Abstract
Background
Severe and persistent mental illnesses in children and adolescents, such as early- onset schizophrenia spectrum (EOSS) disorders and pediatric bipolar disorder (pedBP), are increasingly recognized. Few treatments have demonstrated efficacy in rigorous clinical trials. Enduring response to current medications appears limited. Recently, olanzapine was approved for the treatment of adolescents with schizophrenia or acute manic/mixed episodes in pedBP.
Methods
PubMed searches were conducted for olanzapine combined with pharmacology, schizophrenia, or bipolar disorder. Searches related to schizophrenia and bipolar disorder were limited to children and adolescents. The bibliographies of the retrieved articles were hand-checked for additional relevant studies. The epidemiology, phenomenology, and treatment of EOSS and pedBP, and olanzapine’s pharmacology are reviewed. Studies of olanzapine treatment in youth with EOSS and pedBP are examined.
Results
Olanzapine is efficacious for EOSS and pedBP. However, olanzapine is not more efficacious than risperidone, molindone, or haloperidol in EOSS and is less efficacious than clozapine in treatment-resistant EOSS. No comparative trials have been done in pedBP. Olanzapine is associated with weight gain, dyslipidemia, and transaminase elevations in youth. Extrapyramidal symptoms, neuroleptic malignant syndrome, and blood dyscrasias have also been reported but appear rare.
Conclusions
The authors conclude that olanzapine should be considered a second-line agent in EOSS and pedBP due to its risks for significant weight gain and lipid dysregulation. Awareness of the consistent weight and metabolic changes observed in olanzapine-treated youth focused attention on the potential long-term risks of atypical antipsychotics in youth.
Keywords: early-onset schizophrenia, pediatric bipolar disorder, antipsychotic
The US Food and Drug Administration (FDA) issued an indication for olanzapine in the treatment of adolescents, 13–17 years old, with schizophrenia or acute manic/mixed episodes in bipolar I disorder (BPI) in December 2009. However, the FDA also formally stated that clinicians may “consider prescribing other drugs first in adolescents” given olanzapine’s increased potential for weight gain and hyperlipidemia in adolescents compared with adults. This article reviews the presentation and treatment of early-onset schizophrenia (EOS) and pediatric bipolar disorder (pedBP), the pharmacology and kinetics of olanzapine, the evidence supporting olanzapine’s efficacy in these two serious mental illnesses among children and adolescents, the safety and tolerability of olanzapine in pediatric patients, and the probable role of olanzapine within the pediatric population compared with the adult population. The information presented in this article was gathered through a systematic PubMed review, with searches conducted for olanzapine combined with pharmacology, schizophrenia, or bipolar disorder. Searches related to schizophrenia and bipolar disorder were limited to children and adolescents. The bibliographies of key-retrieved articles were hand-checked for additional relevant studies. Rigorously, controlled data is limited in this population, so observations from nonblinded trials will also be presented. All conclusions are those of the authors and do not reflect specific recommendations of any national organization or government entity.
Early-onset schizophrenia
The incidence of EOS is not well established with epidemiologic studies. However, a number of geographically limited studies focused on hospital admissions have provided consistent estimates of the incidence in children and adolescents. The illness is very uncommon (~0.3/1000) in children younger than 10 years, then increases to approximately 1.3/1000 between ages 10 and 14 years with an even greater increase between 14 and 18 years with incidence rates varying between 2.0 and 5.5/1000. Indeed, at least 5% of those with schizophrenia become ill prior to 14 years of age and up to 20% appear to become ill prior to 18 years of age.1–5 The peak age of onset is between 15 and 20 years in males and between 15 and 25 years in females.6 Modestly more males experience EOS than females (~1.5 males: 1 female).
Diagnostic criteria for EOS are the same as for adult-onset criteria except that psychotic symptoms must be present prior to age 18 years. However, there are some development differences in detailed characteristics of symptoms. Specifically, youth are more likely to have multi-modal hallucinations than adults and frequently personalize their hallucinations giving them names that are frequently stereotyped, such as “Satan,” “my guardian angel,” or the “monster” or derived from visual characteristics, such as the “man with no skin”. They seldom have systematized or bizarre delusional symptoms, though vague paranoia is common. Disorganized thinking is frequently observed.7–10 Younger individuals may have difficulty recognizing their symptoms as abnormal and frequently do not complain spontaneously about hallucinations.11 Youth who will experience EOS frequently show greater premorbid problems with attention, learning, and socialization than individuals who develop schizophrenia as adults. The onset of symptoms in EOS is most often insidious.12,13 Youth with EOS who participate in clinical trials often have more severe psychiatric symptoms, greater neurocognitive deficits, and more pronounced gray matter loss than research participants with adult-onset schizophrenia.14–18 Individuals with EOS are much less likely to have a good outcome than individuals with adult-onset schizophrenia, with the majority having poor or very poor outcomes.13,19–21
Treatment of EOS has traditionally been based on the pharmacologic treatments used for adult-onset schizophrenia. There is little systematic use of psychotherapeutic strategies in this population.22 Efficacy of antipsychotics has been assumed to be generally similar in youth and adults. This view was bolstered by small studies of first-generation antipsychotics (FGAs), including haloperidol, thioridazine, thiothixene, and loxapine that showed relatively high rates of response.9,23,24 However, considerable sedation was also observed, and there were concerns about extrapyramidal symptoms (EPS), which appear to be somewhat more prevalent in youth than adults.25 In the 1990s, a pivotal trial was done showing that youth with treatment-resistant schizophrenia had a robust response to clozapine, the prototypic second-generation antipsychotic (SGA) introduced in 1989.26 However, significant adverse effects were frequent and limited the use of this agent.
As other SGAs have been introduced and approved for use in adults with schizophrenia over the past 20 years (Table 1), including olanzapine in 1996, clinicians embraced them hoping both for greater efficacy and fewer adverse effects than the FGAs or clozapine. There was particularly great enthusiasm for olanzapine because its pharmacologic profile was most similar to clozapine’s and it was less prone to EPS than risperidone, which has more potent dopamine D2 antagonism. Enthusiasm for olanzapine was further increased by a study of olanzapine vs haloperidol in 263 patients with first-episode schizophrenia, who were followed for 2 years.27 Although there were no difference in symptom reduction between the two agents in a last observation carried forward analysis, a mixed-model analysis demonstrated an acute advantage for olanzapine in Positive and Negative Syndrome Scale (PANSS) total score, PANSS negative and general symptoms, and a depression rating scale. Results of the 2-year data found that patients treated with olanzapine were significantly more likely to enter remission (olanzapine 57%, haloperidol 44%, P < 0.036) and continued treatment for a longer period of time (olanzapine 322 days, haloperidol 230 days, P < 0.0085) than those treated with haloperidol.28 Perhaps most importantly, the imaging component of this study found that individuals treated with haloperidol experienced significant decreases in brain gray matter volume, whereas neither those treated with olanzapine nor a healthy control group showed any changes.29 Optimism that olanzapine may have particular advantages for treating youth with psychotic symptoms was also heightened with results published from a small pilot study comparing olanzapine, risperidone, and haloperidol in the treatment of psychotic youth aged 8–20 years.30 This study in the pediatric population found a modest numeric, but not statistically significant, advantage for olanzapine in the response rate (olanzapine 88%, risperidone 74%, haloperidol 53%), premature drop-out (olanzapine 2/16, risperidone 9/19, haloperidol 7/15, P = 0.058), and time to treatment discontinuation (olanzapine 7.4 weeks, risperidone 6.3 weeks, haloperidol 5.7 weeks). However, the same trial also suggested that adverse events with the SGAs may be more common and more severe in youth than in adults. Proponents of olanzapine also focused on results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study in 1,493 adults with chronic schizophrenia. Although there were few clinically significant differences among SGAs and between SGAs and FGAs in the CATIE study, olanzapine had marginally greater benefits as reflected by greater initial reductions in PANSS, greater initial improvements in the Clinical Global Impression (CGI), lower rate of hospitalization due to psychiatric exacerbation, lower discontinuation rates, and longer time to treatment discontinuation.29 As discussed in detail later, the Treatment of Early Onset Schizophrenia Spectrum Disorders (TEOSS) study did not identify any advantage for olanzapine compared with risperidone or molindone in children and adolescents with schizophrenia, 31 suggesting a potential difference in olanzapine’s efficacy during different stages of schizophrenia.
Table 1.
Current FDA indications for second-generation antipsychotics
| Drug | Indication | Approval date |
|---|---|---|
| Aripiprazole | Schizophrenia in adults | November 15, 2002 |
| Maintenance treatment of adult schizophrenia | August 28, 2003 | |
| Acute adult manic/mixed BP1 | September 29, 2004 | |
| Maintenance treatment of adult BP1 | March 1, 2005 | |
| Schizophrenia in 13–17 year olds | October 29, 2007 | |
| Adjunctive treatment in adult major depression | November 16, 2007 | |
| Acute monotherapy or adjunctive therapy with lithium or valproate in manic/mixed episodes of BP1 in 10–17 year olds | February 27, 2008 | |
| Irritability in autism in 6–17 year olds | November 19, 2009 | |
| Asenapine | Schizophrenia in adults BP1 in adults | August 14, 2009 |
| Clozapine | Treatment-resistant schizophrenia | September 26, 1989 |
| Emergent suicidality in schizophrenia or schizoaffective disorder | December 18, 2002 | |
| Olanzapine | Acute psychotic disorders in adults | September 30, 1996 |
| Manic/mixed in BP1 adults March 17, 2000 | ||
| Maintenance treatment in schizophrenia in adults | November 9, 2000 | |
| Adjunctive use with lithium or valproate in acute mania/mixed BP1 | July 10, 2003 | |
| Maintenance treatment in adult BP1 | January 14, 2004 | |
| 13–17 year olds with schizophrenia may consider other drugs first due to weight gain and dyslipidemia | December 4, 2009 | |
| 13–17 year olds with mania/mixed BP1 as monotherapy or adjunct to valproate or lithium may consider other drugs first given weight gain/dyslipidemia | ||
| Quetiapine | Psychotic disorders in adults | September 26, 1997 |
| Monotherapy or adjunctive therapy in BP1 in adults | January 12, 2004 | |
| Major depression associated with BP1 in adults | October 20, 2006 | |
| Maintenance treatment in adult BP1 as adjunct to lithium or valproate | May 13, 2008 | |
| Treatment of schizoprenia in 13–17 year olds | December 2, 2009 | |
| Acute treatment of mania/mixed BP1 in 10–17 year olds either as monotherapy or adjunctive therapy | ||
| Risperidone | Treatment of schizophrenia in adults | December 29, 1993 |
| Long-term treatment of schizophrenia in adults | March 3, 2002 | |
| Monotherapy or adjunctive therapy to lithium or valproate in adults with BP1 | December 4, 2003 | |
| Irritability in autism in 5–16 year olds | October 6, 2006 | |
| Schizophrenia in 13–17 year olds | August 22, 2007 | |
| BP1 in 10–17 year olds | ||
| Ziprasidone | Schizophrenia in adults | February 5, 2001 |
| Monotherapy in manic/mixed in BP1 in adults | August 19, 2004 | |
| Maintenance treatment as adjunct to lithium or valproate in BP1 in adults | November 20, 2009 |
Abbreviations: FDA, US Food and Drug Administration; BP1, bipolar 1 disorder.1
Pediatric bipolar disorder
Similar to EOSS, there are few epidemiologic studies that rigorously examine the incidence of pedBP. The primary exception to this is a recent small study done in which 3,021 community subjects (14–24 years of age) in Germany were reassessed 10 years later.32 This study found that approximately 1.2% of the youth had experienced a manic or hypomanic episode by 12 years of age and approximately 4.5% had experienced a manic or hypomanic episode by 18 years of age. Manic episodes occurred with similar frequency in males and females, whereas hypomanic episodes were about twice as common in females as males and as mania in either gender. Rates dramatically increased during adolescence. Further, 9% of those with a major depressive episode prior to age 17 years subsequently developed bipolar disorder, which was significantly greater than those with later onset of depression. The incidence of manic and hypomanic episodes in this study is greater than the prevalence of bipolar 1 disorder in adults (4.5% lifetime and 2.8 annual),33 which is typically reported in larger epidemiologic studies, likely due to the expert clinical interviewers used in the German study. In a recent multisite treatment study of 3,658 adults with bipolar disorder, 29% reported onset before age 13 and 67% reported onset by age 18.34 A study of 119 individuals consecutively admitted to a psychiatric hospital in Norway with bipolar disorder found that 13.5% had onset during childhood and 61.6% had onset prior to age 20 and that those who reported an affective temperament had earlier onset.35 The period of peak onset appears to be in 12 and 22 years of age.32 It should be noted that there has been approximately a 40-fold increase in the recognition of bipolar disorder in children and adolescents over the past decade in the US.36
Diagnosis of pedBP has been somewhat contentious with many critics arguing that the disorder is misdiagnosed in a large number of youth. The controversy seems related to two major issues.37 First, in children and many adolescents, the pattern of cycling within a given episode and the duration of episodes appear quite different between youth and adults. Youth frequently have a large number of cycles or mood swings within a single episode of illness. Often, this cycling can occur multiple times a day even though there may be months without an extended euthymic period that would signal the end of the episode. In contrast, most adults demonstrate a fairly consistent mood state throughout any given episode. In pedBP, a single episode will often last for a very extended period giving the appearance of a chronic condition, whereas in adults, episodes are usually limited to a few weeks.38,39 Second, children and, to a lesser extent, adolescents frequently experience mixed states of mania and depression, whereas adults less frequently show such states. The sensitivity and specificity of various individual symptoms of mania and their differential manifestations in other childhood diagnoses are eloquently reviewed by Youngstrom et al.37 Further, there are developmental differences within pedBP, with children frequently showing greater comorbidity with attention deficit hyperactivity disorder. Childhood-onset bipolar disorder is associated with greater mood lability, irritability, and hallucinations than adolescent-onset bipolar disorder. However, many highly concerning symptoms, including psychotic symptoms, suicidality, grandiosity, and decreased need for sleep, increase during adolescence, often to a greater extent in those with childhood-onset bipolar disorder than those with adolescent-onset bipolar disorder.40 In addition, there is more controversy about the boundaries of the bipolar spectrum in the pediatric population than in the adult population. Criteria for various potential disorders within the spectrum have been most explicitly defined by Leibenluft and colleagues.41 These criteria subdivide bipolar NOS (Not Otherwise Specified) into categories in which a sufficient number of criteria are not met, symptoms do not appear to have sufficient duration, irritability is primary mood state, and moods are severely dysregulated in the context of many symptoms of hyperarousal but in the absence of psychotic symptoms or elation. The latter category, called severe mood dysregulation, appears most common and is likely to be highly heterogeneous.
