTVL | |||||||||
| 3 (6) | QDSAMYRCASS | PTMREGE | NEQFFGPGTRLTVL | |||||||||
| 3 (6) | LGDSALYLCASSQ | GS | GYTFGSGTRLTVV | |||||||||
| 2 (4) | PSQTSVYFCAS | GPRPL | ETQYFGPGTRLLVL | |||||||||
| 4 | 49 | 49 | 1 (2) | Polyclonal | 50 | 44 | 5 (10) | PEDSALYLCASSQ | GTGV | NEQFFGPGTRLTVL | ||
| 2 (4) | RGDSAVYLCAS | PGQ | SYNEQFFGPGTRLTVL | |||||||||
| 2 (4) | QEDSAVYLCASSL | RLAG | TDTQYFGPGTRLTVL | |||||||||
| 5 | 50 | 49 | 2 (4) | PEDSSFYICSAR | GTG | NEKLFFGSGTQLSVL | 52 | 34 | 12 (23) | PRDSAVYFCASSL | ALTRGREP | GELFFGEGSRLTVL |
| 1 (2) | Polyclonal | 4 (8) | LEDSAMYFCASS | ATGGLR | YNEQFFGPGTRLTVL | |||||||
| 4 (8) | PSQTSVYFCASSD | RYA | TQYFGPGTRLLVL | |||||||||
| 2 (4) | LEDSAMYFCAS | RTGT | NTGELFFGEGSRLTVL | |||||||||
| 6 | 54 | 54 | 1 (2) | Polyclonal | 52 | 37 | 9 (17) | PNQTALYFCATS | QGAV | YEQYFGPGTRLTVT | ||
| 7 (13) | LGDSALYLCASS | PILD | GELFFGEGSRLTVL | |||||||||
| 2 (4) | LGDTAMYLCAT | RQRAG | EKLFFGSGTQLSVL | |||||||||
| 7 | 51 | 51 | 1 (2) | Polyclonal | 54 | 38 | 14 (26) | PSQTSVYFCASS | RTLG | YEQYFGPGTRLTVT | ||
| 4 (7) | SSQTSVYFCAI | VAEWRTS | NEQFFGPGTRLTVL | |||||||||
| 8 | 50 | 50 | 1 (2) | Polyclonal | 46 | 32 | 10 (22) | PNQTSLYFCASSL | FRLASGT | SYEQYFGPGTRLTVT | ||
| 4 (9) | LGDSALYFCASS | VVKGLGD | EQFFGPGTRLTVL | |||||||||
| 2 (4) | RGDSAAYFCASSP | RSGS | TGELFFGEGSRLTVL | |||||||||
| 2 (4) | PSHTSQYLCASSE | GPVE | QYFGPGTRLTVL | |||||||||
| 9 | 48 | 48 | 1 (2) | Polyclonal | 49 | 37 | 7 (14) | TNQTSMYLCASSL | YGDRGH | GYTFGSGTRLTVV | ||
| 5 (10) | QGDSAMYLCASS | SRGGS | TGELFFGEGSRLTVL | |||||||||
| 3 (6) | LGDSAVYFCASS | GEI | SGNTIYFGEGSWLTVV | |||||||||
Oligoclonal: two or more TCRβ V(D)J sequences are the same in two or more individual clones.
Polyclonal: each of the TCRβ V(D)J sequences in 47–54 clones is different.
In all HLA-mismatched pairs we found polyclonal activation of donor CD4+ T cells, because these different clones represented 96–100% of all TCRβ CDR3 sequences analyzed. In one HLA-mismatched pair (Table 2, no. 5), two sequences (4%) were identical, but the remaining 48 (96%) sequences were different. All these alloreactive cells were depleted by using the anti-CD25 IT, because none of the TCRβ CDR3 sequences of the alloreactive cells were the same as the TCRβ CDR3 sequences obtained from the leukemia-reactive cells. In addition, leukemia-reactive CD4+ cells were oligoclonal, representing 4–23% of individual leukemia-reactive clones (Table 2). Again, the other individual TCRβ CDR3 sequences may represent minor clones or immunoregulatory CD4+CD25+ cells. These findings provide direct evidence for the presence of unique donor CD4+ T cell populations that recognize either the normal or leukemic cells but not both.
