Abstract
The transcription factor nuclear factor-κB (NF-κB) is essential for immune and inflammatory responses. NF-κB essential modulator (NEMO) is a scaffolding component of the IκB kinase complex required for NF-κB activation in vitro. Because NF-κB activation is involved in B cell development and function, we set out to determine whether NEMO is required for these processes. NEMO(−/−) mice die very early during embryogenesis, and fetal livers from NEMO(−/−) embryos can not reconstitute either B or T lymphopoiesis in irradiated host mice. We therefore used NEMO(−/−) embryonic stem cells and the OP9 in vitro differentiation system to demonstrate that NEMO is not required for B cell development but plays an important role in B cell survival.
During cellular processes such as hematopoiesis, cell fate is determined through the regulation of transcription. The transcription factor nuclear factor-κB (NF-κB) plays critical roles in the control of inflammation, cell proliferation, and apoptosis (1, 2). NF-κB was first characterized as a transcription factor constitutively binding to the κB motif in the enhancer element within the Ig κ light-chain gene (3, 4). In the cytoplasm of a resting cell, NF-κB is controlled by binding to the inhibitor of κB (IκB) proteins. In response to various cytokines, mitogens, and inflammatory stimuli, serine residues 32 and 36 of IκB-α are phosphorylated and subsequently ubiquitinated for degradation (5, 6). NF-κB then dissociates from IκB, translocates into the nucleus, and activates the transcription of various target genes.
Biochemical studies of NF-κB pathways have shown that the phosphorylation of IκB is carried out by the 700- to 900-kDa IκB kinase (IKK) protein complex (7–9). Two serine-threonine kinases within this complex, IKK-α and -β, have been characterized and shown to be capable of phosphorylating both serines 32 and 36 of IκB-α in vivo and in vitro. IKK-β has 52% amino acid similarity to IKK-α and both kinases contain leucine-zipper (LZ) and helix–loop–helix (HLH) domains. A third component of the IKK complex is NF-κB essential modulator (NEMO)/IKK-γ, first characterized as a protein able to bind to both IKK-α and -β via the LZ and HLH domains (10, 11). NEMO has no intrinsic IκB kinase activity, but biochemical evidence has indicated that NEMO is required for the activation of NF-κB in vitro.
Analyses of gene-targeted mice deficient for subunits of NF-κB or components of the IKK complex have demonstrated the vital role of the NF-κB pathway in development and apoptosis. Fatal liver apoptosis often occurs in the early stages of embryogenesis, precluding the development of hematopoietic progenitors in the fetal liver (12, 13). The IKK-α(−/−) mouse dies immediately after birth due to skin and skeletal malformations (14–16) that are independent of NF-κB activation (17). In contrast, the IKK-β(−/−) mouse dies at embryonic day (E)13.5 due to massive liver apoptosis. IKK-β is also required for the proper activation of NF-κB in response to tumor necrosis factor (TNF)-α and IL-1 in vitro (12, 18). The NEMO(−/−) mouse dies at E12.5 due to liver apoptosis, and NF-κB activation is completely abrogated in NEMO(−/−) embryonic fibroblasts in response to TNF-α, IL-1, or lipopolysaccharide (LPS; ref. 13). Heterozygous female NEMO-deficient mice display skin malformations consistent with recent human genetic studies of NEMO mutations (19, 20).
There is persuasive in vitro and in vivo evidence linking NF-κB activation to B cell development and function (21). However, the early lethal phenotype of the NEMO(−/−) mouse has precluded in vivo studies of the role of NEMO in lymphocyte development. In addition, like the fetal liver of IKK-β(−/−) embryos (22), the fetal liver of NEMO-deficient embryos fails to reconstitute B and T cells in irradiated host bone marrow (D.R., unpublished results). We have therefore used the OP9 in vitro differentiation system in which embryonic stem (ES) cells of a mouse are induced to differentiate into B cells in the presence of OP9 bone marrow cells in vitro (23–25). WT B cells differentiated in this way produce IgM in response to LPS, and their development clearly parallels the natural development of B cells in real bone marrow. In this study, NEMO(−/−) ES cells cocultured with OP9 bone marrow cells underwent normal hematopoiesis, and NEMO(−/−) B lymphocytes developed normally. However, the viability of the NEMO(−/−) IgM+ population was reduced. Our results suggest that NEMO is not required for B cell development but rather plays a vital role in IgM+ B cell survival.
