Introduction
Since the first synthesis of phenylboronic acid in the late 19th century, scientists have been interested in the unique properties associated with boron chemistry. Not until the mid 20th century, however, were the interactions between boronic acids and diol-containing compounds discovered [1]. While the reaction was debated for several years, it soon became apparent that the formation of cyclic boronate esters dominated. In a review published by Shinkai et al. in 1996 [2], numerous synthetic receptors for the optical detection of saccharides based on boronic acid receptors gained notoriety and helped launch a new field of chemical sensors. In 2002, Wang and co-workers discussed work on boronic acid-based sensors [3], leading the group to later publish several articles related to the pH and pKa dependence of boronic acid-diol interactions. It should be noted that while fluorescence-based saccharide detection is not within the scope of this review, significant work has also be reported with that approach [4-9].
Pattern-based sensing, similar to what we now know of the human olfactory and gustatory systems, relies on a selection of cross-reactive semi-selective indicators or receptors, the responses of which are analyzed as a whole in order to gain information on a diverse chemical library. While the concept has been well documented, only recently has pattern-based, differential sensing made its way into the forefront of sensing platforms and has found useful applications to a wide range of analytes, including peptides [10-12], proteins [13], volatile organic compounds [14-16], toxic industrial gases [17,18••], organic compounds in water [19], and many others. Herein we discuss the use of differential sensing for the colorimetric detection of sugars and sugar substitutes.
Interactions between boronic acids and diols
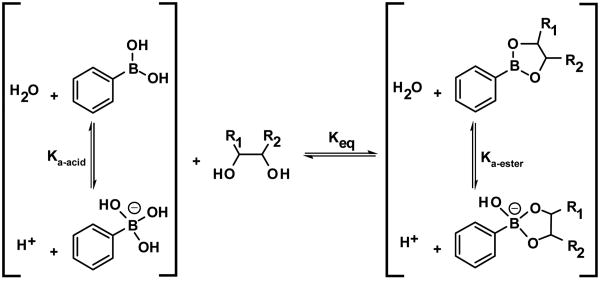
Boronic acids bind strongly with diol-containing compounds with high affinities via boronate ester formation (Figure 1). The reaction, discovered in 1954 by Kuivila et al., has been the basis for many synthetic molecular receptors, but considerable recent efforts have been made to improve the strength of binding, thus paving the way for more sensitive sensing methods for diol-containing targets [20,21]. Shinkai and James have spent the better part of a decade designing new and interesting sensing platforms based on the complexation of the two species [22-24]. The selectivity relies in part on the differences in association constants of different boronic acids with diols resulting in changes in equilibrium between the boronic acid and the cyclic boronate ester.
Figure 1.
Diol adducts of phenylboronic acid. Formation of the cyclic boronate ester can result in one of several detectible changes including pH depression, bond cleavage, indicator displacement, etc., that can result in color changes or changes in color intensity. Image used with permission, [25•].
It has been shown that changing the parameters of the experiment (e.g. solution pH, boronic acid pKa, etc.) can result in drastically different binding constants between boronic acids and diols. As a result, derivatization techniques have been employed to produce a plethora of molecular sensors for saccharides based on phenylboronic acid. In many cases, phenylboronic acids are coupled with chromophores to give a color response to binding of the boronic acid moiety. In this manner, several color changing motifs have been utilized including, indicator displacement [26••,27-28•], boronic acid appended chromophores [24,29-32•], and pH induction [33,34••], and these have been extended to produce differential sensing platforms optimized to human body temperature and physiological pH [23,35-37].
Colorimetric Sensors and Sensing Methods
Indicator Displacement Assays
The use of indicator displacement assays (IDAs) for colorimetric sensing has been pioneered most recently by Anslyn and co-workers [26••,38,39]. The basic principle relies on the affinity for two competing guests to one host. For sensing, the analyte displaces the indicator from the host or receptor (or vice-versa), causing the indicator to change its spectroscopic properties (c.f. Figure 2). For saccharide detection, color-changing indicators containing cis-diols were optimized to create an IDA sugar sensor. Among the most common of these indicators is alizarin red and bromopyrogallol red. IDA inherently represents an equilibrium competition between the indicator and the analyte of interest; this has a potential downside of limiting sensitivity, particularly for lower-affinity analytes.
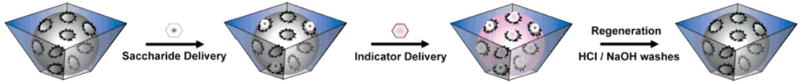
Figure 2.
General scheme for indicator displacement assay. Saccharides are represented by white hexagons and are introduced to the receptor scaffolding, then indicator (represented by blue hexagons. The sensing platform is regenerated by washing in acid and base. Image used with permission, [26••].
