Abstract
Oxidative stress and inflammation are important processes in the progression of Alzheimer's disease (AD). Recent studies have implicated the role of amyloid β-peptides (Aβ) in mediating these processes. In astrocytes, oligomeric Aβ induces the assembly of NADPH oxidase complexes resulting in its activation to produce anionic superoxide. Aβ also promotes production of pro-inflammatory factors in astrocytes. Since low energy laser has previously been reported to attenuate oxidative stress and inflammation in biological systems, the objective of this study was to examine whether this type of laser light was able to abrogate the oxidative and inflammatory responses induced by Aβ. Primary rat astrocytes were exposed to Helium-Neon laser (λ=632.8 nm), followed by the treatment with oligomeric Aβ. Primary rat astrocytes were used to measure Aβ-induced production of superoxide anions using fluorescence microscopy of dihydroethidium (DHE), assembly of NADPH oxidase subunits by the colocalization between the cytosolic p47phox subunit and the membrane gp91phox subunit using fluorescent confocal microscopy, phosphorylation of cytosolic phospholipase A2 (cPLA2), and expressions of pro-inflammatory factors including interleukin-1β (IL-1β) and inducible nitric-oxide synthase (iNOS) using Western blot Analysis. Our data showed that laser light at 632.8 nm suppressed Aβ-induced superoxide production, colocalization between NADPH oxidase gp91phox and p47phox subunits, phosphorylation of cPLA2, and the expressions of IL-1β and iNOS in primary astrocytes. We demonstrated for the first time that 632.8 nm laser was capable of suppressing cellular pathways of oxidative stress and inflammatory responses critical in the pathogenesis in AD. This study should prove to provide the groundwork for further investigations for the potential use of laser therapy as a treatment for AD.
Keywords: phospholipase A2, interleukin-1β, iNOS, NADPH oxidase, oxidative stress, phosphorylation, inflammation
INTRODUCTION
Oxidative stress and inflammation have been implicated in many neurodegenerative diseases including AD (Behl et al., 1994, Simonian and Coyle, 1996, Behl and Holsboer, 1998, Heneka and O'Banion, 2007). Oxidative stress induced by overproduction of reactive oxygen species (ROS) causes damage of basic components in cells, such as lipids (Cini and Moretti, 1995), DNA (Mecocci et al., 1994) and proteins (Smith et al., 1991). In most cell systems, NADPH oxidase (Abramov et al., 2004) and mitochondrial abnormalities (Hirai et al., 2001) are two important sources of ROS. NADPH oxidase is comprised of six subunits (Groemping and Rittinger, 2005, Mizrahi et al., 2006) and its activation is mediated by translocation of the cytosolic subunits (p47phox, p67phox, p40phox and the GTPase Rac) to the plasma membrane subunits (gp91phox and p22phox) (Groemping and Rittinger, 2005, Mizrahi et al., 2006). Activation of NADPH oxidase in astrocytes and microglia results in increased production of superoxide anions, which are toxic to neighboring neurons in AD brains (Shimohama et al., 2000, Qin et al., 2002, Abramov et al., 2004). Oxidative stress also triggers critical downstream pathways including activation of cPLA2, an enzyme responsible for membrane integrity (You et al., 2005, Huber et al., 2006, Sun et al., 2007, Shelat et al., 2008). Consistent with this line of evidence, we have reported that Aβ activates NADPH oxidase to induce ROS and activation of cPLA2 in primary rat astrocytes (Zhu et al., 2006). Activated cPLA2, in turn, targets mitochondria, resulting in mitochondrial dysfunction and further overproduction of ROS (Zhu et al., 2006). Based on these previous studies, it is reasonable to suggest two major mechanisms for Aβ to induce ROS production, initially from NADPH oxidase and subsequently from mitochondria through cPLA2 activation.
There is compelling evidence that inflammation plays a vital role in pathogenesis of AD (Heneka and O'Banion, 2007). Aβ triggers expressions of inflammatory factors including IL-1β and iNOS in glial cells (Akama and Van Eldik, 2000, White et al., 2005). IL-1β is a critical inflammatory cytokine in AD (Griffin and Mrak, 2002) since it can stimulate production of iNOS and other inflammatory cytokines (Lee et al., 1993, Blom et al., 1997).
Low-level laser has been reported to attenuate oxidative stress and inflammation (Karageuzyan et al., 1998, Sakurai et al., 2000, Freitas et al., 2001, Fillipin et al., 2005, Abdel et al., 2007, Lim et al., 2007, Aimbire et al., 2008, Boschi et al., 2008, Hammer et al., 2008, Giuliani et al., 2009). Although the mechanism is yet to be fully understood, this type of light therapy has been used to help tissue repair and wound healing in in vivo models (Whelan et al., 2001, Whelan et al., 2003, Albertini et al., 2007, Correa et al., 2007, Viegas et al., 2007, Aimbire et al., 2008, Reis et al., 2008) and rescue neurons from neurotoxic injuries (Wong-Riley et al., 2005, Liang et al., 2006), implying a variety of promising clinical applications. In this study, we hypothesize that laser has the capability of suppressing Aβ-induced oxidative stress and inflammation in astrocytes, the most abundant cell type in the brain. We tested the effects of low-level laser light at 632.8 nm on Aβ-induced ROS production through the activation of NADPH oxidase, and its downstream pathways involving phosphorylation of cPLA2 and expression of inflammatory factors including IL-1β and iNOS. Information derived from this study should prove to provide groundwork for further investigations on the potential application of laser therapy as a treatment for AD.
EXPERIMENAL PROCEDURES
Chemicals and Reagents
Dulbecco's modified Eagle's medium (DMEM) with high glucose, Ham's F-12 medium, fetal bovine serum (FBS), dihydroethidium (DHE) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were from Invitrogen (Carlsbad, CA). Bovine serum albumin (BSA), dimethyl sulfoxide (DMSO), hexafluoro-2-propanol (HFIP) and poly-D-lysine were from Sigma-Aldrich (St. Louis, MO). Aβ1–42 was from American Peptide (Sunnyvale, CA). gp91 ds-tat Peptide 2, a peptide inhibitor of NADPH oxidase, was from anaSpec (Fremont, CA). Goat polyclonal anti-gp91phox and rabbit polyclonal anti-p47phox were from Santa Cruz Biotechnology (Santa Cruz, CA). Fluorescein-donkey anti-goat antibody, and Texas Red-sheep anti-rabbit antibody were from Abcam (Cambridge, MA).
Cell culture
Primary cortical astrocytes were obtained using a standard stratification/cell-shaking procedure from newborn rat brains. Following the procedure from our previous studies yielded confluent mixed glial cultures within 7–9 days, after which the flasks were shaken at 180 rev./min at room temperature (25°C) for 3h to remove microglial cells (Zhu et al., 2005, Zhu et al., 2006). The purity of these primary rat astrocyte cultures was >95% verified by anti-glial fibrillary acidic protein labeling (data not shown). Astrocytes were cultured onto 35mm dishes or coverslips coated with poly-D-lysine (0.4mg/ml) and fed every 48 h with fresh DMEM culture medium supplemented with 10% FBS. Cells were maintained at 37°C in a 5% CO2 humidified incubator.
