Abstract
Biomaterials are increasingly being used to engineer the biochemical and biophysical properties of the extracellular stem cell microenvironment in order to tailor niche characteristics and direct cell phenotype. To date, stem cell-biomaterial interactions have largely been studied by introducing stem cells into artificial environments, such as 2D cell culture on biomaterial surfaces, encapsulation of cell suspensions within hydrogel materials, or cell seeding on 3D polymeric scaffolds. In this study, microparticles fabricated from different materials, such as agarose, PLGA and gelatin, were stably integrated, in a dose-dependent manner, within aggregates of pluripotent stem cells (PSCs) prior to differentiation as a means to directly examine stem cell-biomaterial interactions in 3D. Interestingly, the presence of the materials within the stem cell aggregates differentially modulated the gene and protein expression patterns of several differentiation markers without adversely affecting cell viability. Microparticle incorporation within 3D stem cell aggregates can control the spatial presentation of extracellular environmental cues (i.e. soluble factors, extracellular matrix and intercellular adhesion molecules) as a means to direct the differentiation of stem cells for tissue engineering and regenerative medicine applications. In addition, these results suggest that the physical presence of microparticles within stem cell aggregates does not compromise PSC differentiation, but in fact the choice of biomaterials can impact the propensity of stem cells to adopt particular differentiated cell phenotypes.
1. Introduction
Pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced pluripotent cells, provide a powerful model system for the study of morphogenic differentiation and represent a potent cell source for regenerative medicine therapies. Maintenance of pluripotency or differentiation of PSCs is dependent on extracellular cues such as autocrine and paracrine signaling, cell-cell and extracellular matrix interactions [1]. Appropriate culture conditions have been developed for the derivation and maintenance of pluripotent mouse [2] and human cells [3] and likewise, manipulation of the stem cell microenvironment can be used to promote lineage specific differentiation [4]. Although simple addition of soluble factors to cell culture medium is a commonly used method for directed differentiation protocols, it represents only a portion of the complex extracellular milieu which directs morphogenesis in the developing embryo.
In order to enhance the efficiency of many differentiation protocols, biomaterials are increasingly being used to engineer the biochemical and biophysical properties of the stem cell microenvironment [5]. Relevant properties of biomaterials such as degradation kinetics, molecular compatibility, porosity, etc., can be engineered to enable spatial-temporal control of extracellular cues presented to stem cells, thereby allowing for the customization needed for the differentiation to a variety of cell types. Studies of biomaterial-stem cell interactions have demonstrated that materials may influence stem cell differentiation even in the absence of delivered biomolecules. Though the exact mechanisms remain the subject of ongoing studies, material properties such as surface chemistry [6, 7] and elasticity [8] have been reported to promote lineage specific differentiation of stem cells. For the most part, stem cell-biomaterial interactions have been examined by introducing stem cells into artificial environments, such as 2D cell culture on biomaterial surfaces [7, 9], encapsulation of cell suspensions within hydrogel materials [6, 10], or cell seeding on 3D polymeric scaffolds [11]. In contrast, incorporation of materials directly within 3D stem cell environments, such as cell spheroids, permits control over the amount and spatial presentation of materials enabling systematic examination of the effects of biomaterials on stem cell differentiation and morphogenesis.
Recent studies have demonstrated the efficacy of morphogen delivery within stem cell spheroids via incorporated biomaterials [12–14]. ESCs mixed with poly(lactic-co-glycolic) acid (PLGA) MPs loaded with retinoic acid (RA) resulted in the homogeneous and organized differentiation of ESC-derived spheroids, referred to as embryoid bodies (EBs) [13]. The observed biological response could not be duplicated by simple soluble application of RA suggesting the importance of spatial presentation of morphogens in the context of EB differentiation. PLGA MPs are well suited for the encapsulation and release of small, hydrophobic molecules, such as RA; however, organic solvents used in fabrication of MPs can adversely affect the bioactivity and efficiency of encapsulation of larger biomolecules such as growth factors [15, 16]. As an alternative, growth factors can be loaded into hydrogel materials, such as agarose [17] or gelatin [18, 19], under aqueous conditions without substantially compromising subsequent bioactivity. Stem cell differentiation protocols utilize a wide variety of molecules delivered with distinct temporal profiles, accordingly, it is advantageous for biomaterials-based strategies to be compatible with a variety of materials so the appropriate molecular compatibility and release kinetics can be engineered for the desired application. As previous studies have been limited to PLGA, the aims of this study were first to develop a method for robust incorporation of various biomaterials within PSC aggregates, and second to characterize the effects of biomaterial incorporation on stem cell differentiation.
Here we examined the incorporation of equal-sized microparticles (MPs) fabricated from different materials (agarose, PLGA and gelatin) within aggregates of PSCs. A forced aggregation technique, previously developed for formation of populations of homogeneously sized EBs [20], was used to coalesce MPs and cells and compared to simple mixing during formation under dynamic culture. The quantities of MPs incorporated within cell aggregates were assessed as a function of the seed ratio of MPs to cells used to form the biomaterial/cell hybrid constructs. The morphology, gene and protein expression patterns of aggregates with different types of incorporated MPs were evaluated to determine the effects of the materials on PSC differentiation. Overall, the controlled incorporation of microparticles within stem cell aggregates represents a new approach to systematically investigate the effects of biomaterials on stem cell phenotypes in 3D.
2. Methods
2.1 ESC culture and aggregate formation
Undifferentiated D3 ESCs were maintained on gelatin-coated tissue culture dishes in DMEM media supplemented with 15% fetal bovine serum and 103 U/ml leukemia inhibitory factor (LIF) (Millipore, Billerica, MA). ESCs were trypsinized into a single cell suspension and aggregates were formed by forced aggregation in AggreWellTM 400 inserts (Stem Cell Technologies, Vancouver, CA) [20] or by rotary orbital culture [21]. Briefly, 1.2 × 106 cells in 0.5 mL of medium were inoculated into AggreWellTM inserts, containing approximately 1200 wells per insert, and centrifuged at 200 × g for 5 minutes to cluster cells in the wells. After 24 hours of culture, cell aggregates were removed from the wells using a wide-bore pipette and transferred to suspension culture on a rotary orbital shaker (40 RPM) to maintain the homogeneity of the population. In order to investigate material incorporation, a second centrifugation of 200 μL of a MP solution at 200 × g for 5 minutes was performed immediately after cell centrifugation. Rotary orbital culture was used for aggregate formation with and without MPs by modifying a method previously described [13]. Uncoated MPs and cells were mixed together in a suspension-culture Petri dish and cultured on a rotary orbital shaker at 40 RPM. Following initial formation, cultures were re-fed with fresh differentiation media (without LIF) every 2 days.