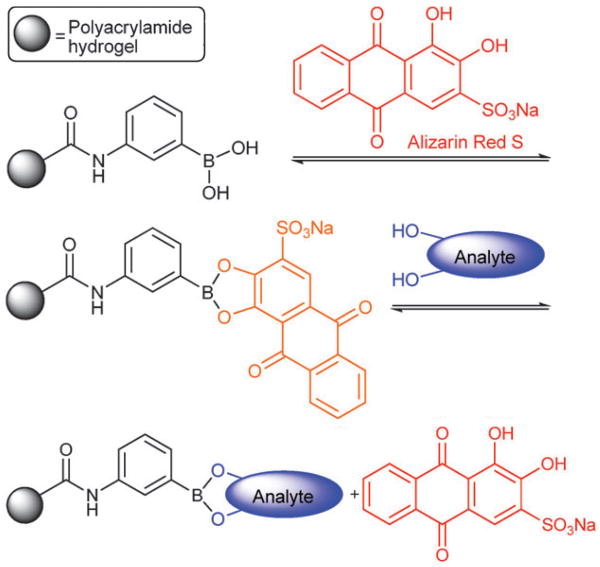
Indicator displacement has recently gained new ground via a sensing motif based on dye displacement using boronate hydrogels [28•]. Making use of a new development, boronate affinity in saccharide electrophoresis, or BASE, that relies on the modulation of saccharide mobility within a flexible porous hydrogel, the group went on to quantitatively detect a wide range of fructose concentrations at physiological pH; the indicator displacement scheme used can be seen in Figure 3. Recently, the synthesis of a similar sensing platform has been reported based on phenylboronic acid derivativization of solid silica supports [27].
Figure 3.
Indicator displacement scheme. Binding and analyte-mediated release of alizarin red-S with hydrogel-bound boronic acid. Image used with permission, [28•].
Boronic-acid Appended Chromophores
Chemosensors
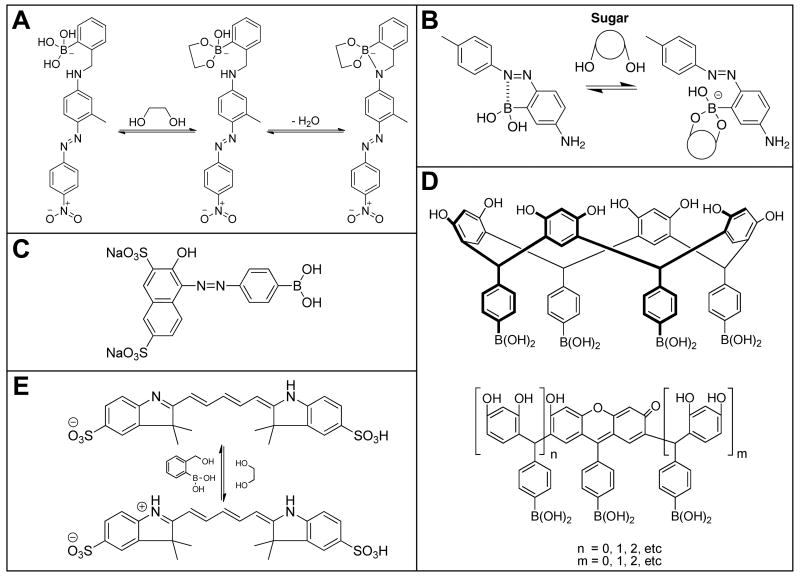
Recently, various colorimetric sensors based on boronic acid appended chromophores have been designed and studied for detection of a host of analyte classes including amines, aldehydes, alcohols, and in particular, cis-diols. Mohr and co-workers have published numerous articles focused on the use of reversible covalent-bonding sensing motifs combining a chromogenic center with a reactive terminus capable of color-change upon reaction with the desired analyte [40-43]. Several chromogenic receptors designed by Shinkai et al. are based on photoinduced electronic transfer (PET) created by complexation between the receptors' boronic acid functionality and saccharides. This strengthens the Lewis acid-Lewis base interaction within the receptor, resulting in PET from the amino nitrogen to the chromophoric center, thereby causing a change in color (Figure 4A) [2,31]. Since a shift in the absorbance of most azo-dyes is directly associated with changes in the environment of one of the bridging nitrogens, some sugar sensors have been designed that make use of a spectral change based on the formation and cleavage of the B—N bond between the boronic acid and the azo linkage (Figure 4B) [30,32•]. In other examples, the boronic acid group is directly attached to the chromophore, whose color changes upon chelation of the boronic acid group to a monosaccharide (see Figure 4C) [29]. In this manner, facile sugar detection has been possible, particularly involving the differentiation of glucose from fructose.
Figure 4.
Representative boronic acid based saccharide sensors. A) Photo-induced electron transfer (PET) method [31], B) B—N bond formation/cleavage method [32•], C) Boronic-acid chelating method [29], D) Macrocyclic ring opening method [44], and E) pH induced color change method [33]. Images used with permission.