Preparation of Aβ1–42
Aβ1–42 (1mg) in the powder form was dissolved in 200μl of HFIP, and the solution was aliquoted into Eppendorf tubes, and after removing HFIP using a speed vacuum apparatus, samples were stored at −20°C until use. The Aβ film left in the tube was resuspended in 2μl DMSO and further diluted in 98 μl Ham's F-12 medium to make a 100μM Aβ1–42 solution. The solution was then sonicated for 1 min and further diluted in DMEM to the final concentration of 5μM for treatments.
Laser irradiation protocol and Aβ treatment
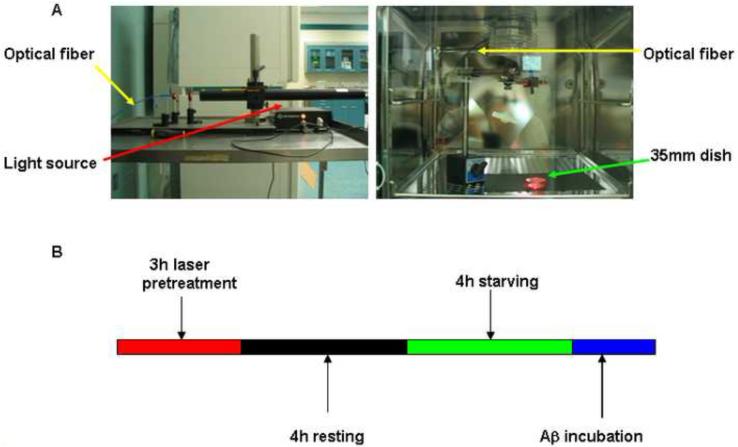
The source of light for irradiation was a helium-neon laser (λ=632.8 nm) with an output power of 15mW. The light source was placed outside of incubator and an optical fiber was used to guide the laser from above onto a 35 mm dish covered with a plastic lid inside the incubator (Fig. 1A). Coverslips coated with poly-D-lysine were in 35 mm dishes if immunostaining was needed for confocal microscopy. The spot size was adjusted to cover the whole dish with area of 10 cm2. The irradiation power density was 1.5mW/cm2. In this study, the irradiation time was 3 h. The total energy was 162 J and energy density was 16.2 J/cm2. Both cell morphology and trypan blue exclusion assay showed that laser irradiation did not cause any ill-effect to cell viability (data not shown). In addition, no detectable change in culture medium temperature was observed during laser irradiation.
Fig. 1. Laser irradiation set-up and protocol.
A: Laser irradiation set-up. Helium-Neon laser (λ=632.8 nm) light source with the power 15mW was placed outside of incubator (A, left). Optical fiber was used to guide laser irradiation onto a 35 mm dish in the incubator (A, right). B: Laser irradiation protocol. For the group with laser pretreatment and Aβ treatment, astrocytes in 35mm dishes and DMEM with 10% FBS were pretreated with laser for 3 h (red). After allowing cells to rest for another 4 h (black), cells were serum starved for 4 h by replacing the DMEM containing 10% FBS with serum-free DMEM (green). Cells were then incubated with Aβ (5μ-M) (blue). For control group, astrocytes were starved for 4 h and incubated with serum-free DMEM. For the group with Aβ treatment alone, astrocytes were starved for 4 h and then incubated with Aβ in DMEM.
Fig. 1B describes the laser irradiation protocol. Briefly, for the group with laser pretreatment and Aβ treatment, astrocytes in 35mm dishes and DMEM with phenol red and 10% FBS were pretreated with laser for 3 h. After allowing cells to rest for another 4 h, cells were serum starved for 4 h by replacing DMEM containing 10% FBS with serum-free DMEM. Cells were then incubated with Aβ (5μM) for 2 h for measuring phosphorylation of cPLA2, superoxide anion production, and colocalization between p47phox and gp91phox, and for 16 h for measuring IL-1β and iNOS. For control group, astrocytes were starved for 4 h and incubated in serum-free DMEM. For the group with Aβ treatment alone, astrocytes were starved for 4 h and then incubated with Aβ in a CO2-incubator without laser installed. For the group with treatment with NADPH oxidase inhibitor (gp91 ds-tat Peptide 2), the inhibitor (1μM) was added into culture medium 1 h before Aβ treatment.
Reactive oxygen species measurement
To quantify superoxide anion production induced by Aβ in astrocytes, cells were starved for 4 h, followed by incubation of cells with both Aβ (5μM) and DHE (20μM) for 2 h. DHE is a cell permeable fluorescent probe. Upon oxidation by superoxide anion, it binds to DNA in nuclei and becomes highly fluorescent (Chapman et al., 2005). Fluorescent intensity measurement of DHE was performed at room temperature using a Nikon TE-2000 U fluorescence microscope with a 20X objective lens. Images were acquired using a CCD camera controlled by a computer running MetaVue imaging software (Universal Imaging, PA). The fluorescent intensity of DHE per cell was measured. Background subtraction was done for all images prior to data analysis.
Confocal immunofluorescence microscopy
After treatments, cells were fixed with 4% paraformaldehyde at 37°C for 30 min. PBS containing 5% BSA was then applied to cells for 1 h to block nonspecific bindings. To label gp91phox at the cell surface, goat polyclonal anti-gp91phox (1:200 dilution) was added and incubated at 4°C without permeabilization. To label p47phox, cells were then permeabilized with 0.1% Triton X-100 in PBS for 5 min. Rabbit polyclonal anti-p47phox (1:200 dilution) in PBS with 1% BSA was then added and incubated at 4°C overnight. This was followed by fluorescent labeling with secondary antibodies (1:500 dilution) at room temperature for 1 h. The secondary antibodies for gp91phox and p47phox were fluorescein-donkey anti-goat antibody, and Texas Red-sheep anti-rabbit antibody, respectively. Secondary antibodies did not show immunostaining in the absence of the primary antibody (data not shown). Confocal immunofluorescence microscopy was performed with an Olympus FV1000 confocal inverted microscope (Tokyo, Japan). Confocal images were acquired with a 60X, numerical aperture 1.2 oil immersion objective lens for colocalization studies between NADPH oxidase subunits, gp91phox and p47phox. Background subtraction was done for all images before analysis. Colocalization images were obtained by suppressing all colors, except yellow, in superimposed images using Adobe Photoshop (Adobe Systems, San Jose, CA). The colocalization of p47phox with gp91phox was quantified by normalizing the intensity of yellow by the intensity of gp91phox.
Western blot analysis
After treatments, the total protein concentration of cell lysate was determined by BCA (bicinchoninic acid) protein assay kit (Pierce Biotechnology, Rockford, IL) according to manufacture's instruction. Equivalent amounts of protein from each sample (e.g., 40 μg) were diluted with Laemmli buffer, boiled for 5 min, subjected to electrophoresis in 7.5% SDS-polyacrylamide gels, and transferred to nitrocellulose membranes. Membranes were blocked for 1 h with 5% (w/v) nonfat dry milk in Tris-buffered saline containing 0.1% (v/v) Tween 20 (TBST) and were incubated overnight at 4°C in 3% (w/v) BSA with 0.02% (w/v) sodium azide in TBST with p-cPLA2 or cPLA2 antibodies (1:1000 dilution; Cell Signaling Technology, Beverly, MA), anti-IL-1β antibody (1:1000 dilution; R&D Systems, Minneapolis, MN), anti-iNOS antibody (1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) and β-actin antibody (Sigma, St. Louis, MO). Membranes were washed three times during a 15-min period with TBST and incubated with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG antibody (1: 5000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) in 5% (w/v) nonfat dry milk in TBST at room temperature for 1 h. After washing with TBST for three times, the membrane was subjected to SuperSignal West Pico Chemiluminescent detection reagents from Pierce (Rockford, IL) to visualize bands. The protein bands detected on x-ray film were quantified using a computer-driven scanner and Quantity One software (Bio-Rad, Hercules, CA).
