Abstract
T cell antigen receptor (TCR) diversity is a critical feature of adaptive immunity. However, restriction of TCR diversity is a potential risk during immune reconstitution by homeostatic proliferation. What peripheral mechanisms are in place to maintain TCR diversity during recovery from lymphopenia? Here, we examine competition between several monoclonal CD4 T cell populations in RAG−/− and TCR Tg RAG−/− environments. The results suggest that specific self ligands constitute a critical limiting resource essential for homeostatic proliferation of naive CD4 T cells. In addition, T cells ignore large numbers of competitors as long as their TCR specificity is different and other non-MHC resources are not limiting. Therefore, the numbers of self ligands expressed in the periphery set the limits on TCR diversity.
The total size of the peripheral T cell population is under strict homeostatic control. Different subsets of T cells, such as αβ versus γδ T cells or naive versus memory T cells, constitute separate compartments and are regulated independently (1–4). A concept of “T cell homeostatic space” emerged in recent years, which suggests that the size of each T cell compartment is controlled by its constituent members. The T cells seem to be under severe pressure to fill this space. Thus, their numbers expand after adoptive transfer into various T cell-deficient hosts and during the recovery phase after partial T cell depletion. Indeed, in these scenarios, the T cell population size can return to normal through homeostatic expansion in the absence of a thymus. However, the precise mechanisms of how T cells perceive absence or availability of homeostatic space remain unclear. There are at least two broad and nonmutually exclusive models that could account for homeostatic proliferation (1, 2, 5, 6). The first model presumes that T cells are in constant competition for limiting stimulatory signals. The second proposes continuous T cell-mediated inhibitory signals. Both models predict little proliferation at the steady state. However, induction of lymphopenia would lead to an increase of stimulatory signals and/or a decrease of inhibitory signals, and drive T cell proliferation until the state of equilibrium is reestablished.
Both models provide a very dynamic picture of T cell homeostasis at equilibrium. Individual T cells are not simply “resting” and awaiting antigen encounter, but are under continuous selective pressure to persist within a limited space occupied by many competitors. Such competition among individual T cells has been demonstrated in mixed bone marrow chimeras and parabionts (7). Different T cells can be ranked in their ability to compete in these systems. Nontransgenic polyclonal T cells dominate over T cell antigen receptor (TCR) transgenic (Tg) T cells, and different TCR Tg populations show a clear hierarchy to become dominant over others. Different T cells can be ranked in their ability to undergo homeostatic proliferation. For example, OT-II and anti-HY TCR Tg T cells are virtually unable to expand in lymphopenic hosts, whereas other TCR Tg T cells display a range of capacities to undergo homeostatic proliferation (5, 7, 8). The apparent heterogeneity in the abilities of different T cells to compete with others raises a potential problem. How does the immune system maintain diversity among its T cells and not become dominated over time by a few of the best-competing clones, especially under conditions of limited thymic output?
One essential signal for homeostatic proliferation is provided by TCR stimulation by self peptide/MHC complexes (1–4, 9). In fact, the heterogeneity among different TCR Tg T cells in their ability to undergo homeostatic proliferation may simply be a reflection of the abundance of peptide/MHC epitopes and strength of their interaction with TCRs (10). However, large numbers of bystander T cells cotransferred into irradiated lymphopenic mice can inhibit homeostatic proliferation independently of their TCR specificity or the ability to undergo homeostatic proliferation on their own (5, 8). These results suggest that self peptide/MHC molecules do not represent a limiting resource for T cells. Instead, T cells may control the size of their compartment through competition for local non-MHC stimulatory factors or direct inhibitory T–T cell interactions (5). The dominance of these non-MHC stimuli may lead to a degree of “democratization” among different T cell clones, whereby those best at proliferation may be restrained by a mediocre majority. This type of regulation by bystander T cells would seem to ensure TCR diversity, at least at a steady state. However, it does not provide a mechanism to limit dominance by a few T cell clones that happen to be the best proliferators under lymphopenic conditions.
To explore this problem, we tested whether resident T cells present in large numbers in TCR Tg RAG−/− mice, but all expressing an identical TCR, would be sufficient to inhibit homeostatic proliferation. Surprisingly, both TCR Tg RAG−/− and plain RAG−/− mice supported similar degrees of homeostatic proliferation as long as the TCR of the resident T cells differed from the TCR of transferred T cells. However, no homeostatic proliferation took place if the TCR of transferred and resident T cells was the same. In addition, the duration of the proliferative burst was very brief and resulted in a relatively small final population of T cells when compared with the baseline numbers of resident T cells seen in TCR Tg RAG−/− mice. These experiments suggest that self peptide/MHC complexes represent the most limiting resource for homeostatic expansion in this simple system. Furthermore, naive T cells of one self peptide/MHC specificity may not need to compete with T cells of another specificity until some other resource required by both T cell populations becomes limiting. Certainly, this limit is not reached in the TCR Tg RAG−/− mice. We propose that the minimal diversity of the TCR repertoire maintained in the absence of the thymus may be determined by the number and density of different self peptide/MHC complexes expressed in the host.
Materials and Methods
Mice.
