Abstract
Biologic scaffold materials composed of mammalian extracellular matrix (ECM) are commonly used for the repair and reconstruction of injured tissues. An important, but unexplored variable of biologic scaffolds is the age of the animal from which the ECM is prepared. The objective of the present study was to compare the structural, mechanical, and compositional properties of small intestinal submucosa (SIS)-ECM harvested from pigs that differed only in age. Degradation product bioactivity of these ECM materials was also examined. Results showed that there are distinct differences in each of these variables among the various age source ECM scaffolds. The strength and growth factors content of ECM from 3 week old animals is less than that of ECM harvested from 12, 26 or >52 week old animals. The elastic modulus of SIS-ECM for 3 week and >52 week old source was less than that of the 12 and 26 week source. Degradation products from all age source ECMs were chemotactic for perivascular stem cells, with the 12 week source the most potent, while the oldest source caused the greatest increase in proliferation. In summary, distinct differences exist in the mechanical, structural, and biologic properties of SIS-ECM harvested from different aged animals.
Keywords: Age/ageing, ECM (extracellular matrix), Mechanical properties, Bioactivity, Growth factors, Stem cell
INTRODUCTION
Biologic scaffold materials composed of mammalian extracellular matrix (ECM) are commonly used for the repair and reconstruction of injured or missing tissues and organs [1]. The mechanical and material properties of ECM scaffolds and the host tissue response to these biomaterials has been shown to depend upon the tissue specificity of the ECM material [2–4], processing methods [5–8], hydration [9], three dimensional configuration [10], and terminal sterilization [11, 12]. The wide spectrum of clinical outcomes possible as a result of these factors has been recently reviewed for ECM scaffold materials commonly used for orthopedic applications [13].
Although the mechanisms of biologic scaffold remodeling and the associated outcomes are only partially understood, there is now convincing evidence that nascent growth factors [14, 15], surface topology and the distribution of surface ligands [16–19], modulation of the host innate immune response [5, 20], and microenvironmental cues including mechanical loading [21, 22] all contribute to the eventual functional outcome. It is generally believed that fetal wound healing results in less scar tissue formation and a more regenerative outcome than the adult response to tissue injury [23–25] and for this reason some biologic scaffolds use source materials that include fetal or neonatal cells [26, 27], or fetal ECM [28]. The effect of donor animal age upon the structural and material properties of the ECM scaffolds produced from these tissues has not been systematically examined.
The objective of the present study is to compare selected structural, mechanical, and compositional properties of an ECM, specifically small intestinal submucosa ECM (SIS-ECM), harvested from pigs that differ only in age. We also investigate the response of select stem cells to the degradation products of these ECM materials.
MATERIALS AND METHODS
Overview of Experimental Design
SIS-ECM was prepared from the jejunum of pigs of 4 different ages: 3 weeks, 12 weeks, 26 weeks, and greater than 52 weeks. This SIS-ECM was subjected to mechanical and materials properties testing, analyzed for growth factor and glycosaminoglycan content, and evaluated for the mitogenic and chemotactic properties of the SIS-ECM degradation products.
Source and Preparation of ECM Material
The jejunum from Whiteshire Hamroc pigs of 4 distinctly different ages (3, 12, 26 and >52 weeks) were harvested immediately following euthanasia (Tissue Source, Lafayette, IN). The animals were of similar genetic heritage, and were raised and kept in identical husbandry conditions including diet and vaccination history. All tissues (intestines) were harvested on the same day and stored on ice prior to processing to create SIS-ECM as previously described [10, 29]. Briefly, the intestines were rinsed with water and the mesenteric tissues were removed. The intestines were split longitudinally and mechanically delaminated to remove the tunica serosa, tunica muscularis externa, and the luminal portion of the tunica mucosa including most of the lamina propria. After delamination, the tunica submucosa and the basilar layer of the tunica mucosa including the muscularis mucosa and the stratum compactum of the lamina propria remained. The material was decellularized with 0.1% peracetic acid (Rochester Midland Corporation, Rochester, NY) followed by multiple rinses with saline and deionized water, and referred to as SIS-ECM. The SIS-ECM material was then frozen and/or lyophilized.
Preparation of SIS-ECM Degradation Products
SIS-ECM from the four age sources was powdered and digested at 10 mg/mL dry weight with 1 mg/mL pepsin (Sigma, St. Louis, MO) in 0.01N HCl for 48 h at 22°C before dialysis at 4°C in phosphate buffered saline using Slide-A-Lyzer Dialysis Cassettes, 3500 MWCO (Pierce, Rockford, IL). Protein concentration was quantified using the BCA protein assay (Thermo, Waltham, MA) against bovine serum albumin. The resultant material was referred to as SIS-ECM degradation products.
Source of Cells and Culture Conditions
Perivascular stem cells were isolated by flow cytometry from human fetal muscle on the basis of expression of CD146 but not CD34, CD45 or CD56 [30]. Isolated cells were cultured in high glucose DMEM (Invitrogen, Carlsbad, CA.) growth medium containing 20% fetal bovine serum (Thermo), 100 U/mL penicillin and 100 mg/mL streptomycin (Sigma) at 37°C in 5% CO2. These cells have been shown to have multipotent differentiation capacity and to be responsive to chemotactic and mitogenic properties of ECM degradation products [30, 31].