Statistical analysis
Data were presented as mean ± SD from at least three independent experiments. Analysis was carried out with one-way ANOVA, followed by Bonferroni's post hoc tests. Values of p< 0.05 are considered to be statistically significant.
RESULTS
Laser light suppressed Aβ-induced superoxide production in primary astrocytes
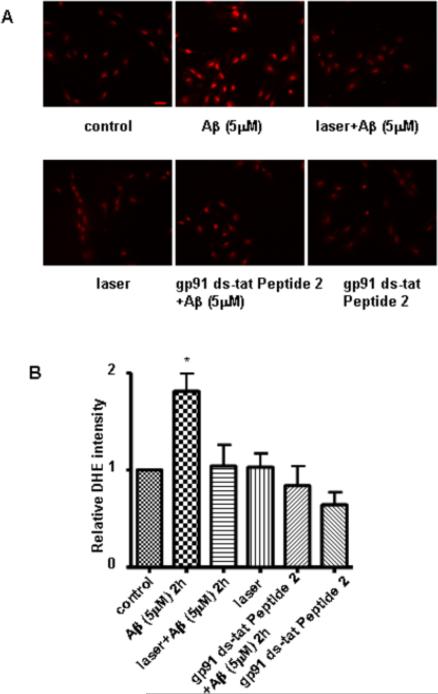
In order to investigate the effects of laser light on Aβ-induced superoxide production, primary rat cortical astrocytes were fluorescently labeled with DHE. We found that stimulation of astrocytes by Aβ for 2 h led to an increase in superoxide anions, as indicated by a significant increase in DHE fluorescent intensity, whereas pretreatment of cells with laser abrogated an Aβ-mediated increase in superoxide anions (Fig. 2). Astrocytes exposed to laser without Aβ treatment showed no effect on DHE intensity (Fig. 2). To verify this technique of measurement for superoxide anions, we demonstrated that NADPH oxidase inhibitor (gp91 ds-tat Peptide 2) suppressed Aβ-mediated increase in DHE intensity. The inhibitor alone had no effect on DHE intensity. In sum, Aβ-induced ROS production in astrocytes through NADPH oxidase activation was suppressed by laser irradiation.
Fig. 2. Laser (632.8nm) suppressed Aβ-induced ROS production in astrocytes.
A: Representative images of astrocytes labeled with dihydroethidium (DHE). Compared to control (A, upper, left), Aβ increased superoxide production (A, upper, middle). Pretreatment with laser suppressed superoxide production induced by Aβ (A, upper, right), but laser alone did not induce superoxide production (A, lower, left). gp91 ds-tat Peptide 2 (NADPH oxidase inhibitor) suppressed Aβ-induced superoxide production in astrocytes (A, lower, middle), but the inhibitor alone did not induce superoxide production (A, lower, right). Scale bar: 40 μm. B: Quantitative analysis of DHE intensity shows that laser (632.8 nm) suppressed Aβ-induced superoxide production in astrocytes. Pretreatment with laser without Aβ treatment did not affect DHE intensity. gp91 ds-tat Peptide 2 suppressed Aβ-induced increase in DHE intensity, but the inhibitor alone did not affect DHE intensity. Data are expressed as percentages of control and mean ± SD from four independent experiments with three replicates per experiment (* p< 0.05).
Laser light suppressed Aβ-induced assembly of NADPH oxidase subunits in primary astrocytes
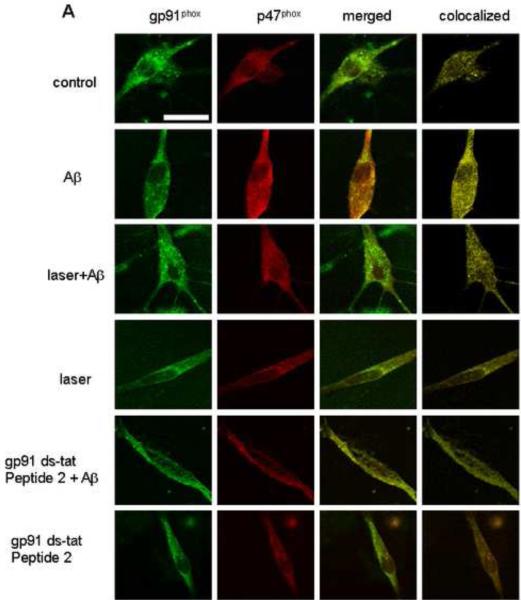
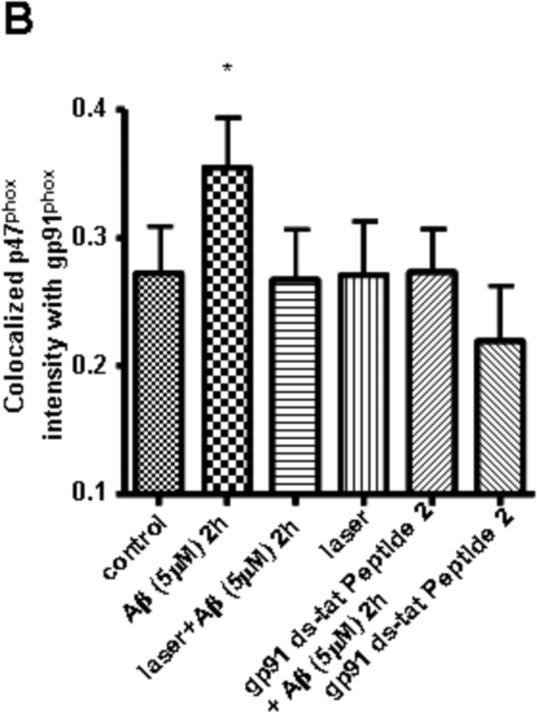
Since ROS production through NADPH oxidase is preceded by translocation of cytosolic subunits to bind with membrane subunits, we tested the effects of laser on colocalization between cytosolic p47phox and membrane-associated gp91phox after treating astrocytes with Aβ. Confocal immunofluorescence microscopy of gp91phox and p47phox subunits showed that stimulation of astrocytes by Aβ for 2 h led to a significant increase in colocalization between these two subunits, while laser pretreatment suppressed the colocalization to the control level (Fig. 3). These results suggest that laser attenuates Aβ-induced NADPH oxidase activation by inhibiting the assembly of its subunits. Astrocytes exposed to laser without Aβ treatment showed no effect on colocalization (Fig. 3). To validate the fluorescent confocal microscopy method for measurement of the colocalization between these two subunits, we demonstrated that NADPH oxidase inhibitor (gp91 ds-tat Peptide 2) suppressed Aβ-mediated increase in colocalization (Fig. 3). The inhibitor alone had no effect on the colocalization. These data suggest that laser attenuates Aβ-induced ROS production through inhibiting NADPH oxidase activation.
Fig. 3. Laser (632.8nm) suppressed Aβ-induced colocalization between NADPH oxidase subunits gp91phox and p47phox in astrocytes.