2.2 Microparticle fabrication and size characterization
Agarose MPs were fabricated using a water-in-oil single emulsion similar to previously described methods [22]. A 3% w/w solution (2 mL) of Ultra-low melt SeaPrep Agarose (Lonza, Rockland, ME) was prepared in deionized (dI) water at 60°C and added drop-wise to 60 mL of corn oil (Sigma Aldrich St. Louis, MO) containing 1 mL Tween 20 (Sigma Aldrich, St. Louis, MO). An emulsion was created by homogenizing at 5000 RPM for 5 minutes using a PT3100 (Kinematica, Switzerland). The emulsion was cooled at 4°C for 20 minutes, agarose MPs were retrieved through centrifugation at 200 × g and rinsed a minimum of 3 times with 25 mL of dI H2O to remove residual oil. Agarose MPs were conjugated to AlexaFluor 488-labeled bovine serum albumin (BSA) using the hetero-bifunctional, photochemical crosslinker Sulfo-Sanpah, N-Sulfosuccinimidyl-6-(4'-azido-2'-nitrophenylamino) hexanoate (Pierce Biotechnology, Rockford, IL), using a modification of a previously described method [23]. A Sulfo-Sanpah solution, 6 mg in 24 μL dimethyl sulfoxide, was mixed into 2 mL 3% w/w agarose solution. The solution was treated with 365nm longwave UV light using a Blak-Ray B-100AP light source (UVP, Upland, CA) for 30 minutes, at a distance of 10 cm, to conjugate the Sulfo-Sanpah to the agarose. Subsequently, a 50 mg/mL solution of AlexaFluor 488 succinimidyl ester (Invitrogen, Carlsbad, CA) labeled BSA was added to the agarose solution and allowed to react overnight. The agarose MPs were rinsed 3 times in 20 mL of dI H2O to remove unbound BSA.
Gelatin MPs were fabricated using a modification of a previously published protocol [18]. Briefly, 2 mL of a 10% w/w solution of gelatin B (Sigma Aldrich) in dI H2O was heated to 55°C, added drop-wise to 60 mL of corn oil, and homogenized at 5000 RPM for 5 minutes to create a water-in-oil emulsion. The emulsion was cooled at 4°C for 10 minutes without mixing, before adding 35 mL of cold acetone to the solution and sonicating the emulsion continuously at 12 W for 1 minute (Sonicator 3000, Misonix, Inc, Farmingdale, NY). The solution was cooled at 4°C for 10 minutes and the MPs were retrieved through centrifugation at 200 × g followed by 3 washes in 25 mL of acetone. The MPs were then crosslinked at room temperature with a 5 mM glutaraldehyde, 0.1% w/w Tween 20 solution in dI H2O under stirred conditions. After 15 hours of crosslinking, the MPs were retrieved by centrifugation and treated with 25mL of 25 mM glycine in dI H2O to block residual aldehyde groups. MPs were washed 3 times in 25 mL dI H2O and labeled either with AlexaFluor 488 or 546 succinimidyl ester in a 0.1 M sodium bicarbonate solution at a pH of 8.3 for 1 hour at room temperature. Fluorescently labeled MPs were washed 3 times in 25 mL of diH2O to remove un-conjugated dye.
PLGA (50:50, Absorbable Polymers International) MPs were fabricated using an oil-in-water single emulsion [13]. PLGA was dissolved in dichloromethane (DCM) (20mg/mL), added to 0.3% PVA, and homogenized at 3000 RPM. The DCM was evaporated for 4 hours at room temperature in a fume hood and MPs were collected by centrifugation at 200 × g. Prior to homogenization, PLGA MPs were fluorescently labeled by adding 50 mg of CellTrackerTM Red (Molecular Probes, Invitrogen, Carlsbad, CA) to the DCM solution. For all materials, MPs were suspended in a small volume of diH2O and frozen at −80°C before lyophilization and storage.
The average size of the MP populations was assayed using a Multisizer 3 Coulter Counter (Beckman Coulter, Fullerton, CA) equipped with a 100 μm aperture with a lower detection limit of 2 μm. MPs were suspended in Isoton II diluent (Beckman Coulter) at a dilution which resulted in the counting of at least 2000 events in a sample size of 0.5 mL.
2.3 Scanning electron microscopy
Samples were dehydrated in graded acetone dilutions and critically point dried using a Polaron E3000 critical point dryer (Quorum Technologies Inc., Guelph, ON, Canada). MPs were sputter coated for 120 seconds at 2.2 kV using a Polaron SC7640 sputter coater and imaged using a Hitachi S-800 scanning electron microscope (Hitachi High Technologies, Pleasanton, CA).
2.4 Microparticle incorporation analysis
After 24 hours of formation, aggregates were removed from the microwells and washed several times in media to separate unincorporated MPs. In order to quantify MP incorporation, volumes chosen to contain on the order of 40–60 aggregates from each experimental condition were sampled and the precise number of aggregates were counted prior to lysing the cells in a 5% SDS solution. MPs were then retrieved from the lysate and quantified using a hemocytometer and normalized to the number of lysed aggregates. Incorporation levels of MPs were assayed in triplicate over 3 separate experiments (n = 9 total for each ratio and condition examined).
The spatial location of fluorescently-labeled MPs within spheroids was analyzed using a LSM 510 microscope (Carl Zeiss, Inc). Cells were labeled with Hoechst dye (1:100) for 15 minutes followed by 3 washes in PBS prior to imaging. Images were obtained at a minimum depth of 30 μm (significantly greater than microparticle diameters) into spheroids for each experimental condition in order to examine the distribution of particles throughout the cell aggregates.
2.5 Cell viability
PSC spheroids were formed as described above, and after 2 and 10 days of differentiation, cell viability was assessed using LIVE/DEAD staining (Invitrogen). Samples were incubated in serum-free, phenol red-free medium containing 1 μM calcein AM and 2 μM ethidium homodimer I at room temperature for 30 minutes. Spheroids were then washed three times with PBS and immediately imaged using confocal microscopy.