Most of the current longitudinal studies of pedBP involve limited follow-up and focus primarily on rates of remission and relapse.42–46 However, adults with childhood-onset bipolar disorder appear to experience significantly worse outcomes, including more rapid relapse, less euthymia, and poorer functioning and quality of life than those with adult-onset bipolar disorder.34 In addition, rates of suicidality and suicide attempts are particularly high in adolescents with bipolar disorder (72%–76% and 31%–44%, respectively).47,48 Suicidality in bipolar youth appears to be increased in those with psychotic symptoms.49
Treatment for pedBP has evolved from treatment of adults with bipolar disorder with specific treatment studies for pedBP limited until the past decade. Until 2007, lithium was the only mood-stabilizing agent specifically approved by the FDA for the treatment of acute mania in adolescents. However, this indication does not reflect specific evaluation of the efficacy and safety of lithium in adolescents. Despite this, valproate was probably the most widely used mood stabilizer in the pediatric population until the SGAs were introduced in the 1990s because of its availability in a sprinkle formulation, more favorable therapeutic index (ratio of toxic dose to therapeutic dose), and less onerous side-effect profile compared to lithium. Carbamazepine was also used to some extent. As other antiepileptic drugs, including oxcarbamazepine, gabapentin, topiramate, and lamotrigine, began to be used in adults, they were embraced for use in the pediatric population because they frequently did not require monitoring of blood levels. Lithium was among the first agents specifically studied in youth. Lithium led to greater reductions in the substance use and improvements in the Clinical Global Impressions Scale than placebo in a sample of 25 adolescents with both bipolar disorder and substance abuse.50 A subsequent open trial found that lithium, in conjunction with an antipsychotic in nearly 50% of the cases, led to reduction in acute manic symptoms in approximately two-thirds of adolescents with acute mania and remission in about one-quarter of the participants.51 Further, although no placebo-controlled trials of lithium for mania have been completed in pedBP, one study evaluated the rapid discontinuation of lithium by replacement with placebo or continuation of lithium over 2 weeks in youth who had responded to therapeutic doses of lithium for at least 4 weeks.52 Surprisingly, more than 50% of the participants in both the lithium and the placebo groups experienced significant symptom exacerbation. In addition, among adolescents with both psychotic and manic symptoms, long-term treatment with antipsychotics in addition to lithium was generally required to maintain the initial response to treatment.53
Other open-label studies in pedBP have described significant responses to valproate and carbamazepine, as well as lithium.54,55 However, in many of these trials, the majority of youth were concurrently taking antipsychotics or other mood stabilizers. In the only randomized but open monotherapy trial of mood stabilizers published to date, more than 50% of the participants did not respond to the initially randomized mood stabilizer though 80% of these responded to combined treatment with two mood stabilizers. 56 Studies exploring discontinuation of one mood stabilizer after stabilization on combination treatment support are consistent with the requirement of combination therapy for many youth with bipolar disorder.57 Significantly, recent placebo-controlled trials of mood stabilizers published to date have failed to demonstrate efficacy of either oxcarbamazepine or valproate.58,59
Antipsychotics were initially used adjunctively in treatment- resistant bipolar disorder in adults. Clozapine appeared particularly effective. Subsequently, other second- generation agents were studied as a treatment of all phases of bipolar disorder, particularly risperidone and olanzapine. Initially, antipsychotics were used as adjunctive treatment to mood stabilizers and appeared to have increased benefit with no major tolerability issues.60 A blinded, placebo-controlled trial of quetiapine adjunctive therapy demonstrated significantly greater reduction in mania scores and greater response rate than valproate monotherapy.61 An open-label trial of olanzapine monotherapy was also very promising.62 Subsequently, a double-blind trial comparing quetiapine with valproate demonstrated superior response and remission rates with quetiapine even though reductions in mean mania scores did not differ.63 Since that time, placebo-controlled trials of each of the atypical antipsychotics other than clozapine – olanzapine,64 aripiprazole,65 risperidone,66 and quetiapine67 – have found that each active agent reduced the Young Mania Rating Scale (YMRS)68 score to a significantly greater extent than placebo over 3 weeks among youth experiencing acute manic/mixed episodes. Based on these results, risperidone and aripiprazole were approved for the acute treatment of mixed and manic episodes of bipolar disorder in youth of 10–17 years old, and olanzapine and quetiapine were approved in adolescents. There has also been one small, double-blind pilot study that failed to show any advantage for quetiapine over placebo in the treatment of depression within pedBP.69 Notably, a large National Institute of Mental Health (NIMH)-sponsored comparative trial of risperidone, valproate, and lithium has been recently completed and should be reported soon. In summary, there is evidence for significant benefit from antipsychotics in the treatment of mixed and manic states in youth and a lack of efficacy of valproate and oxcarbamazepine. There is no empiric evidence yet of an effective treatment for depression or relapse prevention in youth with bipolar disorder.
Olanzapine pharmacology and pharmacokinetics
Olanzapine is a thienobenzodiazepine analog that binds to a large number of neurotransmitter receptors, including the dopamine D1, D2, and D4 receptors, serotonin 5-HT2A, 5-HT2C, 5-HT6, and 5-HT3 receptors, histamine H1 receptor, muscarinic receptors, α- and β-adrenergic receptors, γ-amino butyrate (GABA)a1 receptor, and the benzodiazepine binding sites.70 Its binding profile is more similar to clozapine’s than any other atypical antipsychotic. Olanzapine’s antipsychotic and mood-stabilizing effects are likely the result of potent antagonism at dopamine and serotonin receptors, with affinity constants ranging from 4 nM to 31 nM. It also binds with high affinity to H1 and M1 receptors. The drug binds with moderate affinity to 5-HT3 and M2–5 receptors and binds weakly to GABA, benzodiazepine binding sites, and β-adrenergic receptors. It is also likely that olanzapine regulates various signaling pathways within the brain, including the extracellular signal-related kinases (ERK1/2), particularly with long-term treatment.71 Developmental differences in the binding profile of olanzapine have not been examined to our knowledge. Olanzapine’s metabolic adverse effects have been hypothesized to relate to its histaminergic binding profile.72,73 In addition, olanzapine-associated weight gain has been linked to genetic variations in the 5-HT2A, 5-HT2C, and β3-adrenergic receptor, leptin, and the G-protein β3 subunit genes.74–76 The anticholinergic side effects are likely related to the muscarinic antagonism and the orthostatic hypotension related to antagonism at α-adrenergic receptors.
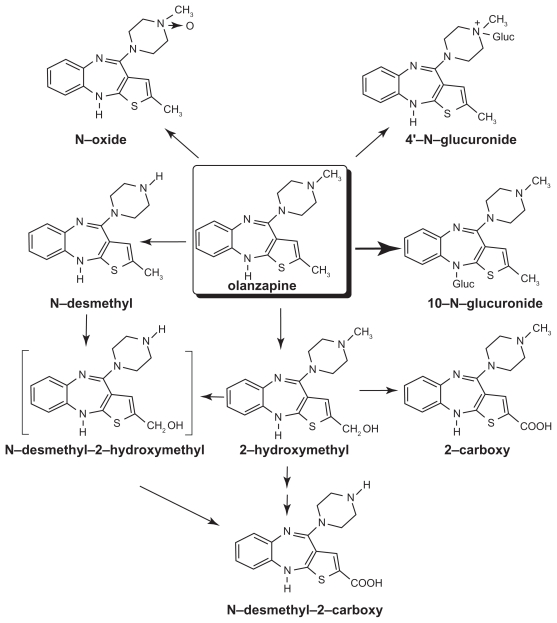
Olanzapine is well absorbed after oral administration and reaches maximal levels in about 5 hours in adults. Eating does not appear to affect olanzapine’s bioavailability. It is excreted in the urine (65%) and feces (35%) over the course of approximately 7 days.77 It is metabolized extensively within the liver undergoing glucuronidation (to yield the primary metabolic products), allylic hydroxylation, oxidation, and dealkylation. Oxidation occurs primarily via the CYP1A2 cytochrome P450 enzyme (Figure 1).78
Figure 1.
Metabolism of olanzapine. The chemical structure and metabolism of olanzapine are shown. Copyright © 1997. Modified with permission from Kassahun K, Mattiuz E, Nyhart E Jr, et al. Disposition and biotransformation of the antipsychotic agent olanzapine in humans. Drug Metab Dispos. 1997;25(1):81–93.77
Levels are affected by smoking (leading to ~30% reduction in plasma level), gender (women have ~85% increased plasma levels), carbamazepine (decreased plasma levels), and fluvoxamine and other selective serotonin reuptake inhibitors (SSRIs) (increased plasma levels).79–84 The elimination half-life ranges from about 27 hours in smokers to 37 hours in nonsmokers. Plasma levels of olanzapine appear to increase slowly over a period of months with a slightly slower time course in women compared with men.81,85 There are no significant differences that appear in bioavailability or half-life of oral tablet and the orally disintegrating form of olanzapine.86
In general, the pharmacokinetics of olanzapine are similar in youth and adults.87,88 Adolescents experience considerable intra-individual variability across time despite stable dosing, which appears somewhat greater in adolescents than adults.85,89 The concentration-to-dose ratio of olanzapine appears generally linear.78 However, adolescents appear to have a higher (~34%) concentration-to-dose ratio than adults even after adjustment for weight.90
Olanzapine efficacy in EOS
The first report of olanzapine treatment in youth with schizophrenia was an open trial done in eight youth with childhood-onset, who had not responded to prior trials with antipsychotics other than clozapine.91 In this study, there was modest improvement in overall symptoms and negative symptoms but no significant change in positive symptoms. A comparison group of 15 treatment-resistant youth exposed to clozapine demonstrated greater benefits only after 6 weeks of treatment. In a trial of 15 children younger than 13 years of age, 10 showed moderate or marked improvements in psychotic symptoms.92 However, all patients who had been previously exposed to antipsychotics did not improve. In contrast, an open-label study of 9 other treatmentresistant children found significant improvements at 12 weeks from baseline in all symptom domains and found that eight of the children sustained improvements over the course of 1 year.93 However, two double-blind studies comparing clozapine and olanzapine in youth with treatment-resistant schizophrenia had similar findings to the initial report.94,95 In each of these trials, clozapine resulted in greater reductions in negative symptoms and, in the most recent trial, greater response rates.