The DO11.10 (11) and HA (12) TCR Tg mice, extensively backcrossed (>15 generations) onto the BALB/c background, were bred onto RAG-2−/− BALB/c background (Taconic Farms). Thy1.1 RAG-2−/− HA TCR Tg mice were generated by breeding with Thy1.1 BALB/c mice generously provided by L. Turka (University of Pennsylvania, Philadelphia). SM-1 TCR Tg RAG-2−/− mice (13), specific for the Salmonella typhimurium flagellin peptide 427–441/I-Ab, were made on the C57BL/6 background, and were generously provided by S. McSorley (University of Connecticut, Farmington). Thy1.1 RAG-2−/− SM-1 TCR Tg and OT-II TCR Tg (14) were generously provided by M. Jenkins (University of Minnesota). OT-II TCR Tg mice were bred onto RAG-1−/− C57BL/6 and Thy1.1 backgrounds (The Jackson Laboratory). All animals were maintained in a specific pathogen-free facility in microisolator cages with filtered air according to National Institutes of Health guidelines. In some experiments, mice received 600 cGy of sublethal irradiation from a cesium source. These mice were maintained on antibiotic water (neomycin sulfate, 2 mg/ml).
Adoptive Transfer.
Unless otherwise specified, donor CD4 T cells collected from secondary lymphoid tissues (axillary, brachial, cervical, mesenteric, inguinal lymph nodes, and spleen) were labeled with 5,6-carboxyfluorescein diacetate succinimyl ester (CFSE; Molecular Probes) by using a technique previously described (15), and injected intravenously at 2–5 × 106 cells per recipient. Competitor polyclonal CD4 T cells were prepared by negative selection by using magnetic microbeads (Miltenyi Biotec, Auburn, CA), depleting cells expressing HSA (CD24), B220, MHC class II, and CD8.
Detection of IL-2 Production by Antigen-Specific T Cells in Vivo.
DO11.10 RAG−/− T cells were adoptively transferred into specified recipients 1 day before the assay. The basic protocol has been described (16, 17). Four hours after the mice were injected with 250 μg of the ovalbumin 323–339 peptide, their spleens were placed in 2% formaldehyde. Single cell suspensions were fixed for 20 min and washed with PBS. The cells were then permeabilized with a buffer containing 0.3% saponin and 25% FCS, and stained with phycoerythrin (PE)-labeled anti-IL-2 (BD PharMingen), CyChrome-labeled anti-CD4 (BD PharMingen), and FITC-labeled KJ1-26 mAbs.
Flow Cytometry.
Recipient mice were killed at indicated time points. Single cell suspensions were prepared separately from spleen and lymph nodes. The T cells were stained by using biotin-labeled mAbs, KJ1-26 or 6.5 (generously provided by H. Levitsky, Johns Hopkins University) followed by SA-PE, PE-labeled anti-Thy1.1 or Thy1.2, and CyChrome-labeled anti-CD4 (BD PharMingen). Dendritic cells (DCs) were released with 20-min Collagenase D digestion (Roche Molecular Biochemicals) and 1-min exposure to 5 mM EDTA. DCs were identified by PE-labeled anti-CD11c mAb, and divided into lymphoid or myeloid subgroups by CyChrome-labeled anti-CD8α mAb (PharMingen). DCs were further stained with FITC-labeled anti-MHC class II, anti-CD80, anti-CD86 mAbs (PharMingen). BrdUrd content was measured by using the BrdUrd Flow Kit (PharMingen); mice were injected i.p. daily with 1 mg of BrdUrd (Sigma) for indicated periods.
Immunohistochemistry.
Spleens were fresh frozen in OCT embedding media (Miles) and sectioned (8 μm) in a cryostat. Glass slides were air-dried, fixed in acetone for 15 min, and rehydrated in PBS. Sections were incubated at room temperature with 1% hydrogen peroxide, followed by blocks with serum and anti-CD16 mAb. Endogenous biotin was blocked by using avidin/biotin blocking kit (Vector Laboratories). Sections were sequentially stained with anti-CD11c, KJ1-26, and B220, and developed by using alkaline phosphatase and peroxide substrates (Vector Laboratories). The slides were dehydrated in ethanol, air dried, and mounted by using VectaMount permanent mounting medium (Vector Laboratories).
Results
Competition for the Self Peptide/MHC Complexes, Not T–T Cell Interactions, Can Limit Homeostatic Proliferation in TCR Tg RAG−/− Mice.
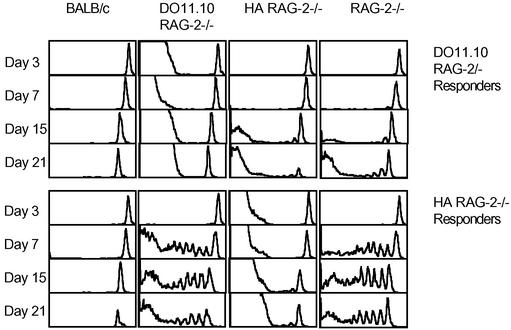
We have bred a number of MHC class II-restricted TCR Tg mice (DO11.10, HA, OT-II, and SM-1) onto the RAG−/− background. All these mice have obviously reduced size of secondary lymphoid organs (lymph nodes and spleen) primarily because they lack all B cells, CD8 T cells, memory CD4 T cells, and γδ T cells. However, they all maintain a relatively large naive CD4 T cell population size (Table 1). To test whether their naive T cell compartments are “full,” we measured the ability of naive T cells to undergo homeostatic proliferation within their secondary lymphoid organs. Mature lymph node cells and splenocytes from DO11.10 RAG−/− or HA RAG−/− transgenics were labeled with CFSE and transferred into normal BALB/c, DO11.10 RAG−/−, HA RAG−/−, and plain RAG−/− recipients. To our surprise, we found that DO11.10 T cells proliferated at least as well in HA RAG−/− as in plain RAG−/− recipients (Fig. 1), but did not undergo homeostatic proliferation in the DO11.10 RAG−/− recipients. Similarly, HA T cells proliferated well in RAG−/− and DO11.10 RAG−/−, but not HA RAG−/− recipients (Figs. 1 and 2A).
Table 1.