Methods
In Vitro Generation of B Cells in ES/OP9 Cocultures.
NEMO(−/−) ES cells were generated as described (13). Control ES cells also contained the NEO cassette and were subjected to G418 selection. The OP9 cell line was originally generated by T. Nakano (Osaka University, Osaka), and tissue culture of OP9 and ES cells was performed as described (25). The medium for the OP9 system was α-MEM containing 15% FCS (HyClone, lot no. FCL13226, FMB15475). For the hematopoietic induction of ES cells, single cell suspensions (5 × 104 cells) of NEMO(−/−) or control ES cells were seeded onto a confluent OP9 monolayer in a 10-cm dish. After 5 days of coculture, the ES cells and OP9 monolayer were trypsinized and a single cell suspension was preplated for 30 min. Nonadherent cells (6 × 105) were transferred to a new OP9 monolayer in a 10-cm dish with the addition of the cytokine Flt3L (10 ng/ml). After 8 days of coculture, the nonadherent differentiating cell suspension was removed from the OP9 monolayer (without disturbing it) by gentle washing with a pipette. The nonadherent differentiating cells were pelleted by centrifugation at 500 × g, resuspended in OP9 medium, and transferred onto fresh confluent OP9 monolayers in 6-well plates. From day 8 on, the culture medium contained both Flt3L (10 ng/ml) and IL-7 (10 ng/ml) to sustain B lymphocyte development. Throughout the coculture period, the medium was changed every other day, and the OP9 layer was replaced every 4 days. To transfer a coculture to new OP9 monolayer, a single cell suspension of both the loosely adherent hematopoietic cells and the spent OP9 monolayer was generated by careful and gentle pipetting in the absence of trypsin. This single cell suspension was filtered through a 70-μm mesh to remove spent OP9 cells, and the filtered hematopoietic cells were transferred to a fresh OP9 monolayer. BR3-Fc was kindly provided by V. Dixit (26). Day 16 NEMO(−/−) cocultures were treated with BR3-Fc (2 μg/ml) for 6 days.
In Vitro Stimulation.
Day 19 ES cell/OP9 cocultures were transferred to fresh wells and either mock-stimulated or treated with 10 μg/ml of LPS (Sigma) for 3 days.
Flow Cytometric Analyses and Cell Sorting.
All antibodies and reagents used for surface and intracellular cytofluorimetric analyses were purchased from PharMingen. Cell staining was detected by flow cytometry with a FACSCalibur (Becton Dickinson) and analyzed with cellquest software. Viability was determined by propidium iodide (PI) staining followed by flow cytometry. All functional analyses were done on viable cells. To sort IgM+/CD19+ and IgM−/CD19+ B cell populations, day 19–21 OP9/ES cocultures were stained with CD19-PE antibody, IgM-APC antibody, and a low concentration of PI (0.2 μg/ml). At least 1 × 105 CD19+/IgM+/PI− cells and 2–3 × 106 CD19+/IgM−/PI− cells were isolated by cell sorting from both NEMO(−/−) and WT cocultures. To analyze the function and developmental transition of B cells, 5 × 104 CD19+/IgM+/PI− cells and 5 × 105 CD19+/IgM−/PI− cells were plated onto 24-well plates containing OP9 monolayers. Each well was either mock-stimulated or treated with LPS (10 μg/ml) for 2 days and analyzed by immunostaining and flow cytometry analysis. The viable cell number for IgM+/B220+ population was calculated by multiplying the percentage of IgM+/B220+ of all live OP9 cocultures from FACS analysis with the total number of viable cells from OP9 coculture.