Quantitative Posttransplant Monitoring of Alloreactive and Leukemia-Reactive T Cells in a Recipient.
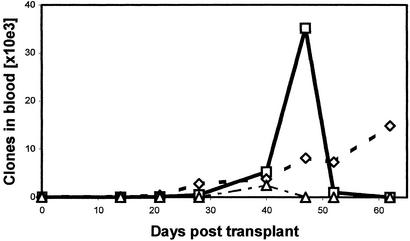
Based on the known sequences of dominant alloreactive and leukemia-reactive CD4+ T cell clones, we were able to construct clone-specific primers and probes to monitor the frequencies of these clones in the peripheral blood of a recipient with refractory AML who was transplanted with hematopoietic stem cells from her HLA-matched sibling donor. Donor cell engraftment of the hematopoietic system was established 30 days after HSCT, but the recipient experienced hematological relapse of her AML at 52 days and died 71 days after the transplant. After the transplant, we were able to study five dominant CD4+ T cell clones using clone-specific quantitative PCR. Three of those clones were detectable and are shown in Fig. 2. The other two clones were undetectable in any of the posttransplant samples. The alloreactive clone (identified originally in 12/55 TCRβ CDR3 sequences in vitro using the clonotypic assay) appeared at low levels in the patient and increased gradually even though the recipient showed no symptoms of GVHD. The leukemia-reactive clone (identified originally in 24/55 TCRβ CDR3 sequences in vitro by using the clonotypic assay) appeared at 28 days and reached its peak value 47 days after the transplant. It then decreased when leukemic relapse was diagnosed 52 days posttransplant and was undetectable at 62 days posttransplant. This suggests that the antileukemia activity of this clone was ineffective at inhibiting tumor growth because (i) it did not expand rapidly enough, (ii) it was of a T cell type that failed to activate a robust cytotoxic T cell response, (iii) it was overwhelmed by rapid growth of leukemic cells, or (iv) the tumor cells were resistant to killing. The third clone that was present in vitro in both the primary and secondary MLR in 3/55 and 4/53 TCRβ CDR3 sequences, respectively, was above the threshold of the quantitative PCR only at 40 days posttransplant. The relevance of this clone is unclear, although as noted it may represent a slowly growing clone that did not express enough CD25 to be eliminated with the IT after the primary MLR.
Figure 2.
Quantitative monitoring of individual clones after HSCT. Clone-specific real-time quantitative PCR was performed with consecutive posttransplant samples from an AML recipient. Absolute numbers of individual alloreactive or leukemia-reactive clones detected in the peripheral blood are shown. □, Leukemia-reactive clone; ⋄, alloreactive clone; ▵, alloreactive and leukemia-reactive clone.
Discussion
This study was designed to determine whether we could identify unique clones of CD4+ T cells that recognized either normal or leukemic cells from the same recipient. Our approach was based on the activation of alloreactive clones or leukemia-reactive clones followed by the sequencing of all the TCRβ CDR3 from each pool of the activated cells. In one instance, these clones were monitored in a transplant recipient. Four major findings emerged from these studies. (i) After a one-way MLR, the selective depletion of alloreactive donor CD25+CD4+ T cell clones by an anti-CD25 IT was highly efficient. The remaining cells could be stimulated with leukemia cells from the same patient. (ii) By sequencing the TCRβ CDR3 from the CD25+CD4+ alloreactive cells and the leukemia-reactive cells, we established that the vast majority of clones that mediated one activity or the other were different from each other. Hence, donor cells that were not stimulated with normal cells but were stimulated with leukemia cells can be physically separated. (iii) In an HLA-matched pair, an oligoclonal population of leukemia-reactive cells was identified, and one of the less-dominant clones was stimulated by both nonleukemic and leukemic cells from the same individual. Hence, this clone escaped the selective depletion with anti-CD25 IT and probably was a slowly growing clone with a low density of CD25. It most likely recognized a minor antigen present on both the normal and leukemic cells from the recipient. These data are in agreement with published studies (6, 11) and provide direct evidence for the presence of three clonal populations: alloreactive, leukemia-reactive, and both alloreactive and leukemia-reactive CD4+ T cells. (iv) The appearance of the alloreactive and leukemia-reactive clones could be followed in a transplant recipient. Importantly, although the leukemia-reactive clone appeared in the recipient, it was unable to prevent leukemic relapse either because it failed to activate a sufficiently robust population of cytotoxic lymphocytes or the tumor cells were growing too rapidly to be controlled by any type of T cell response.