A: Representative confocal images of gp91phox (green) and p47phox (red) in astrocytes. The images of colocalization (yellow) were obtained by superimposing confocal images of corresponding p47phox (red) and gp91phox (green) followed by suppressing all colors except yellow. Scale bar: 10 μm. B: Quantitative analysis of colocalization between gp91phox and p47phox shows that laser (632.8 nm) suppressed Aβ-induced colocalization. Pretreatment with laser without Aβ treatment did not affect colocalization. gp91 ds-tat Peptide 2 (NADPH oxidase inhibitor) suppressed Aβ-induced colocalization, but the inhibitor alone did not affect colocalization. Around 120 cells were used to do quantification for each group. Data are expressed as percentages of control and mean ± SD from four independent experiments with three replicates per experiment (* p< 0.05).
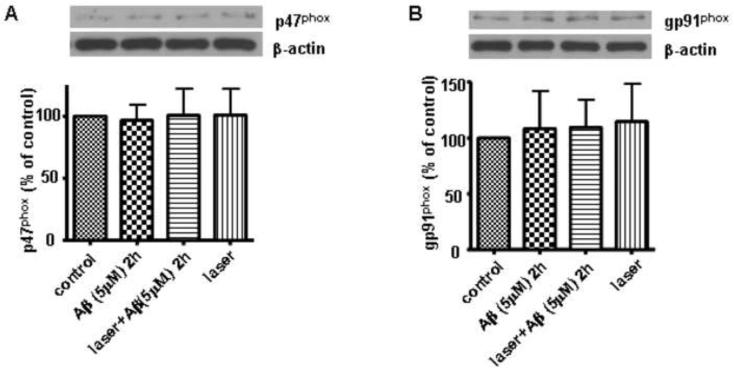
To rule out the possibility that laser and Aβ might have affected the expressions of p47phox and gp91phox, which may also cause changes in the colocalization between p47phox and gp91phox, Western blot analysis showed that laser and Aβ treatments did not change the total expressions of p47phox and gp91phox in astrocytes (Fig. 4).
Fig. 4. Laser (632.8nm) and Aβ did not alter the expression of p47phox and gp91phox in astrocytes.
A: Western blot analysis shows that laser (632.8 nm) and Aβ did not alter the expression of p47phox in astrocytes. B: Western blot analysis shows that laser (632.8 nm) and Aβ did not alter the expression of gp91phox in astrocytes. Data are expressed as percentages of control and mean ± SD from three independent experiments with three replicates per experiment.
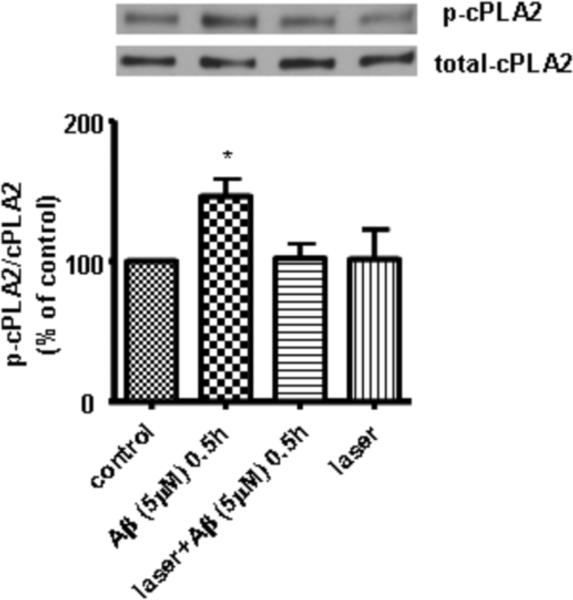
Laser suppressed Aβ-induced phosphorylation of cPLA2 in primary astrocytes
ROS from NADPH oxidase can stimulate downstream signaling pathways including activation of MAPK which further leads to phosphorylation of cPLA2 (Shelat et al., 2008). p-cPLA2 targets mitochondria and triggers further ROS production from mitochondria (Zhu et al., 2006). Since our data suggested that laser suppressed Aβ-induced ROS production through inhibiting NADPH oxidase activation, we hypothesized that laser also suppresses Aβ-induced phosphorylation of cPLA2. In support of our hypothesis, Western blot analysis of p-cPLA2 and cPLA2 showed that stimulation of astrocytes by Aβ for 30 min led to a significant increase in phosphorylation of cPLA2, while laser pretreatment suppressed the phosphorylation to the control level (Fig. 5). Astrocytes exposed to laser without Aβ treatment exerted no effect on phosphorylation of cPLA2 (Fig. 5).
Fig. 5. Laser (632.8nm) suppressed Aβ-induced phosphorylation of cPLA2 in astrocytes.
Western blot analysis shows that laser (632.8 nm) suppressed Aβ-induced phosphorylation of cPLA2 in astrocytes. Data are expressed as percentages of control and mean ± SD from four independent experiments with three replicates per experiment (* p< 0.05).
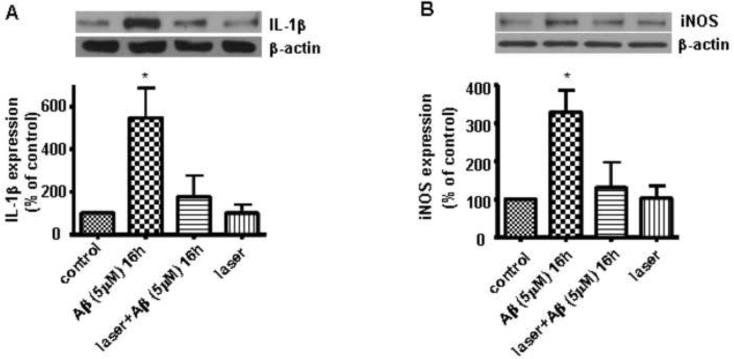
Laser suppressed Aβ-induced expression of pro-inflammatory factors in primary astrocytes
AD is associated with increased inflammatory responses (Griffin et al., 1998), such as increase in IL-1β (Zhu and Qian, 2006). Since there is evidence that low-level light can offer anti-inflammatory effects (Sakurai et al., 2000, Freitas et al., 2001, Lim et al., 2007, Aimbire et al., 2008, Boschi et al., 2008, Hammer et al., 2008), we tested whether laser was capable of suppressing Aβ-induced inflammation in astrocytes. Western blot analyses of IL-1β and iNOS showed that stimulation of astrocytes by Aβ for 16 h led to a significant increase in the expressions of IL-1β and iNOS, while laser pretreatment suppressed the expressions to the control level (Fig. 6). Astrocytes exposed to laser without Aβ treatment showed that laser itself had no effect on expressions of these inflammatory factors (Fig. 6).
Fig. 6. Laser (632.8nm) suppressed Aβ-induced expressions of IL-1β and iNOS in astrocytes.
A: Western blot analysis shows that laser (632.8 nm) suppressed Aβ-induced expression of IL-1β in astrocytes. B: Western blot analysis shows that laser (632.8 nm) suppressed Aβ-induced expression of iNOS in astrocytes. Data are expressed as percentages of control and mean ± SD from four independent experiments with three replicates per experiment (* p< 0.05).