Olanzapine has also been tested in youth with schizophrenia, who are not treatment resistant. The initial open trials for these youth were done in 2003, and included 20 youth (6–15 years old) and 16 adolescents (12–17 years old) respectively.96,97 Both trials showed significant improvements in symptoms, illness severity, and function within 6–8 weeks of initiating treatment. However, improvements in negative symptoms were reported to occur later in the trial that included children and adolescents.97 In the Ross trial, 74% of the youth were considered responders and retained that response status after 1 year of treatment. A similar response rate has been achieved in other open-label trials including a large 6-week trial with an additional 18-week follow-up in 96 adolescents.98,99
Subsequently, olanzapine was openly compared with risperidone and haloperidol in physicians’ choice (nonrandomized) treatment of 43 adolescents with schizophrenia.100 There were no apparent differences in antipsychotic efficacy between the three treatments with significant benefit within 4 weeks of starting treatment. However, haloperidol was associated with greater emergence of depressive symptoms. A more recent trial that involved 16 adolescents with psychotic symptoms treated with olanzapine, 50 treated with risperidone, and 18 treated with quetiapine also failed to detect differences between the antipsychotic efficacy of the agents at 6 months when baseline psychopathology scores were included as a covariate in the analysis.101 However, olanzapine consistently showed the greatest number of reductions in all types of psychotic symptoms. A double-blind, randomized trial of acute psychotic symptoms in 50 youth with either affective or schizophrenic psychoses also failed to show statistically significant differences between treatments.30 In this study, the olanzapine group showed the greatest response rate (88% compared with 74% for risperidone and 53% for haloperidol) and longest duration of treatment. However, the numeric superiority of olanzapine may have partially reflected the preponderance of youth with affective psychoses (69%) in the olanzapine group in contrast with the other two groups where schizophrenic diagnoses were more common. A larger (n = 116), placebo-controlled, randomized trial comparing olanzapine (n = 34) with risperidone (n = 41) and molindone (n = 40) exclusively in youth with EOS, known as the TEOSS trial, again found no statistically significant or numeric differences in symptom reduction between the agents.31,102 However, in contrast to the earlier study, olanzapine treatment was sustained for a numerically shorter period, and numerically fewer participants treated with olanzapine responded than those treated with the other two agents (olanzapine: 34%; risperidone: 46%, molindone: 50%).
One double-blind, placebo-controlled trial of olanzapine has been done in adolescents 13–17 years old with schizophrenia. 103 In this international multisite trial, 72 youth were treated with olanzapine and 35 with placebo. Olanzapine treatment led to much greater and highly statistically significant improvements in the CGI Severity score (P = 0.004), the Brief Psychiatric Rating Scale for Children (BPRS-C)104,105 (P = 0.003), the PANSS total score (P = 0.005), and positive symptom scale (P = 0.002), but not negative symptoms. However, a reviewer at the FDA raised concerns about the study because the positive results of the study were primarily driven by the Russian sites not the US sites.106 Although the BPRS-C symptom reduction seen with olanzapine was similar in both sites (US: −21, Russia: −17), there were dramatic differences in the magnitude of reduction with placebo (US: −15; Russia: −3), resulting in markedly different changes in the P value (US: P = 0.258; Russia: P = 0.003). Importantly, an FDA audit of two Russian sites found no concerns about the conduct of the study. The FDA Director of the Division of Psychiatry Products, Dr. Thomas Laughren, believed that these differences between sites probably reflected differences in the availability of care for youth with schizophrenia between the two countries and greater heterogeneity of the study population at US sites and also believed that olanzapine should be approved in adolescent schizophrenia.
Olanzapine efficacy in pedBP
Olanzapine was initially reported to be useful for treating mania and mixed states in an 8-week, open-label prospective study of 23 youth aged 5–14 years old.62 There was a highly significant improvement in the YMRS107 (−19.0, P < 0.001) and overall response rate (defined as ≥30% reduction in YMRS and CGI-Severity score of mild or less severe) was 61%. Subsequently, DelBello and colleagues108 examined the effects of olanzapine treatment on brain neurochemistry using proton spectroscopy in 19 adolescents with mania, who were scanned prior to beginning treatment and on days 7 and 28 of treatment. Ten healthy control adolescents were scanned to assess normal variability in brain metabolites over 4 weeks. Ten (58%) of the participants had remission of symptoms. All participants showed increases in ventral prefrontal choline. However, those with remission showed significant increases in medial ventral prefrontal N-acetyl aspartate within 7 days of treatment (P = 0.05) and had increased baseline levels of ventral prefrontal choline compared with nonremitters (P < 0.001). Because N-acetyl aspartate is believed to reflect neuronal vitality, this finding suggests potential beneficial changes in brain structure among some adolescents whose mania is treated with olanzapine. The only randomized, placebo-controlled trial of olanzapine for the treatment of manic and mixed states in adolescents (13–17 years old) was conducted by Eli Lilly.64 In that study, 107 youth were treated with olanzapine and 54 with placebo for 3 weeks. The olanzapine group showed significantly greater reduction in the YMRS than the placebo group (olanzapine: −17.7, placebo: −10.0, P < 0.001). Olanzapine also led to dramatically higher response and remission rates than placebo (olanzapine: 48.6% and 35.2%, placebo: 22.2% and 11.1%, P = 0.002 and P = 0.001, respectively). We are unaware of any trials that have compared the efficacy of olanzapine and other mood-stabilizing agents in the pediatric population.
Olanzapine safety and tolerability in youth
Despite the evidence supporting the efficacy of olanzapine in EOS and pedBP, concerns about excessive weight gain with olanzapine emerged early. As olanzapine was used more frequently in youth, additional concerns about metabolic abnormalities, liver function abnormalities, sedation, and prolactin elevations emerged. Subsequently, the safety and tolerability of olanzapine in the pediatric population have been examined in comparison to adults treated with olanzapine. An early postmarketing surveillance study examined the database for olanzapine through March 31, 2000, linked it with patient exposure estimates during the same period provided by Eli Lilly, and divided the analysis into children (birth to 9 years), adolescents (10–19 years), and adults (20 years and older).109 The study found that extrapyramidal syndrome complaint risks were similar across development, and tardive dyskinesia complaint risks were comparable in adolescents and adults. Overrepresented complaints in children included weight gain, liver function abnormalities, sedation and tardive dyskinesia. Overrepresented complaints in adolescents included weight gain, liver function abnormalities, sedation, and prolactin increases. A more recent analysis done by Eli Lilly compared the weight and metabolic data from 454 adolescents aged 13–17 years old exposed to olanzapine (primarily under open conditions) for as long as 32 weeks to the pooled weight data from 7,847 adults treated with olanzapine for up to 32 weeks and pooled metabolic data from adults from four trials.107 The adolescents experienced more frequent and severe weight gain than the adults (>7% weight gain in 65.1% of adolescents and 35.6% of adults, P < 0.001; mean weight gain 7.4 kg in adolescents and 3.2 kg in adults, P < 0.001). Adolescents were also much more likely to develop hyperprolactinemia (adolescents: 55.5%, adults: 29.0%, P < 0.001). However, in contrast, adults had more adverse metabolic changes than the adolescents with 11.8% of adults and 3% of adolescents moving from normal or impaired glucose to high glucose (P < 0.001), and 31%–38% of adults compared with 17%–21% of adolescents developed borderline dyslipidemias (P < 0.001 for all). This likely reflects other lifestyle and metabolic problems including lower insulin reserves in the adults.
The largest and most definitive data review of olanzapine’s safety and tolerability in adolescents, pools all six Eli Lilly-sponsored trials of olanzapine in a total of 179 youth studied under placebo-controlled conditions for up to 6 weeks and 454 youth exposed to olanzapine (primarily under open conditions) for as long as 32 weeks.110 There have also been some trials that compared adverse effects observed in youth treated with olanzapine or other antipsychotics, including data between 45 and 52 weeks of exposure.30,31,93,101,102,111–115 We will examine each of the main adverse effects observed with olanzapine separately.
Weight gain
In the Eli Lilly placebo-controlled adolescent database, youth gained 3.9 kg when treated with olanzapine compared with 0.2 kg when treated with placebo (P < 0.001). In the adolescent-exposure database, the overall weight gain was 7.4 kg, with nearly two-thirds gaining more than 7% of their baseline weight and a 13.3 percentile increase in body mass index (BMI), which is a more appropriate measure of increased size in youth. Overall, 4% of adolescents withdrew from treatment because of weight gain. In examining the time course of weight gain, there appears a marked reduction in the slope of weight gain after 4 weeks of treatment.110 Similar findings of rapid weight gain followed by slower weight gain have been observed in other trials.96,102,111,114
In the comparative studies, olanzapine consistently demonstrated the greatest weight gain both acutely and with more extended use as summarized in Table 2. In almost all cases, it has led to significantly more weight gain than other antipsychotics with a very high level of significance. Further, in contrast with the adult literature, studies that directly compare clozapine and olanzapine treatment in youth have found that clozapine increases weight to a lesser or equivalent extent than olanzapine does.95,113,114 One study examining 65 youth treated with olanzapine, clozapine, or risperidone found elevations in parents’ and patients’ pretreatment BMIs. Female gender and younger age when treated were associated with the magnitude of antipsychotic-associated weight gain independent of medication. Further, individuals with low pretreatment BMIs initially show more rapid weight gain than their heavier peers even though total weight gain with treatment is less.116
Table 2.