Mechanical Testing
Lyophilized SIS-ECM from animals aged 12, 26 or >52 weeks was cut into longitudinal dog bone specimens using an identical template (midsubstance of 40 mm by 8 mm). SIS-ECM from 3 week old animals was narrower than the template and specimens were manually cut to the same length:width ratio of 5 with a mean width of 7.1 +/− 0.6 mm. Specimens were rehydrated for at least 48 h, after which time the width and thickness were measured at three locations prior to uniaxial tensile testing. Specimens were secured to tester grips by placing screws through holes in each end of the specimen to prevent slipping. All specimens were subjected to 10 pre-load cycles at 25.4 mm/min (0.100–0.500 N for SIS-ECM from animals aged 12, 26 or >52 weeks or 0.010–0.050 N for SIS-ECM from 3 week old animals) and immediately tested to failure, also at 25.4 mm/min. Peak load per unit width was calculated using mean width. Ultimate tensile stress was calculated from the peak load, mean width, and mean thickness: σ=F/(w*t). Strain at failure was calculated by dividing the change in length by the original length: ε=ΔL/Lo. Elastic modulus was taken as the tangent modulus (slope of the stress-strain curve) in the range of 20–60% of the ultimate tensile stress: E=0.4*σ/(ε60%σ-ε20%σ). Fourteen samples from each age source were tested.
Thickness Measurement
SIS-ECM from each age source was measured for thickness by a blinded operator applying equivalent compressive force (8.5 +/− 0.5 N) to all samples. Thickness after rehydration was measured by subjecting the specimens to lyophilization and then fully rehydrating the samples by soaking for at least 48 h before measurement by the same operator in the same fashion. Fourteen samples were measured for each age source.
Collagenase Digestion Assay
SIS-ECM from each age source was cut into 1×1 cm squares. Type 1 collagenase (Invitrogen) at 50 U/mL in phosphate buffered saline was added to 5 mg/mL dry weight SIS-ECM and incubated at 37 °C. Samples of the digestion solution were collected hourly and assayed for protein via the BCA protein assay against bovine serum albumin. Statistical comparison was performed at the 5 h time point. The experiment was repeated three times.
Growth Factor Analysis
SIS-ECM from each age source was powdered and suspended at 66 mg/mL in urea-heparin extraction buffer (2 M urea, 5 mg/mL heparin, 50 mM Tris, pH 7.4 and protease inhibitors 1mM phenylmethylsulfonyl fluoride, 5 mM benzamidine, and 10 mM N-ethylmaleimide). The extraction mixture was rocked for 24 h at 4°C and then centrifuged at 12,000 g for 30 mins at 4°C. Supernatants were collected and 6 mL of freshly prepared urea-heparin extraction buffer was added to each pellet, re-incubated for 24 h at 4°C, re-centrifuged and supernatants collected. Supernatants were dialyzed against deionized water and protein concentration was quantified using the BCA protein assay against bovine serum albumin. Concentrations of basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) were determined with the Quantikine bFGF immunoassay and VEGF immunoassay (both R&D Systems, Minneapolis, MN) following the manufacturer’s instructions. Each assay was performed in duplicate and the experiment repeated four times.
Protein Solubilization and SDS-PAGE
Powdered material from each age source was boiled for 10 mins at 2mg material per mL RIPA buffer containing 0.1% SDS, 1% sodium deoxycholate, and 1% NP-40 (Thermo). Solubilized protein was quantified using the BCA protein assay and 3 μg of protein from each age source was resolved on a 7.5% SDS-PAGE gel before staining with Silver Stain Plus (Bio-Rad, Hercules, CA).
Glycosaminoglycan Analysis
Sulfated glycosaminoglycan (sGAG) concentration in SIS-ECM from the different age source was determined using the Blyscan sGAG assay kit (Biocolor Ltd., Carrickfergus, United Kingdom). Samples were prepared by digestion of 50 mg/mL dry weight of each sample with 0.1 mg/mL proteinase K in buffer (10 mM Tris-HCl, pH 8, 100 mM NaCl, 25 mM EDTA) for 48 h at 50°C. Digested samples were assayed following the manufacturer’s instructions. The assay was performed three times. The spatial distribution of GAGs within the materials was examined by histologic methods. SIS-ECM from each age source was fixed in buffered 10% formalin, embedded in paraffin and cut into 5 μm sections, before deparaffinization and staining with Alcian blue at pH 2.5.
Cell Proliferation Assay
Perivascular stem cell proliferation in response to the presence of SIS-ECM degradation products was evaluated via 5-bromo-2′-deoxyuridine (BrdU) ELISA (Roche, Nutley, NJ). The cells were plated at 5×104 cells per well in a standard 96 well plate with 0, 2, 10 or 25 μg/mL SIS-ECM degradation products and BrdU and labeled for 18 h. Relative proliferation was quantified at 370 nm and 492 nm in a plate reader (Molecular Devices, Sunnyvale, CA). The assay was performed in triplicate on three occasions.
Metabolic Activity Assay
Perivascular stem cells were plated at 5×104 cells per well in a standard 96 well plate in medium containing 10% alamarBlue (Biotium, Hayward, CA) and 0, 2 or 25 μg/mL SIS-ECM degradation products. Metabolic activity was quantified fluorescently (Ex. 560 nm/ Em. 590 nm) over a period of 7 h and compared statistically at 7h. Each assay was performed in triplicate on three separate occasions.
Cell Migration Assay
The chemotactic response of perivascular stem cells to SIS-ECM degradation products was quantitatively evaluated using Neuro Probe 48-well micro chambers (Neuro Probe Inc., Gaithersburg, MD). Cells to be assayed were starved for 18 h in media containing 0.5% heat-inactivated FBS. The starved cells were harvested and resuspended in serum free media at a concentration of 6×105 cells/mL, and pre-incubated for 1 h. Polycarbonate PFB chemotaxis filters with a 8 μm pore size (Neuro Probe, Gaithersburg, MD) were coated with 50 μg/mL collagen I (BD Biosciences, San Jose, CA). The migration of 3×104 cells toward 0 or 100 μg/mL of SIS-ECM degradation products was tested for 3 h. Migrated cells were stained with Diff Quik (Dade AG Liederbach, Germany), and 3 magnification fields (20× objective) were counted from each well. Experimental conditions were tested in quadruplicate wells and the average number of migrated cells was determined for each condition. The experiment was repeated six times.