DISCUSSION
The present study demonstrated, for the first time, the ability of laser light at λ = 632.8 nm to suppress Aβ-induced ROS production and inflammatory response in primary rat astrocytes. Specifically, we demonstrated the ability of this laser to suppress Aβ-induced ROS production, colocalization between NADPH oxidase subunits gp91phox and p47phox, phosphorylation of cPLA2, and expression of pro-inflammatory factors including IL-1β and iNOS.
AD is a prominent neurodegenerative disease affecting a large proportion of the aging population (Hebert et al., 2003). Although oxidative stress has been implicated in the progression of this disease, the mechanism for production of ROS has not been clearly elucidated (Gupta et al., 1991, Smith et al., 1991, Behl et al., 1994, Mecocci et al., 1994, Yan et al., 1994, Cini and Moretti, 1995, Forster et al., 1996, Simonian and Coyle, 1996, Smith et al., 1996, Mecocci et al., 1997, Behl and Holsboer, 1998, Schippling et al., 2000, Hamilton et al., 2001, Lovell and Markesbery, 2007). Our previous studies have shown that Aβ triggered NADPH oxidase activation to induce ROS and subsequently phosphorylated cPLA2 in primary astrocytes (Zhu et al., 2006). Furthermore, cPLA2 was shown to cause a decrease in mitochondrial membrane potential (ΔΨm) and result in more ROS production (Zhu et al., 2006). Interestingly, low-level laser (λ=635nm) has been reported to increase ΔΨm and ATP synthesis (Bortoletto et al., 2004). Another study showed that low-level infrared laser protected PC12 cells against oxidative stress through its ability to modulate ΔΨm (Giuliani et al., 2009). In fact, low-level laser therapy has been reported to prevent oxidative stress in rat traumatized Achilles tendon (Fillipin et al., 2005) and Duchenne muscular dystrophy patients (Abdel et al., 2007). Our data showed that 632.8 nm laser suppressed Aβ-induced ROS production (Fig. 1) through inhibiting colocalization of NADPH oxidase subunits, gp91phox and p47phox (Fig. 2). It is known that the interactions of gp91phox with the cytosolic components result in a conformational change in gp91phox, which enables the electron flow from NADPH to oxygen and the generation of superoxide anions (Han et al., 1998, Diebold and Bokoch, 2001). In this study, our results lead to a new hypothesis that low-energy laser light may be capable of causing a conformation change in gp91phox, which inhibits the interaction of gp91phox with the cytosolic components and the generation of superoxide anions. More investigations are needed to further our understanding in the mechanism of inhibiting the assembling of gp91phox with the cytosolic components by low-energy laser light. Consistent with previous studies reporting that phosphorylation of cPLA2 is one of the downstream pathways of NADPH oxidase (Zhu et al., 2006, Shelat et al., 2008), our data also showed that Aβ-induced p-cPLA2 was suppressed by laser pretreatment (Fig. 3). Since cPLA2 is associated with perturbation of mitochondria ΔΨm, results from this study suggested that laser pretreatment reduced ROS from NADPH oxidase and subsequently ROS-induced mitochondrial dysfunction through cPLA2.
Inflammation is an early event of AD and plays critical roles in disease development (Tuppo and Arias, 2005, Wyss-Coray, 2006, Cameron and Landreth, 2010, McNaull et al., 2010). The major players involved in the inflammatory process in AD are microglia and astrocytes (McGeer and McGeer, 1995, 2001, Combs, 2009). In AD, Aβ plaques are surrounded by activated microglia and astrocytes (Wallace et al., 1997, Yin et al., 2006, Bolmont et al., 2008, Koenigsknecht-Talboo et al., 2008, Yan et al., 2009) which have been shown to secrete many pro-inflammatory molecules, such as cytokines, chemokines, prostaglandins, complement proteins and complement inhibitors (McGeer and McGeer, 1995, 2001, Zhang et al., 2009). Among these pro-inflammatory molecules, IL-1β is an important regulator of inflammatory cascades and plays a key role in AD pathogenesis (Griffin and Mrak, 2002). IL-1β is capable of stimulating astrocytes to produce additional pro-inflammatory cytokines such as IL-6, another inflammation marker associated with neurodegeneration (Blom et al., 1997, Fiebich et al., 1998, Griffin et al., 1998). Furthermore, IL-1β triggers activation of NFκB (Cao et al., 1996, Song et al., 1997, Baeuerle, 1998) which is necessary for Aβ-stimulated iNOS induction in astrocytes (Akama et al., 1998, Akama and Van Eldik, 2000). iNOS is responsible for production of nitric oxide (Lee et al., 1993, Weldon et al., 1997) which may be detrimental to neurons (Hu et al., 1997). Low-level laser attenuates inflammation (Sakurai et al., 2000, Freitas et al., 2001, Lim et al., 2007, Aimbire et al., 2008, Boschi et al., 2008, Hammer et al., 2008) and helps tissue repair and wound healing (Whelan et al., 2001, Whelan et al., 2003, Albertini et al., 2007, Correa et al., 2007, Viegas et al., 2007, Reis et al., 2008). However, the effects of laser on Aβ-induced inflammation in astrocytes have not been studied. Here we showed that laser pretreatment suppressed Aβ-induced the expression of IL-1β in astrocytes (Fig. 4A). Consistent with the notion that Aβ stimulation of iNOS in astrocytes is IL-1β dependent (Akama and Van Eldik, 2000), laser also attenuated iNOS induction in astrocytes (Fig. 4B). Since IL-1β is a key player in AD pathogenesis and iNOS activity has detrimental effects on neurons, 632.8 nm laser may also protect neurons against inflammation in AD brain.
Previous studies suggest that low-energy light exerts beneficial effects through modulating gene expressions (Shefer et al., 2002, Eells et al., 2004). Brief treatment with 670 nm light-emitting diode (LED) regulates expression of genes encoding DNA repair protein, antioxidant enzymes and molecular chaperons in retinas of methanol intoxicated rats (Eells et al., 2004). Low-energy laser irradiation (632.8nm, 4.5mW, 3 s) also increases the expression of anti-apoptotic protein Bcl-2 and reduces the expression of pro-apoptotic protein Bax in muscle cell cultures (Shefer et al., 2002).
There has been a large body of compelling evidence suggesting that biological effects of lights could be used to treat neurodegenerative conditions, although the mechanism has yet to be fully understood. In vitro, LED attenuates apoptosis in PC12 cells after exposure to Aβ25–35 (Duan et al., 2003). LED also increases survival and ATP content of neurons and decreases oxidative stress after rotenone-induced toxicity (Liang et al., 2008). In fact, LED also promotes neurite outgrowth of rat cortex in tissue culture (Wollman and Rochkind, 1998). In vivo, near-infrared light exerts neuroprotective effects against rotenone-induced neurotoxicity in rats (Rojas et al., 2008), and these effects was supported by assessing behavioral, morphological and neurochemical changes (Rojas et al., 2008). LED has been shown to induce central and peripheral nerve regeneration after trauma (Anders et al., 1993, Byrnes et al., 2005) and reduce neuroinflammation in rats (Byrnes et al., 2005). In this study, we demonstrated that 632.8 nm laser suppressed Aβ-induced ROS production and inflammation in primary astrocytes. Although we did not investigate the dose response by varying irradiance (W/cm2) and energy density (J/cm2), it is important to note that a biphasic dose response has been demonstrated many times in low level laser therapy research (Huang et al., 2009). These dose dependence studies applied irradiance ranging from 0.7 to 40 mW/cm2 and energy density ranging from 0.18 to 9 J/cm2. While the irradiance (1.5 mW/cm2) was applied in this study, the energy density (16.2 J/cm2) was outside the range of those in previous studies from others. Therefore, one might consider optimizing the use of light by applying various irradiance, and exposure time. Another parameter for optimization is the wavelength, because a longer wavelength is known for a deeper tissue penetration. For example, 810 nm LED light enhances mitochondrial metabolism (Trimmer et al., 2009), and transcranial infrared laser (805 nm) therapy has been reported to improve clinical rating scores after embolic strokes in rabbits (Lapchak et al., 2004). This study serves as a proof-of-principle to provide insights into potential applications of laser therapy for attenuating oxidative and inflammatory responses in AD.