Results
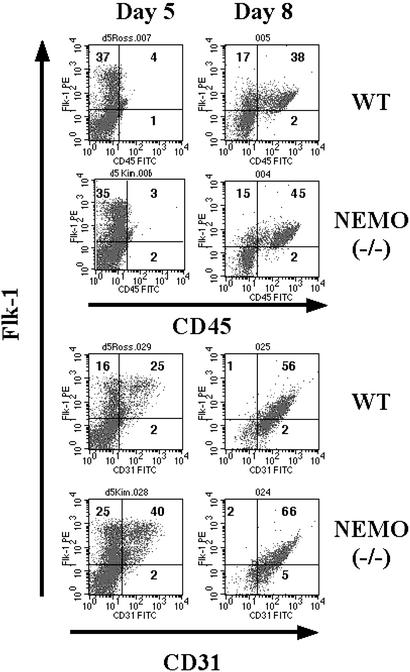
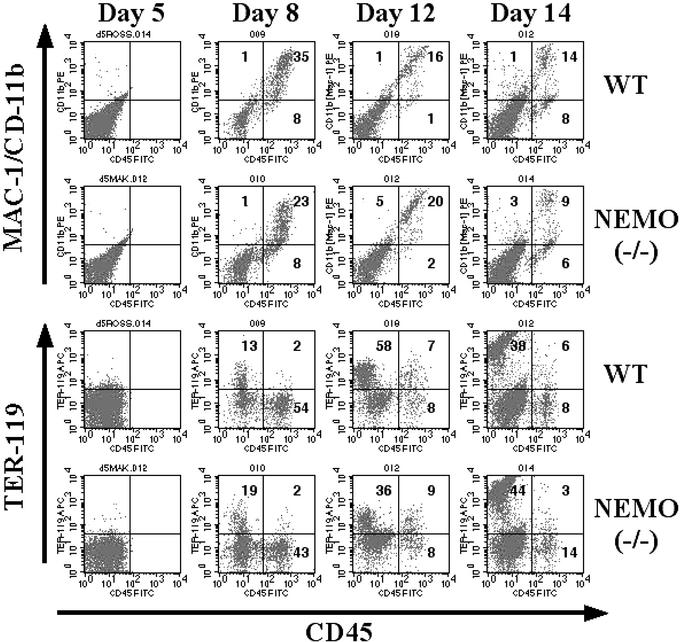
We used the OP9 in vitro differentiation system to generate NEMO-deficient B cells for the study of the role of NEMO in hematopoiesis and lymphopoiesis. The OP9 bone marrow cell line does not produce the cytokine M-CSF, so that lymphopoiesis in these cultures is enhanced at the expense of myelopoiesis (23, 24). ES cells from WT mice cocultured extensively with OP9 cells in the presence of Flt3L and IL-7 give rise to IgM+/B220+ B cells after 19–20 days (25). In this study, NEMO(−/−) ES cells were cocultured with a feeder layer of OP9 cells, and the appearance of B lineage cells was monitored by immunostaining and flow cytometry. After 5 days of coculture, the expression of the early hematopoietic markers Flk-1 and CD31 could be detected to the same level in both WT and NEMO(−/−) cocultures (Fig. 1), indicating the normal formation of endoderm colonies. Surface expression of CD45 on Flk-1+ cells was apparent by day 8, consistent with active hematopoiesis. Mac-1/CD11b and Ter-119 were observed on cell surfaces from day 8 and beyond in both WT and NEMO(−/−) cocultures (Fig. 2). The appearance of Mac1+/CD45+ cells indicates the development of granulocytes and monocytes, whereas Ter-119 is a surface marker for the erythroid lineage. These results suggest that NEMO-deficient ES cells are normal in their potential for monocyte and erythrocyte differentiation.
Figure 1.
Early stages of the in vitro differentiation of WT and NEMO(−/−) ES cells cocultured with OP9 bone marrow cells. Surface expression levels of CD45, Flk-1, and CD31 on days 5 and 8 of coculture were analyzed by immunostaining and flow cytometric analysis. Numbers in quadrants represent percentage of positively staining cells. For all figures, results shown are representative of two independent experiments involving two cocultures per genotype.
Figure 2.
Time course of hematopoietic differentiation in WT and NEMO(−/−) ES/OP9 cocultures. CD45, Mac-1, and Ter-119 surface expression on days 5, 8, 12, and 14 were analyzed as in Fig. 1.
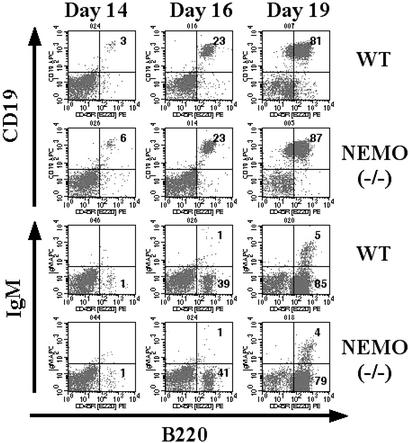
With respect to B lymphocyte development, we observed that cells expressing the B cell markers CD19 and B220 started to emerge in both WT and NEMO(−/−) cocultures on day 14 (Fig. 3 Upper). The NEMO(−/−) CD19+/B220+ population continued to expand through day 19 of coculture, at which point the IgM+ population started to appear (Fig. 3 Lower). This B cell population did not express IgD, CD21, or CD23 (data not shown), indicating that these emerging B cells were not splenic in phenotype but closer to the uneducated and unselected immature B cells derived from bone marrow. Thus, our data show that NEMO(−/−) and control ES cells are equally capable of in vitro B cell development, suggesting that NEMO is not directly required for the transition from a pre-B cell to an immature B cell.