Macrocyclic ring opening and oxidation
A new class of color-changing saccharide sensors was developed earlier this decade by Strongin and coworkers [44,45]. This sensing motif takes advantage of phenylboronic acid appended macrocycles undergoing significant color changes in the presence of carbohydrates as well as glucose phosphates, carboxylic acid and amino sugars. It was found that solutions containing the tetraarylboronic acid resorcinarene macrocycle (Figure 4D), undergo a condensation reaction in situ to form a xanthene chromophore. The authors argue that ring opening of macrocycles to acyclic oligomers could be a prerequisite for xanthene formation from resorcinarenes. In this way, complexation of a sugar with the newly formed acyclic chromophores was shown to semi-selectively discriminate among a family of closely related saccharides.
pH Induced Color Changes
Oddly, one of the least exploited characteristics regarding the reactions of boronic acids with saccharides had been the production of protons resulting from the formation of a cyclic boronate ester. The pH depression associated with the complexation of boronic acids with saccharides is, of course, buffer dependent, and in most of the detection methods mentioned thus far, systems have been strongly buffered. That being said, several groups have developed sugar sensing systems based mostly, if not solely, on induced pH depression. Among these, Chang and co-workers have presented a wonderful piece of work wherein pH indicators were employed to monitor and report the change in solution pH upon the addition of sugars [34••]. More recently, pH reporter dyes have been incorporated with one of several phenylboronic acid candidates, (c.f. Figure 4E) [33]. This new sensor technique can selectively and quantitatively differentiate between fructose and glucose at millimolar concentrations.
Array-based (Pattern-based) Sensing
The detection and identification of chemicals is, fundamentally, supramolecular chemistry and intrinsically relies on the interactions between molecules [46••]. The classification and relative strength of intermolecular interactions is of course very well established and includes Lewis acid-base interactions (the limiting case of which is bond formation and metal ion coordination), Brønsted acid-base interactions, hydrogen-bonding, charge-transfer and π-π molecular complexation, dipolar and multipolar interactions, and van der Waals interaction and physical adsorption. The use of sensors in arrays that probe this full range of intermolecular interactions is essential to the further development of array technology.
Array-based sensing has emerged as a potentially powerful approach toward the detection of chemically diverse analytes. Based on cross-responsive sensor elements, rather than analyte-specific receptors for specific analytes, sensor arrays produce composite responses and it is the composite response that is unique to an odorant or taste, just as in the mammalian olfactory and gustatory systems, respectively. In this design architecture, one receptor responds to many analytes and many receptors respond to any given analyte. It is the distinct pattern of responses produced by the array that provides differential sensing of multiple analytes.
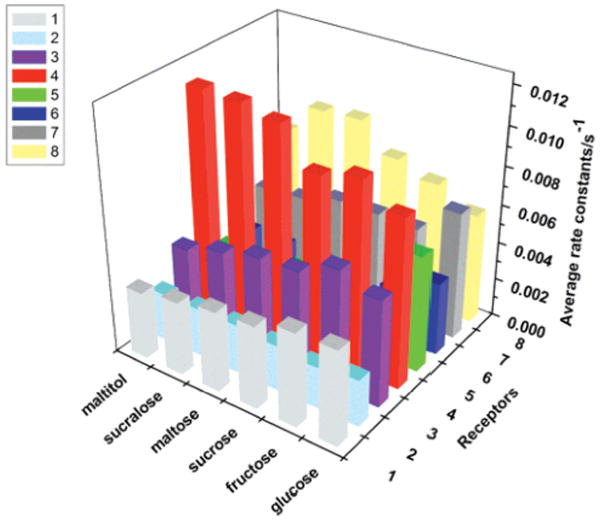
For nearly fifty years, the concept of pattern-based discrimination of a broad range of analytes, also known as “differential sensing”, has emerged as a powerful sensing tool. Recently, Anslyn and co-workers have successfully used arrays of cross-reactive sensors in combination with IDA and kinetic measurements to differentiate among a wide selection of aqueous analytes including, peptides [12], nucleotide phosphates (e.g., ATP, AMP, and GTP) [47], proteins [48], and recently, saccharides. Using boronic acid based peptidic receptors; chemosensors were developed capable of discrimination among a number of monosaccharides, disaccharides, and saccharide derivatives such as sucralose (the sweetening agent used in Splenda brand sweetener) and maltitol (a sugar alcohol derived by the reduction of maltose) [26••]. Multiple liquid wells were assembled using a randomly chosen set of receptors from a combinatorial library of pentapeptidic boronic acids optimized to work at physiological pH. A three-dimensional plot relating analytes, receptors, and average rate constants for indicator adduct displacement is shown in Figure 5. The plot shows how each semi-selective receptor responds to each analyte via rate constants. Indicator displacement assays were carried out, generating a set of response data that, when coupled with dimension-reducing chemometric tools such as linear discriminant analysis (LDA), can classify both structurally similar and chemically diverse analytes.
Figure 5.
Pattern-based saccharide sensing plot. Rate constants are used to assess the responses of each of eight semi-selective receptors with six sugars and sugar adducts. Each saccharide concentration was set to 80 μM in 500 μM HEPES buffer at pH=7.4. The blank resin beads were exposed first to the saccharide solution, then to a brief buffer wash, and finally to the indicator solution (8 μM in 500 μM HEPES buffer at pH=7.4. Image used with permission, [26••].