ACKNOWLEDGMENTS
This work was supported by NIH Grants 1P01 AG18357 and 1R21 NS052385.
Abbreviations
- AD
Alzheimer's disease
- Aβ
amyloid β-peptide
- BCA
bicinchoninic acid
- BSA
Bovine serum albumin
- cPLA2
cytosolic phospholipase A2
- DHE
dihydroethidium
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethyl sulfoxide
- FBS
fetal bovine serum
- HFIP
hexafluoro-2-propanol
- IL-1β
interleukin-1β
- iNOS
inducible nitric-oxide synthase
- LED
light-emitting diode
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdel SE, Abdel-Meguid I, Korraa S. Markers of oxidative stress and aging in Duchene muscular dystrophy patients and the possible ameliorating effect of He:Ne laser. Acta Myol. 2007;26:14–21. [PMC free article] [PubMed] [Google Scholar]
- Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimbire F, Ligeiro de Oliveira AP, Albertini R, Correa JC, Ladeira de Campos CB, Lyon JP, Silva JA, Jr., Costa MS. Low level laser therapy (LLLT) decreases pulmonary microvascular leakage, neutrophil influx and IL-1beta levels in airway and lung from rat subjected to LPS-induced inflammation. Inflammation. 2008;31:189–197. doi: 10.1007/s10753-008-9064-4. [DOI] [PubMed] [Google Scholar]
- Akama KT, Albanese C, Pestell RG, Van Eldik LJ. Amyloid beta-peptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanism. Proc Natl Acad Sci U S A. 1998;95:5795–5800. doi: 10.1073/pnas.95.10.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama KT, Van Eldik LJ. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J Biol Chem. 2000;275:7918–7924. doi: 10.1074/jbc.275.11.7918. [DOI] [PubMed] [Google Scholar]
- Albertini R, Villaverde AB, Aimbire F, Salgado MA, Bjordal JM, Alves LP, Munin E, Costa MS. Anti-inflammatory effects of low-level laser therapy (LLLT) with two different red wavelengths (660 nm and 684 nm) in carrageenan-induced rat paw edema. J Photochem Photobiol B. 2007;89:50–55. doi: 10.1016/j.jphotobiol.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Anders JJ, Borke RC, Woolery SK, Van de Merwe WP. Low power laser irradiation alters the rate of regeneration of the rat facial nerve. Lasers Surg Med. 1993;13:72–82. doi: 10.1002/lsm.1900130113. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA. Pro-inflammatory signaling: last pieces in the NF-kappaB puzzle? Curr Biol. 1998;8:R19–22. doi: 10.1016/s0960-9822(98)70010-7. [DOI] [PubMed] [Google Scholar]
- Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Behl C, Holsboer F. Oxidative stress in the pathogenesis of Alzheimer's disease and antioxidant neuroprotection. Fortschr Neurol Psychiatr. 1998;66:113–121. doi: 10.1055/s-2007-995246. [DOI] [PubMed] [Google Scholar]
- Blom MA, van Twillert MG, de Vries SC, Engels F, Finch CE, Veerhuis R, Eikelenboom P. NSAIDS inhibit the IL-1 beta-induced IL-6 release from human post-mortem astrocytes: the involvement of prostaglandin E2. Brain Res. 1997;777:210–218. doi: 10.1016/s0006-8993(97)01204-3. [DOI] [PubMed] [Google Scholar]
- Bolmont T, Haiss F, Eicke D, Radde R, Mathis CA, Klunk WE, Kohsaka S, Jucker M, Calhoun ME. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci. 2008;28:4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoletto R, Silva NS, Zangaro RA, Pacheco MT, Da Matta RA, Pacheco-Soares C. Mitochondrial membrane potential after low-power laser irradiation. Lasers Med Sci. 2004;18:204–206. doi: 10.1007/s10103-003-0281-7. [DOI] [PubMed] [Google Scholar]
- Boschi ES, Leite CE, Saciura VC, Caberlon E, Lunardelli A, Bitencourt S, Melo DA, Oliveira JR. Anti-Inflammatory effects of low-level laser therapy (660 nm) in the early phase in carrageenan-induced pleurisy in rat. Lasers Surg Med. 2008;40:500–508. doi: 10.1002/lsm.20658. [DOI] [PubMed] [Google Scholar]
- Byrnes KR, Waynant RW, Ilev IK, Wu X, Barna L, Smith K, Heckert R, Gerst H, Anders JJ. Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg Med. 2005;36:171–185. doi: 10.1002/lsm.20143. [DOI] [PubMed] [Google Scholar]
- Cameron B, Landreth GE. Inflammation, microglia, and Alzheimer's disease. Neurobiol Dis. 2010;37:503–509. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- Chapman KE, Sinclair SE, Zhuang D, Hassid A, Desai LP, Waters CM. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L834–841. doi: 10.1152/ajplung.00069.2005. [DOI] [PubMed] [Google Scholar]
- Cini M, Moretti A. Studies on lipid peroxidation and protein oxidation in the aging brain. Neurobiol Aging. 1995;16:53–57. doi: 10.1016/0197-4580(95)80007-e. [DOI] [PubMed] [Google Scholar]
- Combs CK. Inflammation and microglia actions in Alzheimer's disease. J Neuroimmune Pharmacol. 2009;4:380–388. doi: 10.1007/s11481-009-9165-3. [DOI] [PubMed] [Google Scholar]
- Correa F, Lopes Martins RA, Correa JC, Iversen VV, Joenson J, Bjordal JM. Low-level laser therapy (GaAs lambda = 904 nm) reduces inflammatory cell migration in mice with lipopolysaccharide-induced peritonitis. Photomed Laser Surg. 2007;25:245–249. doi: 10.1089/pho.2007.2079. [DOI] [PubMed] [Google Scholar]
- Diebold BA, Bokoch GM. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat Immunol. 2001;2:211–215. doi: 10.1038/85259. [DOI] [PubMed] [Google Scholar]
- Duan R, Zhu L, Liu TC, Li Y, Liu J, Jiao J, Xu X, Yao L, Liu S. Light emitting diode irradiation protect against the amyloid beta 25–35 induced apoptosis of PC12 cell in vitro. Lasers Surg Med. 2003;33:199–203. doi: 10.1002/lsm.10216. [DOI] [PubMed] [Google Scholar]
- Eells JT, Wong-Riley MT, VerHoeve J, Henry M, Buchman EV, Kane MP, Gould LJ, Das R, Jett M, Hodgson BD, Margolis D, Whelan HT. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4:559–567. doi: 10.1016/j.mito.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Fiebich BL, Hull M, Lieb K, Schumann G, Berger M, Bauer J. Potential link between interleukin-6 and arachidonic acid metabolism in Alzheimer's disease. J Neural Transm Suppl. 1998;54:268–278. [PubMed] [Google Scholar]
- Fillipin LI, Mauriz JL, Vedovelli K, Moreira AJ, Zettler CG, Lech O, Marroni NP, Gonzalez-Gallego J. Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med. 2005;37:293–300. doi: 10.1002/lsm.20225. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas AC, Pinheiro AL, Miranda P, Thiers FA, Vieira AL. Assessment of anti-inflammatory effect of 830nm laser light using C-reactive protein levels. Braz Dent J. 2001;12:187–190. [PubMed] [Google Scholar]