Figure 3.
B cell development in WT and NEMO(−/−) ES/OP9 cocultures. Surface expression of B220, CD19, and IgM on days 14, 16, and 19 was analyzed as in Fig. 1.
There are other routes by which NF-κB might be activated in the absence of NEMO. The B cell-specific survival factor B cell activating factor belonging to the tumor necrosis factor family (BAFF)/BlyS is involved in processing the NF-κB p52 precursor in a NEMO-independent manner (26, 27). To investigate whether BAFF was supporting B cell development in NEMO-deficient cells, day 16 WT and NEMO(−/−) cocultures were treated with 2 μg/ml BR3-Fc to block BAFF-mediated NF-κB activation. Both WT and NEMO(−/−) cocultures continued to undergo normal B cell development and show equivalent surface IgM expression (data not shown). It is possible, however, that these conditions were insufficient to block the BAFF signal transduction pathway.
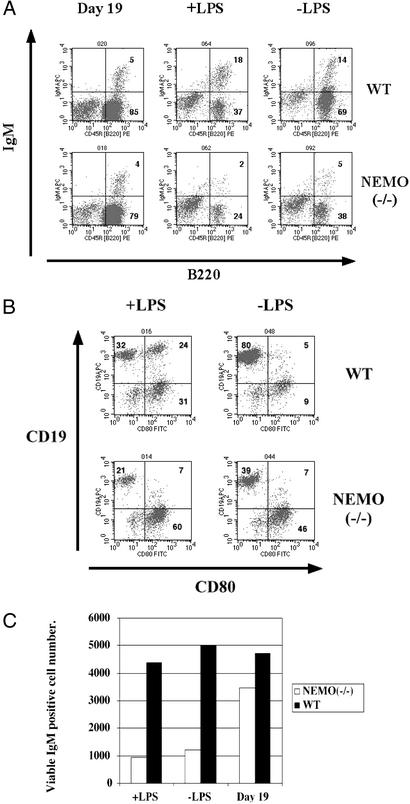
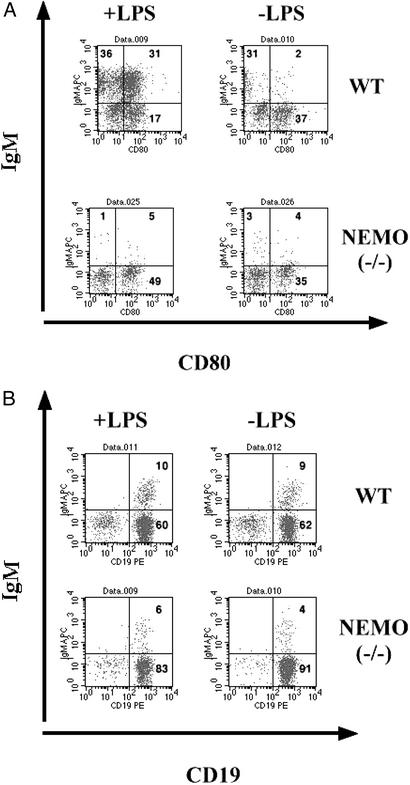
To investigate the role of NEMO in B cell function, WT and NEMO(−/−) day 19 cocultures were treated with a low dose of LPS (10 μg/ml). After 3 days, the surface phenotypes of B cells in the cocultures were analyzed. The IgM+/B220+ population in the NEMO(−/−) coculture was significantly reduced in comparison to the control coculture (Fig. 4A), suggesting either a lack of proliferation or increased apoptosis of NEMO(−/−) B cells in response to LPS. In both cocultures, the B220+ population was depleted when LPS was added, consistent with the decreased cell viability observed in response to LPS. However, although IgM+ B cells appeared in WT cocultures in response to LPS stimulation, the IgM+ population was reduced in the NEMO(−/−) coculture (Fig. 4C). Even without LPS treatment, the IgM+ B cell population was reduced in the NEMO(−/−) coculture. The expression of the activation marker CD80 was also measured in LPS-stimulated WT and NEMO(−/−) cocultures. CD80 is up-regulated on the surface of immature IgM+ WT B cells in response to LPS stimulation (25). Although treatment with LPS (10 μg/ml) resulted in the emergence of CD19+/CD80+ WT cells, no up-regulation of CD80 was observed on cells in the LPS-stimulated NEMO(−/−) coculture (Fig. 4B). This result is consistent with altered proliferation or apoptosis of NEMO(−/−) B cells in response to LPS.