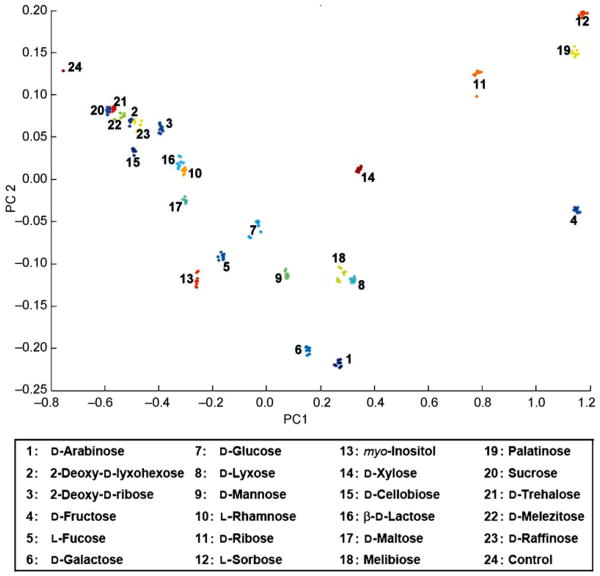
Another example of differential sensing is described by Chang and co-workers, who have recently published their work on a pH change-induced carbohydrate sensing ensemble that allowed for the differential recognition of 23 mono-, di-, and trisaccharides at millimolar concentrations [34•]. 12 dyes were employed in an array of aqueous solutions in well plates, covering a large pH range along with two boronic acids: boric acid and phenylboronic acid. Experiments for each system were carried within a pH range that optimizes the production of the cyclic tetrahedral boronate ester and thus depress the solution pH. A principal component analysis (PCA) scatter plot showing the discrete clusters of saccharides can be seen in Figure 6.
Figure 6.
Principal component analysis (PCA) plot for the identification of 23 carbohydrates at high concentrations (100 mM). Each numbered cluster represents multiple trials for each sugar using 384-well plates of liquid solutions containing one of 12 different pH indicators with one of two boronic acids at non-physiological pH with full spectrophotometric analysis. 98% of discriminatory information was contained within the first two principal components. Cluster plot was designed by combining two sets of data, one for each boronic acid system at a pH optimal to the system. Image used with permission, [34•].
The Suslick research group has been developing colorimetric sensor arrays to detect a myriad of analytes for over a decade. This work started as an application of metalloporphyrins as sensor elements for array-based vapor-sensing [49]. The arrays were broadened to include a wide range of chemically diverse sensors, adding pH indicators (which actually indicate much more than just pH), solvatochromic dyes, and shape-selective receptors to detect and differentiate between chemically diverse analytes [50-52]. We have successfully designed colorimetric sensor arrays capable of differential sensing of volatile organic compounds [14], organics in water [19], amines [53], toxic industrial chemicals (TICs) [17,18••], and even coffees [54], beers [55] and soft drinks [56]. Our previous printing formulations had been based on plasticized hydrophobic colorants that showed resistance to humidity while successfully detecting gaseous analytes and hydrophobic organic analytes in aqueous solutions.
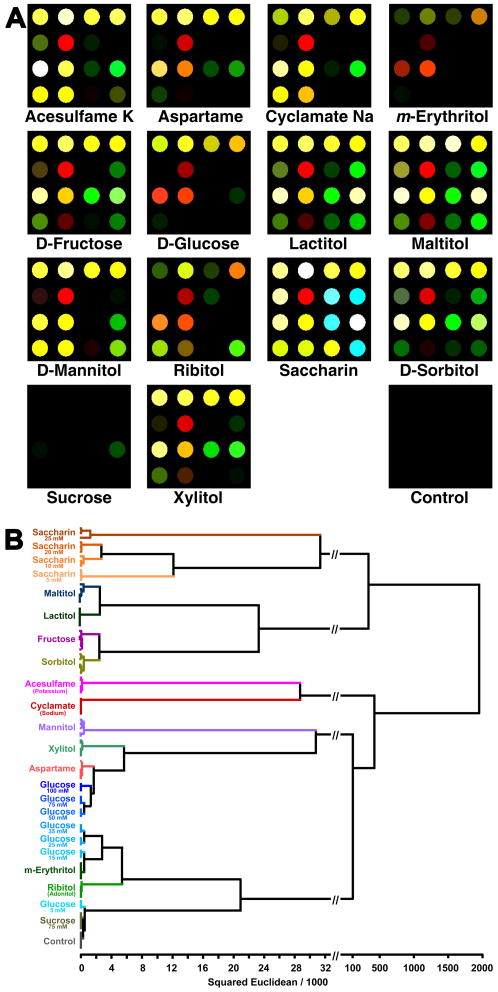
The sensitivity of our earlier hydrophobic sensing platform [19], however, proved to be problematic for the sensing of hydrophilic analytes, including carbohydrates. We determined that rapid and sensitive detection of these classes of analytes requires an immobilization method whereby all chromogenic centers are accessible to analytes while the dyes themselves must remain impervious to leaching or blooming upon exposure to aqueous solutions. After several techniques were tested, we developed a printable sensor array based on nanoporous organically modified polysiloxanes (ormosils) to encapsulate a diverse set of chemically responsive dyes. These nanoporous pigments were printed onto hydrophilic, porous membranes, which permits the user to avoid tedious handling of multiple aqueous solutions in well plates. One simply exposes the analyte solution once to a single printed array and images on an ordinary flat bed scanner. We successfully used this approach to detect and identify 14 structurally similar mono- and disaccharides as well as reduced sugars and sugar substitutes at low millimolar concentrations [25••]. Most of this discrimination was based on the induced solution pH changes from 3-nitrophenylboronic acid-diol complexation an initially neutral pH. Color difference maps are useful in showing how colorimetric sensor arrays can provide “molecular fingerprints” of the analytes tested. As visualized qualitatively in difference maps, discrimination can be easily achieved, as shown in Figure 7A.