Relative rates of proliferation by CD4 T cells in TCR Tg RAG−/− mice at steady state are similar to naive CD4 T cells in normal mice
| Mouse strain | No. of naive CD4 T cells × 10−6 ± SE | Daily rate of BrdUrd incorporation, % BrdUrd + cells ± SE |
|---|---|---|
| C57BL/6 | 41.0 ± 13.1 | 1.46 ± 0.39 |
| BALB/c | 46.0 ± 3.8 | 0.94 ± 0.11 |
| DO11.10 RAG-2−/− | 24.7 ± 1.4 | 0.72 ± 0.29 |
| HA RAG-2−/− | 15.1 ± 3.9 | 0.78 ± 0.07 |
| OT-II RAG-1−/− | 8.9 ± 2.8 | 1.40 ± 0.58 |
| SM-1 RAG-2−/− | 22.4 ± 6.5 | 0.97 ± 0.30 |
Average numbers (from six to nine mice) of naive T cells within spleens and lymph nodes in different strains are indicated. CD45RB expression was used to define naive T cells in normal mice. Mice were injected daily with BrdUrd for 7–10 days. The fraction of naive T cells that incorporated BrdUrd was measured by flow cytometry. An average daily rate of BrdUrd incorporation from four individual mice in each group is shown.
Figure 1.
Homeostatic proliferation of naive CD4 T cells in RAG−/− hosts is not limited by monoclonal competitors expressing a different TCR. CFSE-labeled DO11.10 RAG−/− or HA RAG−/− CD4 T cells were transferred into indicated recipients, 5 × 106 cells per mouse, on day 0. Histograms show CFSE content of transferred cells identified with anti-CD4 and anti-clonotypic mAbs, KJ1-26 and 6.5 for DO11.10 and HA T cells, respectively, in spleens of recipients on indicated days. The data are representative of three independent experiments.
Figure 2.
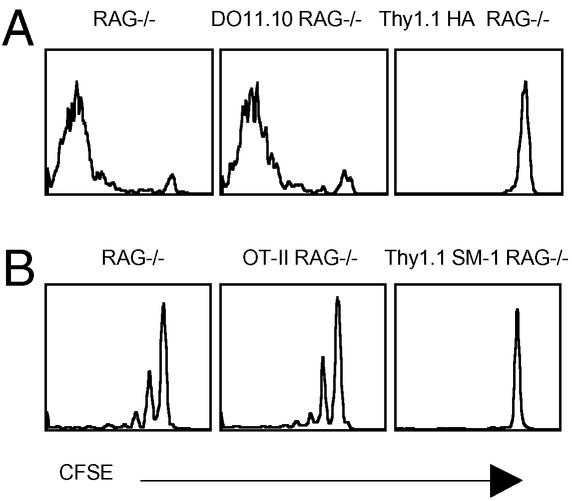
Naive CD4 T cells compete with each other for specific self peptide/MHC complexes. (A) CFSE content of Thy1.2 HA RAG−/− CD4 T cells 7 days after transfer into RAG−/−, DO11.10 RAG−/−, and Thy1.1 HA RAG−/− recipients, 2 × 106 per mouse. (B) CFSE content of Thy1.2 SM-1 RAG−/− CD4 T cells 14 days after transfer into RAG−/−, OT-II RAG−/−, and SM-2 RAG−/− recipients, 5 × 106 cells per mouse. All recipients were Thy1.1 congenics. The results were identical in spleens and lymph nodes. The data are representative of two independent experiments.
These results suggest that endogenous T cells have to compete with each other for access to MHC, although it was formally possible that competition was for access to distinct MHC molecules (I-Ad versus I-Ed) rather than specific peptide/MHC complexes. Therefore, we repeated the experiment by using a different TCR Tg, SM-1 RAG−/−, which was made on the C57BL/6 background. CFSE-labeled SM-1 Thy1.2 cells were transferred into plain RAG−/−, OT-II Thy1.1 RAG−/−, or SM-1 Thy1.1 RAG−/− recipients. The reciprocal experiment, transfer of OT-II cells into different hosts, was not done because OT-II cells are known poor homeostatic proliferators (5, 8). Once again, SM-1 T cells did not proliferate in the SM-1 recipients, but did equally well in the RAG−/− versus OT-II TCR Tg recipients (Fig. 2B). Because C57BL/6 mice lack I-E molecules, we conclude that, in our system, homeostatic proliferation is limited by competition for specific peptide/MHC complexes with bystander T cells.
Interestingly, the DO11.10, HA, and SM-1 T cells had distinct “signatures” of CFSE dilution kinetics (Figs. 1 and 2). DO11.10 T cells were slow to enter cell cycle initially, but proliferated vigorously after the first 2 wk after transfer. In contrast, HA T cells entered cell cycle within the first week, but did not change their CFSE profile significantly thereafter. The reason for these individual differences is unclear. It is possible that a critical number of encounters with self peptide/MHC complexes must occur before commitment to the first cell division, but requirements for further TCR stimulation may be lower for subsequent cell divisions. It is also possible that different T cell clones vary in their requirements for costimulatory signals or ability to respond to growth factors. For example, it has been shown that the extensive proliferation by a fraction of DO11.10 RAG−/− cells could be blocked by cytotoxic T lymphocyte-associated antigen 4 (CTLA-4)-Ig (18), although we found no defect in homeostatic proliferation by DO11.10 CD28−/− T cells (19). In any case, the distinctive signatures of all of the TCR Tg T cells in the experiments reported here were identical in the RAG−/− and TCR Tg RAG−/− recipients. Therefore, endogenous CD4 T cell bystanders were completely ignored unless they had the same TCR.
