Abstract
Three experiments examined the role of the dorsal hippocampus (dHIPP) in occasion setting with diffuse contextual and discrete light stimuli serving as occasion setters in classical fear conditioning with rats. Both sham-operated and dHIPP-lesioned animals readily learned a L→T+, T− serial feature positive discrimination in which a light (L) “set the occasion” for reinforcement of a tone (T+). dHIPP-lesioned animals were deficient, however, in acquiring a similar discrimination in which different contexts (A and B) served as occasion setters, i.e., A(T+) and B(T−). The lesioned animals also failed to discriminate between a context in which a tone had been partially reinforced and a context in which no conditioning had taken place, whereas sham-operated animals froze more to the tone in the conditioned context than in the novel context. Collectively, the data indicate that the dorsal hippocampus is important in processing information about the signaling value of contextual, but not discrete, stimuli.
Keywords: rat, cognition, reinforcement, fear, discrimination
Introduction
Memories are typically separated into declarative (or explicit) memory and non-declarative (or implicit) memory systems based on information content and neuroanatomical substrates (Cohen, Eichenbaum, Deacedo, & Corkin, 1985; Schacter, 1987; Scoville and Milner, 1957; Tulving, 1972; van Strien, Cappaert, & Witter, 2009). Declarative memories depend on the medial temporal lobe structures (such as the hippocampus and surrounding cortical areas) and are thought to comprise relatively complex information (e.g., facts and events), whereas non-declarative memories are thought to be independent of the medial temporal lobe structures and involve simple information (e.g., classical conditioning). However, in a classic study, Richard F. Thompson and colleagues (Berger, Alger, & Thompson, 1976) have demonstrated that the hippocampus is engaged even during simple classical or Pavlovian conditioning and in some cases is required (Solomon, Vander Schaaf, Thompson, & Weisz, 1986). In this special issue of Neurobiology of Learning and Memory commemorating Richard F. Thompson’s 50+ years of memory research, we present a study demonstrating the necessity of the hippocampus in Pavlovian conditioning.
In a typical Pavlovian conditioning procedure, a conditioned stimulus (CS) is contingently paired with an unconditioned stimulus (US) and comes to elicit a conditioned response (CR). While the CS is most often a discrete stimulus such as a tone or a light, it has long been recognized that the context in which conditioning occurs may itself act as a CS and form an association with the US, and thereby come to control a CR (e.g., Hull, 1943; Pavlov, 1927). Most modern theories of Pavlovian conditioning consider context-US associations to be analogous to discrete CS-US associations both in the mechanisms by which they are formed and in the response-eliciting properties that they confer (e.g., Mackintosh, 1975; Pearce & Hall, 1980; Rescorla & Wagner, 1972). Context-US associations have proven to be useful theoretically in accounting for certain challenging observations, such as the “reinstatement” of responding to an extinguished CS by unsignalled US presentations (Rescorla & Heth, 1975; see Bouton & King, 1983) and the importance of CS-US contingency in determining the amount of associative strength that accrues to a CS (see Rescorla & Wagner, 1972).
Nevertheless, several investigators have challenged the simple conditioning view of context as insufficient to account for some of the more interesting effects of context, such as the context-dependence of various conditioning phenomena (e.g., extinction; for reviews see Bouton, 2004; Ji & Maren, 2007). An alternative approach holds that context may serve a modulatory or hierarchical function, in addition to directly controlling a CR, by virtue of its association with the US. Thus, a context may modulate the formation and retrieval of associations between discrete CSs and USs that are paired in its presence (e.g.,; Baeyens et al., 2005; Bouton, 1993, 2004; Hirsh, 1974, 1980; Miller & Schachtman, 1985; Nadel & Willner, 1980; Spear, 1973) in a manner that is relatively independent of any context-US associations that may be assumed to exist (Bouton, 1984; Bouton & King, 1986; Bouton & Swartzentruber, 1986; Swartzentruber, 1991).
This modulatory function of context is reminiscent of the “occasion setting” function of discrete cues trained as features in certain conditional discriminations, such as the serial feature-positive (FP) discrimination (Baeyens et al., 2005; Holland, 1983; Ross & Holland, 1981). In this discrimination a target CS, X, is reinforced when preceded by a feature stimulus, A, and nonreinforced when presented in isolation (i.e., A→X+, X−). In contrast to the simultaneous FP discrimination (in which A and X are presented as a simultaneous compound), which is solved on the basis of direct associations formed between A and the US, the serial FP discrimination (in which A and X are separated by a temporal gap) appears to be solved on the basis of a “gating” or “occasion setting” function carried by A, which acts on an association between X and the US (Holland, 1983, 1992). In other words, A does not directly control a response, but instead modulates or “sets the occasion” (Skinner, 1938) for responding to X.
The apparent correspondence between the modulatory function of contextual stimuli and discrete occasion setters is interesting from a biological perspective. Many current theories of learning and memory assume that the hippocampus (particularly the dorsal region) is important in processing information about contextual-spatial, but not discrete, cues. Considerable evidence supports this assumption. For example, dorsal hippocampal lesions have been found to interfere with the acquisition (Ammassari-Teule, Passino, Restivo, & de Marsanich, 2000; Chen, Kim, Thompson, & Tonegawa, 1996; Kim, Rison, & Fanselow, 1993; Maren & Fanselow, 1997; Paylor, Tracy, Wehner, & Rudy, 1994; Phillips & LeDoux, 1992, 1995; Rudy, 1993; Stiedl, Misane, Spiess, & Ogren, 2000; but see Maren, Aharonov, & Fanselow 1997; Frankland, Cestari, Filipkowski, McDonald, & Silva, 1998; Cho, Friedman and Silva, 1999; Wiltgen, Sanders, Anagnostaras, Sage, & Fanselow, 2006) and retention (Anagnostaras, Maren, & Fanselow, 1999; Frankland et al., 1998; Kim & Fanselow, 1992; Maren et., 1997; Sutherland, O’Brien, & Lehmann, 2008; Wiltgen et al., 2006) of contextual fear conditioning while having relatively no effect on tone fear conditioning (but see complete and ventral hippocampal lesion effects on auditory trace and delay fear conditioning by McEchron, Bouwmeester, Tseng, Weiss, & Disterhoft, 1998 and Richmond et al., 1999, respectively). However, much of the available data concerns the effect of hippocampal lesions on simple conditioning of context–that is, on the formation and retention of context-US associations. The issue of whether the hippocampus is similarly necessary for higher-order, occasion setting functions of context has not been extensively investigated, and the data that do exist are far from unequivocal.
Several studies have indicated that the hippocampus is not necessary for occasion setting by discrete cues (Davidson & Jarrard, 1989; Jarrard & Davidson, 1990, 1991; but see Ross, Orr, Holland, & Berger, 1984), although others have reported certain patterns of impairments (Holland, Lamoureux, Han, & Gallagher, 1999) and enhancements (Han, Gallagher, & Holland, 1998) in the learning of conditional discriminations by hippocampally lesioned animals. If occasion setting by contextual and discrete stimuli are subserved by similar (i.e., extra-hippocampal) brain systems, it is possible that contextual occasion setting may be unaffected by hippocampal lesions despite the apparent importance of the hippocampus for simple conditioning of context. Alternatively, contextual information–which is necessarily complex and multimodal–may by its very nature require higher-order, hippocampal processing, implying that the occasion setting function of context, like the formation of context-US associations, should be vulnerable to the impairing effect of hippocampal lesions.