Reported weight and metabolic changes with pediatric olanzapine treatment
| Parameter study | Treatment duration | Olanzapine N | Observed changes |
P value between drugs | |
|---|---|---|---|---|---|
| Olanzapine | Other agents | ||||
| Weight gain | |||||
| Lilly placebo database110 | 3–6 wk | 179 | 3.9 kg | Placebo 0.2 kg | P < 0.001 |
| Lilly total exposure database110 | up to 32 wk | 450 | 7.4 kg | ||
| Kumra et al91 | 8 wk | 8 | 3.4 kg | ||
| Frazier et al62 | 8 wk | 23 | 5 kg | ||
| Ratzoni et al111 | 12 wk | 21 | 7.2 kg | Risperidone 3.9 kg Haloperidol 1.1 kg |
P = 0.02 |
| Ross et al97 | 6 wk | 19 | 3.8 kg 12.8 kg/12 mo |
||
| Findling et al96 | 8 wk | 16 | 6.5 kg | ||
| Mozes et al93 | 12 wk | 9 | 6.1 kg | ||
| Sikich et al30 | 8 wk | 16 | 7.2 kg | Risperidone 4.9 kg Haloperidol 3.6 kg |
P = 0.0009 |
| Shaw et al94 | 8 wk | 12 | 3.6 kg | Clozapine 3.8 kg | |
| Mozes et al112 | 12 wk | 12 | 5.8 kg | Risperidone 4.5 kg | |
| Fleischhaker et al113 | 6 wk | 15 | 4.6 kg | Risperidone 2.8 kg Clozapine 2.5 kg |
P = 0.03 |
| Quintana et al98 | 10 wk | 16 | 6.2 kg | ||
| Sikich et al31 | 8 wk | 35 | 6.1 kg | Risperidone 3.6 kg Molindone 0.3 kg |
P = 0.0001 |
| Kumra et al95 | 12 wk | 21 | BMI 0.7 kg/m2 | Clozapine 0.7 kg/m2 | |
| Castro-fornieles et al101 | 24 wk | 16 | 11.7 kg | Risperidone 6.1 kg Quetiapine 6.0 kg |
P = 0.02 |
| Dittmann et al99 | 6 wk 24 wk |
96 32 |
5.1 kg 11.7 kg |
||
| Fleischhaker et al114 | 45 wk | 8 | 16.2 kg | Risperidone 7.2 kg Clozapine 9.5 kg |
|
| Correll et al115 | 12 wk | 45 | 8.5 kg | Risperidone 5.3 kg Quetiapine 6.1 kg Aripiprazole 4.4 kg |
|
| Fasting glucose | |||||
| Lilly placebo database110 | 3–6 wk | 179 | 3.6 mg/dL | Placebo −3.6 mg/dL | P < 0.001 |
| Lilly total exposure database110 | up to 32 wk | 450 | 1.8 mg/dL | ||
| Sikich et al30 | 8 wk | 16 | 10.0 mg/dL | Risperidone −7.9 mg/dL Haloperidol −0.3 mg/dL |
|
| Sikich et al31 | 8 wk | 35 | 0.6 mg/dL | Risperidone 1.2 mg/dL Molindone 0.9 mg/dL |
|
| Kumra et al95 | 12 wk | 21 | 3.6 mg/dL | Clozapine 4.5 mg/dL | |
| Correll et al115 | 12 wk | 45 | 3.1 mg/dL | Risperidone 1.1 mg/dL Quetiapine 2.6 mg/dL Aripiprazole 0.5 mg/dL |
|
| HOMA-IR | |||||
| Sikich et al31 | 8 wk | 35 | 1.2 | Risperidone 0 Molindone 0.5 |
|
| Correll et al115 | 12 wk | 45 | 0.62 | Risperidone 0.2 Quetiapine 0.35 Aripiprazole 0.55 |
|
| Total cholesterol | |||||
| Lilly placebo database110 | 3–6 wk | 179 | 11.7 mg/dL | Placebo 0.0 | P = 0.002 |
| Lilly total exposure database110 | up to 32 wk | 450 | 7.8 mg/dL | ||
| Sikich et al31 | 8 wk | 35 | 19.9 mg/dL | Risperidone10.2 mg/dL Molindone 0 mg/dL |
P < 0.002 |
| Kumra et al95 | 12 wk | 21 | 17.2 mg/dL | ||
| Correll et al115 | 12 wk | 45 | 15.6 mg/dL | Risperidone 3.5 mg/dL Quetiapine 9.1 mg/dL Aripiprazole 2.8 mg/dL |
|
| LDL cholesterol | |||||
| Lilly placebo database110 | 3–6 wk | 179 | 7.8 mg/dL | Placebo 0 mg/dL | P = 0.002 |
| Lilly total exposure database110 | up to 32 wk | 450 | 7.8 mg/dL | ||
| Sikich et al30 | 8 wk | 16 | 7.6 mg/dL | Risperidone 2.9 mg/dL Haloperidol 0.1 mg/dL |
|
| Sikich et al31 | 8 wk | 35 | 14.7 mg/dL | Risperidone −9.6 mg/dL Molindone 0.5 mg/dL |
P = 0.003 |
| Correll et al115 | 12 wk | 45 | 11.5 mg/dL | Risperidone 0.2 mg/dL Quetiapine 3.9 mg/dL Aripiprazole 7.4 mg/dL |
|
| Triglycerides | |||||
| Lilly placebo database110 | 3–6 wk | 179 | 26.7 mg/dL | Placebo −8.9 mg/dL | P = 0.007 |
| Lilly total exposure database110 | up to 32 wk | 450 | 26.7 mg/dL | ||
| Sikich et al30 | 8 wk | 16 | 26 mg/dL | Risperidone −2 mg/dL Haloperidol 22 mg/dL |
|
| Sikich et al31 | 8 wk | 35 | 21.6 mg/dL | Risperidone 7.1 mg/dL Molindone −5.8 mg/dL |
|
| Kumra et al95 | 12 wk | 21 | 11.4 mg/dL | Clozapine 16.8 mg/dL | |
| Correll et al115 | 12 wk | 45 | 24.3 mg/dL | Risperidone 9.7 mg/dL Quetiapine 37.0 mg/dL Aripiprazole −2.4 mg/dL |
|
Glucose metabolism
In the Eli Lilly placebo-controlled database, olanzapine-treated youth showed a 3.6 mg/dL (0.2 mmol/L) increase in fasting glucose, whereas placebo-treated youth showed a similarly sized decrease. Although not clinically significant, this finding was highly statistically significant (P < 0.001). Further, 3% of adolescents in the exposure database moved from normal or borderline glucose levels into the diabetic range.110 Increases in fasting glucose with olanzapine treatment ranging from 0.6 to 10 mg/dL have been reported in several other pediatric trials (Table 2).30,31,95 A study of 45 antipsychotic- naïve youth treated with olanzapine found significant increases in glucose, insulin, and insulin resistance (measured with homeostasis model assessment–insulin resistance [HOMA-IR]) with P values between 0.02 and 0.03.115 There have also been multiple case reports of youth developing diabetes during olanzapine treatment.117–120 It is clear that youth are less likely to rapidly develop diabetes as a consequence of olanzapine treatment than adults because most teens have large insulin reserves. It is not yet clear to what extent these increases in glucose persist with sustained treatment.
Lipid metabolism
In the Eli Lilly database, youth treated with olanzapine showed increases in total cholesterol and triglycerides relative to their peers treated with placebo [cholesterol 11.7 mg/dL (0.03 mmol/L) compared to no change and triglycerides 26.7 mg/dL (0.03 mmol/L) compared to a decrease of 8.9 mg/dL (−0.1 mmol/L); P = 0.002 and P = 0.007, respectively]. Those in the exposure database showed identical changes. Approximately one-fifth of the patients developed borderline elevations in total cholesterol (>200 and <240 mg/dL), low-density lipoproteins (LDL cholesterol >130 and <160 mg/dL), and triglycerides (>150 and <200 mg/dL), and 17.8% developed hypertriglyceridemia.110 Interestingly, changes in lipid levels were slightly less in the study of antipsychotic-naïve youth ranging from 11.5 mg/dL for LDL cholesterol to 24.3 mg/dL for triglycerides with all P values less than 0.004.115 Similar changes have been reported in other small studies that included individuals previously exposed to antipsychotics (Table 2).30,31,95 Again there is limited information about the long-term course of these lipid changes.
Liver dysfunction
The Eli Lilly databases also report significant elevations in liver enzymes associated with olanzapine treatment. Alanine aminotransferase (ALT) increased by 20.0 and 21.4 U/L, aspartate aminotransferase (AST) increased by 6.4 U/L and 8.2 U/L, and γ-glutamyltransferase (GGT) increased by 7.5 and 9.0 U/L in the placebo-controlled and exposure databases, respectively (P ≤ 0.002 for each comparison). In contrast, total bilirubin decreased significantly (−1.7 and −1.1 μmol/L) in the two databases. Similar increases in ALT and AST have been reported in other pediatric studies of olanzapine.30,31 In the TEOSS trial, such elevations were more common in youth treated with olanzapine than those treated with either risperidone or molindone. In this study, there also appeared to be a tendency for these increases to diminish somewhat with continuing treatment.102 Eight (1.8%) of the 454 participants in the Lilly adolescent exposure database discontinued treatment due to liver function problems. These elevations may reflect nonalcoholic steatohepatitis (nonalcoholic fatty liver disease), although imaging studies and long-term follow-up is not yet available. In a retrospective chart review of youth treated with olanzapine alone, divalproex alone, or the combination of olanzapine and divalproex, 59% of those treated with olanzapine alone had at least 1 elevated hepatic enzyme over an average of 8 months of treatment while only 26% of those treated with divalproex alone did. However, all of those treated with the combination experienced at least 1 elevation and 42% of these had persistent elevations throughout the treatment period. One of the youth treated with both agents developed clinically significant steatohepatitis that resolved when the olanzapine was stopped.121
Hyperprolactinemia
In adults, olanzapine has not been associated with significant increases in prolactin. However, children and adolescents appear very sensitive to the prolactin-increasing effects of all antipsychotics, including olanzapine.122 The Eli Lilly placebo databases report acute prolactin increases of 11.4 μg/L with olanzapine compared with a slight decrease with placebo (P < 0.001). In the longer-term exposure database, the increase is much more significant at 23.0 μg/L compared with a decrease of −4.2 μg/L in the pooled adult data (P = 0.004). Interestingly, fewer female adolescents experienced prolactin elevations than male adolescents. Similar changes have been described in other clinical trials.30,31 Further, Wudarsky reported that 7 of 10 youth treated with olanzapine had elevations above the upper limits of normal for their age and gender. However, prolactin elevations with risperidone have consistently been found to be more extreme than with olanzapine in pediatric patients.30,31 It appears that olanzapine plasma concentration is significantly correlated with serum prolactin levels.123 However, most clinical trials do not report high rates of prolactin-related adverse events, such as menstrual irregularities, gynecomastia, or galactorrhea. The large Eli Lilly exposure database identified such problems in only 0.6%–4.2% of participants. The long-term consequences of moderate elevations in prolactin during adolescence are unknown.
Sedation
Most pediatric trials of olanzapine report high rates of sedation ranging from about 40% to 91%.30,31,95,96,100 In the Eli Lilly databases, the incidence appears somewhat lower (17.4%–19.0%). However, if the reported rates of somnolence are generally additive to those of sedation, the rates move into the 40% range reported in other studies. Unfortunately, sedation often does not resolve in individuals who continue long-term treatment.100,102 Sedation can have significant impact upon a youth’s ability to participate in school and other age-appropriate activities. Less than 1% of youth in Eli Lilly’s databases discontinued participation due to sedation.
Extrapyramidal symptoms
Almost all types of extrapyramidal adverse effects, including parkinsonian symptoms, dystonia, dyskinesia, tardive dyskinesia, akathisia, and neuroleptic malignant syndrome (NMS), have been reported in youth treated with olanzapine. However, these adverse events appear to occur much less often with olanzapine than with other antipsychotics including aripiprazole in youth. The incidence in the Lilly exposure database was 2.3% for parkinsonian symptoms, 5.5% for akathisia, and 1.4% for dyskinetic movements. Generally, those symptoms that do occur are minimal to mild. In comparative studies, olanzapine is consistently associated with fewer and less-severe extrapyramidal adverse events than risperidone, haloperidol, or molindone.30,31,100 There is 1 case report of persistent dystonic movements emerging during olanzapine treatment and persisting up to 6 weeks after switching to clozapine treatment.124 However, very few children and adolescents have been maintained for an extended period on a single antipsychotic or followed for sufficiently long periods to rigorously assess the medication-specific incidence of this late appearing and persistent adverse effect.
Neuroleptic malignant syndrome
NMS is a rare, potentially fatal, and idiosyncratic drug reaction that often occurs early in the course of antipsychotic treatment. NMS previously has been associated with the use of classical high-potency neuroleptics.125,126 Case reports suggest that NMS resulting from treatment with olanzapine and other SGAs has a more insidious course than NMS precipitated by first-generation high-potency agents. In addition, temperature elevations may emerge late in the course. Cognitive symptoms including agitation and delirium may be more prominent. However, there have been two case reports of classic NMS occurring during olanzapine monotherapy,127,128 one with olanzapine overdose,129 and several in the context of polypharmacy with other psychotropics (as described in two recent reviews).126,130
Hematologic effects
Although clozapine is best known for its risk of hematologic abnormalities, there are many case reports of neutropenia being caused by other antipsychotics as well. Olanzapine and ziprasidone seem to be more likely than the other newer antipsychotics to cause such abnormalities. 131 There are some case reports where olanzapine was the only medication being taken.132 Consequently, the FDA required a label change noting the risk of neutropenia and leukopenia with olanzapine treatment in August 2009. The label notes that this risk appears to be heightened in individuals with a history of blood dyscrasias or low white blood cell counts, particularly those induced by other drugs or those on drugs that have similar effects. In such cases, the patient’s blood count should be monitored frequently when treatment is initiated and treatment should be interrupted if the absolute neutrophil count drops below 1000 cells/cm3. It is unknown whether the risk for neutropenia is greater in the pediatric population than in the adult population.