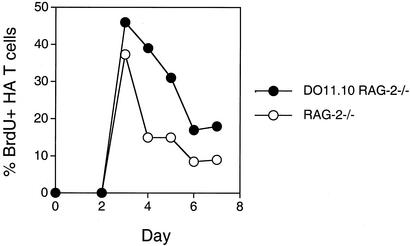
Despite the different latency periods before entering the first cell cycle displayed by the different TCR Tg T cells, kinetics of changes in CFSE profiles suggested that, in all cases, the proliferative bursts were of short duration. This impression was further confirmed by the following BrdUrd-labeling experiment (Fig. 3). HA T cells were transferred on sequential days into plain RAG−/− and DO11.10 RAG−/− recipients. BrdUrd was administered i.p. a day before killing. BrdUrd incorporation was maximal on day 3 after the adoptive transfer and declined significantly over the next several days in both recipients (Fig. 3). The total population of HA cells in the spleen stabilized at a mere one percent of the resident DO11.10 T cell population after completion of the proliferative burst. The final population size was similarly small after transfer of DO11.10 T cells and SM-1 cells into plain RAG−/− mice or TCR Tg RAG−/− mice. Furthermore, as reported previously (18, 20), we found that transferring decreasing numbers of TCR Tg T cells increased the extent of homeostatic proliferation (data not shown). The most likely explanation for this result is that homeostatic proliferation stops when peptide/MHC complexes become limiting. In contrast, the population size of resident T cells in the TCR Tg RAG−/− is maintained at much higher levels because of continuous thymic output and potentially altered requirements for peptide/MHC stimulation for survival versus expansion (21–25).
Figure 3.
Individual TCR Tg populations undergo a brief burst of homeostatic proliferation in RAG−/− mice and ignore resident TCR Tg cells with a different TCR. HA RAG−/− CD4 T cells, 5 × 106 cells per mouse, were transferred into plain RAG−/− or DO11.10 RAG−/− recipients on different days before injection with BrdUrd. All mice were killed 24 h after BrdUrd injection. BrdUrd content in the HA RAG−/− CD4 T cells was measured by flow cytometry. Only 1–2% of resident DO11.10 T cells incorporated BrdUrd. The data are representative of three independent experiments.
The pattern of relatively brief proliferative bursts and variable periods of latency before initiation of proliferation seen with the monoclonal TCR Tg populations contrasts with steady and progressive proliferation seen with polyclonal CD4 T cells. In fact, homeostatic proliferation of polyclonal CD4 T cells into T cell-deficient animals results in complete reconstitution of CD4 T cell numbers (2, 26). Indeed, we observed this behavior with the polyclonal CD4 T cells in our experiments and found similar or better proliferation of polyclonal cells in TCR Tg RAG−/− hosts as in plain RAG−/− hosts (data not shown). It is likely that the polyclonal population is made up of T cells with a full range of proliferative patterns; thus, the overall rate of homeostatic proliferation seems relatively slow and steady because the patterns of all individual T cells are averaged. In addition, the polyclonal CD4 T cells are stimulated by much greater number of peptide/MHC complexes and therefore their population can reach much greater size.
DC Function Is Comparable in RAG-2−/− and TCR Tg RAG-2−/− Mice.
One possible difficulty in making comparisons between plain RAG-2−/− and TCR Tg RAG-2−/− environments is potential effects of bystander T cells on the antigen-presenting cell (APC) function. One could envision that T cell–DC interactions may improve the lymphoid architecture or promote DC function. It is also possible that TCR Tg RAG−/− may be exposed to a greater number of environmental antigens from the gut due to improved maturation and sampling ability by the Peyer's patches (27). However, we found little evidence that any of these considerations were playing an important role in our system. Immunohistology of RAG−/− and TCR Tg RAG−/− spleens showed well-organized white pulp around central arterioles (data not shown). Myeloid (CD8a−) and lymphoid (CD8a+) DCs were present in similar proportions in both kinds of mice and were distributed similarly [lymphoid DCs located centrally and myeloid DCs peripherally with respect to the central arterioles (28)]. Transferred T cells localized in areas dominated by the lymphoid DCs in the TCR Tg RAG−/− and RAG−/− mice. In addition, surface expression of class II MHC, CD80, and CD86 molecules by myeloid and lymphoid DCs was equivalent in normal BALB/c, RAG−/−, and TCR Tg RAG−/− animals (data not shown).
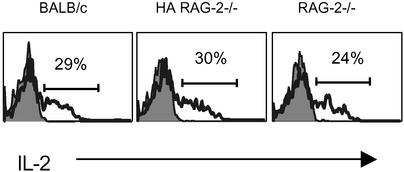
Although we could not find an obvious phenotypic difference in the DC compartments among the strains of mice used in our experiments, we also tested whether T cells can sense a difference in APC function toward cognate antigen in different hosts. We focused on early T cell responses after antigen encounter such as production of IL-2. DO11.10 RAG−/− T cells were transferred into RAG−/−, HA RAG−/−, and BALB/c recipients. The next day, a time point much earlier that the start of homeostatic proliferation for DO11.10 cells, all recipients were given a bolus i.v. injection of the ovalbumin peptide. We have shown previously that antigen-specific T cells respond in total synchrony toward this type of antigenic stimulation and that measurements of biochemical activity in the T cells can be conducted within minutes directly ex vivo (16, 29). In fact, all three environments were essentially identical in the amplitude and kinetics of cytokine production by antigen-specific T cells (Fig. 4).
Figure 4.
APCs present peptide antigen with comparable efficiency in normal, RAG−/−, and TCR Tg RAG−/− mice. Ovalbumin peptide was injected into mice that 1 day prior were transferred DO11.10 RAG−/− cells, 5 × 106 per mouse. Spleens were removed 4 h after the peptide injection and fixed in 2% formaldehyde. Heavy line histograms show intracellular staining of CD4+KJ1-26+ cells for IL-2. Shaded histograms show peptide uninjected controls. The experiment is representative of at least five independent experiments.