While no study to date, to our knowledge, has directly compared the effect of dorsal hippocampal lesions on occasion setting by discrete and contextual cues, a few authors have examined the hippocampal dependence of certain conditional contextual discriminations that are similar to those sometimes used in the examinations of occasion setting with explicit stimuli. For example, a biconditional contextual discrimination (in which a target stimulus, X, is reinforced in context A but nonreinforced in context B, whereas a second target, Y, is reinforced in context B but nonreinforced in context A; i.e., A[X+], B[X−], A[Y−], B[Y+]) has been the subject of several studies that have, nevertheless, produced contrasting results: some authors (Hall, Purves, & Bonardi, 1996; McDonald et al., 1997) have reported that hippocampally lesioned animals are able to solve the discrimination as well as controls, and others (Good & Honey, 1991) have found substantial impairments. Other studies have examined the context specificity of conditioning (often taken to reflect an occasion setting or retrieval function of context; see Bouton, 1993, 2004) under various circumstances and, again, failed to agree as to whether hippocampally lesioned animals are lacking in contextual control of responding (e.g., Good, de Hoz, & Morris, 1998; Good & Honey, 1991; Holt & Maren, 1999; Honey & Good, 1993; Penick & Solomon, 1991) or are not different from control animals (e.g., Hall et al., 1996; McDonald et al., 1997; Wilson, Brooks, & Bouton, 1995).
The present series of experiments were designed to examine the question of the dorsal hippocampal dependence of occasion setting by contextual and discrete cues more directly (schematically summarized in Table 1). Experiment 1 first established the hippocampal dependence of contextual occasion setting by determining the effect of dorsal hippocampal (dHIPP) lesions on contingent contextual modulation of responding. It may be argued that, while contingent contextual modulation requires the intact hippocampus, incidental contextual modulation does not (e.g., Frankland et al., 1998; Young, Bohenek, & Fanselow, 1994). Even though this possibility is unresolved, given the unfavorable outcome of certain studies designed specifically to bear on the question of the hippocampal dependence of incidental and contingent contextual conditioning (Good et al., 1998; Phillips & LeDoux, 1994), Experiment 2 was designed to further examine the dHIPP role in incidental contextual modulation. Experiment 3 then compared the effect of dHIPP lesions on occasion setting by contextual and discrete stimuli. This experiment examined the performance of a discrete FP discrimination (in which a light set the occasion for responding to a tone) in the context in which the discrimination was trained and in a novel context. This design permits, for the first time, a within--subjects comparison of the effect of dHIPP lesions on contextual and discrete occasion setting. It may thus represent the most powerful test to date of the (often cited but ill-supported) notion that all types of occasion setting are hippocampus-dependent.
Table 1.
Design of Experiments 1–3
| Experiment | Training |
Test |
||
|---|---|---|---|---|
| Context A | Context B | Context A | Context B | |
| 1 | T+ | T− | T | T |
| 2 | T+, T− | T | T | |
| 3 | L→ T+, T− | L→T, T, L | L→T, T, L | |
Note. T and L refer to tone and light CSs. + and − indicate reinforced and nonreinforced trials.
Materials and Methods
Animals
Animals were experimentally naive male Long-Evans rats (250–300 g) were individually housed in standard plastic cages in a climate-controlled vivarium with ad libitum access to food and water. Animals were maintained on a 12-hr light:dark cycle (lights on at 7AM). All test procedures were conducted during the light phase of the cycle and completed at Yale University, New Haven, CT (J.J.K.’s former laboratory).
Surgery
The rats were anesthetized with ketamine HC1 (30 mg/kg) and xylazine (2.5 mg/kg) and placed in a stereotaxic apparatus. A stainless steel insect pin (#00), insulated with epoxy except for 0.5 mm at the tip, was lowered into the dorsal hippocampus (coordinates: 2.8 mm posterior to bregma, ± 2.0 mm lateral to midline, and 4.0 mm ventral to the surface of the skull). Lesions were produced in 30 animals by passing 1.5 mA anodal current for 15 sec (Grass Instruments, West Warwick, RI). Thirty control animals were treated identically except that no current was passed to create a lesion. Animals were adapted to handling and transportation procedures each day during a 7 day postoperative recovery period.
Apparatus
Training and testing took place in two modular operant test chambers (both 27 cm width × 28 cm length × 30.5 cm height) equipped with a speaker module (Coulbourn Instruments, Allentown, PA), located in an acoustic isolation room. The floor of each chamber was composed of 16 stainless steel bars (4.5 mm diameter) spaced 17.5 mm center-to-center and wired to a Coulbourn Precision Regulated Animal Shocker. The grid floor and base pan were washed thoroughly with tap water and completely dried prior to conditioning and testing.
Procedure
Experiment 1
Conditioning took place over ten consecutive days. On Day 1 and subsequent odd days, the rats (n = 20) were placed into Context B (denoted safe context), which was comprised of the following features: the side walls of the modular operant chamber were aluminum, while the front and back walls were clear Plexiglas covered with black and white checkered wallpaper; the tone and light modules were on the right side wall; the overhead light in the acoustic isolation room was off; the grid floor was covered by a Plexiglas plate scattered with sawdust; and the chamber walls were wiped with 20% ethanol. In the safe context the animals were exposed to ten presentations of a 15-s tone (2 KHz, 80 dB), which were separated by a 3-min inter-trial interval (ITI). On Day 2 and subsequent even days of training, the rats were placed into Context A (denoted shock context) which was comprised of the following features: all four walls of the modular operant chamber were clear Plexiglas; the tone and light modules were on the left side wall; the overhead light in the acoustic isolation room was on; the grid floor was exposed; and the chamber walls were wiped with 5% ammonium hydroxide. In the shock context the animals were exposed to ten presentations of the same 15-sec tone, which now overlapped and coterminated with a 1-sec footshock (0.5 mA). The ITI was 3 minutes. Upon completion of each day’s training session, the rats were returned to their home cages.
Three days after the completion of training, a test was conducted in the safe context (Context B) in which freezing in the presence of the tone was assessed. The test consisted of a 1-min baseline period followed by 8 minutes of continuous tone. Three days subsequent to this test, a second, identical test was conducted in the shock context (Context A). This order of testing (i.e., a test in the safe context followed by a test in the shock context) was chosen because it provides the most sensitive measure of conditioned tone fear in the safe context.