Figure 4.
Impaired survival and mitogenic response of IgM+/B220+ cells from stimulated NEMO(−/−) cocultures. (A) On day 19, WT and NEMO(−/−) ES/OP9 cocultures were either mock-stimulated (−LPS) or treated with LPS (10 μg/ml) for 3 days. Each coculture was analyzed by flow cytometry to determine the relative percentage of IgM+/B220+ cells. (B) Surface expression of the activation marker CD80. On day 19, NEMO(−/−) and WT cocultures were either mock-stimulated or treated with LPS (10 μg/ml) for 3 days. The percentage of CD19+/CD80+ cells was determined by flow cytometry. (C) The number of viable IgM+ B cells in cocultures of A as calculated by immunostaining and flow cytometry.
We next investigated the viability of CD19+/IgM+ cells isolated from WT and NEMO(−/−) cocultures by cell sorting. For both WT and NEMO(−/−) cocultures, ≈1 × 105 CD19+/IgM+ cells and 2 × 106 CD19+/IgM− cells were recovered. Approximately 5 × 104 CD19+/IgM+ cells and 5 × 105 CD19+/IgM− cells were then plated onto OP9 monolayers, and the cultures were treated with 10 μg/ml of LPS for 48 h. The up-regulation of CD80 on CD19+/IgM+ cells was determined by flow cytometry. Isolated CD19+/IgM+ WT B cells both up-regulated CD80 and expanded their numbers in response to LPS (Fig. 5A). Isolated CD19+/IgM+ NEMO(−/−) B cells were greatly reduced in number, however, whether or not they were stimulated with LPS, demonstrating that isolated NEMO(−/−) IgM+ B cells are inherently less viable than controls. However, isolated CD19+/IgM− B cells from either LPS-treated coculture showed an equal capacity to differentiate into CD19+/IgM+ B cells (Fig. 5B). Thus, NEMO is not required for the surface IgM expression but is essential for normal B cell survival.
Figure 5.
Impaired survival of isolated NEMO-deficient immature B cells. (A) Surface expression of the activation marker CD80. On day 19, CD19+/IgM+ cells were isolated from NEMO(−/−) and WT cocultures by cell sorting. Isolated CD19+/IgM+ B cells were either mock-stimulated or treated with LPS (10 μg/ml) for 2 days. The percentage of IgM+/CD80+ cells was determined by immunostaining and flow cytometry. (B) Surface expression of IgM. On day 19, CD19+/IgM− cells were isolated from NEMO(−/−) and WT cocultures by cell sorting. Isolated CD19+/IgM− B cells were stimulated as in A. The percentage of IgM+/CD19+ cells was determined by immunostaining and flow cytometry.
Discussion
The NF-κB pathway is directly involved in inflammatory and immune responses (1). Recent biochemical and genetic evidence has revealed NEMO to be a critical regulator of NF-κB activation in response to tumor necrosis factor α and IL-1, because NEMO recruits both IKK-α and -β (10, 11). However, the role of NF-κB in lymphocytes has been very difficult to analyze because of the early lethal phenotypes of mice deficient for subunits of NF-κB itself or components of its pathways. In addition, when fetal liver cells from mice lacking both the p50 and Rel A subunits of NF-κB are transferred to irradiated host mice, no reconstitution of hematopoietic progenitors in bone marrow is observed (28). This defect is not cell-autonomous, because the phenotype can be rescued in the presence of WT hematopoietic cells. That a population of B cells has appeared in a host reconstituted with fetal liver lacking p50 and Rel A suggests that at least these subunits of NF-κB are not required for B cell development. However, these results do not exclude a possible role for either p52 or Rel B in B cell development. A recent report has also suggested that IKK-α and its function in p52 processing are required for B cell development and functions (29). Thus, the role of NF-κB in B cell development remains unresolved. We believe that the study of NEMO may provide useful insights into this question.