Homeostatic Proliferation of Naive CD4 T Cells in Sublethally Irradiated Normal Mice Versus Unirradiated RAG−/− Mice.
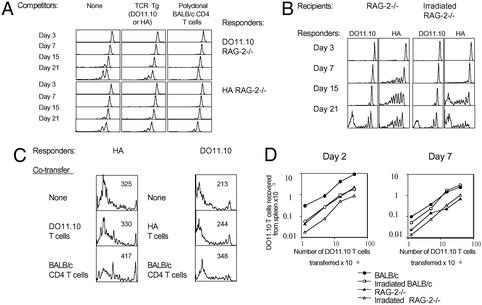
Our results stand in apparent contradiction to previous work on homeostatic proliferation that used sublethally irradiated mice as lymphopenic hosts. For example, it was found by Surh and coworkers (5, 8) that cotransfer of large numbers of competitor naive T cells does inhibit homeostatic proliferation of T cells with unrelated TCR specificity. Therefore, we performed a similar experiment by using CFSE-labeled HA or DO11.10 RAG−/− populations as “responders” and sublethally irradiated BALB/c mice as hosts (Fig. 5A). Large numbers of purified BALB/c CD4 T cells or DO11.10 or HA RAG−/− cells were used as competitors. The most striking difference between using sublethally irradiated normal mice and unirradiated RAG−/− mice is the considerably lesser degree of homeostatic proliferation seen in the former (Fig. 1 versus Fig. 5A). In an attempt to understand this result, we tested several operational variables.
Figure 5.
Homeostatic proliferation of naive CD4 T cells is greater in RAG−/− than irradiated normal recipients and cannot be inhibited by cotransferred nonspecific T cells. (A) CFSE content of DO11.10 (Upper) or HA (Lower) RAG−/− CD4 T cells (5 × 106 per mouse), transferred into normal irradiated (600 cGy) BALB/c mice, was followed over the indicated time course. The responder T cells were transferred alone or together with 50 × 106 competitors that were either polyclonal BALB/c CD4 T cells or TCR Tg cells (HA T cells as competitors for DO11.10 responders, and DO11.10 T cells for HA responders). This is one of two representative experiments. (B) Homeostatic proliferation of DO11.10 or HA T cells was measured in normal RAG−/− mice or irradiated (600 cGy) RAG−/− mice. (C) Large numbers of competitor cells (50 × 106 per mouse) were cotransferred along with HA or DO11.10 responders into RAG−/− mice. CFSE content of HA cells was measured on day 7 after transfer, and CFSE content of DO11.10 cells was measured on day 20 after transfer. The numbers shown in the upper right of each histogram represent geometric mean fluorescence intensity. This is one of two representative experiments. (D) Varying numbers of DO11.10 T cells were transferred into indicated recipients. The graphs show numbers of DO11.10 T cells recovered from spleens on days 2 or 7 after transfer.
Sublethal irradiation of RAG−/− recipients resulted in substantial loss of APCs (data not shown), and slowed down initial homeostatic proliferation of the HA T cells (Fig. 5B). Little effect was observed on the normally slower DO11.10 T cells. By week 3, the HA T cells divided more times in the irradiated RAG−/− hosts. This, as shown below, was most likely due to the limited ability of irradiated lymphoid organs to accommodate transferred T cells, and the smaller starting population in the irradiated hosts ultimately divided out more times.
Cotransfers of large numbers of bystander TCR Tg RAG−/− T cells had no effect on homeostatic proliferation of responders in RAG−/− recipients, whereas cotransfers of normal polyclonal CD4 T had a reproducible, but relatively trivial effect (Fig. 5C). As previously reported, we did observe that cotransfers of normal CD4 T cells inhibited homeostatic proliferation in the sublethally irradiated normal mice (Fig. 5A). However, the ability of TCR Tg RAG−/− bystander T cells to inhibit homeostatic proliferation was more limited or nonexistent. It is important to note that recipient mice have a limited ability to accommodate transferred T cells (Fig. 5D). To test the “parking” efficiency in different hosts, we used DO11.10 RAG−/− T cells, which begin homeostatic proliferation late. Initially, normal BALB/c recipients contained the most transferred DO11.10 cells. However, unlike the irradiated BALB/c mice, normal recipients lost the majority of transferred cells within a week. Ultimately, sublethally irradiated normal mice could accommodate the most cells, which may provide at least a partial reason for greater effects of cotransferred bystander T cells in sublethally irradiated as compared with RAG−/− mice. In fact, previous studies (8) have shown that limited numbers of cotransferred bystander T cells are ineffective at restraining homeostatic expansion in sublethally irradiated mice.
There are multiple differences between lymphopenic environments induced by RAG deficiency versus irradiation. Therefore, a number of explanations that will be discussed below can be offered to explain the lesser degree of homeostatic proliferation seen in the latter. However, our results suggest that self ligands can be a limiting resource, even in the irradiation model of lymphopenia, because polyclonal CD4 T cells were more efficient in their ability to inhibit homeostatic proliferation of a monoclonal TCR Tg population.
Discussion
One fundamental property of adaptive immunity is the ability to respond to a large number of unknown and unpredictable antigens. This task is achieved through maintenance of a large population of lymphocytes expressing unique antigen receptors. Continuous generation of new T cells in the thymus that can replace senescent T cells in the periphery is one important mechanism of maintaining T cell diversity. However, thymic output declines with age, and supply of new T cells can become limiting. Nevertheless, the total size of the T cell population is well maintained throughout life even though the individual is expected to experience multiple episodes of transient lymphopenia after various viral infections (30–33). Undoubtedly, the ability of naive T cells to undergo homeostatic expansion is an important mechanism that allows maintenance of T cell numbers. However, mere maintenance of T cell numbers is unlikely to benefit the host unless it is accompanied by sufficient T cell diversity.