- Giuliani A, Lorenzini L, Gallamini M, Massella A, Giardino L, Calza L. Low infra red laser light irradiation on cultured neural cells: effects on mitochondria and cell viability after oxidative stress. BMC Complement Altern Med. 2009;9:8. doi: 10.1186/1472-6882-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WS, Mrak RE. Interleukin-1 in the genesis and progression of and risk for development of neuronal degeneration in Alzheimer's disease. J Leukoc Biol. 2002;72:233–238. [PMC free article] [PubMed] [Google Scholar]
- Griffin WS, Sheng JG, Royston MC, Gentleman SM, McKenzie JE, Graham DI, Roberts GW, Mrak RE. Glial-neuronal interactions in Alzheimer's disease: the potential role of a `cytokine cycle' in disease progression. Brain Pathol. 1998;8:65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Hasan M, Chander R, Kapoor NK. Age-related elevation of lipid peroxidation products: diminution of superoxide dismutase activity in the central nervous system of rats. Gerontology. 1991;37:305–309. doi: 10.1159/000213277. [DOI] [PubMed] [Google Scholar]
- Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci U S A. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer KD, Hillwig ML, Neighbors JD, Sim YJ, Kohut ML, Wiemer DF, Wurtele ES, Birt DF. Pseudohypericin is necessary for the light-activated inhibition of prostaglandin E2 pathways by a 4 component system mimicking an Hypericum perforatum fraction. Phytochemistry. 2008;69:2354–2362. doi: 10.1016/j.phytochem.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CH, Freeman JL, Lee T, Motalebi SA, Lambeth JD. Regulation of the neutrophil respiratory burst oxidase. Identification of an activation domain in p67(phox) J Biol Chem. 1998;273:16663–16668. doi: 10.1074/jbc.273.27.16663. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Heneka MT, O'Banion MK. Inflammatory processes in Alzheimer's disease. J Neuroimmunol. 2007;184:69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Ferreira A, Van Eldik LJ. S100beta induces neuronal cell death through nitric oxide release from astrocytes. J Neurochem. 1997;69:2294–2301. doi: 10.1046/j.1471-4159.1997.69062294.x. [DOI] [PubMed] [Google Scholar]
- Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J, Furnkranz A, Bochkov VN, Patricia MK, Lee H, Hedrick CC, Berliner JA, Binder BR, Leitinger N. Specific monocyte adhesion to endothelial cells induced by oxidized phospholipids involves activation of cPLA2 and lipoxygenase. J Lipid Res. 2006;47:1054–1062. doi: 10.1194/jlr.M500555-JLR200. [DOI] [PubMed] [Google Scholar]
- Karageuzyan KG, Sekoyan ES, Karagyan AT, Pogosyan NR, Manucharyan GG, Sekoyan AE, Tunyan AY, Boyajyan VG, Karageuzyan MK. Phospholipid pool, lipid peroxidation, and superoxide dismutase activity under various types of oxidative stress of the brain and the effect of low-energy infrared laser irradiation. Biochemistry (Mosc) 1998;63:1226–1232. [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J, Meyer-Luehmann M, Parsadanian M, Garcia-Alloza M, Finn MB, Hyman BT, Bacskai BJ, Holtzman DM. Rapid microglial response around amyloid pathology after systemic anti-Abeta antibody administration in PDAPP mice. J Neurosci. 2008;28:14156–14164. doi: 10.1523/JNEUROSCI.4147-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Wei J, Zivin JA. Transcranial infrared laser therapy improves clinical rating scores after embolic strokes in rabbits. Stroke. 2004;35:1985–1988. doi: 10.1161/01.STR.0000131808.69640.b7. [DOI] [PubMed] [Google Scholar]
- Lee SC, Dickson DW, Liu W, Brosnan CF. Induction of nitric oxide synthase activity in human astrocytes by interleukin-1 beta and interferon-gamma. J Neuroimmunol. 1993;46:19–24. doi: 10.1016/0165-5728(93)90229-r. [DOI] [PubMed] [Google Scholar]
- Liang HL, Whelan HT, Eells JT, Meng H, Buchmann E, Lerch-Gaggl A, Wong-Riley M. Photobiomodulation partially rescues visual cortical neurons from cyanide-induced apoptosis. Neuroscience. 2006;139:639–649. doi: 10.1016/j.neuroscience.2005.12.047. [DOI] [PubMed] [Google Scholar]
- Liang HL, Whelan HT, Eells JT, Wong-Riley MT. Near-infrared light via light-emitting diode treatment is therapeutic against rotenone- and 1-methyl-4-phenylpyridinium ion-induced neurotoxicity. Neuroscience. 2008;153:963–974. doi: 10.1016/j.neuroscience.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W, Lee S, Kim I, Chung M, Kim M, Lim H, Park J, Kim O, Choi H. The anti-inflammatory mechanism of 635 nm light-emitting-diode irradiation compared with existing COX inhibitors. Lasers Surg Med. 2007;39:614–621. doi: 10.1002/lsm.20533. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic Acids Res. 2007;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation, autotoxicity and Alzheimer disease. Neurobiol Aging. 2001;22:799–809. doi: 10.1016/s0197-4580(01)00289-5. [DOI] [PubMed] [Google Scholar]
- McNaull BB, Todd S, McGuinness B, Passmore AP. Inflammation and anti-inflammatory strategies for Alzheimer's disease--a mini-review. Gerontology. 2010;56:3–14. doi: 10.1159/000237873. [DOI] [PubMed] [Google Scholar]
- Mecocci P, Beal MF, Cecchetti R, Polidori MC, Cherubini A, Chionne F, Avellini L, Romano G, Senin U. Mitochondrial membrane fluidity and oxidative damage to mitochondrial DNA in aged and AD human brain. Mol Chem Neuropathol. 1997;31:53–64. doi: 10.1007/BF02815160. [DOI] [PubMed] [Google Scholar]
- Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer's disease. Ann Neurol. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- Mizrahi A, Berdichevsky Y, Ugolev Y, Molshanski-Mor S, Nakash Y, Dahan I, Alloul N, Gorzalczany Y, Sarfstein R, Hirshberg M, Pick E. Assembly of the phagocyte NADPH oxidase complex: chimeric constructs derived from the cytosolic components as tools for exploring structure-function relationships. J Leukoc Biol. 2006;79:881–895. doi: 10.1189/jlb.1005553. [DOI] [PubMed] [Google Scholar]
- Qin L, Liu Y, Cooper C, Liu B, Wilson B, Hong JS. Microglia enhance beta-amyloid peptide-induced toxicity in cortical and mesencephalic neurons by producing reactive oxygen species. J Neurochem. 2002;83:973–983. doi: 10.1046/j.1471-4159.2002.01210.x. [DOI] [PubMed] [Google Scholar]
- Reis SR, Medrado AP, Marchionni AM, Figueira C, Fracassi LD, Knop LA. Effect of 670-nm laser therapy and dexamethasone on tissue repair: a histological and ultrastructural study. Photomed Laser Surg. 2008;26:307–313. doi: 10.1089/pho.2007.2151. [DOI] [PubMed] [Google Scholar]