2.6 Gene expression analysis
RNA was extracted from spheroids at various time points for up to 14 days of differentiation with the RNeasy Mini kit (Qiagen Inc, Valencia, CA). Samples analyzed using the Mouse Embryonic Stem Cell array (PAMM-081, SA Biosciences, Frederick, MD) were converted to complimentary DNA using the RT2 First Strand Kit (Qiagen Inc), loaded in the prefabricated 96-well array plates and analyzed using real time PCR (MyIQ cycler, BioRad). Genesis software version 1.7.5 (http://genome.tugraz.at/genesisclient/genesisclient_download.shtml) was used to construct a heat map of log2 transformed PCR array data [24]. Hierarchical clustering was performed using Euclidean distance and average linkage clustering.
For all other gene expression analysis, RNA was converted to complimentary DNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) and analyzed using real time PCR (MyIQ cycler, BioRad). Forward and reverse primers for Oct-4, MLC-2V, Pax6, AFP, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed with Beacon Designer software (sequences and conditions are given in Table 1) and purchased from Invitrogen. Oct-4 gene expression was calculated with respect to undifferentiated ESC expression levels using the Pfaffl method [25]. Pax6, MLC-2V, and AFP expression in treated samples were normalized to GAPDH expression levels and compared to untreated spheroid expression.
Table 1.
Primers and conditions used for PCR.
| Gene | Forward Sequence | Reverse Sequence | Melt Temperature |
|---|---|---|---|
| GAPDH | GCCTTCCGTGTTCCTACC | GCCTGCTTCACCACCTTC | 55.0 |
| Oct-4 | CCGTGTGAGGTGGAGTCTGGAG | GCGATGTGAGTGATCTGCTGTAGG | 64.0 |
| Pax-6 | ACGGCATGTATGATAAACTAAG | GCTGAAGTCGCATCTGAG | 58.0 |
| MLC-2V | GACCATTCTCAACGCATTCAAG | CTTCTCCGTGGGTAATGATGG | 58.0 |
| AFP | CACACCCGCTTCCCTCATCC | TTCTTCTCCGTCACGCACTGG | 64.0 |
2.7 Histology and immunostaining
PSC spheroids were collected, fixed in 10% formalin, embedded in Histogel (Thermo Scientific), processed and paraffin embedded. Sections of 5 μm in thickness were deparaffinized before staining with hematoxylin and eosin (H&E). Histological samples were imaged using a Nikon 80i upright microscope and a SPOT Flex camera (15.2 64MP Shifting Pixel, Diagnostic Instruments). For whole-mount immunofluorescent staining, spheroids were permeabilized in a 1.5% Triton X-100 BSA solution for 30 minutes, incubated with primary antibody in 2% BSA solution overnight at 4°C, rinsed with PBS and incubated with a secondary antibody for 4 hours. Antibodies and concentrations used were: mouse monoclonal, anti-α-sarcomeric actin (5c5, Sigma, St. Louis, MO) (1:500) with an AlexaFluor 488 conjugated, goat–anti-mouse secondary (Invitrogen) (1:200), and rabbit polyclonal anti-human alpha-fetoprotein (AFP) (Dako, Glostrup, Denmark) (1:200) with a goat–anti-rabbit, FITC-conjugated secondary (Invitrogen) (1:200). Spheroids were counterstained with Hoechst (1:100), washed 3 times in PBS and imaged using a Zeiss LSM 510 confocal microscope (Carl Zeiss Inc.).
2.8 Statistical analysis
Experimental values are reported as mean ± standard deviation (n≥3). Statistical analysis was determined using one or two way ANOVA coupled with Tukey’s post hoc analysis using Systat (v12, Systat Software Inc.). P-values < 0.05 were considered statistically significant.
3. Results
3.1 Microparticle formation
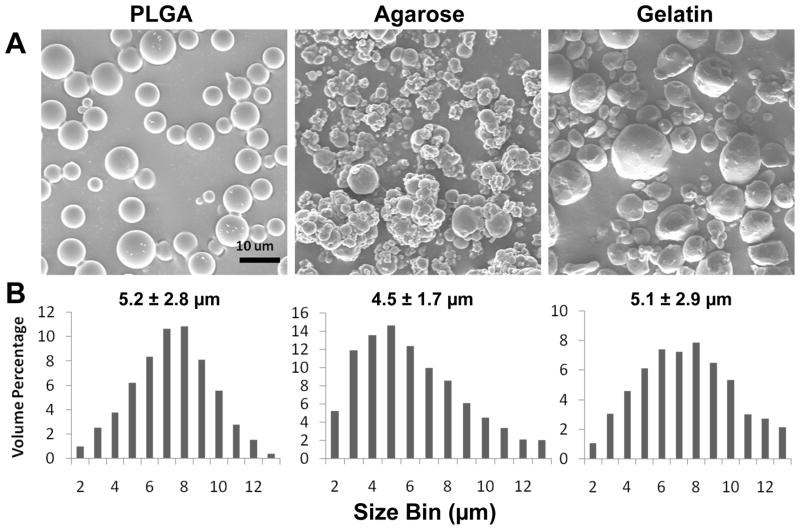
SEM micrographs indicated that MPs from each of the materials exhibited similar round shapes and smooth topological surface morphologies. The hydrogel MPs are likely to appear smaller when analyzed by SEM due to the necessary dehydration of the samples, prior to imaging, thus the diameter of the different MPs was analyzed using a Coulter Counter in an aqueous buffer in order to obtain more accurate size measurements. The average diameters of the PLGA, agarose and gelatin MPs were determined to be 5.2 ± 2.8, 4.5 ± 1.7 and 5.1 ± 2.9 μm, respectively (Figure 1).
Figure 1. MP characterization.
PLGA, agarose and gelatin MPs were fabricated using a single oil-in-water or water-in-oil emulsion to create similarly sized MPs. MPs were generally round with smooth surfaces as indicated by scanning electron microscope images. Coulter counter size analysis of MPs in aqueous conditions revealed average diameters of the MPs to be between 4.5 – 5.2 μm. Scale bar = 10 μm.