Individual T cells vary greatly in their ability to undergo homeostatic proliferation, which may be secondary to the diversity in relative abundance of different self peptide/MHC complexes and their affinity for different TCRs. However, there are potentially serious drawbacks to this system design. First, TCR diversity will become limited if a few “best” T cell clones take over the available T cell space. Second, because the best competitors are likely those with highest affinity for self antigens, there is further risk that these best T cell clones may cause autoimmunity. The reality of both concerns is supported by animal model and clinical data. Restriction of the TCR repertoire and oligoclonal T cell expansion is seen in neonatally thymectomized mice (34), and in mice that possess a limited TCR repertoire (35). Similar findings along with autoantibodies are seen in myasthenia gravis patients within several years of their thymectomy (36). Many animal models demonstrate emergence of autoimmunity after repopulation of T cell-deficient animals with naive T cells (37–39). Multiple human connective tissue disorders are associated with lymphopenia (40–43). Autoimmunity can also accompany lymphopenia caused by HIV infection (44, 45), and be triggered by highly active anti-retroviral therapy (46).
Our results suggest that availability of particular self peptide/MHC complexes capable of stimulating a subpopulation of T cells can limit the size that subpopulation can achieve in the course of homeostatic expansion. According to this model, the naive T cell compartment may be subdivided into multiple subcompartments defined by the specificity of their TCRs for certain self ligands. Some subcompartments may be relatively small and some may be large depending on the abundance of the particular self peptide/MHC complexes. Furthermore, a particular TCR may be stimulated by several different self peptide/MHC complexes, and conversely any given self peptide/MHC complex may stimulate a number of different TCRs. In our system, it is likely that HA and DO11.10, as well as OT-II and SM-1 TCRs, recognize different sets of self peptide/MHC complexes, and therefore do not have to compete with each other.
We found no evidence that presence of large numbers of resident TCR Tg T cells had any effect on homeostatic proliferation unless they shared the self peptide/MHC specificity with the responder T cells. Thus, resident T cells did not deliver direct inhibitory signals, or consume some non-MHC factors provided by APCs or stromal components of the lymphoid organs. This result along with the greater extent of homeostatic proliferation by CD4 T cells in RAG−/− hosts contrasts with experiments that used irradiated normal recipient hosts and cotransferred T cell competitors, where bystander T cells inhibit homeostatic proliferation without any apparent competition for self peptide/MHC (5, 8). Many differences exist between the models of lymphopenia created by sublethal irradiation versus RAG deficiency and could potentially account for this apparent discrepancy.
First, in our experiments the relative numbers of T cells attempting to undergo homeostatic proliferation was smaller in the RAG−/− than in the irradiated normal mice. This result is due to the number of transferred T cells that could be accommodated by the lymphoid tissues of RAG−/− mice was smaller, and the starting population of T cells in the irradiated normal mice consisted of transferred and residual resident T cells. It is also likely that some critical factor(s) other than MHC may be more limiting acutely after irradiation. For example, we did see a significant reduction in the numbers of APCs. It is possible that levels of essential growth and survival factors, such as IL-7, could also become acutely decreased. Therefore, it is easily plausible that these general non-MHC resources would be more limiting in the sublethal irradiation model, especially with larger numbers of bystander T cells that would have to compete for them, at least initially. It is interesting to note that homeostatic proliferation was somewhat delayed in the irradiated RAG−/− mice, and the ability of bystander T cells to inhibit homeostatic proliferation of polyclonal T cell responders was most convincingly shown to occur at relatively early time points (5, 8).
Second, the balance among various cytokines is likely to be different in irradiated and RAG−/− mice. Tissue damage induced by ionizing radiation is associated with production of various proinflammatory cytokines (47). Certain proinflammatory cytokines, such as IL-12, have been shown to enhance homeostatic proliferation (48). However, irradiation has also been shown to lead to induction and activation of transforming growth factor (TGF)-β (49, 50), a pluripotent immunosuppressive cytokine with antiproliferative properties. TGF-β may be directly released by the apoptotic cells (51), and it may be produced along with other immunosuppressive molecules by persisting APCs after ingestion of apoptotic bodies (52, 53). These acute changes in the cytokine balance may affect homeostatic proliferation of T cells and provide another explanation for the differences seen when compared with RAG−/− hosts.
Regulatory cells might also be important in control of homeostatic proliferation. The best-characterized naturally occurring regulatory T cells are CD25+CD4 T cells (54–56). These cells can prevent autoimmunity induced by naive polyclonal CD4 T cells after adoptive transfer into T cell-deficient hosts. Whereas their regulatory mechanisms are still poorly understood, it is formally possible that one of their roles is control of homeostatic proliferation (26). Some regulatory cells may persist after sublethal irradiation. In addition, it is possible that the ability of large numbers of bystander T cells to inhibit homeostatic proliferation is attributable to a small fraction of regulatory T cells that are also contained within the non-RAG−/− TCR Tg CD4 T cell pool (57).