Statistical Analysis
A one-way analysis of variance (ANOVA) was used to determine any differences between groups for each assay with a Tukey’s post hoc test used to determine differences between pairs, with p<0.05 considered significant. For mechanical properties testing, data set outliers (no more than one value per group) were excluded when greater than two standard deviations away from the mean. All statistical analysis used SPSS Statistical Analysis Software (SPSS, IBM, Chicago, IL, USA).
RESULTS
Physical Characterization of SIS-ECM from Four Different Age Sources
Mechanical Analysis
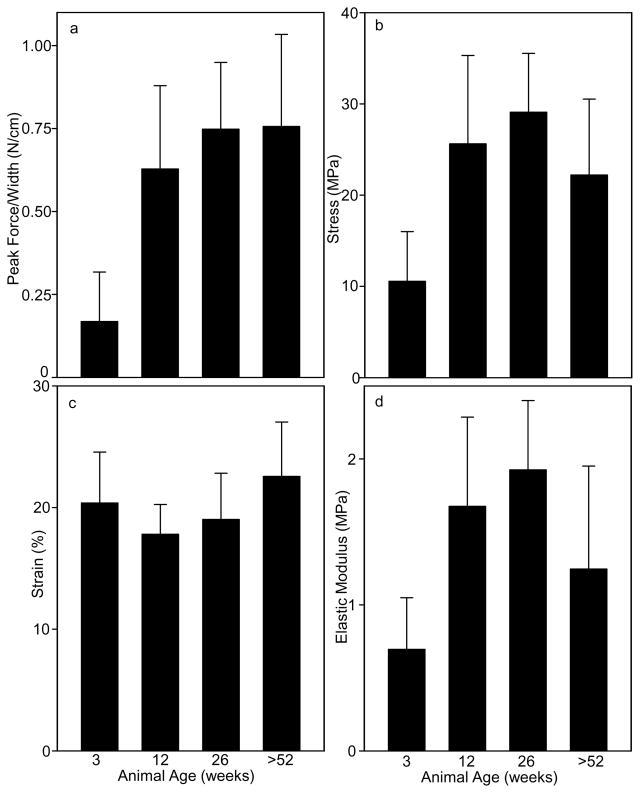
Uniaxial tensile testing showed differences in SIS-ECM from the various age sources. SIS-ECM from 3 week old animals had lower peak load per unit width (p<0.001) compared to all other sources (Fig. 1a). SIS-ECM from 12, 26 and >52 week old animals failed at higher stress (p<0.002) compared to SIS-ECM from 3 week old animals (Fig. 1b). SIS-ECM from animals aged >52 weeks failed at higher strain (p=0.016) compared to SIS-ECM from 12 week old animals (Fig. 1c). SIS-ECM from 12 or 26 week old animals had higher elastic moduli (p<0.018) compared to SIS-ECM from 3-week-old animals, and SIS-ECM from 26 week old animals had a higher elastic modulus (p=0.035) compared to SIS-ECM from animals aged >52 weeks (Fig. 1d).
Fig. 1. Mechanical Properties of Porcine SIS-ECM Derived from Animals of Different Ages.
Longitudinal SIS-ECM specimens were tensile tested to failure in uniaxial tension and mechanical properties were calculated from specimen dimensions and load-extension data. (a) Peak load per unit width was lower for SIS-ECM from 3 week old animals than all other sources. (b) Ultimate tensile stress was higher for SIS-ECM from 12 or 26 week old animals compared to 3 week old animals and higher for SIS-ECM from 26 week old animals compared to animals aged >52 weeks. (c) Strain at failure was higher for SIS-ECM from animals aged >52 weeks compared to 12 or 26 week old animals. (d) Elastic modulus was higher for SIS-ECM from 12 or 26 week old animals compared to 3-week-old animals and higher for SIS-ECM from 26 week old animals compared to animals aged >52 weeks. Data are means of 13 determinations with S.D.
Thickness Measurement and Collagenase Digestion
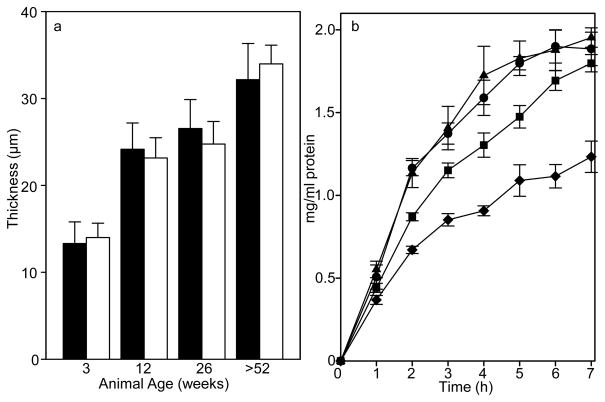
The SIS-ECM material becomes substantially thicker with age. The 3 week old SIS-ECM was thinner (p<0.001) than all other ages, while the >52 week old SIS-ECM was thicker (p<0.001) (Fig. 2a). There was no statistical difference between the thickness of the 12 week old and the 26 week old SIS-ECM. Lyophilization and rehydration did not alter the thickness of any of the different age materials. The speed of remodeling in vivo and strength over time of an implanted device will in part be determined by the resistance of the device to enzymatic degradation. Samples of each age source material were incubated in collagenase and aliquots of solubilized protein collected hourly (Fig. 2b). SIS-ECM from all age source materials released protein from the material. The 3 and 12 week old source materials released more protein, more rapidly, than the older source materials (p<0.005). The >52 week old source material released less protein than any other source (p<0.002). After 24 h in the digestion solution the SIS-ECM from the 3, 12, and 26 week old source was no longer visible, while the >52 week material still appeared mostly intact.
Fig. 2. Thickness and Collagenase Resistance of SIS-ECM from Different Age Sources.
(a) SIS-ECM from the >52 week old source was thicker and 3 week old thinner than 12 or 26 week old source material. Thickness was measured at three locations for 13 samples from each age source. SIS-ECM from each age source which had never been lyophilized (black bars) or lyophilized and then fully rehydrated (white bars) was measured for thickness. Data are means with S.D. (b) SIS-ECM from older animals was more resistant to type I collagenase digestion. Squares of SIS-ECM material were digested with collagenase and the protein released into the digestion solution measured for 3 week old (circles), 12 week old (triangles), 26 week old (squares) or >52 week old (diamonds) source material. Data are means of triplicate determinations with S.D. The assay was performed on three occasions with similar trends observed.