3.2 Material incorporation using rotary orbital culture
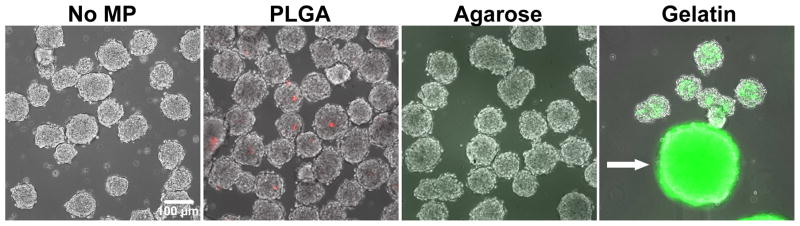
Rotary spheroid formation has been used previously by our lab as a method to control the incorporation of ECM-coated PLGA MPs within EBs [12, 13], and was therefore tested initially as a method to incorporate various uncoated MPs within the aggregates. However, simple mixing of ESCs with uncoated PLGA or agarose MPs using rotary culture resulted in limited incorporation of the materials within aggregates (Figure 2B, C). Out of nearly 150 aggregates analyzed, 57% did not contain any PLGA MPs, 42% contained 1 to 5 PLGA MPs, and only a single aggregate contained more than 5 PLGA MPs. Agarose MP incorporation within aggregates was even lower, with no aggregates containing more than one MP and <5% containing a single agarose MP. In contrast, rotary orbital mixing of ESCs with gelatin MPs resulted in 100% of the aggregates examined containing 10+ gelatin MPs each; however, the extent of incorporation was rather heterogeneous and large (~1mm diameter) gelatin MP-cell aggregates were frequently observed (Figure 2D).
Figure 2. Rotary culture results in variable MP incorporation within PSC aggregates.
Fluorescently labeled MPs (CellTrackerTM Red incorporated or AlexaFluor® 488 succinimidyl ester conjugated) were mixed with ESCs at a ratio of 2:1 and cultured on a rotary orbital shaker at 40 rpm for 48 hours to promote aggregate formation. Non-adhesive materials (PLGA and agarose) were not efficiently incorporated as demonstrated by a lack of punctuate fluorescent particles. Gelatin, an adhesive material, was incorporated efficiently within the aggregates, however, the formation of large masses of MPs and cells (arrow) were observed along with smaller sized aggregates. Scale bar = 100 μm.
3.3 Material incorporation using forced aggregation
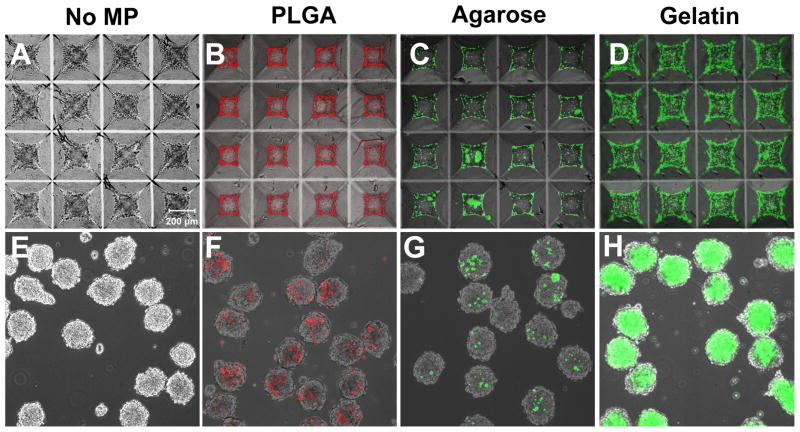
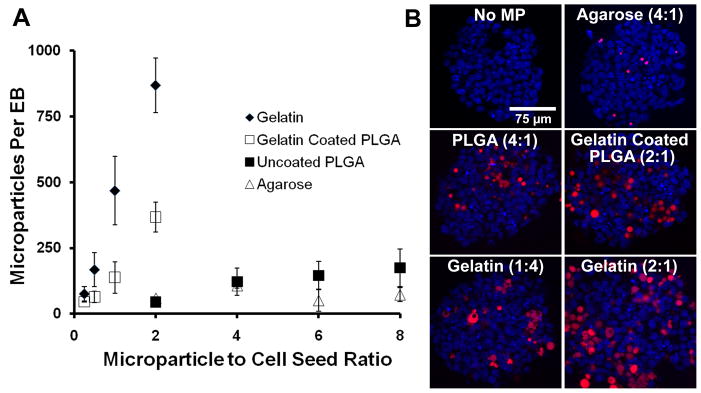
Simple mixing of MPs and PSCs did not result in efficient incorporation of the various materials assayed; therefore forced aggregation of MPs and PSCs in microwells was investigated as an alternative means to incorporate materials within aggregates. Using the same 2:1 ratio of MPs to cells as used in rotary formation, increased incorporation of each material in homogeneously sized aggregates was observed (Figure 3E–H). Analysis of the fluorescent signal within aggregates indicated that gelatin MP-treated aggregates contained the most incorporated MPs and agarose MP-treated aggregates contained the fewest MPs per aggregate. In contrast to rotary culture, all aggregates formed through forced aggregation contained more than one MP for each material investigated. Because the efficiency of incorporation was noticeably different across material groups, the average number of MPs incorporated per aggregate was analyzed as a function of the seed ratio of MPs to cells for a range of MP values with a fixed number of 1.2 × 106 cells/insert (1000 ESCs/microwell) (Figure 4A). The incorporation of non-adhesive materials (PLGA and agarose) was characterized by a gradual increase allowing for incorporation of up to approximately 125 MPs per aggregate over the range of seed ratios examined, whereas gelatin MPs were incorporated with much greater efficiency. In order to determine if incorporation was dependent on the adhesivity of the material surface, the incorporation of gelatin-coated PLGA MPs was also analyzed. The profile of gelatin-coated PLGA MP incorporation resembled that of gelatin MPs and was greater than uncoated PLGA MPs. Optical sectioning of aggregates indicated that MPs were incorporated throughout the aggregate and not simply localized to the surface of the aggregates (Figure 4B). In PLGA and gelatin-treated samples, some aggregation of MPs was observed within spheroids, whereas aggregation of incorporated agarose MPs was not observed. Together these results indicate that forced aggregation can be used to control the extent of incorporation and distribution of adhesive and non-adhesive biomaterial MPs within PSC aggregates.
Figure 3. Forced aggregation of PSCs and MPs results in uniform material incorporation within spheroids.
ESCs centrifuged alone (A), or with fluorescently labeled MPs (B–D) in microwells formed spheroids after 24 hours of culture. Spheroids were removed from the wells and thereafter maintained in rotary orbital suspension culture at 40 rpm (bottom row). Uniform incorporation of materials within populations of PSC aggregates was observed (E–H). Scale bar = 200 μm.