Experiment 2
The animals were trained similarly to those of Experiment 1, except that conditioning was conducted in a single training context (which corresponded to Context A, the shock context, of Experiment 1). Conditioning took place over ten consecutive days. On Day 1 and subsequent odd days, the rats (n = 20) experienced the “safe” paradigm: they were exposed to ten presentations of a 15-s tone, which were separated by a 3-min ITI. On Day 2 and subsequent even days of training, the rats experienced the “shock” paradigm: they were exposed to ten presentations of the same 15-s tone, which now overlapped and co-terminated with a 1-sec (0.5 mA) footshock. The ITI was 3 minutes. Upon completion of each day’s training session, the rats were returned to their home cages.
Three days after the completion of training, a test was conducted in a novel context (which corresponded to Context B, the safe context, of Experiment 1) in which freezing in the presence of the tone was assessed. The test consisted of a 1-min baseline period followed by 8 minutes of continuous tone. Three days subsequent to this test, a second, identical test was conducted in the conditioning context (Context A).
Experiment 3
Conditioning was conducted in a single training context (which corresponded to Context A, the shock context, of Experiment 1) and took place over ten consecutive days. On Day 1 and subsequent odd days, the rats (n = 40) experienced the safe paradigm: they were exposed to ten presentations of a 15-s tone, which were separated by a 3-min ITI. On Day 2 and subsequent even days of training, the rats experienced the shock paradigm: they were exposed to ten presentations of a 15-s light (occasion setter), immediately followed by a 15-s tone, which overlapped and co-terminated with a 1-s footshock. The ITI was 3 minutes. Upon completion of each day’s training session, the rats were returned to their home cages.
Three days after the completion of training, a test was conducted in either a novel context (for half of the animals of each group; the novel context corresponded to Context B, the safe context, of Experiment 1) or the training context (for the remaining animals of each group) in which freezing in the presence of the tone was assessed. The test consisted of a 1-min baseline period, followed by a 15-s light, followed by 8 minutes of continuous tone. Three days subsequent to this light-tone test, a second test was conducted in the same context, and consisted of a 1-min baseline period followed by 8 minutes of continuous tone. Three days after this tone test, a third test was given in the same context, and consisted of a 1-min baseline period followed by 8 minutes of continuous light.
Behavioral Data Collection
The experimental events were controlled, and the data collected, by an IBM-PC computer equipped with the Coulbourn LabLinc Habitest Universal Linc System. A 24-cell infrared activity monitor, which detects movement in the x, y, and z axes of the animal’s emitted infrared (1300 nm) body heat image, was mounted on the top of each chamber and used to assess freezing behavior (cf., Lee & Kim, 1998). In brief, the total time of inactivity exhibited by each animal was measured using a computer program, and freezing was defined as inactivity lasting ≥ 3 sec. Any period of inactivity lasting less than 3 sec was recorded as general activity. (Freezing scores obtained via infrared monitoring and observer time sampling methods [cf., Kim, DeCola, Landeira-Fernandez, & Fanselow, 1991] have been consistently and robustly correlated.)
Histology
At the completion of behavioral testing, the animals were overdosed with ketamine HC1 and xylazine and perfused intracardially with 0.9% saline followed by 10% buffered formalin. The brains were removed and stored in 10% formalin for at least 2 weeks before slicing. Coronal sections (60 μm) were taken through the extent of the lesion, mounted on gelatinized slides, and stained with Prussian blue and cresyl violet dyes. The histological reconstruction of the lesions was assessed by an observer who was blind to the behavioral data.
Data Analysis
Freezing data were analyzed using repeated-measures multivariate analysis of variance (ANOVA), with the between-subjects factor group (sham vs. lesion), and within-subjects factors minute, context (shock vs. safe; training vs. novel) and test (tone, light, light-tone). The level of statistical significance was p < 0.05.
Results
Histological Results
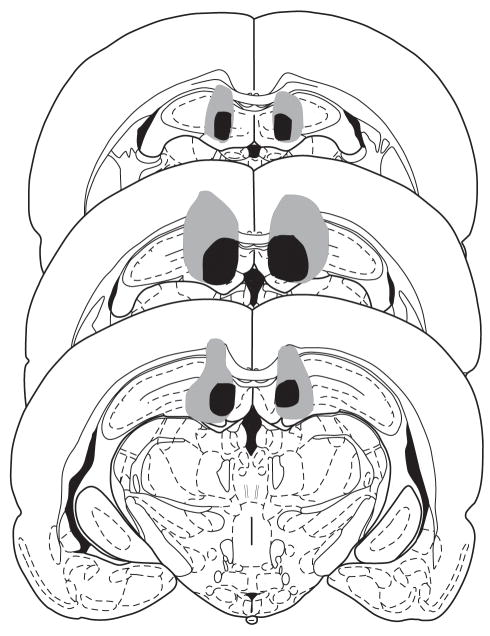
Figure 1 presents a reconstruction of the minimum and maximum extent of the damage in animals from all three experiments with lesions of the dHIPP. Hippocampal damage was found mainly in medial portions of the dorsal hippocampal formation (areas CA1 and dentate gyrus), but there were also small amounts of damage to the overlying cortex.
Figure 1.
Minimum (black) and maximum (gray) extent of dorsal hippocampal lesions in animals from Experiments 1–3. The lesions were reconstructed on successive coronal sections (−2.30, −3.60, and −4.16 mm bregma) from Paxinos and Watson (1997). Reprinted with permission from Elsevier ©1997.
Behavioral Results
Experiment 1
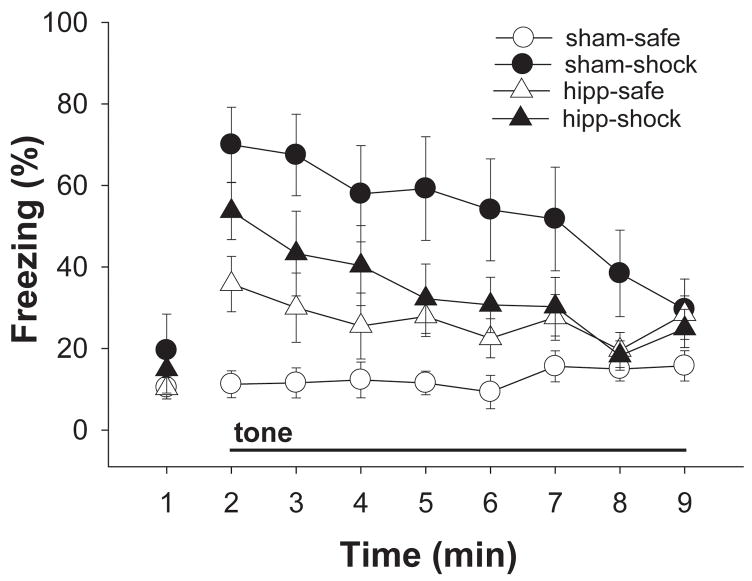
Experiment 1 used an analogue of the serial FP discrimination in which a context, rather than a discrete feature, set the occasion for responding to a target cue: A(X+), B(X−). Figure 2 depicts the mean percent freezing exhibited by both groups of animals during 1 minute of baseline and 8 minutes of continuous tone in the tests in the safe and shock contexts. No group or context differences were apparent in baseline freezing (largest F = 1.61; all p values > 0.05). The groups did, however, perform differently during the 8 minutes of continuous tone. An ANOVA revealed significant main effects of context (F(1,18) = 20.41, p < 0.01) and minute (F(7,126) = 5.77, p < 0.01), a significant context × group interaction (F(1,18) = 10.69, p < 0.01) and a significant context × minute interaction (F(7,126) = 6.08, p < 0.01). No other main effects or interactions were significant (all F values < 1). The significant context × group interaction demonstrates that the discrimination between the contexts was not equally evident in the lesion and sham groups. Tests of simple main effects (using the error term appropriate to each comparison; Howell, 1997) revealed that the sham-operated animals froze reliably more to the tone in the shock context than in the safe context (F(1,9) = 24.22, p < 0.01), whereas the dHIPP-lesioned animals did not freeze differentially to the tone in the two contexts (F(1,9) = 1.04, p > 0.05).