Because fetal liver cells from NEMO(−/−) embryos fail to reconstitute T and B lymphocytes in the bone marrow of host mice, we have used the ES cell/OP9 coculture differentiation system to examine B cell development. We have shown that NEMO-deficient ES cells can undergo normal hematopoiesis in the OP9 in vitro system. Interestingly, we did not observe perturbations in either monocyte development or erythropoiesis. The increased myelopoiesis reported after fetal liver reconstitution of the p50/Rel A double-mutant mouse was not observed in our system. Moreover, B cell development from NEMO(−/−) ES cells was just as efficient as that from control ES cells, indicating that there was no intrinsic defect in the hematopoietic differentiation capacity of NEMO-deficient ES cells. Our data thus support the hypothesis that the mutant fetal liver environment as a whole is responsible for the lack of hematopoietic progenitors in NEMO(−/−) mice, and that NEMO itself is not essential for hematopoiesis.
It has long been speculated that NF-κB activation is required for the generation of immature B cells. Mice deficient for any of the NF-κB subunits (p50, p52, Rel A, or Rel B) all show some type of B cell defect (30–33). These defects range from reduced proliferation in response to mitogen, to impaired isotype switching defects and decreased Ig production. In addition, there are clear dosage effects of NF-κB activation on these various B cell defects. Importantly, a lack of NF-κB activation induces the apoptosis of B cells (34–36). NF-κB activation depends on IκB degradation and thus on the IKK complex. Accordingly, mice deficient for the IKK-α component of the IKK complex show B cell defects. In addition, IgM+ B cells are reduced in both the bone marrow and spleen of irradiated host animals reconstituted with IKK-α-deficient fetal liver, and mature IKK-α-deficient B cells show impaired survival and reduced IgM and IgG production (37). Analysis of an IKK-α “knock-in” mutant mouse demonstrated that IKK-α is involved in processing p52 and that the B cell defect of this mouse is independent of NF-κB activation (29). In contrast, fetal liver from IKK-β(−/−) mice can reconstitute myelopoiesis in irradiated host animals but T and B lymphopoiesis does not occur (22). In the presence of WT bone marrow cells, fetal liver from IKK-β(−/−) can partially restore T lymphocyte development. Mice completely deficient for the last IKK component, NEMO, exhibit fatal liver apoptosis, whereas heterozygotes have skin malformations (13, 19, 20). Analysis of B cell-specific NEMO-deficient mice demonstrated that NEMO is required for the maintenance of peripheral B cell populations (38). Our data suggest that, although NEMO is not required for the normal development of immature IgM+ B cells, it is very important for the viability of immature IgM+ B cells. It is still formally possible that a NEMO-independent pathway of NF-κB activation is responsible for the transition of pre-B cells into IgM+ B cells. However, our results are significant in that a major component of the IKK complex controlling NF-κB activation has been found to be dispensable for B cell development. That the viability of NEMO-deficient IgM+ B cells is reduced in comparison to control B cells (Fig. 5A) supports a role for NEMO in the survival of immature IgM+ B cells.
Acknowledgments
We thank Mary Saunders for scientific editing and Irene Ng for excellent administrative assistance. We thank Dr. V. Dixit for providing us with BR3-FC. R.N.A.L.M.-M. is supported by the Canadian Institutes of Health Research (CIHR). J.C.Z.-P. is a CIHR Scientist and is supported by the National Cancer Institute of Canada (NCI). This work was supported by CIHR and NCI.