Figure 4. MP incorporation is controlled in a dose-dependent manner.
Incorporation was analyzed as a function of MP to cell seed ratio (A). Adhesive materials (gelatin and gelatin-coated PLGA) were incorporated more efficiently than non-adhesive materials (agarose and PLGA). The presence of fluorescently labeled particles throughout the spheroids was confirmed by confocal microscopy (shown at a depth of 30 μm) (B). Scale bar = 75 μm.
3.4 Microparticle effects on viability and differentiation
The effects of MP incorporation on PSC viability and differentiation were examined for aggregates containing approximately equal amounts of different types of MPs. Formation conditions which resulted in ~125 MPs per spheroid (1 MP for every 8 cells) were chosen for subsequent studies and corresponded to the following MP to cell seed ratios: agarose 4:1, PLGA 4:1, and gelatin 1:4 (as indicated in Figure 3). In addition, in order to investigate the effects of increased MP incorporation, the increased dynamic range of gelatin incorporation was exploited to include a fourth MP experimental group of 2:1 gelatin, which resulted in an average incorporation of ~800 MPs per spheroid (4 MP for every 5 cells).
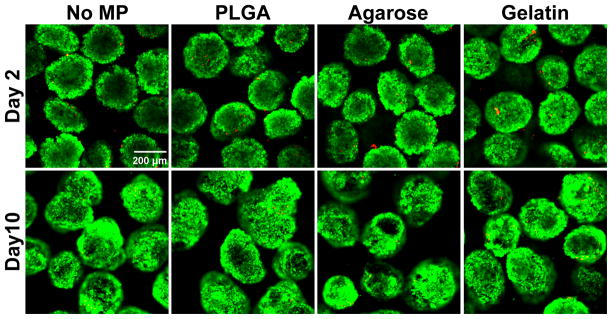
PSC aggregates cultured with materials were collected at days 2 and 10 of differentiation and stained for live and dead cells (Figure 5). At both time points, spheroids contained few dead cells and no differences were observed across any of the experimental conditions suggesting that the presence of materials did not negatively affect cell viability at early or later stages of culture.
Figure 5. Cell viability is not adversely affected by MP incorporation.
Cell viability was assayed after 2 (top row) and 10 (bottom row) days of differentiation using LIVE/DEAD® stain. Live cells were labeled with calcein AM (green) and dead cells were labeled with ethidium homodimer (red). Few dead cells were observed in any of the groups at either of the time points examined.
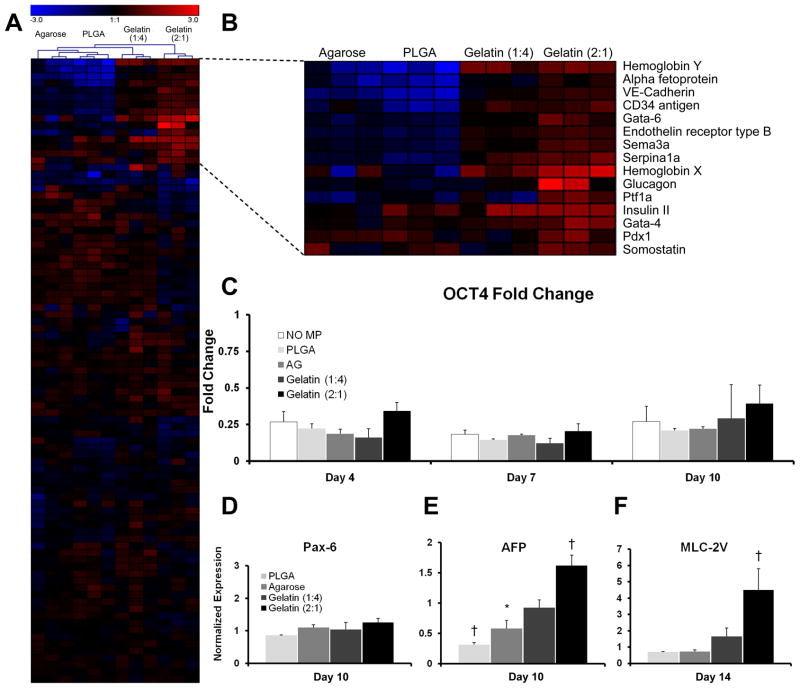
Global gene expression analysis of PSC aggregates after 10 days of suspension culture was performed for 84 distinct genes initially using PCR SuperArrays (Figure 6A). Overall, relatively modest differences were observed for the majority of genes examined within any of the experimental groups compared to spheroids that lacked MP incorporation. Interestingly, hierarchical clustering of the resulting data indicated that each of the replicate samples grouped most closely together, reflecting a common gene expression profile among spheroids treated with the same type of material. Significant differences (p ≤ 0.05) in the expression of 34 of the genes assayed (40%) were noted in at least one of the MP-treated groups compared to untreated spheroids; this level of significance corresponds to a probability of no more than 2 falsely identified genes. The majority of the genes affected by incorporation of different MPs were associated with endoderm (AFP, Gata-6, Serpina1a, Glucagon, and Ptf1a) or mesoderm (Hemoglobin Y, VE-Cadherin, CD34 antigen, Hemoglobin X) differentiation, whereas few differences in pluripotent or ectoderm lineage markers were observed. Thus, the gene expression profile of PSC aggregates is sensitive to the incorporation of different materials even after normalization of MP size and number are taken into account.
Figure 6. Gene expression is modulated by the presence of different materials.
SuperArray analysis (A) at day 10 of differentiation demonstrated that the presence of different materials within PSC aggregates significantly (p<.05) modulated the gene expression of 40% (34/84) of the genes examined. Magnified view of heat map (B) results displayed the top 15 genes with the greatest fold changes relative to aggregates that lacked MPs. Oct-4 gene expression (C) was not significantly different between any of the experimental groups at any of the time points examined (days 4, 7 and 10). Pax-6 gene expression was not significantly altered, whereas AFP (E) and myosin light chain 2-ventricle (MLC-2V) (F) were modulated by the presence of different materials at days 10 and 14, respectively. n=3 for all experiments. * = p ≤ 0.05, † = p ≤ 0.005.