Figure 2.
Mean percent freezing (±SEM) during 1 minute of baseline measurement and 8 minutes of continuous tone in the “safe” and “shock” contexts of Experiment 1.
Experiment 2
Two animals died following surgery and one was excluded from further data analyses because of an inaccurate lesion placement, leaving 9 animals in the lesion group and 8 in the sham group.
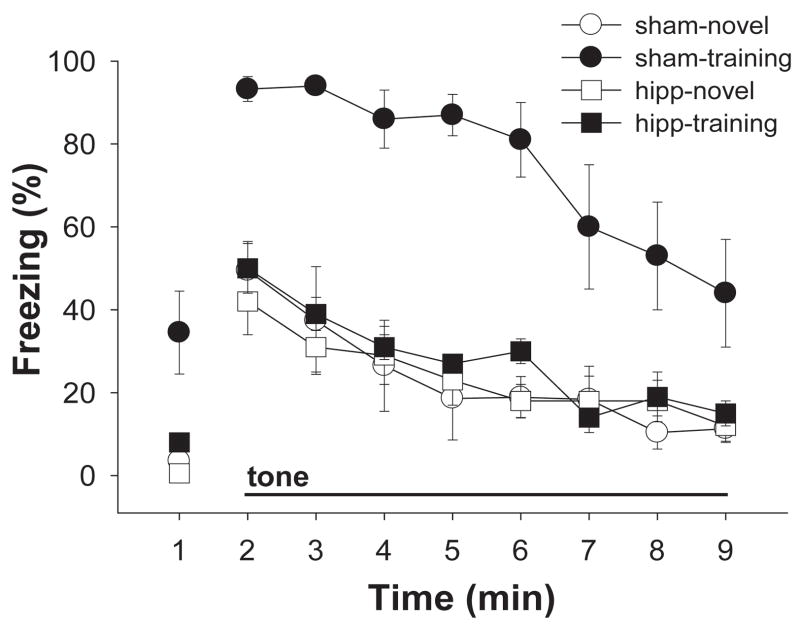
Figure 3 presents the mean percent freezing exhibited by both groups of animals during 1 minute of baseline and 8 minutes of continuous tone in the tests in the training and novel contexts. The sham-operated animals exhibited robust freezing during the baseline period in the training context but not in the novel context, whereas the dHIPP-lesioned animals exhibited little baseline freezing in either context. Similarly, the sham-operated animals exhibited considerably more freezing to the tone in the training context than in the novel context, while the dHIPP-lesioned animals froze indiscriminately to the tone in the two contexts.
Figure 3.
Mean percent freezing (±SEM) during 1 minute of baseline measurement and 8 minutes of continuous tone in the training and novel contexts of Experiment 2.
An ANOVA was used to compare baseline freezing across groups and contexts. There was a significant main effect of group (F(1,15) = 6.67, p < 0.05), and of context (F(1,15) = 19.08, p < 0.01), and a significant group × context interaction (F(1,15) = 6.64, p < 0.05). Tests of simple main effects revealed that both groups froze more in the trained context than in the novel context (Fs = 11.22 and 17.19 for sham and lesion groups, respectively; all p values < 0.05), but that the sham group froze reliably more than the lesion group in the trained context (F(1,16) = 6.86, p < 0.05), and not in the novel context (F(1,16) = 2.01, p > 0.05). Overall, however, very little freezing was observed in either group and in either context during the baseline period.
An ANOVA was used to assess freezing during the 8-minute tone test with group, context, and minute as factors. There were significant main effects of context (F(1,15) = 33.09, p < 0.01), minute (F(7,105) = 24.78, p < 0.01), and group (F(1,15) = 14.06, p < 0.01), and a significant context × group interaction (F(1,15) = 23.82, p < 0.01). No other interactions were significant (largest F = 2.01; all p values > 0.05). The context × group interaction suggests that the discrimination between the two contexts was not equal in the two groups. Tests of simple main effects revealed that the sham-operated animals froze significantly more to the tone in the trained context than in the novel context (F(1,7) = 37.95, p < 0.01), while the dHIPP-lesioned animals did not respond differentially to the tone in the two contexts (F(1,8) < 1).
Experiment 3
Experiment 3 compared the effect of dHIPP-lesions on occasion setting by contextual and discrete cues. Since lesions of the dorsal hippocampus impair contextual occasion setting (Experiments 1 and 2), these same lesions should also impair occasion setting by discrete cues if it is true that the two forms of modulation share a similar neural basis.
Two animals died following surgery and one was excluded from the further data analyses because of an inaccurate lesion placement, leaving 19 animals in the lesion group (9 tested in the novel context and 10 tested in the trained context) and 18 animals in the sham group (9 tested in each context).
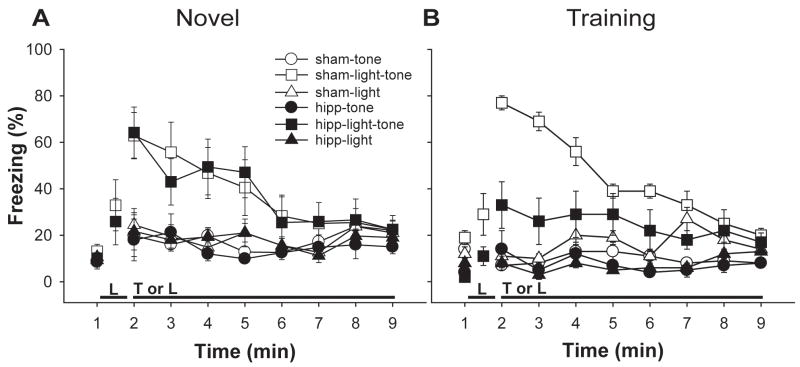
Panel A of Figure 4 presents the mean percent freezing exhibited during 1 minute of baseline, 15 seconds of light, and 8 minutes of continuous tone or light in the light-tone, tone, and light tests for those animals tested in the novel context. These data were analyzed using multiple ANOVAs comparing group performance during the baseline period, the light feature, and the tests of freezing to the tone, the light, and the tone that followed the light feature. An ANOVA with group and test as factors assessed freezing during the baseline periods preceding the tone, light, and light-tone tests. There were no main effects of group and test, and no group × test interaction (all F values < 1). Similarly, a one-way ANOVA comparing group performance during the 15-s light feature revealed no group difference (F < 1). The data from the tone, light, and light-tone tests were analyzed using an ANOVA with group, test, and minute as factors. There were significant main effects of test (F(2,34) = 17.39, p < 0.01) and minute (F(7,119) = 4.71, p < 0.01), and a significant test × minute interaction (F(14,238) = 4.04, p < 0.01). No other main effects or interactions reached significance (all F values < 1). Tests of simple main effects revealed that the animals generally froze more in the light-tone test than in the light test (F(1,18) = 17.83, p < 0.01), or the tone test (F(1,18) = 19.60, p < 0.01), and that they also froze more in the light test than in the tone test, (F(1,18) = 6.61, p < 0.05).