Overdose
In adult studies, Lily data of over 3,100 subjects showed that the largest ingestion was 300 mg and that person did well with supportive care. However, there are multiple other reports of fatalities that some believe are related to olanzapine. 133–135 There have been at least two fatalities in children who have overdosed on olanzapine.136 Often, individuals with olanzapine overdoses develop EPS or NMS, respiratory symptoms, and mental status changes.129,137–139 Overdoses in pediatric patients are reviewed by Theisen.140 Management is primarily supportive and symptoms may take several days to a few weeks to fully resolve.
Olanzapine use in the pediatric population
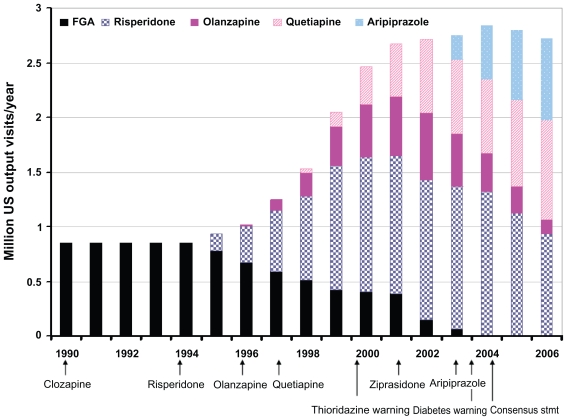
The pattern of olanzapine’s use over time in the US pediatric population relative to other antipsychotics is shown in Figure 2. When olanzapine was approved by the FDA in 1996, it almost immediately began being used in the pediatric population.140–149 However, its use never became as prevalent as risperidone’s. Quetiapine’s use was of similar magnitude by 2001. However, as data about olanzapine-associated weight gain emerged, the FDA issued requirements that SGA labels warn about the weight and metabolic risks; the American Diabetes Association and the American Psychiatric Association released a consensus statement indicating that olanzapine and clozapine had the greatest risk for such side effects and olanzapine use in the pediatric population quickly diminished to 5% or less of the total antipsychotics prescribed.146,148
Figure 2.
Patterns of antipsychotic use in the pediatric population of the United States. Following the introduction of each second-generation antipsychotic except clozapine, there has been rapid use within the pediatric population. However, risperidone, the first agent introduced after clozapine, has consistently been used more widely than the other agents. Further, safety considerations appear to have limited use of clozapine, ziprasidone, and earlier in 2003, olanzapine. This figure is a synthesis of data from multiple sources.135–140,142 Number of prescriptions and proportion related to each agent are approximate.
Evidence from the CATIE trial that showed a slight advantage of olanzapine over other first-line antipsychotics in total duration of treatment and symptom response and from a first-episode study showing neuroimaging benefits compared with haloperidol, as well as the similarities between the binding profiles of olanzapine and clozapine, led some clinicians to use olanzapine in a few youth with very severe or persistent psychotic symptoms.27,29,150 Youth with bipolar disorder are even less likely to be treated with olanzapine. In a recent study of pediatric bipolar treatment, it was used less often than any other antipsychotic (including FGAs).151
Olanzapine appears to be used more frequently in some parts of Europe but still to a lesser extent than either risperidone or quetiapine. In the CAFEPS longitudinal naturalistic study of youth with positive psychotic symptoms about 15% were treated with olanzapine.101 In Canada, use is also somewhat greater than in the United States with about 6000 children (~22% of those who are treated with antipsychotics) receiving prescriptions for olanzapine annually between 2003 and 2006.147 In countries outside the United States, aripiprazole appears to be less frequently used, so olanzapine and quetiapine are likely to be viewed as the antipsychotics with the least risk for EPS.
Conclusions
Olanzapine has clear efficacy for pedBP and EOSS. However, it also appears to have very great potential for promoting weight gain, increased insulin secretion, and dyslipidemia in children and adolescents. These adverse risks appear to be remarkably increased relative to other antipsychotics with the possible exception of clozapine. In the light of this extreme risk and because there is no compelling evidence that olanzapine has greater antipsychotic efficacy than other agents in either unselected youth with EOSS and other psychotic illnesses or in treatment-resistant EOSS, we argue that olanzapine should not be considered a first-line treatment for EOSS. Because there is clear evidence that clozapine has superior efficacy to olanzapine in treatment-resistant EOSS, we feel clinicians and families should seriously consider a trial of clozapine rather than olanzapine in such youth. There are no comparative studies examining the mood-stabilizing or antimanic properties of olanzapine relative to other SGAs. In the absence of any empiric data regarding comparative efficacy, olanzapine’s heightened risks for weight gain and metabolic dysregulation suggest to the authors that it should not be considered a first-line treatment for pedBP either. However, because there is no treatment of choice for youth with pedBP, who have failed to respond to multiple first-line treatments, olanzapine might have a role in treating such children and adolescents.
Although olanzapine is unlikely to be used frequently in the pediatric population, it remains an alternative for some individuals. Such individuals might include those who are underweight, have no family history of diabetes or dyslipidemia, or are particularly sensitive to the extrapyramidal side effects of antipsychotics with more potent dopamine D2 receptor binding. These conclusions are supported by the FDA’s recent decision to approve olanzapine for the treatment of adolescents with schizophrenia or manic/mixed states in bipolar disorder, provided clinicians and families carefully consider prescribing other agents first due to olanzapine’s marked risks for significant weight gain and lipid dysregulation in adolescents.
Olanzapine has played a critical role in alerting scientists and the public to the metabolic consequences of most antipsychotics, particularly within children and adolescents. The consistent weight and metabolic changes observed in olanzapine-treated youth spurred investigations of such effects among youth treated with other antipsychotics and provided a strong impetus for long-term studies of antipsychotic tolerability in youth.
Footnotes
Disclosures
Dr Maloney has received software for a computer intervention in schizophrenia from Posit Science. Dr Sikich receives research funding from NIMH, NIH, Foundation of Hope, Case Western Reserve University (subcontract from NICHD), NY Institute for Mental Hygiene Research (subcontract from NIMH), and Bristol Myers-Squibb. She is participating or has participated in clinical trials with Otsuka, Bristol Myers- Squibb, Neuropharm, Curemark, and Seaside Pharmaceuticals. She also received medication for clinical trials from Eli Lilly, Janssen, and Bristol Myers-Squibb, and software for a computer intervention in schizophrenia from Posit Science. She has served as a consultant for Sanofi Aventis within the past 2 years. She has given CME talks that have been indirectly supported by Bristol Myers-Squibb.
References
- 1.Hafner H, Nowotny B. Epidemiology of early-onset schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1995;245:80–92. doi: 10.1007/BF02190734. [DOI] [PubMed] [Google Scholar]
- 2.Maziade M, Gingras N, Rodrigue C, et al. Long-term stability of diagnosis and symptom dimensions in a systematic sample of patients with onset of schizophrenia in childhood and early adolescence. I: nosology, sex and age of onset. Br J Psychiatry. 1996;169(3):361–370. doi: 10.1192/bjp.169.3.361. [DOI] [PubMed] [Google Scholar]
- 3.Thomsen PH. Schizophrenia with childhood and adolescent onset – a nationwide register-based study. Acta Psychiatr Scand. 1996;94(3):187–193. doi: 10.1111/j.1600-0447.1996.tb09847.x. [DOI] [PubMed] [Google Scholar]
- 4.Schimmelmann BG, Conus P, Cotton S, McGorry PD, Lambert M. Pretreatment, baseline, and outcome differences between early-onset and adult-onset psychosis in an epidemiological cohort of 636 first-episode patients. Schizophr Res. 2007;95:1–3. 1–8. doi: 10.1016/j.schres.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Luoma S, Hakko H, Ollinen T, Jarvelin MR, Lindeman S. Association between age at onset and clinical features of schizophrenia: the Northern Finland 1966 birth cohort study. Eur Psychiatry. 2008;23(5):331–335. doi: 10.1016/j.eurpsy.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Hafner H, Riecher A, Maurer K, Loffler W, Munk-Jorgensen P, Stromgren E. How does gender influence age at first hospitalization for schizophrenia? A transnational case register study. Psychol Med. 1989;19(4):903–918. doi: 10.1017/s0033291700005626. [DOI] [PubMed] [Google Scholar]
- 7.Remschmidt HE, Schulz E, Martin M, Warnke A, Trott GE. Childhood-onset schizophrenia: history of the concept and recent studies. Schizophr Bull. 1994;20(4):727–745. doi: 10.1093/schbul/20.4.727. [DOI] [PubMed] [Google Scholar]
- 8.Russell AT. The clinical presentation of childhood-onset schizophrenia. Schizophr Bull. 1994;20(4):631–646. doi: 10.1093/schbul/20.4.631. [DOI] [PubMed] [Google Scholar]
- 9.Spencer EK, Campbell M. Children with schizophrenia: diagnosis, phenomenology, and pharmacotherapy. Schizophr Bull. 1994;20(4):713–725. doi: 10.1093/schbul/20.4.713. [DOI] [PubMed] [Google Scholar]
- 10.Werry JS, McClellan JM, Andrews LK, Ham M. Clinical features and outcome of child and adolescent schizophrenia. Schizophr Bull. 1994;20(4):619–630. doi: 10.1093/schbul/20.4.619. [DOI] [PubMed] [Google Scholar]
- 11.Schaeffer JL, Ross RG. Childhood-onset schizophrenia: premorbid and prodromal diagnostic and treatment histories. J Am Acad Child Adolesc Psychiatry. 2002;41(5):538–545. doi: 10.1097/00004583-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Eggers C, Bunk D. The long-term course of childhood-onset schizophrenia: a 42-year followup. Schizophr Bull. 1997;23:105–117. doi: 10.1093/schbul/23.1.105. [DOI] [PubMed] [Google Scholar]
- 13.Ropcke B, Eggers C. Early-onset schizophrenia: a 15-year follow-up. Eur Child Adolesc Psychiatry. 2005;14(6):341–350. doi: 10.1007/s00787-005-0483-6. [DOI] [PubMed] [Google Scholar]
- 14.Basso MR, Nasrallah HA, Olson SC, Bornstein RA. Cognitive deficits distinguish patients with adolescent- and adult- onset schizophrenia. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10(2):107–112. [PubMed] [Google Scholar]
- 15.Giedd JN, Jeffries NO, Blumenthal J, et al. Childhood-onset schizophrenia: progressive brain changes during adolescence. Biol Psychiatry. 1999;46(7):892–898. doi: 10.1016/s0006-3223(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 16.Tuulio-Henriksson A, Partonen T, Suvisaari J, Haukka J, Lonnqvist J. Age at onset and cognitive functioning in schizophrenia. Br J Psychiatry. 2004;185:215–219. doi: 10.1192/bjp.185.3.215. [DOI] [PubMed] [Google Scholar]
- 17.Biswas P, Malhotra S, Malhotra A, Gupta N. Comparative study of neuropsychological correlates in schizophrenia with onset in childhood, adolescence and adulthood. Eur Child Adolesc Psychiatry. 2006;15(6):360–366. doi: 10.1007/s00787-006-0542-7. [DOI] [PubMed] [Google Scholar]
- 18.Frazier JA, McClellan J, Findling RL, et al. Treatment of early- onset schizophrenia spectrum disorders (TEOSS): demographic and clinical characteristics. J Am Acad Child Adolesc Psychiatry. 2007;46(8):979–988. doi: 10.1097/chi.0b013e31807083fd. [DOI] [PubMed] [Google Scholar]