Our system has tested the ultimate limits of how the immune system ensures some degree of TCR diversity among naive CD4 T cells in the absence of thymus. A RAG−/− mouse is a model of lymphopenia where most general resources such as APCs, stromal cells, and critical growth factors are relatively abundant, and regulatory T cells are absent. In this model, self ligands represent the most critically limiting resource. The emergence of a restricted TCR repertoire in some of the clinical scenarios described above suggests that these limits may also be tested in clinical practice. The complexity and clinical significance of various lymphopenic states are only beginning to be understood and appreciated. So far, the most attention has been focused on HIV-1 infection, where IL-7 levels increase proportionately to the degree of T cell depletion (58–60), a situation that may be similar to the RAG−/− model of lymphopenia. However, reconstitution of the immune system presents unique challenges in many other clinical settings. The apparent ability of the immune system to recover after different natural and therapeutic insults suggests existence of multiple, perhaps imperfect, mechanisms to ensure TCR diversity and self tolerance. These are certain to continue be a focus of intense investigations.
Acknowledgments
We thank Drs. Elizabeth Ingulli and Andrea Itano for discussions and comments on the manuscript. This work was supported by National Institutes of Health Award 1 KO8 DK02784-01 and the Phileona Foundation (A.K.), and by National Institutes of Health Award AI38903 (to S.C.J.).
Abbreviations
- CFSE
5,6-carboxyfluorescein diacetate succinimyl ester
- DC
dendritic cell
- TCR
T cell antigen receptor
- Tg
transgenic
- PE
phycoerythrin
- APC
antigen-presenting cell
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Goldrath A W, Bevan M J. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 2.Freitas A A, Rocha B. Annu Rev Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- 3.Marrack P, Bender J, Hildeman D, Jordan M, Mitchell T, Murakami M, Sakamoto A, Schaefer B C, Swanson B, Kappler J. Nat Immunol. 2000;1:107–111. doi: 10.1038/77778. [DOI] [PubMed] [Google Scholar]
- 4.Surh C D, Sprent J. Microbes Infect. 2002;4:51–56. doi: 10.1016/s1286-4579(01)01509-x. [DOI] [PubMed] [Google Scholar]
- 5.Dummer W, Ernst B, LeRoy E, Lee D-S, Surh C D. J Immunol. 2001;166:2460–2468. doi: 10.4049/jimmunol.166.4.2460. [DOI] [PubMed] [Google Scholar]
- 6.Goldrath A W. Microbes Infect. 2002;4:539–545. doi: 10.1016/s1286-4579(02)01570-8. [DOI] [PubMed] [Google Scholar]
- 7.Freitas A A, Agenes F, Coutinho G C. Eur J Immunol. 1996;26:2640–2649. doi: 10.1002/eji.1830261115. [DOI] [PubMed] [Google Scholar]
- 8.Ernst B, Lee D-S, Chang J M, Sprent J, Surh C D. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 9.Prlic M, Jameson S C. Microbes Infect. 2002;4:531–537. doi: 10.1016/s1286-4579(02)01569-1. [DOI] [PubMed] [Google Scholar]
- 10.Ge G, Rao V P, Cho B K, Eisen H N, Chen J. Proc Natl Acad Sci USA. 2000;98:1728–1733. doi: 10.1073/pnas.98.4.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy K M, Heimberger A B, Loh D Y. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 12.Kirberg J, Baron A, Jakob S, Rolink A, Karjalaine K, von Boehmer H. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McSorley S J, Asch S, Costalonga M, Reinhardt R L, Jenkins M K. Immunity. 2002;16:325–328. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 14.Barnden M J, Allison J, Heath W R, Carbone F R. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 15.Lyons A B, Parish C R. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 16.Merica R, Khoruts A, Pape K A, Reinhardt R L, Jenkins M K. J Immunol. 2000;164:4551–4557. doi: 10.4049/jimmunol.164.9.4551. [DOI] [PubMed] [Google Scholar]
- 17.Thorstenson K M, Khoruts A. J Immunol. 2001;167:188–195. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 18.Gudmundsdottir H, Turka L A. J Immunol. 2001;167:3699–3707. doi: 10.4049/jimmunol.167.7.3699. [DOI] [PubMed] [Google Scholar]
- 19.Prlic M, Blazar B R, Khoruts A, Zell T, Jameson S C. J Immunol. 2001;167:5664–5668. doi: 10.4049/jimmunol.167.10.5664. [DOI] [PubMed] [Google Scholar]
- 20.Cho B K, Rao V P, Ge Q, Eisen H N, Chen J. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bender J, Mitchell T, Kappler J, Marrack P. J Exp Med. 1999;190:367–374. doi: 10.1084/jem.190.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seddon B, Legname G, Tomliseon P, Zamoyska R. Science. 2000;290:127–131. doi: 10.1126/science.290.5489.127. [DOI] [PubMed] [Google Scholar]
- 23.Dorfman J R, Stefanova I, Yasutomo K, Germain R N. Nat Immunol. 2000;1:329–335. doi: 10.1038/79783. [DOI] [PubMed] [Google Scholar]
- 24.Clarke S R M, Rudensky A Y. J Immunol. 2000;165:2458–2464. doi: 10.4049/jimmunol.165.5.2458. [DOI] [PubMed] [Google Scholar]
- 25.Polic B, Kunkel D, Scheffold A, Rajewsky K. Proc Natl Acad Sci USA. 2001;98:8744–8749. doi: 10.1073/pnas.141218898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Annacker O, Burlen-Defranoux O, Pimenta-Araujo R, Cumano A, Bandeira A. J Immunol. 2000;164:3573–3580. doi: 10.4049/jimmunol.164.7.3573. [DOI] [PubMed] [Google Scholar]