Temporal gene expression analysis of the pluripotent marker Oct-4 (Figure 6B), as well as several germ lineage markers (AFP, Myosin light-chain 2 ventricle (MLC-2V), Pax6, Figure 6C) was performed using qRT-PCR over 14 days of culture. In all of the conditions examined, Oct-4 expression decreased significantly after 4 days of differentiation and no significant differences were observed between any of the experimental groups. Pax-6 expression was not affected by the presence of materials; however, consistent with PCR array data, significant differences in mesoderm and endoderm marker expression were detected. MLC-2V expression was significantly (p < 0.05) increased by 4.5 fold in gelatin 2:1 spheroids at day 14 compared to untreated spheroids. Agarose and PLGA spheroids expressed 1.7 and 3.2 fold less AFP at day 10, respectively, while gelatin 2:1 spheroids expressed AFP at a 1.6 fold higher level than untreated controls (Figure 6C).
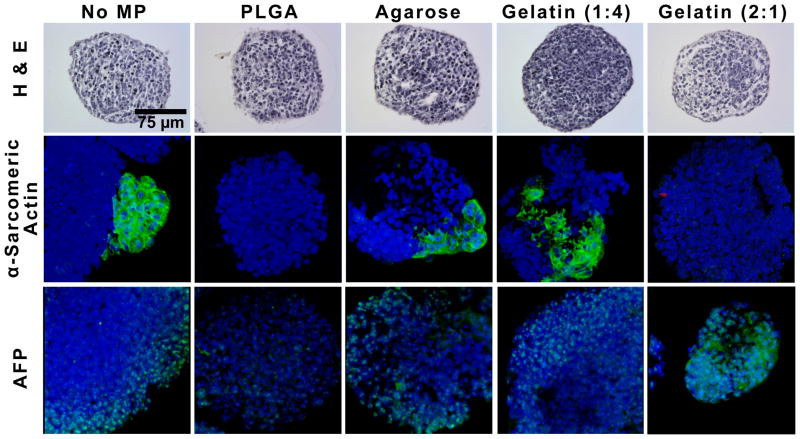
Incorporation of MPs did not appear to have any gross effects on aggregate formation or morphology through 14 days of differentiation in suspension culture. Immunostaining of the spatial distribution of phenotypic markers was assessed within the aggregates to further characterize differentiation as hematoxylin and eosin staining of cross-sections of spheroids revealed no observable differences in morphology (Figure 7, day 7 shown). Whole-mount immunofluorescent staining was performed on alpha-sarcomeric actin (cardiac mesoderm) and AFP since gene expression differences were almost exclusively found in mesoderm and endoderm markers. The observed spatial distributions of mesoderm-like and endoderm-like phenotypes were altered within the different treated groups. In untreated spheroids, positive expression of alpha-sarcomeric actin, a cardiac muscle actin, was localized to small, concentrated regions on the spheroid exterior, and similar expression patterns were observed in spheroids with agarose MPs. In contrast, alpha-sarcomeric actin staining was not observed in PLGA and gelatin 2:1 spheroids at the same time point (14 days), whereas gelatin 1:4 spheroids displayed positive expression in larger sections including the interior sections. In day 14 spheroids, AFP expression was localized to the outer layer of cells for most of the experimental groups, in agreement with previous literature reports that endoderm differentiation is primarily localized to the periphery of EBs by this stage of differentiation [26, 27]. However, in gelatin 2:1 spheroids, more homogeneous AFP expression was observed throughout and was not limited to the cells at the periphery. Altogether the gene and phenotypic marker expression data demonstrate that materials incorporated within PSC aggregates during formation influence differentiation through 14 days of culture.
Figure 7. Spatial distribution of cell phenotypes is altered by the presence of materials.
Hematoxylin and eosin staining of day 7 PSC spheroids with or without different MPs did not exhibit gross differences in morphology (top row). At day 14 of differentiation, the spatial distribution of α-sarcomeric actin and AFP within EBs was assessed. α-sarcomeric actin expression in gelatin (1:4) aggregates was observed throughout, while positive expression was localized to the periphery in untreated and agarose-treated spheroids and no positive expression was observed in PLGA- and gelatin 2:1-treated spheroids. AFP expression was localized to the periphery of all experimental groups with the exception of gelatin 2:1 spheroids, where expression was detected throughout the cell aggregates. Scale bar = 75 μm.
4. Discussion
In this study, the effects of PLGA, agarose and gelatin MPs (of equal size) incorporated within pluripotent stem cell aggregates were directly examined and compared. Rotary aggregate formation, previously used to incorporate gelatin-coated PLGA MPs, was not sufficient to homogeneously incorporate the three materials equally and therefore we developed a forced aggregation technique that enabled dose-dependent control of MP incorporation for each of the materials. The incorporation efficiency of the materials varied according to relative adhesivity and therefore, the appropriate aggregate formation conditions for each material were determined empirically in order to normalize for equivalent numbers of MPs/spheroid to make fair evaluations of material effects. No significant differences were observed in the formation of aggregates or cell viability in any of the experimental groups compared to cell aggregates alone, although gene and protein expression of phenotypic markers indicated that even at low incorporation levels (~1 MP/8 cells) the differentiation of PSCs to mesoderm and endoderm lineages was significantly affected. Interestingly, none of the genes assayed were uniformly up-regulated or down-regulated by MP-treated spheroids, suggesting that the differences in gene expression were related to specific material characteristics and not simply the presence of foreign particles. While the gross morphology of the MP-treated aggregates was not distinguishable from untreated aggregates, the spatial distribution of certain differentiated phenotypes was altered, suggesting that the biomaterials themselves influence the extracellular microenvironment. The adhesive and elastic properties of the biomaterials examined, both of which have been linked to stem cell fate determination [8, 28], vary widely between PLGA, agarose and gelatin. Overall, the results of this study indicate that PSC differentiation within spheroids is sensitive to various types of naïve biomaterials and that forced aggregation can be used as a platform for direct study of stem cell-biomaterial interactions in 3D culture.