Characterization of ECM Composition in Four Different Age Sources of SIS-ECM
Growth Factor Analysis and Glycosaminoglycan Quantification
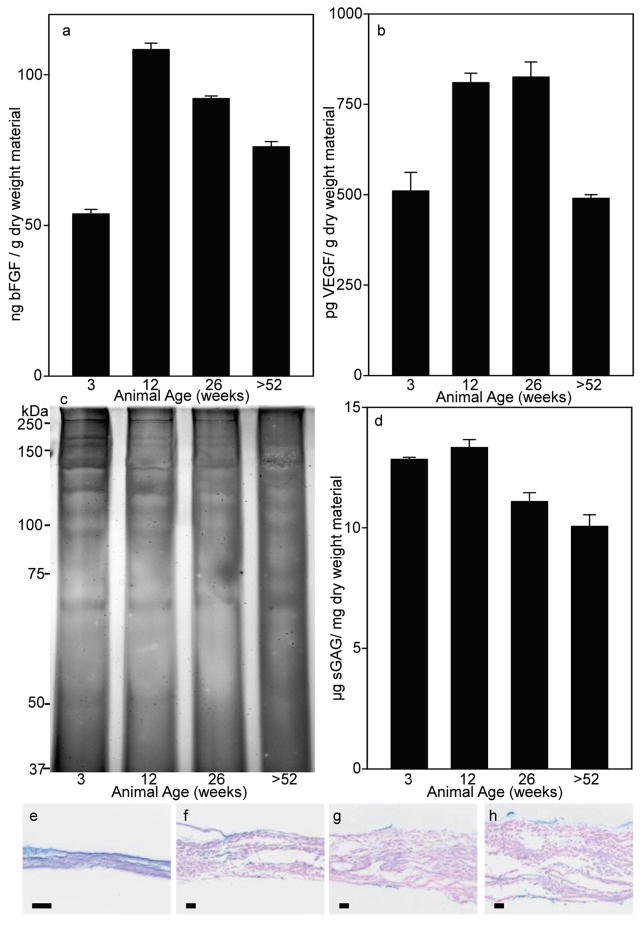
Samples from each age source were analyzed for the concentration of growth factors bFGF and VEGF. SIS-ECM from the 3 week old source had less bFGF protein per mg dry weight of material (p<0.001) than any other age source (Fig. 3a). The 12 week source SIS-ECM had the greatest amount (p<0.011) with the amount of bFGF protein per mg dry weight material then decreasing with age (26 weeks less than 12 weeks p=0.011, >52 weeks less than 26 weeks p=0.004). The VEGF analysis showed that the 3 and >52 week source SIS-ECM had less VEGF protein (p<0.001) than the 12 or 26 week old source SIS-ECM, which had equivalent amounts of VEGF (Fig. 3b). No major differences in solubilized proteins were determined among the four different ages of SIS-ECM from SDS PAGE (Fig. 3c).
Fig. 3. Growth Factor and sGAG Quantification.
(a) SIS-ECM from the 12 and 26 week old sources had more bFGF and (b) VEGF than the 3 or >52 week old source materials. Data are means of duplicate determinations with S.D. The assay was performed on four occasions with similar trends observed. (c) Protein from each different age source of SIS-ECM was solubilized and 3 μg was resolved via 7.5% SDS PAGE and visualized by silver staining. The pattern of protein bands shows no major differences between each age source. (d) SIS-ECM from 3 and 12 week old source animals had more sGAGs than the older animal source materials. Data are means of triplicate determinations with S.D. The assay was performed on three occasions with similar trends observed. (e–h) SIS-ECM was stained with Alcian blue to detect sGAGs from (e) 3 weeks, (f) 12 weeks, (g) 26 weeks and (h) >52 weeks source material. Black bar = 50 μm.
Samples from each age source were analyzed for the presence of sGAG (Fig. 3d). SIS-ECM from the 3 and 12 week old sources contained more sGAG per mg dry weight material (p<0.001) than the 26 or >52 week old source. The >52 week old source material SIS-ECM contained less sGAG than the 26 week old source material (p=0.027). The 3 and 12 week old source material contained equivalent amounts of sGAG. Staining of transverse tissue sections of SIS-ECM from each age source with Alcian blue to determine GAG spatial distribution showed that most of the GAGs are on the inner and outer edges of the material, with the 3 and 12 week old material (Fig. 3e and f respectively) showing more positive staining within material than the 26 or >52 week material (Fig. 3g and h respectively).