Abbreviations
- NF-κB
nuclear factor-κB
- NEMO
NF-κB essential modulator
- IκB
inhibitor of κB
- IKK
IκB kinase
- LPS
lipopolysaccharide
- ES
embryonic stem
- PI
propidium iodide
References
- 1.Baeuerle P A, Baltimore D. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 2.Barnes P J, Karin M. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 3.Sen R, Baltimore D. Cell. 1986;46:705–716. [PubMed] [Google Scholar]
- 4.Sen R, Baltimore D. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 5.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 6.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 8.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 9.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 10.Yamaoka S, Courtois G, Bessia C, Whiteside S T, Weil R, Agou F, Kirk H E, Kay R J, Israel A. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 11.Rothwarf D M, Zandi E, Natoli G, Karin M. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 12.Li Z W, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudolph D, Yeh W C, Wakeham A, Rudolph B, Nallainathan D, Potter J, Elia A J, Mak T W. Genes Dev. 2000;14:854–862. [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Lu Q, Hwang J Y, Buscher D, Lee K F, Izpisua-Belmonte J C, Verma I M. Genes Dev. 1999;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, Akira S. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Baud V, Oga T, Kim K I, Yoshida K, Karin M. Nature. 2001;410:710–714. doi: 10.1038/35070605. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Van Antwerp D, Mercurio F, Lee K F, Verma I M. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt-Supprian M, Bloch W, Courtois G, Addicks K, Israel A, Rajewsky K, Pasparakis M. Mol Cell. 2000;5:981–992. doi: 10.1016/s1097-2765(00)80263-4. [DOI] [PubMed] [Google Scholar]
- 20.Makris C, Godfrey V L, Krahn-Senftleben G, Takahashi T, Roberts J L, Schwarz T, Feng L, Johnson R S, Karin M. Mol Cell. 2000;5:969–979. doi: 10.1016/s1097-2765(00)80262-2. [DOI] [PubMed] [Google Scholar]
- 21.Baeuerle P A, Henkel T. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 22.Senftleben U, Li Z W, Baud V, Karin M. Immunity. 2001;14:217–230. doi: 10.1016/s1074-7613(01)00104-2. [DOI] [PubMed] [Google Scholar]
- 23.Nakano T, Kodama H, Honjo T. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 24.Nakano T, Kodama H, Honjo T. Science. 1996;272:722–724. doi: 10.1126/science.272.5262.722. [DOI] [PubMed] [Google Scholar]
- 25.Cho S K, Webber T D, Carlyle J R, Nakano T, Lewis S M, Zúñiga-Pflücker J C. Proc Natl Acad Sci USA. 1999;96:9797–9802. doi: 10.1073/pnas.96.17.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayagaki N, Yan M, Seshasayee D, Wang H, Lee W, French D M, Grewal I S, Cochran A G, Gordon N C, Yin J, et al. Immunity. 2002;17:515–524. doi: 10.1016/s1074-7613(02)00425-9. [DOI] [PubMed] [Google Scholar]
- 27.Claudio E, Brown K, Park S, Wang H, Siebenlist U. Nat Immunol. 2002;3:958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 28.Horwitz B H, Scott M L, Cherry S R, Bronson R T, Baltimore D. Immunity. 1997;6:765–772. doi: 10.1016/s1074-7613(00)80451-3. [DOI] [PubMed] [Google Scholar]
- 29.Senftleben U, Cao Y, Xiao G, Greten F R, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun S C, Karin M. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 30.Snapper C M, Zelazowski P, Rosas F R, Kehry M R, Tian M, Baltimore D, Sha W C. J Immunol. 1996;156:183–191. [PubMed] [Google Scholar]
- 31.Sha W C, Liou H C, Tuomanen E I, Baltimore D. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 32.Doi T S, Takahashi T, Taguchi O, Azuma T, Obata Y. J Exp Med. 1997;185:953–961. doi: 10.1084/jem.185.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caamano J H, Rizzo C A, Durham S K, Barton D S, Raventos-Suarez C, Snapper C M, Bravo R. J Exp Med. 1998;187:185–196. doi: 10.1084/jem.187.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grumont R J, Rourke I J, O'Reilly L A, Strasser A, Miyake K, Sha W, Gerondakis S. J Exp Med. 1998;187:663–74. doi: 10.1084/jem.187.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grumont R J, Rourke I J, Gerondakis S. Genes Dev. 1999;13:400–411. doi: 10.1101/gad.13.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu M, Lee H, Bellas R E, Schauer S L, Arsura M, Katz D, FitzGerald M J, Rothstein T L, Sherr D H, Sonenshein G E. EMBO J. 1996;15:4682–4690. [PMC free article] [PubMed] [Google Scholar]
- 37.Kaisho T, Takeda K, Tsujimura T, Kawai T, Nomura F, Terada N, Akira S. J Exp Med. 2001;193:417–426. doi: 10.1084/jem.193.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasparakis M, Schmidt-Supprian M, Rajewsky K. J Exp Med. 2002;196:743–752. doi: 10.1084/jem.20020907. [DOI] [PMC free article] [PubMed] [Google Scholar]