Figure 7.
Colorimetric sensor array for sugars. A) Difference maps (ΔRGB) for 14 representative natural and artificial sweeteners (10 mM) and one control, and B) Hierarchical clustering analysis dendrogram for aforementioned sweeteners; zero misclassifications were observed. Image used with permission, [25•].
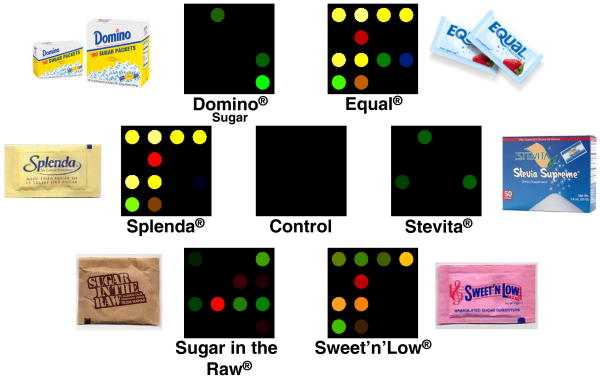
For display of the discrimination among the sweeteners and sugars, we use a standard chemometric approach, hierarchical cluster analysis (HCA), which is based on the grouping of the analyte vectors according to their spatial distances in their full vector space [57]. HCA has the advantages of being model-free (unlike, for examples, linear discriminant analysis or neural nets) and of using the full dimensionality of the data. As shown in Figure 7B, HCA generates dendrograms based on clustering of the array response data. Using HCA, we showed that our array was capable of discrimination of the analytes with discreet clustering and no misclassifications among 14 different analytes in 75 trials. As an extension of this work, we were also able to test our array's performance in real-world situations using common table-top sweetener packets readily found in any restaurant or café, both in aqueous solution and in sweetened iced-tea. Differential sensing among these analytes was observed without error. Color difference maps representing the arrays response to one serving of several representative sweeteners and a control are shown in Figure 8.
Figure 8.
Color difference maps for six commonly used natural and artificial sweetener packets and one control. One packet of each analyte was dissolved in 4 oz. of weakly buffered 5 mM, 3-nitrophenylboronic acid solution (pH 7.45) and scanned for 5 min after exposure. The color range is expanded from 3 bits to 8 bits per color (RGB range of 3-10 expanded to 0-255). Image used with permission, [25•].
Conclusion and Future Endeavors
In recent years, we have seen substantial progress in the design, optimization, and application of semi-selective, cross-responsive indicators for the differential sensing of a host of analytes, including sugars, the focus of this overview. By manipulating the old and well understood reaction of boronic acids with diols, scientists have developed arrays of chemoresponsive chromophores for the detection and identification of sugars and sugar analogs. Using biomimetic pattern-based sensing similar to the mammalian olfactory and gustatory systems, the development of electronic noses and electronic tongues based on colorimetric sensor arrays are providing advancements in sensitivity and pattern-based selectivity above and beyond that which nature provides.
Acknowledgments
We would like to thank Dr. Sung Lim for helpful discussions as our work in this area developed. This work was supported through the NIH Genes, Environment and Health Initiative through award U01ES016011.