- 19.Jarbin H, Ott Y, Von Knorring AL. Adult outcome of social function in adolescent-onset schizophrenia and affective psychosis. J Am Acad Child Adolesc Psychiatry. 2003;42(2):176–183. doi: 10.1097/00004583-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Fleischhaker C, Schulz E, Tepper K, Martin M, Hennighausen K, Remschmidt H. Long-term course of adolescent schizophrenia. Schizophr Bull. 2005;31(3):769–780. doi: 10.1093/schbul/sbi014. [DOI] [PubMed] [Google Scholar]
- 21.Remschmidt H, Martin M, Fleischhaker C, et al. Forty-two-years later: the outcome of childhood-onset schizophrenia. J Neural Transm. 2007;114(4):505–512. doi: 10.1007/s00702-006-0553-z. [DOI] [PubMed] [Google Scholar]
- 22.Sikich L. Individual and school-based interventions. In: Findling R, Schluz S, editors. Schizophrenia in Adolescents and Children: Assessment, Neurobiology and Treatment. Baltimore (MD): John Hopkins University Press; 2005. pp. 257–287. [Google Scholar]
- 23.Pool D, Bloom W, Mielke DH, Roniger JJ, Jr, Gallant DM. A controlled evaluation of loxitane in seventy-five adolescent schizophrenic patients. Curr Ther Res Clin Exp. 1976;19(1):99–104. [PubMed] [Google Scholar]
- 24.Realmuto GM, Erickson WD, Yellin AM, Hopwood JH, Greenberg LM. Clinical comparison of thiothixene and thioridazine in schizophrenic adolescents. Am J Psychiatry. 1984;141:440–442. doi: 10.1176/ajp.141.3.440. [DOI] [PubMed] [Google Scholar]
- 25.Keepers GA, Clappison VJ, Casey DE. Initial anticholinergic prophylaxis for neuroleptic-induced extrapyramidal syndromes. Arch Gen Psychiatry. 1983;40(10):1113–1117. doi: 10.1001/archpsyc.1983.01790090075012. [DOI] [PubMed] [Google Scholar]
- 26.Kumra S, Frazier JA, Jacobsen LK, et al. Childhood-onset schizophrenia. A double-blind clozapine-haloperidol comparison. Arch Gen Psychiatry. 1996;53(12):1090–1097. doi: 10.1001/archpsyc.1996.01830120020005. [DOI] [PubMed] [Google Scholar]
- 27.Lieberman JA, Tollefson G, Tohen M, et al. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160(8):1396–1404. doi: 10.1176/appi.ajp.160.8.1396. [DOI] [PubMed] [Google Scholar]
- 28.Green AI, Lieberman JA, Hamer RM, et al. Olanzapine and haloperidol in first episode psychosis: two-year data. Schizophr Res. 2006;86:1–3. 234–243. doi: 10.1016/j.schres.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 30.Sikich L, Hamer RM, Bashford RA, Sheitman BB, Lieberman JA. A pilot study of risperidone, olanzapine, and haloperidol in psychotic youth: a double-blind, randomized, 8-week trial. Neuropsychopharmacology. 2004;29(1):133–145. doi: 10.1038/sj.npp.1300327. [DOI] [PubMed] [Google Scholar]
- 31.Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165(11):1420–1431. doi: 10.1176/appi.ajp.2008.08050756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beesdo K, Hofler M, Leibenluft E, Lieb R, Bauer M, Pfennig A. Mood episodes and mood disorders: patterns of incidence and conversion in the first three decades of life. Bipolar Disord. 2009;11(6):637–649. doi: 10.1111/j.1399-5618.2009.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perlis RH, Dennehy EB, Miklowitz DJ, et al. Retrospective age at onset of bipolar disorder and outcome during two-year follow-up: results from the STEP-BD study. Bipolar Disord. 2009;11(4):391–400. doi: 10.1111/j.1399-5618.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oedegaard KJ, Syrstad VE, Morken G, Akiskal HS, Fasmer OB. A study of age at onset and affective temperaments in a Norwegian sample of patients with mood disorders. J Affect Disord. 2009;118:1–3. 229–233. doi: 10.1016/j.jad.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 36.Moreno C, Laje G, Blanco C, Jiang H, Schmidt AB, Olfson M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032–1039. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- 37.Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10(1 Pt 2):194–214. doi: 10.1111/j.1399-5618.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geller B, Tillman R, Bolhofner K. Proposed definitions of bipolar I disorder episodes and daily rapid cycling phenomena in preschoolers, school-aged children, adolescents, and adults. J Child Adolesc Psychopharmacol. 2007;17(2):217–222. doi: 10.1089/cap.2007.0017. [DOI] [PubMed] [Google Scholar]
- 39.Tillman R, Geller B. Definitions of rapid, ultrarapid, and ultradian cycling and of episode duration in pediatric and adult bipolar disorders: a proposal to distinguish episodes from cycles. J Child Adolesc Psychopharmacol. 2003;13(3):267–271. doi: 10.1089/104454603322572598. [DOI] [PubMed] [Google Scholar]
- 40.Birmaher B, Axelson D, Strober M, et al. Comparison of manic and depressive symptoms between children and adolescents with bipolar spectrum disorders. Bipolar Disord. 2009;11(1):52–62. doi: 10.1111/j.1399-5618.2008.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160(3):430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 42.Geller B, Tillman R, Craney JL, Bolhofner K. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry. 2004;61(5):459–467. doi: 10.1001/archpsyc.61.5.459. [DOI] [PubMed] [Google Scholar]
- 43.Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Dev Psychopathol. 2006;18(4):1023–1035. doi: 10.1017/S0954579406060500. [DOI] [PubMed] [Google Scholar]
- 44.Rende R, Birmaher B, Axelson D, et al. Psychotic symptoms in pediatric bipolar disorder and family history of psychiatric illness. J Affect Disord. 2006;96:1–2. 127–131. doi: 10.1016/j.jad.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 45.Strober M, Birmaher B, Ryan N, et al. Pediatric bipolar disease: current and future perspectives for study of its long-term course and treatment. Bipolar Disord. 2006;8(4):311–321. doi: 10.1111/j.1399-5618.2006.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DelBello MP, Hanseman D, Adler CM, Fleck DE, Strakowski SM. Twelve-month outcome of adolescents with bipolar disorder following first hospitalization for a manic or mixed episode. Am J Psychiatry. 2007;164(4):582–590. doi: 10.1176/ajp.2007.164.4.582. [DOI] [PubMed] [Google Scholar]
- 47.Lewinsohn P, Seeley J, Klein D. Bipolar disorder in adolescents: epidemiology and suicidal behavior. In: Geller B, DelBello MP, editors. Bipolar Disorder in Childhood and Early Adolescence. New York: Guilford; 2003. pp. 7–24. [Google Scholar]
- 48.Axelson D, Birmaher B, Strober M, et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(10):1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- 49.Caetano SC, Olvera RL, Hunter K, et al. Association of psychosis with suicidality in pediatric bipolar I, II and bipolar NOS patients. J Affect Disord. 2006;91(1):33–37. doi: 10.1016/j.jad.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Geller B, Cooper TB, Sun K, et al. Double-blind and placebocontrolled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry. 1998;37(2):171–178. doi: 10.1097/00004583-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Kafantaris V, Coletti DJ, Dicker R, Padula G, Kane JM. Lithium treatment of acute mania in adolescents: a large open trial. J Am Acad Child Adolesc Psychiatry. 2003;42(9):1038–1045. doi: 10.1097/01.CHI.0000070247.24125.24. [DOI] [PubMed] [Google Scholar]
- 52.Kafantaris V, Coletti DJ, Dicker R, Padula G, Pleak RR, Alvir JM. Lithium treatment of acute mania in adolescents: a placebo-controlled discontinuation study. J Am Acad Child Adolesc Psychiatry. 2004;43(8):984–993. doi: 10.1097/01.chi.0000129223.89433.74. [DOI] [PubMed] [Google Scholar]
- 53.Kafantaris V, Coletti DJ, Dicker R, Padula G, Kane JM. Adjunctive antipsychotic treatment of adolescents with bipolar psychosis. J Am Acad Child Adolesc Psychiatry. 2001;40(12):1448–1456. doi: 10.1097/00004583-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 54.Wagner KD, Weller EB, Carlson GA, et al. An open-label trial of divalproex in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1224–1230. doi: 10.1097/00004583-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 55.Pavuluri MN, Henry DB, Carbray JA, Naylor MW, Janicak PG. Divalproex sodium for pediatric mixed mania: a 6-month prospective trial. Bipolar Disord. 2005;7(3):266–273. doi: 10.1111/j.1399-5618.2005.00204.x. [DOI] [PubMed] [Google Scholar]
- 56.Kowatch RA, Suppes T, Carmody TJ, et al. Effect size of lithium, divalproex sodium, and carbamazepine in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2000;39(6):713–720. doi: 10.1097/00004583-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Findling RL, McNamara NK, Gracious BL, et al. Combination lithium and divalproex sodium in pediatric bipolarity. J Am Acad Child Adolesc Psychiatry. 2003;42(8):895–901. doi: 10.1097/01.CHI.0000046893.27264.53. [DOI] [PubMed] [Google Scholar]
- 58.Wagner KD, Kowatch RA, Emslie GJ, et al. A double-blind, randomized, placebo-controlled trial of oxcarbazepine in the treatment of bipolar disorder in children and adolescents. Am J Psychiatry. 2006;163(7):1179–1186. doi: 10.1176/ajp.2006.163.7.1179. [DOI] [PubMed] [Google Scholar]
- 59.Wagner KD, Redden L, Kowatch RA, et al. A double-blind, randomized, placebo-controlled trial of divalproex extended-release in the treatment of bipolar disorder in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48(5):519–532. doi: 10.1097/CHI.0b013e31819c55ec. [DOI] [PubMed] [Google Scholar]
- 60.Pavuluri MN, Henry DB, Carbray JA, Sampson G, Naylor MW, Janicak PG. Open-label prospective trial of risperidone in combination with lithium or divalproex sodium in pediatric mania. J Affect Disord. 2004;82(Suppl 1):S103–111. doi: 10.1016/j.jad.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 61.Delbello MP, Schwiers ML, Rosenberg HL, Strakowski SM. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1216–1223. doi: 10.1097/00004583-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 62.Frazier JA, Biederman J, Tohen M, et al. A prospective open-label treatment trial of olanzapine monotherapy in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol. 2001;11(3):239–250. doi: 10.1089/10445460152595568. [DOI] [PubMed] [Google Scholar]
- 63.Barzman DH, DelBello MP, Adler CM, Stanford KE, Strakowski SM. The efficacy and tolerability of quetiapine versus divalproex for the treatment of impulsivity and reactive aggression in adolescents with co-occurring bipolar disorder and disruptive behavior disorder(s) J Child Adolesc Psychopharmacol. 2006;16(6):665–670. doi: 10.1089/cap.2006.16.665. [DOI] [PubMed] [Google Scholar]
- 64.Tohen M, Kryzhanovskaya L, Carlson G, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry. 2007;164(10):1547–1556. doi: 10.1176/appi.ajp.2007.06111932. [DOI] [PubMed] [Google Scholar]
- 65.Findling RL, Nyilas M, Forbes RA, et al. Acute treatment of pediatric bipolar I disorder, manic or mixed episode, with aripiprazole: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2009;70(10):1441–1451. doi: 10.4088/JCP.09m05164yel. [DOI] [PubMed] [Google Scholar]
- 66.Haas M, Delbello MP, Pandina G, et al. Risperidone for the treatment of acute mania in children and adolescents with bipolar disorder: a randomized, double-blind, placebo-controlled study. Bipolar Disord. 2009;11(7):687–700. doi: 10.1111/j.1399-5618.2009.00750.x. [DOI] [PubMed] [Google Scholar]
- 67.FDA_website. [Accessed Sep 15, 2009]. fda.gov/downloads/Drugs/DevelopmentApprovalProcess?DevelopmentResources/UCM163241.pdf. Published 2007.