Mitogenic, Metabolic, and Chemotactic Properties of SIS-ECM on Perivascular Stem Cells
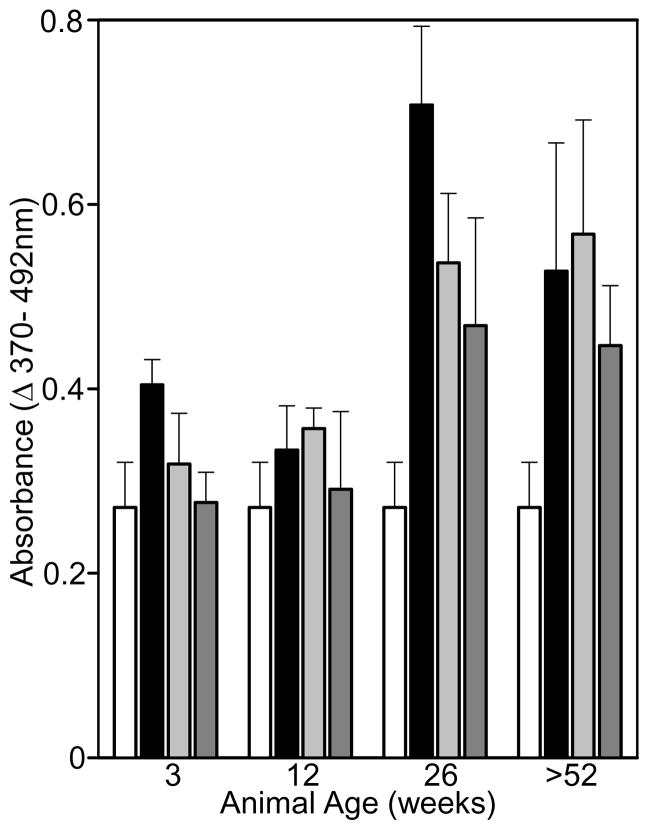
Degradation products derived from ECM are believed to play important roles in the recruitment and mitogenesis of selected cell types [32]. The effects of a pepsin digest of the different age source SIS-ECM materials on key processes of tissue remodeling, such as stem cell proliferation and recruitment, were therefore investigated. SIS-ECM degradation products at 2 μg/mL (black bars) from all age sources increased the proliferation of the stem cells (p<0.001) compared to untreated controls (Fig. 4). At 2 μg/mL, the SIS-ECM degradation products from the 26 and >52 week old source increased the proliferation of the perivascular stem cells more than the younger age source materials (p<0.009). SIS-ECM degradation products at 10 and 25 μg/mL also increased the proliferation of the perivascular stem cells (p<0.010) compared to the untreated control for all age sources except the 3 week old source material. The 26 and >52 week source materials increased the proliferation of the stem cells when compared to the 3 week old source material (p<0.009), but not the 12 week old animal source SIS-ECM. The 26 and >52 week old SIS-ECM degradation products caused an equivalent increase in proliferation at all concentrations tested.
Fig. 4. Proliferation of Perivascular Stem Cells Exposed to Different Age Source SIS-ECM.
SIS-ECM degradation products from all source materials increased proliferation, with the 26 and >52 week source material increasing proliferation the most. Perivascular stem cells were inoculated into media containing 0 (white bars), 2 (black bars), 10 (light grey bars), or 25 (dark grey bars) μg/mL SIS-ECM degradation products from the different age source materials and incubated for 18h. DNA synthesis was monitored via BrdU incorporation immunoassay and quantified colormetrically at 370 and 492 nm. Data are means of triplicate determinations with S.D. The assay was performed on three occasions with similar trends observed.
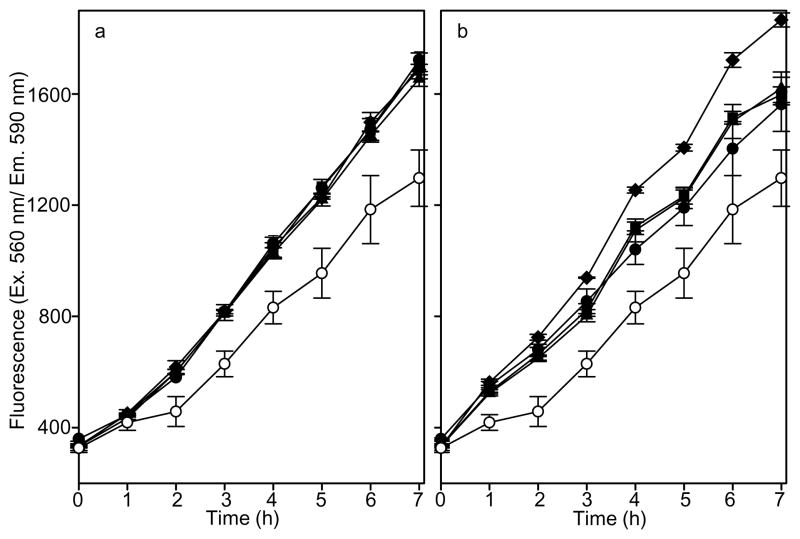
The increase in metabolism of perivascular stem cells exposed to the different age sources of SIS-ECM degradation products was monitored for 7 h (Fig. 5). The SIS-ECM degradation products from all age sources increased the metabolism of the perivascular stem cells (p<0.001) at all concentrations tested. At 25 μg/mL (Fig. 5b), the SIS-ECM degradation products from the >52 week old source caused a greater increase in metabolism when compared to the SIS-ECM degradation products from 3, 12, or 26 week old animals (p<0.001). There was no difference in the increase in metabolism between the age sources of SIS-ECM degradation products at 2 μg/mL or between the 3, 12, or 26 week old animals at 25 μg/mL.
Fig. 5. Metabolism of Perivascular Stem Cells Exposed to Different Age Source SIS-ECM.
SIS-ECM degradation products from all source materials increased metabolism, with the >52 week source material increasing metabolism the most. Perivascular stem cells were inoculated into media containing alamarBlue and (a) 2 μg/mL or (b) 25 μg/mL SIS-ECM degradation products from 3 week old (black circles), 12 week old (black triangles), 26 week old (black squares) or >52 week old (black diamonds). Perivascular stem cell metabolism without SIS-ECM (white circles) is shown as a control. AlamarBlue metabolism was quantified fluorescently (Ex. 560 nm/ Em. 590 nm) over 7h. Data are means of triplicate determinations with S.D. The assay was performed on three occasions with similar trends observed.