A large body of evidence supports the existence of different T cells that are reactive with either normal or malignant cells. In the allogeneic setting, it is possible to identify T cell lines that react with leukemic blasts but not with normal hematological cells from the same individual (23). In HLA-identical siblings, cytotoxic T lymphocytes that kill leukemic or nonleukemic targets have also been isolated (24). Furthermore, experiments in mice have demonstrated clearly that GVL and GVH reactivity are separable (25). In another study, clones with three different reactivities were identified: clones reacting only against normal PBMCs, clones reacting only against leukemic cells, and clones reacting against both (6). This finding was also confirmed in another in vitro model where allogeneic T cells selectively killed Philadelphia chromosome-bearing human leukemia lines (26). Similar results have been demonstrated by using minor histocompatibility antigen-specific cytotoxic T cells in both animal and human studies (18, 27, 28). In an animal model it has been shown that donor T cells primed against a single known minor histocompatibility antigen caused no GVHD but produced a curative antileukemic response (27). Most of these studies focused on cytotoxic CD8+ T cells but not the CD4+ T cells. Many of these CD4+ T cells, and particularly those of the T helper 1 type may be involved in activating CD8+ T cells. In addition, CD4+ T cells may have cytotoxic activity by virtue of the cytokines that they secrete (18, 29, 30). In published studies, the identification of clonal T cell responses has been accomplished by flow cytometry using mAbs directed against TCRβ V families or by TCRβ CDR3 spectratyping (11, 31). In the case of flow cytometry, the series of mAbs is not complete enough to analyze all TCRβ V families, and in the case of spectratyping, TCRβ V subfamilies but not individual T cell clones within these families can be identified and monitored (11). Using tetramers for tracking specific T cell clones requires prior knowledge of the antigen and is restricted by certain HLA class I molecules (32).
In our study we could identify all individual TCRβ CDR3 sequences based on antigen stimulation in an MLR and anchored RT-PCR. Thus, the amplified products comprise all of the TCRβ CDR3 sequences expressed in the alloreactive or leukemia-reactive cells. These individual TCRβ CDR3 sequences most likely represent the entire spectrum of CD4+ T cell clones from the donor activated by either normal or malignant cells from the host. The polyclonal alloresponse in HLA-mismatched pairs is not surprising because of the large numbers of different HLA and minor histocompatibility antigens present on cells from the recipient. In the case of the HLA-matched pair, the alloresponse was oligoclonal because of fewer differences in histocompatibility antigens on the cells of the donor versus the host. This finding is in agreement with data obtained from another 10 recipients undergoing HSCT from their HLA-matched siblings where the alloresponses were also oligoclonal.‖ When allodepleted donor cells were restimulated with leukemic cells there was an oligoclonal expansion of CD4+ T cells in both the HLA-mismatched and HLA-matched pairs. All the leukemia-reactive clones and the alloreactive clones from the same HLA-mismatched pairs were different. The results of our studies strongly suggest that allodepleted T cell transplants should exert GVL effects but rarely cause GVHD. Thus far, data from one clinical trial using IT-depleted cells support this possibility (33). Further clinical trials will be required to confirm and extend these findings in different transplant settings. Finally, the results of our studies suggest that the expansion and reinfusion of these GVL-specific CD4+ T cells may be useful for immunotherapeutic purposes.