- 27.Golovkina T V, Shlomchik M, Hannum L, Chervonsky A. Science. 1999;286:1965–1968. doi: 10.1126/science.286.5446.1965. [DOI] [PubMed] [Google Scholar]
- 28.Steinman R M, Pack M, Inaba K. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 29.Zell T, Khoruts A, Ingulli E, Bonnevier J L, Mueller D L, Jenkins M K. Proc Natl Acad Sci USA. 2001;98:10805–10810. doi: 10.1073/pnas.191567898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson M J, Higgins P G, Davis L R, Willman J S, Jones S E, Kidd I M, Pattison J R, Tyrrell D A. J Infect Dis. 1985;152:257–265. doi: 10.1093/infdis/152.2.257. [DOI] [PubMed] [Google Scholar]
- 31.Yuen K Y, Chan P K, Peiris M, Tsang D N, Que T L, Shortridge K F, Cheung P T, To W K, Ho E T, Sung R, Cheng A F. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 32.Tumpey T M, Lu X, Morken T, Zaki S R, Katz J M. J Virol. 2000;74:6105–6116. doi: 10.1128/jvi.74.13.6105-6116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada H, Kobune F, Sato T A, Kohama T, Takeuchi Y, Abe T, Takayama N, Tsuchiya T, Tashiro M. Arch Virol. 2000;145:905–920. doi: 10.1007/s007050050683. [DOI] [PubMed] [Google Scholar]
- 34.La Gruta N L, van Driel I R, Gleeson P A. Eur J Immunol. 2000;30:3380–3386. doi: 10.1002/1521-4141(2000012)30:12<3380::AID-IMMU3380>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 35.Correia-Neves M, Waltzinger C, Mathis D, Benoist C. Immunity. 2001;14:21–32. doi: 10.1016/s1074-7613(01)00086-3. [DOI] [PubMed] [Google Scholar]
- 36.Gerli R, Paganelli R, Cossarizza A, Muscat C, Piccolo G, Barbieri D, Mariotti S, Monti D, Bistoni O, Raiola E, et al. J Allergy Clin Immunol. 1999;103:865–872. doi: 10.1016/s0091-6749(99)70431-8. [DOI] [PubMed] [Google Scholar]
- 37.Bonomo A, Kehn P J, Shevach E M. Immunol Today. 1995;16:61–67. doi: 10.1016/0167-5699(95)80089-1. [DOI] [PubMed] [Google Scholar]
- 38.Gleeson P A, Toh B H, van Driel I R. Immunol Rev. 1996;149:97–125. doi: 10.1111/j.1600-065x.1996.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 39.Annacker O, Powrie F. Microbes Infect. 2002;4:567–574. doi: 10.1016/s1286-4579(02)01574-5. [DOI] [PubMed] [Google Scholar]
- 40.Utsinger P D, Yount W J. J Rheumatol. 1976;3:355–366. [PubMed] [Google Scholar]
- 41.Kirtava Z, Blomberg J, Bredberg A, Henriksson G, Jacobsson L, Manthorpe R. Clin Exp Rheumatol. 1995;13:609–616. [PubMed] [Google Scholar]
- 42.Mirzayan M J, Schmidt R E, Witte T. Rheumatology. 2000;39:1316–1319. doi: 10.1093/rheumatology/39.12.1316. [DOI] [PubMed] [Google Scholar]
- 43.Quintero-Del-Rio A I, Bacino D, Kelly J, Aberle T, Harley J B. Cell Mol Biol. 2001;47:1223–1227. [PubMed] [Google Scholar]
- 44.Itescu S. Curr Opin Rheumatol. 1996;8:346–353. doi: 10.1097/00002281-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Kaye B R. Clin Rev Allergy Immunol. 1996;14:385–416. doi: 10.1007/BF02771754. [DOI] [PubMed] [Google Scholar]
- 46.Jubault V, Penfornis A, Schillo F, Hoen B, Izembart M, Timsit J, Kazatchikine M D, Gilquin J, Viard J P. J Clin Endocrinol Metab. 2000;85:4254–4257. doi: 10.1210/jcem.85.11.6988. [DOI] [PubMed] [Google Scholar]
- 47.Ferrara J L. Curr Opin Immunol. 1993;5:794–799. doi: 10.1016/0952-7915(93)90139-j. [DOI] [PubMed] [Google Scholar]
- 48.Kieper W C, Prlic M, Schmidt C S, Mescher M F, Jameson S C. J Immunol. 2001;166:5515–5521. doi: 10.4049/jimmunol.166.9.5515. [DOI] [PubMed] [Google Scholar]
- 49.Barcellos-Hoff M H. Cancer Res. 1993;53:3880–3886. [PubMed] [Google Scholar]
- 50.Ehrhart E J, Segarini P, Tsang M L, Carroll A G, Barcellos-Hoff M H. FASEB J. 1997;11:991–1002. doi: 10.1096/fasebj.11.12.9337152. [DOI] [PubMed] [Google Scholar]
- 51.Chen W, Frank M E, Jin W, Wahl S M. Immunity. 2001;14:715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 52.Fadok V A, Bratton D L, Rose D M, Pearson A, Ezekewitz R A, Henson P M. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 53.Fadok V A, Chimini G. Semin Immunol. 2001;13:365–372. doi: 10.1006/smim.2001.0333. [DOI] [PubMed] [Google Scholar]
- 54.Sakaguchi S. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 55.Shevach E M. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 56.Maloy K J, Powrie F. Nat Immunol. 2001;2:816–823. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 57.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 58.Fry T J, Connick E, Faloon J, Lederman M M, Liewehr D J, Spritzler J, Steinber S M, Wood L V, Yarchoan R, Zuckerman J, et al. Blood. 2001;97:2983–2990. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 59.Llano A, Barretina J, Cutierrez A, Blanco J, Cabrera C, Clotet B, Este J A. J Virol. 2001;75:10319–10325. doi: 10.1128/JVI.75.21.10319-10325.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Napolitono L A, Grant R M, Deeks S G, Schmidt D, De Rosa S C, Herzenberg L A, Herndier B G, Andersson J, McCune J M. Nat Med. 2001;7:73–79. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]