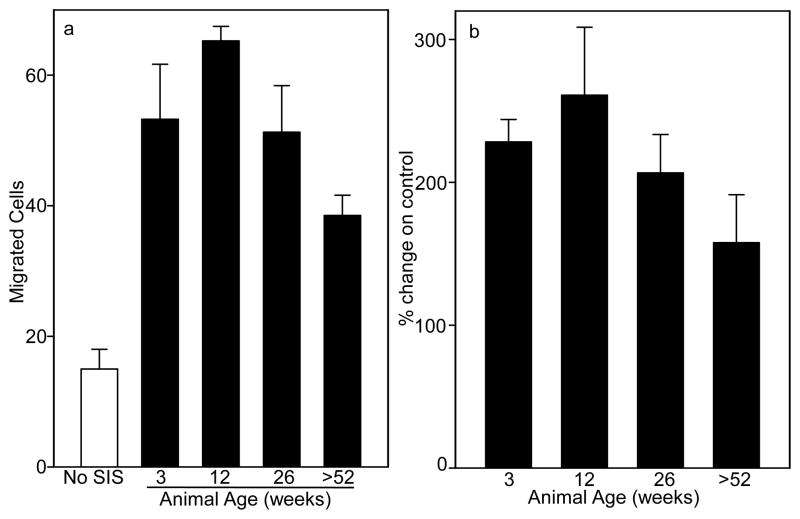
The SIS-ECM degradation products from all age sources are chemotactic for perivascular stem cells (Fig. 6, p<0.001). The migration of perivascular stem cells toward the SIS-ECM degradation products from 12 week old animals was increased over SIS-ECM degradation products from 3, 26, and >52 week old animals (p<0.033). The migration of cells toward SIS-ECM degradation products from >52 week old animals was less than for SIS-ECM degradation products from the other age animals (p<0.001). The migration of perivascular stem cells toward SIS-ECM degradation products from 3 and 26 old animals was equivalent.
Fig. 6. Migration of Perivascular Stem Cells Exposed to Different Age Source SIS-ECM.
The SIS-ECM degradation products from 12 week old animals caused greater migration and >52 week old animals less migration of perivascular stem cells than SIS-ECM from 3 or 26 week old animals. (a) The migration of perivascular stem cells toward 0 (white bar) or 100μg/mL (black bars) of the SIS-ECM degradation products from the different age source material was measured in a chemotaxis chamber for 3h. The y-axis represents the number of migrated cells in three 20× magnification fields. The data are means of quadruplicate determinations with S.D. The assay was performed on six occasions with similar trends observed. (b) Average of averages from six cell migration experiments showing fold migration change from 0 μg/mL SIS-ECM control. Error bars are S.D.
DISCUSSION
Biomaterials composed of allogeneic or xenogeneic ECM are now commonly used for reconstruction of a variety of injured or missing tissues and organs [1]. However, clinical outcomes have varied considerably [13]. Many variables may contribute to these disparate outcomes, including the age of the animal from which the tissue is harvested. The results of the present study confirm that differences exist in the composition, structure, and mechanical properties of SIS-ECM prepared from tissues harvested from different aged animals. The present study represents the first systematic comparison of biologic scaffold materials that differ only in the age of the animal from which the materials were manufactured.
Ultimate stress, strain at failure, and elastic modulus of SIS-ECM in this study were similar to values obtained in other studies of SIS-ECM [33, 34]. Single-layer SIS-ECM samples in this study and multi-layer SIS-ECM samples previously reported by other groups had a peak force per unit width within an order of magnitude of each other [35]. Peak force per unit width and elastic modulus of SIS-ECM in this study were also within an order of magnitude of some polymer surgical meshes [36], as was strain at failure [37]. Peak load per unit width and sample thickness were generally proportional to one another (Fig. 1a and Fig. 2a), the only exception being SIS-ECM from the oldest animals. The differences between the mechanical properties of SIS-ECM from animals aged >52 weeks and other age sources, was likely caused by changes in tissue organization because differences in protein composition between the sources appear to be subtle and were not consistent with the content of macromolecules such as growth factors and sGAGs (Fig. 3). The thickness of the SIS-ECM materials depends upon the technique of measurement. Under compressive force (8.5 +/−0.5N) we found that the materials have mean thicknesses between 12 (3 week source) and 33 (>52 week source) μm (Fig. 2a); however via histology we observed thicknesses ranging from 33 to 350 μm (Fig. 3e–h). The increased thickness observed via histology reflects the expansion of the material caused by histological processing. Previously reported values for SIS-ECM have been 100 [38] to 150μm [39].
There is a significant difference between the in vitro degradation rates of the different ages of SIS-ECM (Fig. 2b) which may have an effect upon the in vivo remodeling process of the device. The 12 and 26 week old source SIS-ECM degraded at different rates even though they had equivalent thicknesses (Fig. 2a). This indicates a change in the SIS-ECM, which is further continued in the >52 week source SIS-ECM. Age-related changes of ECM post synthesis were first reported in rat tail collagen fibers which underwent age related crosslinking [40] due to the Maillard reaction, a non-enzymatic modification of tissue proteins by reducing sugars. This process results in collagen fibers which are resistant to collagenase digestion [41, 42]. A longer lasting implanted SIS-ECM device may be beneficial in some instances, and selection of the correct age or combination of SIS-ECM ages may allow the tuning of the device to a particular application.
The SIS-ECM prepared from all age sources modified the behavior of the perivascular stem cells, increasing their metabolism, proliferation and causing them to migrate towards the SIS-ECM degradation products (Fig. 4–6). These cells are believed to be a precursor for mesenchymal stem cells and therefore of particular interest because their multipotency and wide distribution throughout the body makes them a possible source for multiple different tissues that may require repair and regeneration [30, 43]. The SIS-ECM biomaterials contain growth factors and sGAGs which are involved in cell signaling events and promote cell migration and proliferation [44–47]. The levels of growth factors and sGAGs reported here are very similar to levels previously reported for SIS-ECM [48]. After in vitro digestion with pepsin to generate the SIS-ECM degradation products it is unlikely that much if any active growth factor remains. The observed increase in proliferation, metabolism, and migration and of perivascular stem cells (Fig. 4–6) is likely caused by matricryptic peptides, derived from ECM during the process of degradation. These peptides can modulate biologic events including the recruitment of stem cells [49]. Such matrikines are generated as a result of ECM degradation and are a possible source of the effects seen here. Although the specific mediators of these events are not known, a head to head comparison of degradation products derived from the four different age sources of ECM shows that the >52 weeks age source has less potent chemotactic effects than the ECM from younger pigs, but both the >52 week and the 26 week age source result in greater mitogenic effects compared to the other age sources. This difference illustrates the complexities of cell-matrikine interactions and is likely to be dependent on proteinase-specific cleavage of ECM peptides during degradation.