Figure 4.
Panel A. Mean percent freezing (±SEM) during the tone, light, and light-tone tests of Experiment 3, in animals tested in the novel context. Panel B. Mean percent freezing (±SEM) during the tone, light, and light-tone tests of Experiment 3, in animals tested in the training context.
Panel B of Figure 4 presents the data from the analogous tests conducted in the trained context. Analysis of the baseline freezing revealed a significant main effect of group (F(1,16) = 15.08, p < 0.01), but no main effect of test (F(2,32) = 2.97, p > 0.05), or group × test interaction (F < 1). There was no main effect of group during the 15-s light feature (F(1,17) = 3.14, p > 0.05). During the tone, light, and tone preceded by light tests, there were significant main effects of group (F(1,16) = 11.65, p < 0.01), of test (F(2,32) = 38.66, p < 0.01), and of minute (F(7,112) = 4.93, p < 0.01). There were significant interactions between group and test (F(2,32) = 4.20, p < 0.05), group and minute (F(7,112) = 2.20, p < 0.05), and test and minute (F(14,224) = 7.46, p < 0.01), and a significant group × minute × test interaction (F(14,224) = 3.68, p < 0.01). Tests of simple main effects revealed that the animals generally froze more in the light-tone test than in the light test (F(1,17) = 31.94, p < 0.01), or the tone test (F(1,17) = 38.44, p < 0.01), whereas there was no difference in freezing between the tone and light tests, (F(1,17) = 3.40, p > 0.05). Comparisons of the groups’ performance in each test confirmed that the groups did not differ in the tone test (F(1,17) < 1), but that the sham-operated animals did freeze more in the light test (F(1,17) = 8.37, p < 0.05), and the light-tone test (F(1,17) = 7.51, p < 0.05), than did the dHIPP-lesioned animals.
Discussion
The present series of experiments had two objectives: first, to determine whether contextual occasion setting, like other forms of contextual conditioning, is hippocampus-dependent; and second, to compare the effect of dorsal hippocampal lesions on occasion setting by discrete and contextual cues. Animals with dHIPP-lesions were found to be impaired in learning a discrimination in which contextual modulation is necessary for appropriate performance (Experiment 1), and in expressing any degree of contextual specificity of responding, a measure of “incidental” contextual modulation (Experiment 2). dHIPP-lesioned animals were not impaired, however, in learning a serial FP discrimination in which a light set the occasion for responding to a tone, although again they were lacking in contextual modulation of performance (Experiment 3). Collectively, these data indicate that the dorsal hippocampus is important in processing information about the signaling value of contextual, but not discrete, stimuli.
These findings join a large body of evidence implicating the hippocampus in the processing of contextual information for certain conditioning phenomena (e.g., Penick & Solomon, 1991), whether that information is relevant to simple conditioning of context (i.e., the formation of context-US associations) or higher-order contextual conditioning (i.e., contextual occasion setting; see Holland & Bouton, 1999, but see also Rudy, 2009). Nevertheless, this conclusion has not been universally accepted (Benoit, Davidson, & Chan, 1999; Good & Honey, 1997; McNish, Gewirtz, & Davis, 1997); particularly with respect to the hippocampal-dependence of contextual occasion setting, there are a number of studies that suggest otherwise. For example, among the studies that have examined the effect of hippocampal lesions on the solution of a biconditional contextual discrimination, only one of them (Good & Honey, 1991) reported an impairment, while others (Good et al., 1998; Hall et al., 1996; McDonald et al., 1997) did not. This type of discrimination is quite similar to the contextual analogue of the FP discrimination that we examined in Experiment 1 because it consists of two concurrent contextual FP discriminations, arranged such that each of the contexts signals reinforcement of one target and nonreinforcement of the other.
Given our finding that the solution of the contextual FP discrimination is devastated by dorsal hippocampal lesions, it is not at all clear why the apparently more complex contextual biconditional discrimination should be spared. Studies that make use of other measures of contextual modulation, such as the context specificity of responding, have also reported conflicting findings (Fox & Holland, 1998; Good & Honey, 1991; Good et al., 1998; Hall & Honey, 1989; Hall et al., 1996; Holt & Maren, 1999; Honey & Good, 1993; Penick & Solomon, 1991; McDonald et al., 1997; Wilson et al., 1995). There are sometimes clear procedural differences that might account for the discrepancies, such as the use of aversive versus appetitive conditioning procedures (compare, e.g., Fox & Holland, 1998; Wilson et al., 1995), the method used to create and/or the ultimate extent of the hippocampal lesions (compare, e.g., Good & Honey, 1991; Good et al., 1998), or the use of different training and/or testing protocols (compare, e.g., Good & Honey, 1991; Hall et al., 1996). Different outcomes are, perhaps, to be expected with differences in lesion type and/or experimental design. However, differences among studies that are identical in all respects except for the choice of an appetitive or aversive paradigm are puzzling.
In fact, these types of differences have also been notable in studies examining the hippocampal-dependence of simple contextual conditioning, and are the source of much of the controversy surrounding the claim that the formation of context-US associations requires the intact hippocampus. With appetitive conditioning procedures, the contextual conditioning, which occurs when the unsignaled US is repeatedly presented within a context, is seldom disrupted by hippocampal lesions (Fox & Holland, 1998; Good & Honey, 1991; Honey & Good, 1993); whereas with aversive fear conditioning procedures, the contextual conditioning produces mixed results (Chen et al., 1996; Kim et al., 1993; Maren, Aharonov & Fanselow, 1997; McNish et al., 1997; Wiltgen et al., 2006), which have been postulated as a compensatory conditioning to ‘elements’ of context in the absence of the hippocampus (Gonzalez, Quinn, & Fanselow, 2003; Rudy, Huff, & Matus-Amat, 2004). Some have suggested that appetitive USs might be less readily associated with contextual stimuli than are aversive USs (Fox & Holland, 1998). However, there are inconsistencies even among studies using similar conditioning paradigms. One might appeal to lesion type and extent as at least partially accounting for the discrepant results of these studies. Two appetitive conditioning studies that reported no impairment used neurotoxic lesions (Good et al., 1998; McDonald et al., 1997), whereas the one that found a deficit used electrolytic lesions (Good & Honey, 1991). However, a third study finding no impairment also used electrolytic lesions (Hall et al., 1996), and almost exactly replicated the experimental design used by Good and Honey (1991). In this instance, then, the impact of an argument based on procedural details is considerably lessened.