Footnotes
Summary of recent advances: While the complexes between boronic acids and diols have been studied for decades, researchers continue to design new and interesting methods to use these interactions to produce saccharide sensors that are more sensitive and selective. Herein we discuss how the use of pattern-based colorimetric arrays from a collection of cross-reactive sensors have been developed as new differential sensing platforms for sugars and related saccharides.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Kuivila HG, Keough AH, Soboczenski EJ. Areneboronates from diols and polyols. J Org Chem. 1954;19:780–783. [Google Scholar]
- 2.James TD, Sandanayake KRAS, Shinkai S. Saccharide sensing with molecular receptors based on boronic acid. Angew Chem Int Ed. 1996;35:1911–1922. [Google Scholar]
- 3.Wang W, Gao X, Wang B. Boronic acid-based sensors. Curr Org Chem. 2002;6:1285–1317. [Google Scholar]
- 4.James TD, Linnane P, Shinkai S. Fluorescent saccharide receptors: a sweet solution to the design, assembly and evaluation of boronic acid derived PET sensors. Chem Commun. 1996:281–288. [Google Scholar]
- 5.Peng B, Qin Y. Lipophilic Polymer Membrane Optical Sensor with a Synthetic Receptor for Saccharide Detection. Anal Chem. 2008;80:6137–6141. doi: 10.1021/ac800946p. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Barragan I, Costa-Fernandez JM, Sanz-Medel A. Tailoring the pH response range of fluorescent-based pH sensing phases by sol-gel surfactants co-immobilization. Sens Actuators, B. 2005;B107:69–76. [Google Scholar]
- 7.Schiller A, Wessling RA, Singaram B. A Fluorescent Sensor Array for Saccharides Based on Boronic Acid Appended Bipyridinium Salts. Angew Chem Int Ed. 2007;46:6457–6459. doi: 10.1002/anie.200701888. [DOI] [PubMed] [Google Scholar]
- 8.Tan J, Wang HF, Yan XP. Discrimination of Saccharides with a Fluorescent Molecular Imprinting Sensor Array Based on Phenylboronic Acid Functionalized Mesoporous Silica. Anal Chem. 2009;81:5273–5280. doi: 10.1021/ac900484x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Gao X, Hardcastle K, Wang B. Water-soluble fluorescent boronic acid compounds for saccharide sensing: substituent effects on their fluorescence properties. Chem Eur J. 2006;12:1377–1384. doi: 10.1002/chem.200500982. [DOI] [PubMed] [Google Scholar]
- 10.Rochat S, Gao J, Qian X, Zaubitzer F, Severin K. Cross-Reactive Sensor Arrays for the Detection of Peptides in Aqueous Solution by Fluorescence Spectroscopy. Chem Eur J. 2010;16:104–113. S104/101–S104/136. doi: 10.1002/chem.200902202. [DOI] [PubMed] [Google Scholar]
- 11.Zhang T, Edwards NY, Bonizzoni M, Anslyn EV. The Use of Differential Receptors to Pattern Peptide Phosphorylation. J Am Chem Soc. 2009;131:11976–11984. doi: 10.1021/ja9041675. [DOI] [PubMed] [Google Scholar]
- 12.Collins BE, Anslyn EV. Pattern-based peptide recognition. Chem Eur J. 2007;13:4700–4708. doi: 10.1002/chem.200700153. [DOI] [PubMed] [Google Scholar]
- 13.Miranda OR, Chen HT, You CC, Mortenson DE, Yang XC, Bunz UHF, Rotello VM. Enzyme-Amplified Array Sensing of Proteins in Solution and in Biofluids. J Am Chem Soc. 2010;132:5285–5289. doi: 10.1021/ja1006756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janzen MC, Ponder JB, Bailey DP, Ingison CK, Suslick KS. Colorimetric Sensor Arrays for Volatile Organic Compounds. Anal Chem. 2006;78:3591–3600. doi: 10.1021/ac052111s. [DOI] [PubMed] [Google Scholar]
- 15.Jiang H, Wang Y, Ye Q, Zou G, Su W, Zhang Q. Polydiacetylene-based colorimetric sensor microarray for volatile organic compounds. Sens Actuators, B. 2010;B143:789–794. [Google Scholar]
- 16.Kostesha NV, Alstrom TS, Johnsen C, Nilesen KA, Jeppesen JO, Larsen J, Jakobsen MH, Boisen A, Vo-Dinh T, Lieberman RA, et al. Development of the colorimetric sensor array for detection of explosives and volatile organic compounds in air. Proc SPIE Int Soc Opt Eng. 2010;7673:76730I–76730I-76739. [Google Scholar]
- 17.Feng L, Musto CJ, Kemling JW, Lim SH, Suslick KS. A colorimetric sensor array for identification of toxic gases below permissible exposure limits. Chem Commun. 2010;46:2037–2039. doi: 10.1039/b926848k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••18.Lim SH, Feng L, Kemling JW, Musto CJ, Suslick KS. An optoelectronic nose for the detection of toxic gases. Nature Chem. 2009;1:562–567. doi: 10.1038/nchem.360. [DOI] [PMC free article] [PubMed] [Google Scholar]; A colorimetric sensor array made from cross-reactive nanoporous pigments was developed for rapid and sensitive detection and identification of 20 different toxic industrial chemicals (TICs).