Previous studies of stem cell-biomaterial interactions have been performed using monolayer culture or cell encapsulation [6, 7]. Stem cells cultured on spotted biomaterial arrays can be used for high-throughput screening of various surfaces and in this manner materials may be identified for further study in more complex 3D systems. Monolayer culture has been used previously to identify polymer combinations which supported the growth and differentiation of human ESCs [7]. Encapsulation of cells within biomaterials is low-throughput in comparison to monolayer arrays, but allows for study of the biomaterial effects on stem cell differentiation in a 3D suspension-culture. Mesenchymal stem cell differentiation, for example, can be influenced by the chemical moiety presented in the hydrogel in which they are encapsulated [6]. Encapsulation works particularly well with single cell suspensions, where each cell is surrounded by the biomaterial, however; with respect to spheroid culture, encapsulation presents the material surface only to the cells on the aggregate exterior. The distinction of MP-based strategies is that the material can be incorporated in a controlled manner throughout cell spheroids allowing for the study of biomaterials directly within a 3D stem cell aggregate environment. Although the forced aggregation approach detailed in this study was originally developed for the production of homogeneous EBs, it has recently been successfully applied to form spheroids of mesenchymal stem cells (MSCs) as well [29]. Thus, the method of controlled incorporation of biomaterials within cell aggregates described herein could be applied to other cell types cultured as spheroids, such as MSCs, neural stem cells or tumor cells [30], for studies of directed differentiation or the influence of microenvironment on cell fate.
The materials examined in this study were chosen to represent different classes of biomaterials that have been used in conjunction with stem cells either as molecular or cellular delivery vehicles. PLGA is a hydrolytically-degradable, synthetic copolymer, commonly used for controlled release of small, hydrophobic molecules. Agarose is a polysaccharide thermo-sensitive hydrogel material that is largely considered non-adhesive and is not degraded by mammalian cells, however it can be functionalized in a variety of manners for controlled presentation of biomolecules, such as growth factors [31, 32] and short peptide sequences [33]. Gelatin is an enzymatically degradable hydrogel material formulated from denatured collagen that is dually capable of growth factor delivery and direct binding to cells via integrin receptor ligation. The three materials can be used alone or in combination to deliver a variety of morphogens or growth factors with different kinetics, yet it is notable that even in the absence of loading with exogenous molecules, the results presented within this study indicate that naïve materials themselves can influence the relative differentiation of PSC aggregates.
PLGA is composed of acidic monomers, consequently, the hydrolytic degradation of the polymer can result in acidification of the local environment [34]. Although the effects of pH on ESC differentiation have not been extensively studied, it has been reported that below pH 7.0, mouse ESC proliferation is decreased and at a pH of 6.7 Oct-4 expression is reduced [35]. PLGA MP treatment at the levels studied here did not result in any differences in cell viability nor in relative changes to Oct-4 expression (Figure 6B), suggesting that the local pH levels may not be decreased significantly within the spheroids during the culture period examined. This is supported by previous studies of the degradation kinetics of 50:50 PLGA, which have reported that degradation is limited to between 10 and 15% of the original mass during the first 2 weeks of degradation [36, 37]. Longer-term studies using PLGA for stem cell cultures may need to take into account acidification of the stem cell microenvironment.
Integrins are known to play critical roles in cellular differentiation, migration and proliferation [38]. Likewise, growth of ESCs on extracellular matrix proteins such as laminin has been used to direct neural differentiation of human ESCs mediated through α6β1 integrin signaling [39]. In addition to integrin signaling effects, it is possible that the addition of adhesive materials within the spheroids can affect the mobility of cells. Time lapse microscopy and cell tracking have demonstrated that cells are very mobile within early stage embryos [40] as well as EBs [41], a characteristic which may be modulated by the presence of adhesive MPs within PSC aggregates. Tracking of Disabled-2 (Dab2) positive, primitive endoderm cells suggests that endoderm differentiation may occur throughout aggregates during the earliest stages of differentiation and as cells are passively migrating throughout the spheroid, Dab2 mediated polarization enables primitive endoderm cells to create a non-adherent apical interface and remain preferentially at the exterior surface of EBs throughout differentiation [41]. We observed endoderm, identified as AFP+, cells on the exterior of untreated, PLGA and agarose-treated spheroids. However, in aggregates containing an increased concentration of gelatin MPs, positive AFP expression was observed throughout. In aggregates with a high adhesive MP to cell ratio, more cells are exposed to adhesive biomaterial surface within the spheroid and it is possible that cell adhesion to the biomaterial could restrict movement and disrupt the normal migration patterns of PSCs undergoing differentiation. In future studies, incorporation of adhesive ECM-derived MPs within PSC aggregates could be used to specifically examine the effects of cell movement on early differentiation events, such as endoderm formation, primitive streak development and the resulting spatial distributions of differentiated cell phenotypes.
5. Conclusions
We have developed a forced aggregation technique to control the incorporation of biomaterials within PSC aggregates, without the need for surface modification of the materials. This advance allows for direct study of stem cell-biomaterial interactions in a 3D model system of PSC differentiation. The results of this study demonstrate that PSC differentiation is sensitive to the presence of biomaterials in the extracellular microenvironment, suggesting that biomaterials incorporated within stem cell spheroids can be used in conjunction with other methods (i.e. soluble molecule delivery, mechanical forces) to direct the differentiation of stem cells for tissue engineering and regenerative medicine applications.