The practical value of the findings herein depends upon the desired characteristics of the ECM device to be manufactured. For example, the design of scaffold materials that must withstand substantial mechanical loading after in vivo implantation and remodel into load-bearing or force-generating tissues are likely to require material properties that have the greatest ultimate tensile strength and elastic modulus. The present study shows that the animal from which the material is harvested should be at least 12 weeks of age but older animals provide no advantage with regard to this particular mechanical property (Fig. 1). Somewhat surprisingly, the pigs aged >52 weeks yield SIS-ECM with lower elastic modulus compared to 12 or 26 week old animal source. The in vitro degradation studies suggest that SIS-ECM scaffolds may persist longer in vivo if manufactured from animals aged >52 weeks (Fig. 2b). If bFGF and VEGF presence is a major concern, then the raw material should be harvested from animals at least 12 weeks of age (Fig. 3a and b), but the sGAG content is greatest in ECM prepared from the youngest age sources (3 and 12 weeks, Fig. 3d).
Although the methodology of the present study minimized the inherent biologic variability that exists in materials harvested from an animal source, there are limitations that must be noted in the interpretation of the results. First, the results represent the findings from a single source of ECM material; specifically porcine SIS-ECM. These results cannot necessarily be generalized to all ECM materials. Second, the mechanical and materials property testing was limited to uniaxial tensile testing. Other tests of mechanical and material properties, such as biaxial testing, ball burst strength, and suture retention strength may be more relevant for selected clinical applications and in vivo remodeling. Third, the in vivo biologic activity of ECM scaffold materials may not be related to in vitro tests of a particular stem cell type subjected to a mixture of ECM degradation products generated by pepsin digestion. However, in an effort to understand the variables that may affect a different clinical outcome, the isolation of a single variable, such as the age of the animal from which source material is harvested, is essential.
CONCLUSIONS
It is clear that the age of the source animal makes a difference to the properties of the SIS-ECM scaffold material. Selection of the appropriate age source material for a particular application should be a considered criterion. This study addresses one of the important variables to be considered in the design and manufacturing of biologic devices composed of mammalian ECM, the age of the source animal. The age of the animal from which the material is harvested has implications for the devices physical properties (Fig. 1 and 2), compositional makeup (Fig. 3), and the mitogenesis (Fig. 4), metabolism (Fig. 5), and migration (Fig. 6) of stem cells.
Future investigations in an animal model implanted with the different age sources of SIS-ECM are warranted, as well as experiments to determine the extent to which these findings can be generalized to other biologic scaffold materials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26(4):507–523. doi: 10.1016/j.cpm.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Sellaro TL, Ranade A, Faulk DM, McCabe GP, Dorko K, Badylak SF, et al. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng Part A. 2009;16(3):1075–1082. doi: 10.1089/ten.tea.2008.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Little D, Guilak F, Ruch DS. Ligament-derived matrix stimulates a ligamentous phenotype in human adipose-derived stem cells. Tissue Eng Part A. 2010;16(7):2307–2319. doi: 10.1089/ten.tea.2009.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakayama KH, Batchelder CA, Lee CI, Tarantal AF. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng Part A. 2010;16(7):2207–2216. doi: 10.1089/ten.tea.2009.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14(11):1835–1842. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 6.Flynn LE, Prestwich GD, Semple JL, Woodhouse KA. Proliferation and differentiation of adipose-derived stem cells on naturally derived scaffolds. Biomaterials. 2008;29(12):1862–1871. doi: 10.1016/j.biomaterials.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Rieder E, Kasimir MT, Silberhumer G, Seebacher G, Wolner E, Simon P, et al. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg. 2004;127(2):399–405. doi: 10.1016/j.jtcvs.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30(8):1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5(1):1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Freytes DO, Badylak SF, Webster TJ, Geddes LA, Rundell AE. Biaxial strength of multilaminated extracellular matrix scaffolds. Biomaterials. 2004;25(12):2353–2361. doi: 10.1016/j.biomaterials.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Hoburg AT, Keshlaf S, Schmidt T, Smith M, Gohs U, Perka C, et al. Effect of electron beam irradiation on biomechanical properties of patellar tendon allografts in anterior cruciate ligament reconstruction. Am J Sports Med. 2010;38(6):1134–1140. doi: 10.1177/0363546509361161. [DOI] [PubMed] [Google Scholar]
- 12.Freytes DO, Stoner RM, Badylak SF. Uniaxial and biaxial properties of terminally sterilized porcine urinary bladder matrix scaffolds. J Biomed Mater Res B Appl Biomater. 2008;84(2):408–414. doi: 10.1002/jbm.b.30885. [DOI] [PubMed] [Google Scholar]
- 13.Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19(3):467–476. doi: 10.1016/j.jse.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Hodde JP, Record RD, Liang HA, Badylak SF. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium. 2001;8(1):11–24. doi: 10.3109/10623320109063154. [DOI] [PubMed] [Google Scholar]
- 15.Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67(4):478–491. [PubMed] [Google Scholar]