- 19.Zhang C, Suslick KS. A Colorimetric Sensor Array for Organics in Water. J Am Chem Soc. 2005;127:11548–11549. doi: 10.1021/ja052606z. [DOI] [PubMed] [Google Scholar]
- 20.Springsteen G, Wang B. A detailed examination of boronic acid-diol complexation. Tetrahedron. 2002;58:5291–5300. [Google Scholar]
- 21.Yan J, Springsteen G, Deeter S, Wang B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols-it is not as simple as it appears. Tetrahedron. 2004;60:11205–11209. [Google Scholar]
- 22.James TD, Phillips MD, Shinkai S. Boronic Acids in Saccharide Recognition. Cambridge, U.K: Royal Society of Chemistry; 2006. [Google Scholar]
- 23.Koumoto K, Shinkai S. Colorimetric sugar sensing method useful in “neutral” aqueous media. Chemistry Letters. 2000:856–857. [Google Scholar]
- 24.Koumoto K, Takeuchi M, Shinkai S. Design of a visualized sugar sensing system utilizing a boronic acid-azopyridine interaction. Supramolecular Chemistry. 1998;9:203–210. [Google Scholar]
- ••25.Musto CJ, Lim SH, Suslick KS. Colorimetric Detection and Identification of Natural and Artificial Sweeteners. Anal Chem. 2009;81:6526–6533. doi: 10.1021/ac901019g. [DOI] [PMC free article] [PubMed] [Google Scholar]; Colorimetric sensor solid-state arrays made from nanoporous pigments were used to differentiate 14 sugars, sugar alcohols, and artificial sweeteners at low millimolar concentrations, imaged with an ordinary flat bed scanner. Real-world applications of the array are demonstrated using tea sweetened with coffee-house sweeteners.
- ••26.Edwards NY, Sager TW, McDevitt JT, Anslyn EV. Boronic Acid Based Peptidic Receptors for Pattern-Based Saccharide Sensing in Neutral Aqueous Media, an Application in Real-Life Samples. J Am Chem Soc. 2007;129:13575–13583. doi: 10.1021/ja073939u. [DOI] [PubMed] [Google Scholar]; This comprehensive work describes, in detail, the workings of indicator displacement assays as they relate to the differential sensing of sugars and sugar substitutes.
- 27.Kurczewska J, Schroeder G. Modified silica surface by phenylboronic acid derivatives as effective sugar sensor. Cent Eur J Chem. 2009;7:697–701. [Google Scholar]
- •28.Ma WMJ, Pereira Morais MP, D'Hooge F, van den Elsen JMH, Cox JPL, James TD, Fossey JS. Dye displacement assay for saccharide detection with boronate hydrogels. Chem Commun. 2009:532–534. doi: 10.1039/b814379j. [DOI] [PubMed] [Google Scholar]; An interesting semi-solid state indicator displacement assay. Easily synthesized hydrogel spheres incorporate boronate units that function as sugar sensors.
- 29.DiCesare N, Lakowicz JR. New color chemosensors for monosaccharides based on azo dyes. Org Lett. 2001;3:3891–3893. doi: 10.1021/ol016813p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egawa Y, Gotoh R, Niina S, Anzai Ji. Ortho-azo substituted phenylboronic acids for colorimetric sugar sensors. Bioorg Med Chem Lett. 2007;17:3789–3792. doi: 10.1016/j.bmcl.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 31.Ward CJ, Ashton PR, James TD, Patel P. A molecular colour sensor for monosaccharides. Chem Commun. 2000:229–230. [Google Scholar]
- •32.Egawa Y, Gotoh R, Seki T, Anzai Ji. Sugar response of boronic acid-substituted azobenzene dye-modified polymer. Mater Sci Eng, C. 2009;29:115–118. [Google Scholar]; A culmination of several studies performed by the group. In this latest publication, multilayer films based on boronic acid appended azo-dyes were synthesized and employed to differentiate glucose from fructose.
- 33.Kim Y, H SA, Weissleder R, Tung CH. Sugar sensing based on induced pH changes. Chem Commun. 2007:2299–2301. doi: 10.1039/b700741h. [DOI] [PubMed] [Google Scholar]
- •34.Lee JW, Lee JS, Chang YT. Colorimetric identification of carbohydrates by a pH indicator/pH change inducer ensemble. Angew Chem Int Ed. 2006;45:6485–6487. doi: 10.1002/anie.200602055. [DOI] [PubMed] [Google Scholar]; A pattern-based sugar-sensing technique relying on the pH depression at non-physiological pH associated with cyclic boronate ester formation when diols interact with boronic acids.