- 68.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 69.DelBello MP, Chang K, Welge JA, et al. A double-blind, placebocontrolled pilot study of quetiapine for depressed adolescents with bipolar disorder. Bipolar Disord. 2009;11(5):483–493. doi: 10.1111/j.1399-5618.2009.00728.x. [DOI] [PubMed] [Google Scholar]
- 70.Bymaster FP, Calligaro DO, Falcone JF, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14(2):87–96. doi: 10.1016/0893-133X(94)00129-N. [DOI] [PubMed] [Google Scholar]
- 71.Fumagalli F, Frasca A, Sparta M, Drago F, Racagni G, Riva MA. Longterm exposure to the atypical antipsychotic olanzapine differently upregulates extracellular signal-regulated kinases 1 and 2 phosphorylation in subcellular compartments of rat prefrontal cortex. Mol Pharmacol. 2006;69(4):1366–1372. doi: 10.1124/mol.105.019828. [DOI] [PubMed] [Google Scholar]
- 72.Wirshing DA, Wirshing WC, Kysar L, et al. Novel antipsychotics: comparison of weight gain liabilities. J Clin Psychiatry. 1999;60(6):358–363. [PubMed] [Google Scholar]
- 73.Kroeze WK, Hufeisen SJ, Popadak BA, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28(3):519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 74.Templeman LA, Reynolds GP, Arranz B, San L. Polymorphisms of the 5-HT2C receptor and leptin genes are associated with antipsychotic drug-induced weight gain in Caucasian subjects with a first-episode psychosis. Pharmacogenet Genomics. 2005;15(4):195–200. doi: 10.1097/01213011-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Ujike H, Nomura A, Morita Y, et al. Multiple genetic factors in olanzapine- induced weight gain in schizophrenia patients: a cohort study. J Clin Psychiatry. 2008;69(9):1416–1422. doi: 10.4088/jcp.v69n0909. [DOI] [PubMed] [Google Scholar]
- 76.Gunes A, Melkersson KI, Scordo MG, Dahl ML. Association between HTR2C and HTR2A polymorphisms and metabolic abnormalities in patients treated with olanzapine or clozapine. J Clin Psychopharmacol. 2009;29(1):65–68. doi: 10.1097/JCP.0b013e31819302c3. [DOI] [PubMed] [Google Scholar]
- 77.Kassahun K, Mattiuz E, Nyhart E, Jr, et al. Disposition and biotransformation of the antipsychotic agent olanzapine in humans. Drug Metab Dispos. 1997;25(1):81–93. [PubMed] [Google Scholar]
- 78.Kando JC, Shepski JC, Satterlee W, Patel JK, Reams SG, Green AI. Olanzapine: a new antipsychotic agent with efficacy in the management of schizophrenia. Ann Pharmacother. 1997;31(11):1325–1334. doi: 10.1177/106002809703101110. [DOI] [PubMed] [Google Scholar]
- 79.Lucas RA, Gilfillan DJ, Bergstrom RF. A pharmacokinetic interaction between carbamazepine and olanzapine: observations on possible mechanism. Eur J Clin Pharmacol. 1998;54(8):639–643. doi: 10.1007/s002280050527. [DOI] [PubMed] [Google Scholar]
- 80.Callaghan JT, Bergstrom RF, Ptak LR, Beasley CM. Olanzapine. Pharmacokinetic and pharmacodynamic profile. Clin Pharmacokinet. 1999;37(3):177–193. doi: 10.2165/00003088-199937030-00001. [DOI] [PubMed] [Google Scholar]
- 81.Kelly DL, Conley RR, Tamminga CA. Differential olanzapine plasma concentrations by sex in a fixed-dose study. Schizophr Res. 1999;40(2):101–104. doi: 10.1016/s0920-9964(99)00053-5. [DOI] [PubMed] [Google Scholar]
- 82.Hiemke C, Peled A, Jabarin M, et al. Fluvoxamine augmentation of olanzapine in chronic schizophrenia: pharmacokinetic interactions and clinical effects. J Clin Psychopharmacol. 2002;22(5):502–506. doi: 10.1097/00004714-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 83.Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64(18):1917–1921. doi: 10.2146/ajhp060414. [DOI] [PubMed] [Google Scholar]
- 84.Spina E, de Leon J. Metabolic drug interactions with newer antipsychotics: a comparative review. Basic Clin Pharmacol Toxicol. 2007;100(1):4–22. doi: 10.1111/j.1742-7843.2007.00017.x. [DOI] [PubMed] [Google Scholar]
- 85.Darby JK, Pasta DJ, Wilson MG, Herbert J. Long-term therapeutic drug monitoring of risperidone and olanzapine identifies altered steady-state pharmacokinetics: a clinical, two-group, naturalistic study. Clin Drug Investig. 2008;28(9):553–564. doi: 10.2165/00044011-200828090-00002. [DOI] [PubMed] [Google Scholar]
- 86.Markowitz JS, DeVane CL, Malcolm RJ, et al. Pharmacokinetics of olanzapine after single-dose oral administration of standard tablet versus normal and sublingual administration of an orally disintegrating tablet in normal volunteers. J Clin Pharmacol. 2006;46(2):164–171. doi: 10.1177/0091270005283839. [DOI] [PubMed] [Google Scholar]
- 87.Grothe DR, Calis KA, Jacobsen L, et al. Olanzapine pharmacokinetics in pediatric and adolescent inpatients with childhood-onset schizophrenia. J Clin Psychopharmacol. 2000;20(2):220–225. doi: 10.1097/00004714-200004000-00015. [DOI] [PubMed] [Google Scholar]
- 88.Theisen FM, Haberhausen M, Schulz E, et al. Serum levels of olanzapine and its N-desmethyl and 2-hydroxymethyl metabolites in child and adolescent psychiatric disorders: effects of dose, diagnosis, age, sex, smoking, and comedication. Ther Drug Monit. 2006;28(6):750–759. doi: 10.1097/01.ftd.0000249950.75462.7f. [DOI] [PubMed] [Google Scholar]
- 89.Bachmann CJ, Haberhausen M, Heinzel-Gutenbrunner M, Remschmidt H, Theisen FM. Large intraindividual variability of olanzapine serum concentrations in adolescent patients. Ther Drug Monit. 2008;30(1):108–112. doi: 10.1097/FTD.0b013e3181633429. [DOI] [PubMed] [Google Scholar]
- 90.Aichhorn W, Marksteiner J, Walch T, et al. Age and gender effects on olanzapine and risperidone plasma concentrations in children and adolescents. J Child Adolesc Psychopharmacol. 2007;17(5):665–674. doi: 10.1089/cap.2006.0045. [DOI] [PubMed] [Google Scholar]
- 91.Kumra S, Jacobsen LK, Lenane M, et al. Childhood-onset schizophrenia: an open-label study of olanzapine in adolescents. J Am Acad Child Adolesc Psychiatry. 1998;37(4):377–385. doi: 10.1097/00004583-199804000-00015. [DOI] [PubMed] [Google Scholar]
- 92.Sholevar EH, Baron DA, Hardie TL. Treatment of childhood-onset schizophrenia with olanzapine. J Child Adolesc Psychopharmacol. 2000;10(2):69–78. doi: 10.1089/cap.2000.10.69. [DOI] [PubMed] [Google Scholar]
- 93.Mozes T, Greenberg Y, Spivak B, Tyano S, Weizman A, Mester R. Olanzapine treatment in chronic drug-resistant childhood-onset schizophrenia: an open-label study. J Child Adolesc Psychopharmacol. 2003;13(3):311–317. doi: 10.1089/104454603322572642. [DOI] [PubMed] [Google Scholar]
- 94.Shaw P, Sporn A, Gogtay N, et al. Childhood-onset schizophrenia: A double-blind, randomized clozapine-olanzapine comparison. Arch Gen Psychiatry. 2006;63(7):721–730. doi: 10.1001/archpsyc.63.7.721. [DOI] [PubMed] [Google Scholar]
- 95.Kumra S, Kranzler H, Gerbino-Rosen G, et al. Clozapine and “high-dose” olanzapine in refractory early-onset schizophrenia: a 12-week randomized and double-blind comparison. Biol Psychiatry. 2008;63(5):524–529. doi: 10.1016/j.biopsych.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 96.Findling RL, McNamara NK, Youngstrom EA, Branicky LA, Demeter CA, Schulz SC. A prospective, open-label trial of olanzapine in adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry. 2003;42(2):170–175. doi: 10.1097/00004583-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 97.Ross RG, Novins D, Farley GK, Adler LE. A 1-year open-label trial of olanzapine in school-age children with schizophrenia. J Child Adolesc Psychopharmacol. 2003;13(3):301–309. doi: 10.1089/104454603322572633. [DOI] [PubMed] [Google Scholar]
- 98.Quintana H, Wilson MS, 2nd, Purnell W, Layman AK, Mercante D. An open-label study of olanzapine in children and adolescents with schizophrenia. J Psychiatr Pract. 2007;13(2):86–96. doi: 10.1097/01.pra.0000265765.25495.e0. [DOI] [PubMed] [Google Scholar]
- 99.Dittmann RW, Meyer E, Freisleder FJ, et al. Effectiveness and tolerability of olanzapine in the treatment of adolescents with schizophrenia and related psychotic disorders: results from a large, prospective, open-label study. J Child Adolesc Psychopharmacol. 2008;18(1):54–69. doi: 10.1089/cap.2006.0137. [DOI] [PubMed] [Google Scholar]
- 100.Gothelf D, Apter A, Reidman J, et al. Olanzapine, risperidone and haloperidol in the treatment of adolescent patients with schizophrenia. J Neural Transm. 2003;110(5):545–560. doi: 10.1007/s00702-002-0803-7. [DOI] [PubMed] [Google Scholar]
- 101.Castro-Fornieles J, Parellada M, Soutullo CA, et al. Antipsychotic treatment in child and adolescent first-episode psychosis: a longitudinal naturalistic approach. J Child Adolesc Psychopharmacol. 2008;18(4):327–336. doi: 10.1089/cap.2007.0138. [DOI] [PubMed] [Google Scholar]
- 102.Findling R, Johnson J, McClellan J, et al. Double-blind maintenance safety and effectiveness findings from the Treatment of Early-Onset Schizophrenia Spectrum Study (TEOSS) J Am Acad Child Adolesc Psychiatry. 2010;49(6):583–594. doi: 10.1016/j.jaac.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kryzhanovskaya L, Schulz SC, McDougle C, et al. Olanzapine versus placebo in adolescents with schizophrenia: a 6-week, randomized, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(1):60–70. doi: 10.1097/CHI.0b013e3181900404. [DOI] [PubMed] [Google Scholar]
- 104.Overall JE, Pfefferbaum B. The Brief Psychiatric Rating Scale for Children. Psychopharmacol Bull. 1982;18(2):10–16. 1978; 133:429–435. [PubMed] [Google Scholar]
- 105.Hughes CW, Rintelmann J, Emslie GJ, Lopez M, MacCabe N. A revised anchored version of the BPRS-C for childhood psychiatric disorders. J Child Adolesc Psychopharmacol. Spring. 2001;11(1):77–93. doi: 10.1089/104454601750143500. [DOI] [PubMed] [Google Scholar]
- 106.Laughren T. Memorandum on recommendation for approvable actions for Zyprexa Pediatric Supplements for bipolar disorder (acute mania) and schizophrenia. [pdf from website] Apr 29, 2007. [Accessed Feb 10, 2010]. Available from: http://www.fda.gov/downloads/Drugs/DevelopmentApproval-Process/DevelopmentResources/UCM163360.pdf. Published 2010.