Our finding that the solution of a discrete, serial FP discrimination is not affected by dorsal hippocampal lesions also joins a literature that is marked by inconsistencies. In the first study to consider the effect of hippocampal lesions on occasion setting, Ross, Orr, Holland, and Berger (1984) reported that aspiration lesions of the hippocampal formation prevented the acquisition and eliminated the retention of a serial FP discrimination. However, subsequent work using neurotoxic hippocampal lesions failed to replicate the impairment in either acquisition (Jarrard & Davidson, 1990) or retention (Davidson & Jarrard, 1989). Moreover, when the performance of groups of rats with aspiration and neurotoxic lesions was directly compared, the aspiration-lesioned group was found to be impaired but the neurotoxic-lesioned group was not (Jarrard & Davidson, 1991), leading to the conclusion that occasion setting is subserved by extra-hippocampal structures that are damaged with aspiration but not neurotoxic lesions. More recently, the question of the hippocampal-dependence of simple occasion setting has again been raised in light of findings that the learning of serial FP or feature-negative (FN) discriminations may, under some circumstances, be either enhanced (Han et al., 1998) or impaired (Holland et al., 1999) by neurotoxic lesions of the hippocampus proper. It is worth noting that the observed enhancements occurred in situations in which the inter-trial interval (ITI) was relatively short (30 or 60 seconds), unlike the 3 minute ITI used in our Experiment 3, and that the impairments were primarily observed in the learning of a FN discrimination, which the present study did not consider. Thus, our finding that the learning of a discrete serial FP discrimination is not affected by hippocampal lesions is consistent with the bulk of the data collected to date.
Studies that use contextual FP discrimination have provided perhaps the strongest evidence to date for an occasion setting function of context distinct from simple contextual conditioning. For example, Bouton and Swartzentruber (1986) demonstrated that the solution of the contextual FP discrimination is not readily explainable by appeal to simple context-US associations. Using a conditioned suppression paradigm, these authors found that response suppression in the presence of the target was reliably greater in the reinforced context (A), and reliably smaller in the nonreinforced context (B), than in a novel context (C), indicating that both contexts modulated responding. However, subsequent tests of conditioned excitation and inhibition failed to reveal any simple associative tendencies held by either context. Moreover, extended nonreinforced exposure to context A after discrimination training had little impact on subsequent discrimination performance, strongly suggesting that the occasion setting ability of a context is largely independent of its simple associative value.
An alternative interpretation of the outcome of Experiment 1 is possible, considering the mechanisms by which contexts may come to act as occasion setters. It is commonly held that, in order for a discrete cue to become an occasion setter, two criteria must be met: first, an appropriate temporal and/or salience relationship must exist between feature and target (see, e.g., Holland, 1986, 1989), and second, an “informational” relationship must be established in which the target cue is uniquely reinforced or nonreinforced in the presence of the feature (see Swartzentruber, 1995, for a review). The second criterion is most easily and effectively met through the use of a discrimination procedure in which, for example, the target is reinforced in the presence of the feature but nonreinforced in its absence (i.e., the FP discrimination). However, there is evidence that discrimination training is not necessary for a feature cue to become an occasion setter (Bonardi, 1992; Hall & Mondragon, 1998; Wagner & Brandon, 2000). All that is needed, by these accounts, is that the feature signals the reinforcement (or nonreinforcement, as the case may be) of the target stimulus (see Bonardi, 2007; Bonardi & Jennings, 2009). That the establishment of contextual occasion setting may similarly not require discrimination training is suggested by the contextual specificity of certain types of conditioning (e.g., latent inhibition; see, e.g., Hall & Honey, 1989) in which discrimination training is not involved (Hall & Mondragon, 1998).
Frohardt, Guarraci, and Bouton (2000) examined the hippocampal role in renewal effect and used the context as the occasion setter. Their finding indicated the complete hippocampal lesions do not disrupt the renewal effect. One might argue that this finding conflicts with the present results from Experiment 1. In addition to the difference in the extent of the lesions in the hippocampus, the renewal effect in their study was tested after several extinction sessions. However, our contextual occasion setting was tested during the extinction phase. In fact, like similar freezing responses for the hippocampal group between the safe and shock contexts in our experiment, the performance of hippocampus-lesioned group in the Frohardt et al. study (2000) showed comparable suppression ratio of bar-pressing for the novel and trained contexts throughout the extinction trials.
The novel context test in Experiment 2 was conducted on an odd day that corresponds to the safe paradigm of the ten days of training; the trained context test was done on an even day for shock paradigm of training. Therefore, one might argue that control rats learned the ‘safe-shock’ alternating training contingency and used the previous session type as an occasion setter. However, this is unlikely in that the same training schedule was used in Experiment 3 and the control group showed robust freezing (sham-light-tone) on the odd day.
An important finding from the discrete, serial FP discrimination study (Experiment 3) is that, even though a single explicit CS is regarded to be a more difficult indicator to be conditioned for serial FP discrimination than simple contextual CS, the dHIPP-lesioned group successfully exhibited freezing during light-tone presentations in the novel context. Therefore, the impairment of occasion-setting in the trained context isn’t likely due to the disrupted role of the dorsal hippocampus in solving difficult (or complex) associative learning. In addition, the possibility that the light worked as a simple excitor rather than the occasion setter prior to tone is also unlikely because the separate presentation of light by itself didn’t increase the level of freezing.
On a theoretical level, the findings of the present study indicate that the dorsal hippocampus is not primarily involved in the representation of conditional (Hirsh, 1974, 1980) or configural (Gluck & Myers, 1993; Rudy, 2009; Sutherland & Rudy, 1989) relations among stimuli, at least not in the simple manner assumed by most early theories. If this were the case, then one would expect that the learning of both the discrete FP discrimination and its contextual analogue would be impaired by dHIPP lesions. However, the deficit that we observed was selective to the contextual discrimination.
Other theories have assumed that the processing of information relevant to discrete and contextual cues occurs in different areas of the hippocampal formation (Myers, Gluck, & Granger, 1995; Zackheim, Myers, & Gluck, 1998), or that hippocampal lesions may be expected to have quite different effects on the learning of discrete and contextual conditional discriminations as a function of the qualitatively different temporal encoding demanded by discrete (phasic) and contextual (tonic) cues (Schmajuk & Buhusi, 1997). Our data are consistent with either possibility. As Han et al. (1998) have noted, it seems clear that quantitative, real-time models such as these are necessary to capture the complexity of hippocampal lesion effects on various conditioning phenomena, as simpler models have not been able to do so with similar accuracy.
In summary, although the functions of the hippocampus in classical conditioning remain incompletely characterized, the extensive amount of research to date and the continued pursuit of clarity in this topic are acknowledgements to the significance of Richard F. Thompson and his colleagues’ pioneering work.
Acknowledgments
The authors would like to thank Zachary Katz and Seth Altman for their assistance in data collection, and Karyn M. Myers for valuable contributions in the data analyses and on an earlier version of this article.