- 35.Boduroglu S, El Khoury JM, Reddy DV, Rinaldi PL, Hu J. A colorimetric titration method for quantification of millimolar glucose in a pH 7.4 aqueous phosphate buffer. Bioorg Med Chem Lett. 2005;15:3974–3977. doi: 10.1016/j.bmcl.2005.05.075. [DOI] [PubMed] [Google Scholar]
- 36.Dowlut M, Hall DG. An Improved Class of Sugar-Binding Boronic Acids, Soluble and Capable of Complexing Glycosides in Neutral Water. J Am Chem Soc. 2006;128:4226–4227. doi: 10.1021/ja057798c. [DOI] [PubMed] [Google Scholar]
- 37.Mulla HR, Agard NJ, Basu A. 3-Methoxycarbonyl-5-nitrophenyl boronic acid: high affinity diol recognition at neutral pH. Bioorg Med Chem Lett. 2004;14:25–27. doi: 10.1016/j.bmcl.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Wright AT, Anslyn EV. Differential receptor arrays and assays for solution-based molecular recognition. Chem Soc Rev. 2006;35:14–28. doi: 10.1039/b505518k. [DOI] [PubMed] [Google Scholar]
- 39.Zhang T, Anslyn EV. A colorimetric boronic acid based sensing ensemble for carboxy and phospho sugars. Org Lett. 2006;8:1649–1652. doi: 10.1021/ol060279+. [DOI] [PubMed] [Google Scholar]
- 40.Mohr GJ. New chromoreactands for the detection of aldehydes, amines and alcohols. Sens Actuators, B. 2003;B90:31–36. [Google Scholar]
- 41.Mohr GJ. Chromo- and fluororeactands: indicators for detection of neutral analytes by using reversible covalent-bond chemistry. Chem Eur J. 2004;10:1082–1090. doi: 10.1002/chem.200305524. [DOI] [PubMed] [Google Scholar]
- 42.Mohr GJ. Covalent bond formation as an analytical tool to optically detect neutral and anionic analytes. Sens Actuators, B. 2005;B107:2–13. [Google Scholar]
- 43.Mohr GJ. New chromogenic and fluorogenic reagents and sensors for neutral and ionic analytes based on covalent bond formation - a review of recent developments. Anal Bioanal Chem. 2006;386:1201–1214. doi: 10.1007/s00216-006-0647-3. [DOI] [PubMed] [Google Scholar]
- 44.Jiang S, Escobedo JO, Kim KK, Alptuerk O, Samoei GK, Fakayode SO, Warner IM, Rusin O, Strongin RM. Stereochemical and regiochemical trends in the selective detection of saccharides. J Am Chem Soc. 2006;128:12221–12228. doi: 10.1021/ja063651p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He M, Johnson RJ, Escobedo JO, Beck PA, Kim KK, St Luce NN, Davis CJ, Lewis PT, Fronczek FR, Melancon BJ, et al. Chromophore formation in resorcinarene solutions and the visual detection of mono- and oligosaccharides. J Am Chem Soc. 2002;124:5000–5009. doi: 10.1021/ja017713h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••46.Anslyn EV. Supramolecular analytical chemistry. J Org Chem. 2007;72:687–699. doi: 10.1021/jo0617971. [DOI] [PubMed] [Google Scholar]; A compelling review of recent advances in analytical chemistry using synthetic receptors meant to mimic natural receptors. Several techniques were discussed including indicator displacement assays(IDA), and photo-induced electron transfer (PET), and their employment as differential receptors.
- 47.McCleskey SC, Griffin MJ, Schneider SE, McDevitt JT, Anslyn EV. Differential receptors create patterns diagnostic for ATP and GTP. J Am Chem Soc. 2003;125:1114–1115. doi: 10.1021/ja021230b. [DOI] [PubMed] [Google Scholar]
- 48.Wright AT, Griffin MJ, Zhong Z, McCleskey SC, Anslyn EV, McDevitt JT. Differential receptors create patterns that distinguish various proteins. Angew Chem Int Ed. 2005;44:6375–6378. doi: 10.1002/anie.200501137. [DOI] [PubMed] [Google Scholar]
- 49.Rakow NA, Suslick KS. A colorimetric sensor array for odor visualization. Nature. 2000;406:710–713. doi: 10.1038/35021028. [DOI] [PubMed] [Google Scholar]
- 50.Suslick KS. An optoelectronic nose: seeing smells by means of colorimetric sensor arrays. MRS Bulletin. 2004;29:720–725. doi: 10.1557/mrs2004.209. [DOI] [PubMed] [Google Scholar]
- 51.Suslick KS, Bailey DP, Ingison CK, Janzen M, Kosal MA, McNamara WB, III, Rakow NA, Sen A, Weaver JJ, Wilson JB, et al. Seeing Smells: Development of an Optoelectronic Nose. Quimica Nova. 2007;30:677–681. [Google Scholar]
- 52.Suslick KS, Rakow NA, Sen A. Colorimetric sensor arrays for molecular recognition. Tetrahedron. 2004;60:11133–11138. [Google Scholar]
- 53.Rakow NA, Sen A, Janzen MC, Ponder JB, Suslick KS. Molecular Recognition and Discrimination of Amines with a Colorimetric Array. Angew Chem Int Ed. 2005;44:4528–4532. doi: 10.1002/anie.200500939. [DOI] [PubMed] [Google Scholar]
- 54.Suslick BA, Feng L, Suslick KS. Discrimination of Complex Mixtures by a Colorimetric Sensor Array: Coffee Aromas. Anal Chem. 2010;82:2067–2073. doi: 10.1021/ac902823w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang C, Bailey DP, Suslick KS. Colorimetric Sensor Arrays for the Analysis of Beers: A Feasibility Study. J Agric Food Chem. 2006;54:4925–4931. doi: 10.1021/jf060110a. [DOI] [PubMed] [Google Scholar]
- 56.Zhang C, Suslick KS. Colorimetric Sensor Array for Soft Drink Analysis. J Agric Food Chem. 2007;55:237–242. doi: 10.1021/jf0624695. [DOI] [PubMed] [Google Scholar]
- 57.Johnson RA, Wichern DW. Applied Multivariate Statistical Analysis. 6th. Prentice Hall; 2007. [Google Scholar]