- 107.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 108.DelBello MP, Cecil KM, Adler CM, Daniels JP, Strakowski SM. Neurochemical effects of olanzapine in first-hospitalization manic adolescents: a proton magnetic resonance spectroscopy study. Neuropsychopharmacology. 2006;31(6):1264–1273. doi: 10.1038/sj.npp.1300950. [DOI] [PubMed] [Google Scholar]
- 109.Woods SW, Martin A, Spector SG, McGlashan TH. Effects of development on olanzapine-associated adverse events. J Am Acad Child Adolesc Psychiatry. 2002;41(12):1439–1446. doi: 10.1097/00004583-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 110.Kryzhanovskaya LA, Robertson-Plouch CK, Xu W, Carlson JL, Merida KM, Dittmann RW. The safety of olanzapine in adolescents with schizophrenia or bipolar I disorder: a pooled analysis of 4 clinical trials. J Clin Psychiatry. 2009;70(2):247–258. doi: 10.4088/jcp.08m03538. [DOI] [PubMed] [Google Scholar]
- 111.Ratzoni G, Gothelf D, Brand-Gothelf A, et al. Weight gain associated with olanzapine and risperidone in adolescent patients: a comparative prospective study. J Am Acad Child Adolesc Psychiatry. 2002;41(3):337–343. doi: 10.1097/00004583-200203000-00014. [DOI] [PubMed] [Google Scholar]
- 112.Mozes T, Ebert T, Michal SE, Spivak B, Weizman A. An open-label randomized comparison of olanzapine versus risperidone in the treatment of childhood-onset schizophrenia. J Child Adolesc Psychopharmacol. 2006;16(4):393–403. doi: 10.1089/cap.2006.16.393. [DOI] [PubMed] [Google Scholar]
- 113.Fleischhaker C, Heiser P, Hennighausen K, et al. Weight gain associated with clozapine, olanzapine and risperidone in children and adolescents. J Neural Transm. 2007;114(2):273–280. doi: 10.1007/s00702-006-0602-7. [DOI] [PubMed] [Google Scholar]
- 114.Fleischhaker C, Heiser P, Hennighausen K, et al. Weight gain in children and adolescents during 45 weeks treatment with clozapine, olanzapine and risperidone. J Neural Transm. 2008;115(11):1599–1608. doi: 10.1007/s00702-008-0105-9. [DOI] [PubMed] [Google Scholar]
- 115.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gebhardt S, Haberhausen M, Heinzel-Gutenbrunner M, et al. Antipsychotic-induced body weight gain: predictors and a systematic categorization of the long-term weight course. J Psychiatr Res. 2009;43(6):620–626. doi: 10.1016/j.jpsychires.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 117.Domon SE, Webber JC. Hyperglycemia and hypertriglyceridemia secondary to olanzapine. J Child Adolesc Psychopharmacol. 2001;11(3):285–288. doi: 10.1089/10445460152595603. [DOI] [PubMed] [Google Scholar]
- 118.Selva KA, Scott SM. Diabetic ketoacidosis associated with olanzapine in an adolescent patient. J Pediatr. 2001;138(6):936–938. doi: 10.1067/mpd.2001.114016. [DOI] [PubMed] [Google Scholar]
- 119.Bloch Y, Vardi O, Mendlovic S, Levkovitz Y, Gothelf D, Ratzoni G. Hyperglycemia from olanzapine treatment in adolescents. J Child Adolesc Psychopharmacol. 2003;13(1):97–102. doi: 10.1089/104454603321666234. [DOI] [PubMed] [Google Scholar]
- 120.Courvoisie HE, Cooke DW, Riddle MA. Olanzapine-induced diabetes in a seven-year-old boy. J Child Adolesc Psychopharmacol. 2004;14(4):612–616. doi: 10.1089/cap.2004.14.612. [DOI] [PubMed] [Google Scholar]
- 121.Gonzalez-Heydrich J, Raches D, Wilens TE, Leichtner A, Mezzacappa E. Retrospective study of hepatic enzyme elevations in children treated with olanzapine, divalproex, and their combination. J Am Acad Child Adolesc Psychiatry. 2003;42(10):1227–1233. doi: 10.1097/00004583-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 122.Wudarsky M, Nicolson R, Hamburger SD, et al. Elevated prolactin in pediatric patients on typical and atypical antipsychotics. J Child Adolesc Psychopharmacol. 1999;9(4):239–245. doi: 10.1089/cap.1999.9.239. [DOI] [PubMed] [Google Scholar]
- 123.Alfaro CL, Wudarsky M, Nicolson R, et al. Correlation of antipsychotic and prolactin concentrations in children and adolescents acutely treated with haloperidol, clozapine, or olanzapine. J Child Adolesc Psychopharmacol. 2002;12(2):83–91. doi: 10.1089/104454602760219126. [DOI] [PubMed] [Google Scholar]
- 124.Kumra S, Jacobsen LK, Lenane M, et al. Case series: spectrum of neuroleptic-induced movement disorders and extrapyramidal side effects in childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 1998;37(2):221–227. doi: 10.1097/00004583-199802000-00016. [DOI] [PubMed] [Google Scholar]
- 125.Picard LS, Lindsay S, Strawn JR, Kaneria RM, Patel NC, Keck PE., Jr Atypical neuroleptic malignant syndrome: diagnostic controversies and considerations. Pharmacotherapy. 2008;28(4):530–535. doi: 10.1592/phco.28.4.530. [DOI] [PubMed] [Google Scholar]
- 126.Neuhut R, Lindenmayer JP, Silva R. Neuroleptic malignant syndrome in children and adolescents on atypical antipsychotic medication: a review. J Child Adolesc Psychopharmacol. 2009;19(4):415–422. doi: 10.1089/cap.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abu-Kishk I, Toledano M, Reis A, Daniel D, Berkovitch M. Neuroleptic malignant syndrome in a child treated with an atypical antipsychotic. J Toxicol Clin Toxicol. 2004;42(6):921–925. doi: 10.1081/clt-200035214. [DOI] [PubMed] [Google Scholar]
- 128.Mendhekar DN, Duggal HS. Persistent amnesia as a sequel of olanzapine- induced neuroleptic malignant syndrome. J Neuropsychiatry Clin Neurosci. 2006;18(4):552–553. doi: 10.1176/jnp.2006.18.4.552. [DOI] [PubMed] [Google Scholar]
- 129.Cohen LG, Fatalo A, Thompson BT, Di Centes Bergeron G, Flood JG, Poupolo PR. Olanzapine overdose with serum concentrations. Ann Emerg Med. 1999;34(2):275–278. doi: 10.1016/s0196-0644(99)70243-x. [DOI] [PubMed] [Google Scholar]
- 130.Croarkin PE, Emslie GJ, Mayes TL. Neuroleptic malignant syndrome associated with atypical antipsychotics in pediatric patients: a review of published cases. J Clin Psychiatry. 2008;69(7):1157–1165. doi: 10.4088/jcp.v69n0716. [DOI] [PubMed] [Google Scholar]
- 131.Delieu JM, Horobin RW, Duguid JK. Exploring the relationship of drug-induced neutrophil immaturity and haematological toxicity to drug chemistry using quantitative structure-activity models. Med Chem. 2009;5(1):7–14. doi: 10.2174/157340609787049307. [DOI] [PubMed] [Google Scholar]
- 132.Stubner S, Grohmann R, Engel R, et al. Blood dyscrasias induced by psychotropic drugs. Pharmacopsychiatry. 2004;37(Suppl 1):S70–78. doi: 10.1055/s-2004-815513. [DOI] [PubMed] [Google Scholar]
- 133.Elian AA. Fatal overdose of olanzepine. Forensic Sci Int. 1998;91(3):231–235. doi: 10.1016/s0379-0738(97)00195-3. [DOI] [PubMed] [Google Scholar]
- 134.Gerber JE, Cawthon B. Overdose and death with olanzapine: two case reports. Am J Forensic Med Pathol. 2000;21(3):249–251. doi: 10.1097/00000433-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 135.Chue P, Singer P. A review of olanzapine-associated toxicity and fatality in overdose. J Psychiatry Neurosci. 2003;28(4):253–261. [PMC free article] [PubMed] [Google Scholar]
- 136.Antia SX, Sholevar EH, Baron DA. Overdoses and ingestions of second-generation antipsychotics in children and adolescents. J Child Adolesc Psychopharmacol. 2005;15(6):970–985. doi: 10.1089/cap.2005.15.970. [DOI] [PubMed] [Google Scholar]
- 137.Chambers RA, Caracansi A, Weiss G. Olanzapine overdose cause of acute extrapyramidal symptoms. Am J Psychiatry. 1998;155(11):1630–1631. [PubMed] [Google Scholar]
- 138.Catalano G, Cooper DS, Catalano MC, Butera AS. Olanzapine overdose in an 18-month-old child. J Child Adolesc Psychopharmacol. 1999;9(4):267–271. doi: 10.1089/cap.1999.9.267. [DOI] [PubMed] [Google Scholar]
- 139.Kochhar S, Nwokike JN, Jankowitz B, Sholevar EH, Abed T, Baron DA. Olanzapine overdose: a pediatric case report. J Child Adolesc Psychopharmacol. 2002;12(4):351–353. doi: 10.1089/104454602762599907. [DOI] [PubMed] [Google Scholar]
- 140.Theisen FM, Grabarkiewicz J, Fegbeutel C, Hubner A, Mehler-Wex C, Remschmidt H. Olanzapine overdose in children and adolescents: two case reports and a review of the literature. J Child Adolesc Psychopharmacol. 2005;15(6):986–995. doi: 10.1089/cap.2005.15.986. [DOI] [PubMed] [Google Scholar]
- 141.Cooper WO, Hickson GB, Fuchs C, Arbogast PG, Ray WA. New users of antipsychotic medications among children enrolled in TennCare. Arch Pediatr Adolesc Med. 2004;158(8):753–759. doi: 10.1001/archpedi.158.8.753. [DOI] [PubMed] [Google Scholar]
- 142.Aparasu RR, Bhatara V. Antipsychotic prescribing trends among youths, 1997–2002. Psychiatr Serv. 2005;56(8):904. doi: 10.1176/appi.ps.56.8.904. [DOI] [PubMed] [Google Scholar]
- 143.Patel NC, Crismon ML, Hoagwood K, et al. Trends in the use of typical and atypical antipsychotics in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2005;44(6):548–556. doi: 10.1097/01.chi.0000157543.74509.c8. [DOI] [PubMed] [Google Scholar]
- 144.Cooper WO, Arbogast PG, Ding H, Hickson GB, Fuchs DC, Ray WA. Trends in prescribing of antipsychotic medications for US children. Ambul Pediatr. 2006;6(2):79–83. doi: 10.1016/j.ambp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 145.Olfson M, Blanco C, Liu L, Moreno C, Laje G. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry. 2006;63(6):679–685. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- 146.Aparasu RR, Bhatara V. Patterns and determinants of antipsychotic prescribing in children and adolescents, 2003–2004. Curr Med Res Opin. 2007;23(1):49–56. doi: 10.1185/030079906X158075. [DOI] [PubMed] [Google Scholar]
- 147.Alessi-Severini S, Biscontri RG, Collins DM, Kozyrskyj A, Sareen J, Enns MW. Utilization and costs of antipsychotic agents: a Canadian population-based study, 1996–2006. Psychiatr Serv. 2008;59(5):547–553. doi: 10.1176/ps.2008.59.5.547. [DOI] [PubMed] [Google Scholar]
- 148.Constantine R, Tandon R. Changing trends in pediatric antipsychotic use in Florida’s Medicaid program. Psychiatr Serv. 2008;59(10):1162–1168. doi: 10.1176/ps.2008.59.10.1162. [DOI] [PubMed] [Google Scholar]
- 149.Rani F, Murray ML, Byrne PJ, Wong IC. Epidemiologic features of antipsychotic prescribing to children and adolescents in primary care in the United Kingdom. Pediatrics. 2008;121(5):1002–1009. doi: 10.1542/peds.2007-2008. [DOI] [PubMed] [Google Scholar]
- 150.Lieberman JA, Tollefson GD, Charles C, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62(4):361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- 151.Potter MP, Liu HY, Monuteaux MC, et al. Prescribing patterns for treatment of pediatric bipolar disorder in a specialty clinic. J Child Adolesc Psychopharmacol. 2009;19(5):529–538. doi: 10.1089/cap.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]