- Rojas JC, Lee J, John JM, Gonzalez-Lima F. Neuroprotective effects of near-infrared light in an in vivo model of mitochondrial optic neuropathy. J Neurosci. 2008;28:13511–13521. doi: 10.1523/JNEUROSCI.3457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y, Yamaguchi M, Abiko Y. Inhibitory effect of low-level laser irradiation on LPS-stimulated prostaglandin E2 production and cyclooxygenase-2 in human gingival fibroblasts. Eur J Oral Sci. 2000;108:29–34. doi: 10.1034/j.1600-0722.2000.00783.x. [DOI] [PubMed] [Google Scholar]
- Schippling S, Kontush A, Arlt S, Buhmann C, Sturenburg HJ, Mann U, Muller-Thomsen T, Beisiegel U. Increased lipoprotein oxidation in Alzheimer's disease. Free Radic Biol Med. 2000;28:351–360. doi: 10.1016/s0891-5849(99)00247-6. [DOI] [PubMed] [Google Scholar]
- Shefer G, Partridge TA, Heslop L, Gross JG, Oron U, Halevy O. Low-energy laser irradiation promotes the survival and cell cycle entry of skeletal muscle satellite cells. J Cell Sci. 2002;115:1461–1469. doi: 10.1242/jcs.115.7.1461. [DOI] [PubMed] [Google Scholar]
- Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, Sun AY, Simonyi A, Sun GY. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J Neurochem. 2008;106:45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Tanino H, Kawakami N, Okamura N, Kodama H, Yamaguchi T, Hayakawa T, Nunomura A, Chiba S, Perry G, Smith MA, Fujimoto S. Activation of NADPH oxidase in Alzheimer's disease brains. Biochem Biophys Res Commun. 2000;273:5–9. doi: 10.1006/bbrc.2000.2897. [DOI] [PubMed] [Google Scholar]
- Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer's. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- Song HY, Regnier CH, Kirschning CJ, Goeddel DV, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-kappaB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci U S A. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GY, Horrocks LA, Farooqui AA. The roles of NADPH oxidase and phospholipases A2 in oxidative and inflammatory responses in neurodegenerative diseases. J Neurochem. 2007;103:1–16. doi: 10.1111/j.1471-4159.2007.04670.x. [DOI] [PubMed] [Google Scholar]
- Trimmer PA, Schwartz KM, Borland MK, De Taboada L, Streeter J, Oron U. Reduced axonal transport in Parkinson's disease cybrid neurites is restored by light therapy. Mol Neurodegener. 2009;4:26. doi: 10.1186/1750-1326-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Viegas VN, Abreu ME, Viezzer C, Machado DC, Filho MS, Silva DN, Pagnoncelli RM. Effect of low-level laser therapy on inflammatory reactions during wound healing: comparison with meloxicam. Photomed Laser Surg. 2007;25:467–473. doi: 10.1089/pho.2007.1098. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Geddes JG, Farquhar DA, Masson MR. Nitric oxide synthase in reactive astrocytes adjacent to beta-amyloid plaques. Exp Neurol. 1997;144:266–272. doi: 10.1006/exnr.1996.6373. [DOI] [PubMed] [Google Scholar]
- Weldon DT, Maggio JE, Mantyh PW. New insights into the neuropathology and cell biology of Alzheimer's disease. Geriatrics. 1997;52(Suppl 2):S13–16. [PubMed] [Google Scholar]
- Whelan HT, Buchmann EV, Dhokalia A, Kane MP, Whelan NT, Wong-Riley MT, Eells JT, Gould LJ, Hammamieh R, Das R, Jett M. Effect of NASA light-emitting diode irradiation on molecular changes for wound healing in diabetic mice. J Clin Laser Med Surg. 2003;21:67–74. doi: 10.1089/104454703765035484. [DOI] [PubMed] [Google Scholar]
- Whelan HT, Smits RL, Jr., Buchman EV, Whelan NT, Turner SG, Margolis DA, Cevenini V, Stinson H, Ignatius R, Martin T, Cwiklinski J, Philippi AF, Graf WR, Hodgson B, Gould L, Kane M, Chen G, Caviness J. Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg. 2001;19:305–314. doi: 10.1089/104454701753342758. [DOI] [PubMed] [Google Scholar]
- White JA, Manelli AM, Holmberg KH, Van Eldik LJ, Ladu MJ. Differential effects of oligomeric and fibrillar amyloid-beta 1–42 on astrocyte-mediated inflammation. Neurobiol Dis. 2005;18:459–465. doi: 10.1016/j.nbd.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Wollman Y, Rochkind S. In vitro cellular processes sprouting in cortex microexplants of adult rat brains induced by low power laser irradiation. Neurol Res. 1998;20:470–472. doi: 10.1080/01616412.1998.11740550. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Yan P, Bero AW, Cirrito JR, Xiao Q, Hu X, Wang Y, Gonzales E, Holtzman DM, Lee JM. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J Neurosci. 2009;29:10706–10714. doi: 10.1523/JNEUROSCI.2637-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SD, Chen X, Schmidt AM, Brett J, Godman G, Zou YS, Scott CW, Caputo C, Frappier T, Smith MA, et al. Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proc Natl Acad Sci U S A. 1994;91:7787–7791. doi: 10.1073/pnas.91.16.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin KJ, Cirrito JR, Yan P, Hu X, Xiao Q, Pan X, Bateman R, Song H, Hsu FF, Turk J, Xu J, Hsu CY, Mills JC, Holtzman DM, Lee JM. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J Neurosci. 2006;26:10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You HJ, Woo CH, Choi EY, Cho SH, Yoo YJ, Kim JH. Roles of Rac and p38 kinase in the activation of cytosolic phospholipase A2 in response to PMA. Biochem J. 2005;388:527–535. doi: 10.1042/BJ20041614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Hu X, Qian L, Wilson B, Lee C, Flood P, Langenbach R, Hong JS. Prostaglandin E2 released from activated microglia enhances astrocyte proliferation in vitro. Toxicol Appl Pharmacol. 2009;238:64–70. doi: 10.1016/j.taap.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Lai Y, Shelat PB, Hu C, Sun GY, Lee JC. Phospholipases A2 mediate amyloid-beta peptide-induced mitochondrial dysfunction. J Neurosci. 2006;26:11111–11119. doi: 10.1523/JNEUROSCI.3505-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Tan KS, Zhang X, Sun AY, Sun GY, Lee JC. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J Cell Sci. 2005;118:3695–3703. doi: 10.1242/jcs.02507. [DOI] [PubMed] [Google Scholar]
- Zhu F, Qian C. Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1beta and inducible nitric oxide synthase in the rat model of Alzheimer's disease. BMC Neurosci. 2006;7:78. doi: 10.1186/1471-2202-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]