This work was supported by Grant M97-17 from the Whitehall Foundation and NIMH (MH64457) to J.J.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ammassari-Teule M, Passino E, Restivo L, de Marsanich B. Fear conditioning in C57/BL/6 and DBA/2 mice: variability in nucleus accumbens function according to the strain predisposition to show contextual- or cue-based responding. European Journal of Neuroscience. 2000;12:4467–4474. [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: Within-subjects examination. Journal of Neuroscience. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens F, Vansteenwegen D, Beckers T, Hermans D, Kerkhof I, De Ceulaer A. Extinction and renewal of Pavlovian modulation in human sequential feature positive discrimination learning. Learning & Memory. 2005;12:178–192. doi: 10.1101/lm.89905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit SC, Davidson TL, Chan KH. Pavlovian conditioning and extinction of context cues and punctate CSs in rats with ibotenate lesions of the hippocampus. Psychobiology. 1999;27:26–39. [Google Scholar]
- Berger TW, Alger B, Thompson RF. Neuronal substrate of classical conditioning in the hippocampus. Science. 1976;192:483–485. doi: 10.1126/science.1257783. [DOI] [PubMed] [Google Scholar]
- Bonardi C. Occasion setting without feature-positive discrimination training. Learning and Motivation. 1992;23:343–367. [Google Scholar]
- Bonardi C. Occasion setting is specific to the CS-US association. Learning and Motivation. 2007;38:208–228. [Google Scholar]
- Bonardi C, Jennings D. Learning about associations: evidence for a hierarchical account of occasion setting. Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:440–445. doi: 10.1037/a0014019. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Differential control by context in the inflation and reinstatement paradigms. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:56–74. [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning & Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, King DA. Contextual control of the extinction of conditioned fear: Tests for the associative value of the context. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:248–265. [PubMed] [Google Scholar]
- Bouton ME, King DA. Effect of context on performance to conditioned stimuli with mixed histories of reinforcement and nonreinforcement. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:4–15. [Google Scholar]
- Bouton ME, Swartzentruber D. Analysis of the associative and occasion-setting properties of contexts participating in a Pavlovian discrimination. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:333–350. [Google Scholar]
- Chen C, Kim JJ, Thompson RF, Tonegawa S. Hippocampal lesions impair contextual fear conditioning in two strains of mice. Behavioral Neuroscience. 1996;110:1177–1180. doi: 10.1037//0735-7044.110.5.1177. [DOI] [PubMed] [Google Scholar]
- Cho YH, Friedman E, Silva AJ. Ibotenate lesions of the hippocampus impair spatial learning but not contextual fear conditioning in mice. Behavioural Brain Research. 1999;98:77–87. doi: 10.1016/s0166-4328(98)00054-0. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H, Deacedo BS, Corkin S. Different memory systems underlying acquisition of procedural and declarative knowledge. Annals of the New York Academy of Sciences. 1985;444:54–71. doi: 10.1111/j.1749-6632.1985.tb37579.x. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Jarrard LE. Retention of concurrent conditional discriminations in rats with ibotenate lesions of hippocampus. Psychobiology. 1989;17:49–60. [Google Scholar]
- Fox GD, Holland PC. Neurotoxic hippocampal lesions fail to impair reinstatement of an appetitively conditioned response. Behavioral Neuroscience. 1998;112:255–260. [PubMed] [Google Scholar]
- Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behavioral Neuroscience. 1998;112:863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- Frohardt RJ, Guarraci FA, Bouton ME. The effects of neurotoxic hippocampal lesions on two effects of context after fear extinction. Behavioral Neuroscience. 2000;114:227–240. doi: 10.1037//0735-7044.114.2.227. [DOI] [PubMed] [Google Scholar]
- Gluck M, Myers C. Hippocampal mediation of stimulus representation: A computational theory. Hippocampus. 1993;3:491–516. doi: 10.1002/hipo.450030410. [DOI] [PubMed] [Google Scholar]
- Gonzalez F, Quinn JJ, Fanselow MS. Differential effects of adding and removing components of a context on the generalization of conditional freezing. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:78–83. [PubMed] [Google Scholar]
- Good M, de Hoz L, Morris RGM. Contingent versus incidental context processing during conditioning: Dissociation after excitotoxic hippocampal plus dentate gyrus lesions. Hippocampus. 1998;8:147–159. doi: 10.1002/(SICI)1098-1063(1998)8:2<147::AID-HIPO7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Good M, Honey RC. Conditioning and contextual retrieval in hippocampal rats. Behavioral Neuroscience. 1991;105:499–509. doi: 10.1037//0735-7044.105.4.499. [DOI] [PubMed] [Google Scholar]
- Good M, Honey RC. Dissociable effects of selective lesions to hippocampal subsystems on exploratory behavior, contextual learning, and spatial learning. Behavioral Neuroscience. 1997;111:487–493. doi: 10.1037//0735-7044.111.3.487. [DOI] [PubMed] [Google Scholar]
- Hall G, Honey RC. Contextual effects in conditioning, latent inhibition, and habituation: Associative and retrieval functions of contextual cues. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:232–241. [Google Scholar]
- Hall G, Mondragon E. Contextual control as occasion setting. In: Schmajuk NA, Holland PC, editors. Occasion setting: Associative learning and cognition in animals. Washington, DC: APA; 1998. pp. 199–222. [Google Scholar]
- Hall G, Purves D, Bonardi C. Contextual control of conditioned responding in rats with dorsal hippocampal lesions. Behavioral Neuroscience. 1996;110:933–945. doi: 10.1037//0735-7044.110.5.933. [DOI] [PubMed] [Google Scholar]
- Han JS, Gallagher M, Holland P. Hippocampal lesions enhance configural learning by reducing proactive interference. Hippocampus. 1998;8:138–146. doi: 10.1002/(SICI)1098-1063(1998)8:2<138::AID-HIPO6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hirsh R. The hippocampus and contextual retrieval of information from memory: A theory. Behavioral Biology. 1974;12:421–444. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- Hirsh R. The hippocampus, conditional operations, and cognition. Physiological Psychology. 1980;8:175–182. [Google Scholar]
- Holland PC. Occasion setting in Pavlovian feature positive discriminations. In: Commons ML, Herrnstein RJ, Wagner AR, editors. Quantitative analyses of behavior: Discrimination processes. Cambridge, MA: Balinger; 1983. pp. 183–206. [Google Scholar]
- Holland PC. Temporal determinants of occasion setting in feature-positive discriminations. Animal Learning and Behavior. 1986;14:111–120. [Google Scholar]
- Holland PC. Occasion setting with simultaneous compounds in rats. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:183–193. [PubMed] [Google Scholar]
- Holland PC. Occasion setting in Pavlovian conditioning. In: Medin D, editor. The Psychology of Learning and Motivation. San Diego, CA: Academic Press; 1992. pp. 69–125. [Google Scholar]
- Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Current Opinion in Neurobiology. 1999;9:195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Holland PC, Lamoureux JA, Han JS, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9:143–157. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Holt W, Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. Journal of Neuroscience. 1999;19:9054–9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey RC, Good M. Selective hippocampal lesions abolish the contextual specificity of latent inhibition and conditioning. Behavioral Neuroscience. 1993;107:23–33. doi: 10.1037//0735-7044.107.1.23. [DOI] [PubMed] [Google Scholar]