Acknowledgments
We thank Dr. Laurie Davis for helping us set up the MLR; Dr. Victor Ghetie for preparing the IT; and Drs. Michael Bennett and Jonathan Uhr for reviewing the paper. We acknowledge Ms. Shannon Flowers for assistance in preparing the manuscript. These studies were partially supported by the Leukemia Association of North Central Texas.
Abbreviations
- HSCT
hematopoietic stem cell transplantation
- GVH
graft versus host
- GVHD
GVH disease
- GVL
graft versus leukemia
- AML
acute myeloid leukemia
- TCR
T cell receptor
- CDR3
complementarity-determining region 3
- MLR
mixed leukocyte reaction
- IT
immunotoxin
- PBMC
peripheral blood mononuclear cell
Footnotes
Michálek, J., Collins, R. H., Hill, B. J. & Vitetta, E. S. (2001) Blood 98, 664a (abstr.).
Michálek, J., Collins, R. H. & Hill, B. J. (2000) Blood 96, 312b (abstr.).
References
- 1.Ferrara J L M, Deeg H J. N Engl J Med. 1991;324:667–674. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 2.Murphy W J, Blazar B. Curr Opin Immunol. 1999;11:509–515. doi: 10.1016/s0952-7915(99)00002-3. [DOI] [PubMed] [Google Scholar]
- 3.Horowitz M M, Gale R P, Sondel P M. Blood. 1991;75:555–562. [PubMed] [Google Scholar]
- 4.Barrett A J, Malkovaska V. Br J Haematol. 1996;93:754–761. doi: 10.1046/j.1365-2141.1996.d01-1713.x. [DOI] [PubMed] [Google Scholar]
- 5.Barrett A J, Mavroudis D, Tisdale J, Molldrem J, Clave E, Dunbar C, Cottler-Fox M, Phang S, Carter C, Okunnieff P, et al. Bone Marrow Transplant. 1998;21:543–551. doi: 10.1038/sj.bmt.1701131. [DOI] [PubMed] [Google Scholar]
- 6.van Lochem E, de Gast B, Goulmy E. Bone Marrow Transplant. 1992;10:181–183. [PubMed] [Google Scholar]
- 7.Champlin R. Exp Hematol (Charlottesville, Va) 1995;23:1148–1153. [PubMed] [Google Scholar]
- 8.Weiden P L, Flournoy N, Thomas E D, Prentice R L, Fefer A, Buckner C D, Storb R. N Engl J Med. 1979;300:1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 9.Nash R, Storb R. Curr Opin Immunol. 1996;8:674–680. doi: 10.1016/s0952-7915(96)80085-9. [DOI] [PubMed] [Google Scholar]
- 10.Kolb H J, Schattenberg A, Goldman J M, Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A, Verdonck L, Niederwieser D, et al. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 11.Maslanka K, Piatek T, Gorski J, Yassai M. Hum Immunol. 1995;44:28–34. doi: 10.1016/0198-8859(95)00056-a. [DOI] [PubMed] [Google Scholar]
- 12.Collins R H, Jr, Shpilberg O, Drobyski W R, Porter D L, Giralt S, Champlin R, Goodman S A, Wolff S N, Hu W, Verfaillie C, et al. J Clin Oncol. 1997;15:433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 13.Bar B M A M, Schattenberg A, Mensink E J B M. J Clin Oncol. 1993;11:513–519. doi: 10.1200/JCO.1993.11.3.513. [DOI] [PubMed] [Google Scholar]
- 14.MacKinnon S, Papadopoulos E B, Carabasi M H. Blood. 1995;86:1261–1265. [PubMed] [Google Scholar]
- 15.Jiang Y Z, Mavroudis D, Dermime S, Molldrem J, Hensel N F, Barrett A J. Bone Marrow Transplant. 1997;19:899–903. doi: 10.1038/sj.bmt.1700769. [DOI] [PubMed] [Google Scholar]