Acknowledgments
Financial support was provided by funding from the National Science Foundation (CBET 0651739), the National Institutes of Health R01 (GM088291) and the Canadian Institutes of Health Research (CIHR; MOP57885). ABL is supported by a NIH training grant (GM008433) as well as funding from the Goizueta Foundation. MU is supported by a St. George’s Society Fellowship at the McEwen Centre for Regenerative Medicine. PWZ is a Canada Research Chair in Stem Cell Bioengineering. RLC was supported by a GAANN fellowship from the Center of Drug Design, Development and Delivery. We are grateful for electron microscopy training and facilities provided by The Center for Nanostructure Characterization at Georgia Tech as well as use of core facilities provided by the Parker H. Petit Institute of Bioengineering and Bioscience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 3.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 4.Bratt-Leal AM, Carpenedo RL, McDevitt TC. Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnol Prog. 2009;25:43–51. doi: 10.1002/btpr.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–41. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater. 2008;7:816–23. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotechnol. 2004;22:863–6. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 8.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 9.Valamehr B, Jonas SJ, Polleux J, Qiao R, Guo S, Gschweng EH, et al. Hydrophobic surfaces for enhanced differentiation of embryonic stem cell-derived embryoid bodies. Proc Natl Acad Sci U S A. 2008;105:14459–64. doi: 10.1073/pnas.0807235105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dang SM, Gerecht-Nir S, Chen J, Itskovitz-Eldor J, Zandstra PW. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells. 2004;22:275–82. doi: 10.1634/stemcells.22-3-275. [DOI] [PubMed] [Google Scholar]
- 11.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci U S A. 2003;100:12741–6. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpenedo RL, Seaman SA, McDevitt TC. Microsphere size effects on embryoid body incorporation and embryonic stem cell differentiation. J Biomed Mater Res A. 2010;94:466–75. doi: 10.1002/jbm.a.32710. [DOI] [PubMed] [Google Scholar]
- 13.Carpenedo RL, Bratt-Leal AM, Marklein RA, Seaman SA, Bowen NJ, McDonald JF, et al. Homogeneous and organized differentiation within embryoid bodies induced by microsphere-mediated delivery of small molecules. Biomaterials. 2009;30:2507–15. doi: 10.1016/j.biomaterials.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira L, Squier T, Park H, Choe H, Kohane DS, Langer R. Human embryoid bodies containing nano- and microparticulate delivery vehicles. Adv Mater. 2008;20:2285–91. [Google Scholar]
- 15.Fu K, Griebenow K, Hsieh L, Klibanov AM, Langer R. FTIR characterization of the secondary structure of proteins encapsulated within PLGA microspheres. J Control Release. 1999;58:357–66. doi: 10.1016/s0168-3659(98)00192-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhu G, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly (lactide- co-glycolide) Nat Biotechnol. 2000;18:52–7. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 17.Moribe K, Nomizu N, Izukura S, Yamamoto K, Tozuka Y, Sakurai M, et al. Physicochemical, morphological and therapeutic evaluation of agarose hydrogel particles as a reservoir for basic fibroblast growth factor. Pharm Dev Technol. 2008;13:541–7. doi: 10.1080/10837450802309661. [DOI] [PubMed] [Google Scholar]
- 18.Tabata Y, Hijikata S, Muniruzzaman M, Ikada Y. Neovascularization effect of biodegradable gelatin microspheres incorporating basic fibroblast growth factor. J Biomater Sci Polym Ed. 1999;10:79–94. doi: 10.1163/156856299x00298. [DOI] [PubMed] [Google Scholar]
- 19.Holland TA, Tabata Y, Mikos AG. In vitro release of transforming growth factor-beta 1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. J Control Release. 2003;91:299–313. doi: 10.1016/s0168-3659(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 20.Ungrin MD, Joshi C, Nica A, Bauwens C, Zandstra PW. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS ONE. 2008;3:e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpenedo RL, Sargent CY, McDevitt TC. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells. 2007;25:2224–34. doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- 22.Wang N, Wu XS. Preparation and characterization of agarose hydrogel nanoparticles for protein and peptide drug delivery. Pharm Dev Technol. 1997;2:135–42. doi: 10.3109/10837459709022618. [DOI] [PubMed] [Google Scholar]
- 23.Dodla MC, Bellamkonda RV. Anisotropic scaffolds facilitate enhanced neurite extension in vitro. J Biomed Mater Res A. 2006;78:213–21. doi: 10.1002/jbm.a.30747. [DOI] [PubMed] [Google Scholar]
- 24.Sturn A, Quackenbush J, Trajanoski Z. Genesis: cluster analysis of microarray data. Bioinformatics. 2002;18:207–8. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- 25.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Li X, Eswarakumar VP, Seger R, Lonai P. Fibroblast growth factor (FGF) signaling through PI 3-kinase and Akt/PKB is required for embryoid body differentiation. Oncogene. 2000;19:3750–6. doi: 10.1038/sj.onc.1203726. [DOI] [PubMed] [Google Scholar]
- 27.Esner M, Pachernik J, Hampl A, Dvorak P. Targeted disruption of fibroblast growth factor receptor-1 blocks maturation of visceral endoderm and cavitation in mouse embryoid bodies. Int J Dev Biol. 2002;46:817–25. [PubMed] [Google Scholar]
- 28.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markway BD, Tan GK, Brooke G, Hudson JE, Cooper-White JJ, Doran MR. Enhanced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in low oxygen environment micropellet cultures. Cell Transplant. 2010;19:29–42. doi: 10.3727/096368909X478560. [DOI] [PubMed] [Google Scholar]
- 30.Sutherland RM. Cell and environment interactions in tumor microregions - the multicell spheroid model. Science. 1988;240:177–84. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- 31.Rahman N, Purpura KA, Wylie RG, Zandstra PW, Shoichet MS. The use of vascular endothelial growth factor functionalized agarose to guide pluripotent stem cell aggregates toward blood progenitor cells. Biomaterials. 2010 doi: 10.1016/j.biomaterials.2010.07.040. [DOI] [PubMed] [Google Scholar]
- 32.Yu XJ, Dillon GP, Bellamkonda RV. A laminin and nerve growth factor-laden three-dimensional scaffold for enhanced neurite extension. Tissue Eng. 1999;5:291–304. doi: 10.1089/ten.1999.5.291. [DOI] [PubMed] [Google Scholar]
- 33.Bellamkonda R, Ranieri JP, Aebischer P. Laminin oligopeptide derivatized agarose gels allow 3-dimensional neurite extension in-vitro. J of Neurosci Res. 1995;41:501–9. doi: 10.1002/jnr.490410409. [DOI] [PubMed] [Google Scholar]
- 34.Fu K, Pack DW, Klibanov AM, Langer R. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm Res. 2000;17:100–6. doi: 10.1023/a:1007582911958. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhry MA, Bowen BD, Piret JM. Culture pH and osmolality influence proliferation and embryoid body yields of murine embryonic stem cells. Biochem Eng J. 2009;45:126–35. [Google Scholar]
- 36.Kenley RA, Lee MO, Mahoney TR, Sanders LM. Poly(lactide-co-glycolide) decomposition kinetics invivo and invitro. Macromolecules. 1987;20:2398–403. [Google Scholar]
- 37.Reed AM, Gilding DK. Biodegradable polymers for use in surgery - poly(glycolic)-poly(lactic acid) homo and co-polymers: 2. Invitro Degradation. Polymer. 1981;22:494–8. [Google Scholar]
- 38.Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–98. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- 39.Ma W, Tavakoli T, Derby E, Serebryakova Y, Rao MS, Mattson MP. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell. 2006;10:615–24. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 41.Rula ME, Cai KQ, Moore R, Yang DH, Staub CM, Capo-Chichi CD, et al. Cell autonomous sorting and surface positioning in the formation of primitive endoderm in embryoid bodies. Genesis. 2007;45:327–38. doi: 10.1002/dvg.20298. [DOI] [PubMed] [Google Scholar]