- Howell DC. Statistical methods for psychology. Belmont, CA: Wadsworth; 1997. [Google Scholar]
- Hull CL. Principles of behavior: An introduction to behavior theory. New York, NY: Appleton-Century-Crofts; 1943. [Google Scholar]
- Jarrard LE, Davidson TL. Acquisition of concurrent conditional discriminations in rats with ibotenate lesions of hippocampus and subiculum. Psychobiology. 1990;18:68–73. [Google Scholar]
- Jarrard LE, Davidson TL. On the hippocampus and learned conditional responding: Effects of aspiration versus ibotenate lesions. Hippocampus. 1991;1:107–117. doi: 10.1002/hipo.450010110. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- Kim JJ, DeCola JP, Landeira-Fernandez J, Fanselow MS. N-methyl-D-aspartate receptor antagonist APV blocks acquisition but not expression of fear conditioning. Behavioral Neuroscience. 1991;105:126–133. doi: 10.1037//0735-7044.105.1.126. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behavioral Neuroscience. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear conditioned rats. Journal of Neuroscience. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the association of stimuli with reinforcement. Psychological Review. 1975;82:276–298. [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behavioral Brain Research. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiology of Learning Memory. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, Murphy RA, Guarraci FA, Gortler JR, White NM, Baker AG. Systematic comparison of the effects of hippocampal and fornix-fimbria lesions on the acquisition of three configural discriminations. Hippocampus. 1997;7:371–388. doi: 10.1002/(SICI)1098-1063(1997)7:4<371::AID-HIPO3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McNish KA, Gewirtz JS, Davis M. Evidence of contextual fear after lesions of the hippocampus: A disruption of freezing but not fear potentiated startle. Journal of Neuroscience. 1997;17:93539360. doi: 10.1523/JNEUROSCI.17-23-09353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RR, Schachtman TR. The several roles of context at the time of retrieval. In: Balsam PD, Tomie A, editors. Context and learning. Hillsdale, NJ: Erlbaum; 1985. pp. 167–194. [Google Scholar]
- Myers CE, Gluck MA, Granger R. Dissociation of hippocampal and entorhinal function in associative learning: A computational approach. Psychobiology. 1995;23:116–138. [Google Scholar]
- Nadel L, Willner J. Context and conditioning: A place for space. Physiological Psychology. 1980;8:218–228. [Google Scholar]
- Pavlov IP. Conditioned Reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Paylor R, Tracy R, Wehner J, Rudy JW. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behavioral Neuroscience. 1994;108:810–817. doi: 10.1037//0735-7044.108.4.810. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Reviews. 1980;87:532–552. [PubMed] [Google Scholar]
- Penick S, Solomon PR. Hippocampus, context, and conditioning. Behavioral Neuroscience. 1991;105:611–617. doi: 10.1037//0735-7044.105.5.611. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contributions of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learning & Memory. 1994;1:34–44. [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Lesions of the fornix but not the entorhinal or perirhinal cortex interfere with contextual fear conditioning. Journal of Neuroscience. 1995;15:5308–5315. doi: 10.1523/JNEUROSCI.15-07-05308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. Journal of Experimental Psychology: Animal Behavior Processes. 1975;1:88–96. [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current theory and research. New York, NY: Appleton Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JNP, Feldon J, Bannerman DM. Dissociating context and space within the hippocampus: Effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behavioral Neuroscience. 1999;113:1189–1203. doi: 10.1037/0735-7044.113.6.1189. [DOI] [PubMed] [Google Scholar]
- Ross RT, Holland PC. Conditioning of simultaneous and serial feature-positive discriminations. Animal Learning & Behavior. 1981;9:293–303. [Google Scholar]
- Ross RT, Orr WB, Holland PC, Berger TW. Hippocampectomy disrupts acquisition and retention of learned conditional responding. Behavioral Neuroscience. 1984;98:211–225. doi: 10.1037//0735-7044.98.2.211. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behavioral Neuroscience. 1993;107:887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal – hippocampal system. Learning & Memory. 2009;16:573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neuroscience and Biobehavioral Reviews. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Implicit memory: history and current status. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13:501–518. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- Schmajuk NA, Buhusi CV. Stimulus configuration, occasion setting, and the hippocampus. Behavioral Neuroscience. 1997;111:235–258. doi: 10.1037//0735-7044.111.2.235. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF. The behavior of organisms. New York, NY: Appleton-Century-Crofts; 1938. [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behavioral Neuroscience. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Spear NE. Retrieval of memory in animals. Psychological Review. 1973;80:163–194. [Google Scholar]
- Stiedl O, Misane I, Spiess J, Ogren SO. Involvement of the 5-HT1A receptors in classical fear conditioning in C57BL/6J Mice. Journal of Neuroscience. 2000;15:8515–8527. doi: 10.1523/JNEUROSCI.20-22-08515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, O’Brien J, Lehmann H. Absence of systems consolidation of fear memories after dorsal, ventral, or complete hippocampal damage. Hippocampus. 2008;18:710–718. doi: 10.1002/hipo.20431. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Rudy JW. Configural association theory: The role of the hippocampal formation in learning, memory, and amnesia. Psychobiology. 1989;17:129–144. [Google Scholar]
- Swartzentruber D. Blocking between occasion setters and contextual stimuli. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:163–173. doi: 10.1037//0097-7403.17.2.163. [DOI] [PubMed] [Google Scholar]
- Swartzentruber D. Modulatory mechanisms in Pavlovian conditioning. Animal Learning & Behavior. 1995;23:123–143. [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. New York: Academic Press; 1972. pp. 381–403. [Google Scholar]
- van Strien NM, Cappaert NLM, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nature Reviews. Neuroscience. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- Wagner AR, Brandon SE. A componential theory of Pavlovian conditioning. In: Mowrer RR, Klein SB, editors. Handbook of contemporary learning theories. Hillsdale, NJ: Erlbaum; 2000. pp. 23–64. [Google Scholar]
- Wilson A, Brooks DC, Bouton ME. The role of the rat hippocampal system in several effects of context in extinction. Behavioral Neuroscience. 1995;109:828–836. doi: 10.1037//0735-7044.109.5.828. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. Journal of Neuroscience. 2006;26:5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SL, Bohenek DL, Fanselow MS. NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: immunization against amnesia by context preexposure. Behavioral Neuroscience. 1994;108:19–29. doi: 10.1037//0735-7044.108.1.19. [DOI] [PubMed] [Google Scholar]
- Zackheim J, Myers C, Gluck M. A temporally sensitive recurrent network model of occasion setting. In: Schmajuk NA, Holland PC, editors. Occasion setting: Associative learning and cognition in animals. Washington, DC: APA; 1998. pp. 319–342. [Google Scholar]