- 16.Epperson D E, Margolis D A, McOlash L, Janczak T, Barrett J. Br J Haematol. 2001;114:57–62. doi: 10.1046/j.1365-2141.2001.02879.x. [DOI] [PubMed] [Google Scholar]
- 17.Claret E J, Alyea E P, Orsini E, Pickett C C, Collins H A, Wang Y, Neuberg D, Soiffer R, Ritz J. J Clin Invest. 1997;100:855–866. doi: 10.1172/JCI119601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falkenburg J H, Marijit W A F, Heemskerk M H M, Willemze R. Curr Opin Hematol. 2002;9:497–502. doi: 10.1097/00062752-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Douek D C, Betts M R, Brenchley J M. J Immunol. 2002;168:3099–3104. doi: 10.4049/jimmunol.168.6.3099. [DOI] [PubMed] [Google Scholar]
- 20. Michalek, J., Collins, R. H., Hill, B. J., Brenchley, J. M. & Douek, D. C. (2003) Lancet, in press. [DOI] [PubMed]
- 21.Montagna D, Yvon E, Calcaterra V, Comoli P, Locatelli F, Maccario R, Fisher A, Cavazzana-Calvo M. Blood. 1999;93:3550–3557. [PubMed] [Google Scholar]
- 22. Solomon, S. R., Tran, T., Carter, C. S., Donelly, S., Hensel, N., Schindler, J., Bahceci, E., Ghetie, V., Michalek, J., Mavroudis, D., Read, E. J., et al. (2003) Cytotherapy, in press. [DOI] [PubMed]
- 23.Salomo M, Steinmann J, Glass B, Herwartz C, Uharek L, Gassmann W, Muller-Ruchholtz W. Bone Marrow Transplant. 1995;15:179–186. [PubMed] [Google Scholar]
- 24.Hoffmann T, Theobald M, Bunjes D, Weiss M, Heimpel H, Heit W. Bone Marrow Transplant. 1993;12:18. [PubMed] [Google Scholar]
- 25.Glass B, Uharek L, Gassmann W, Focks B, Bolouri H, Loeffler H, Muller-Ruchholtz W. Ann Hematol. 1992;64:255–259. doi: 10.1007/BF01695466. [DOI] [PubMed] [Google Scholar]
- 26.Oettel K R, Wesly O H, Albertini M R, Hank J A, Iliopolis O, Sosman J A, Voelkerding K, Wu S Q, Clark S S, Sondel P M. Blood. 1994;83:3390–3402. [PubMed] [Google Scholar]
- 27.Fontaine P, Roy-Proulx G, Knafo L, Baron C, Roy D-C, Perreault C. Nat Med. 2001;7:789–794. doi: 10.1038/89907. [DOI] [PubMed] [Google Scholar]
- 28.Dickenson A M, Wang X N, Sviland L, Vyth-Dreese F A, Jackson G H, Schumacher T N. Nat Med. 2002;8:410–414. doi: 10.1038/nm0402-410. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Y Z, Kanfer E J, Macdonald D, Cullis J O, Goldman J M, Barrett A J. Bone Marrow Transplant. 1991;8:253–258. [PubMed] [Google Scholar]
- 30.Faber L M, van Luxemburg-Heijs S A, Veenhof W F, Willemze R, Falkenburg J H. Blood. 1995;86:2821–2828. [PubMed] [Google Scholar]
- 31.Verfuerth S, Peggs K, Vyas P, Barnett L, O'Reilly R J, Mackinin S. Blood. 2000;95:3990–3995. [PubMed] [Google Scholar]
- 32.Altman J D, Moss P A, Goulder P J, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 33.Andre-Schmutz I, Le Deist F, Hacein-Bey-Abina S, Vitetta E, Schindler J, Chedeville G, Vilmer E, Fischer A, Cavazzana-Calvo M. Lancet. 2002;360:130–137. doi: 10.1016/S0140-6736(02)09413-8. [DOI] [PubMed] [Google Scholar]