- 16.Nelson CM, Bissell MJ. Modeling dynamic reciprocity: Engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Seminars in Cancer Biology. 2005;15(5):342–352. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70(9–10):537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gassmann PEA, Haier J. Role of tumor cell and adhesion and migration in organ-specific metastasis formation. Onkologie. 2004;27(6):577–582. doi: 10.1159/000081343. [DOI] [PubMed] [Google Scholar]
- 19.Brown BN, Barnes CA, Kasick RT, Michel R, Gilbert TW, Beer-Stolz D, et al. Surface characterization of extracellular matrix scaffolds. Biomaterials. 2010;31(3):428–437. doi: 10.1016/j.biomaterials.2009.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20(2):109– 116. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodde JP, Badylak SF, Shelbourne KD. The effect of range of motion on remodeling of small intestinal submucosa (SIS) when used as an Achilles tendon repair material in the rabbit. Tissue Eng. 1997;3(1):27–37. [Google Scholar]
- 22.Boruch AV, Nieponice A, Qureshi IR, Gilbert TW, Badylak SF. Constructive remodeling of biologic scaffolds is dependent on early exposure to physiologic bladder filling in a canine partial cystectomy model. J Surg Res. 2010;161(2):217–225. doi: 10.1016/j.jss.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Moulin V, Plamondon M. Differential expression of collagen integrin receptor on fetal vs. adult skin fibroblasts: implication in wound contraction during healing. Br J Dermatol. 2002;147(5):886–892. doi: 10.1046/j.1365-2133.2002.04975.x. [DOI] [PubMed] [Google Scholar]
- 24.Buchanan EP, Longaker MT, Lorenz HP. Fetal skin wound healing. Adv Clin Chem. 2009;48:137–161. doi: 10.1016/s0065-2423(09)48006-5. [DOI] [PubMed] [Google Scholar]
- 25.Coolen NA, Schouten KC, Boekema BK, Middelkoop E, Ulrich MM. Wound healing in a fetal, adult, and scar tissue model: a comparative study. Wound Repair Regen. 2010;18(3):291–301. doi: 10.1111/j.1524-475X.2010.00585.x. [DOI] [PubMed] [Google Scholar]
- 26.Eaglstein WH, Falanga V. Tissue engineering and the development of Apligraf, a human skin equivalent. Clin Ther. 1997;19(5):894–905. doi: 10.1016/s0149-2918(97)80043-4. [DOI] [PubMed] [Google Scholar]
- 27.Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. Current status in wound healing. Am J Clin Dermatol. 2001;2(5):305–313. doi: 10.2165/00128071-200102050-00005. [DOI] [PubMed] [Google Scholar]
- 28.Zerris VA, James KS, Roberts JB, Bell E, Heilman CB. Repair of the dura mater with processed collagen devices. J Biomed Mater Res B Appl Biomater. 2007;83(2):580–588. doi: 10.1002/jbm.b.30831. [DOI] [PubMed] [Google Scholar]
- 29.Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989;47(1):74–80. doi: 10.1016/0022-4804(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 30.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Tottey S, Corselli M, Jeffries EM, Londono R, Peault B, Badylak SF. Extracellular matrix degradation products and low oxygen conditions enhance the regenerative potential of perivascular stem cells. Tissue Eng Part A. 2010 doi: 10.1089/ten.tea.2010.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, Heber-Katz E, et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15(3):605–614. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 33.Feng C, Xu YM, Fu Q, Zhu WD, Cui L, Chen J. Evaluation of the biocompatibility and mechanical properties of naturally derived and synthetic scaffolds for urethral reconstruction. J Biomed Mater Res A. 2010;94(1):317–325. doi: 10.1002/jbm.a.32729. [DOI] [PubMed] [Google Scholar]
- 34.Dora CD, Dimarco DS, Zobitz ME, Elliott DS. Time dependent variations in biomechanical properties of cadaveric fascia, porcine dermis, porcine small intestine submucosa, polypropylene mesh and autologous fascia in the rabbit model: implications for sling surgery. J Urol. 2004;171(5):1970–1973. doi: 10.1097/01.ju.0000121377.61788.ad. [DOI] [PubMed] [Google Scholar]
- 35.Konstantinovic ML, Lagae P, Zheng F, Verbeken EK, De Ridder D, Deprest JA. Comparison of host response to polypropylene and non-cross-linked porcine small intestine serosal-derived collagen implants in a rat model. BJOG. 2005;112(11):1554–1560. doi: 10.1111/j.1471-0528.2005.00688.x. [DOI] [PubMed] [Google Scholar]
- 36.Hollinsky C, Sandberg S, Koch T, Seidler S. Biomechanical properties of lightweight versus heavyweight meshes for laparoscopic inguinal hernia repair and their impact on recurrence rates. Surg Endosc. 2008;22(12):2679–2685. doi: 10.1007/s00464-008-9936-6. [DOI] [PubMed] [Google Scholar]
- 37.Jones KA, Feola A, Meyn L, Abramowitch SD, Moalli PA. Tensile properties of commonly used prolapse meshes. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(7):847–853. doi: 10.1007/s00192-008-0781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badylak SF, Kropp B, McPherson T, Liang H, Snyder PW. Small intestinal submucosa: a rapidly resorbed bioscaffold for augmentation cystoplasty in a dog model. Tissue Eng. 1998;4(4):379–387. doi: 10.1089/ten.1998.4.379. [DOI] [PubMed] [Google Scholar]
- 39.Demling R, Niezgoda JA, Haraway GD, Mostow EN. Small intestinal submucosa wound matrix and full-thickness venous ulcers: preliminary results. Wounds. 2004;16 (1):18–22. [Google Scholar]
- 40.Verzar F. Aging of the collagen fiber. Int Rev Connect Tissue Res. 1964;2:243–300. doi: 10.1016/b978-1-4831-6751-0.50012-4. [DOI] [PubMed] [Google Scholar]
- 41.Robert L, Labat-Robert J. Aging of connective tissues: from genetic to epigenetic mechanisms. Biogerontology. 2000;1(2):123–131. doi: 10.1023/a:1010048014925. [DOI] [PubMed] [Google Scholar]
- 42.Verzijl N, DeGroot J, Oldehinkel E, Bank RA, Thorpe SR, Baynes JW, et al. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem J. 2000;350(Pt 2):381–387. [PMC free article] [PubMed] [Google Scholar]
- 43.Chen CW, Montelatici E, Crisan M, Corselli M, Huard J, Lazzari L, et al. Perivascular multi-lineage progenitor cells in human organs: Regenerative units, cytokine sources or both? Cytokine & Growth Factor Reviews. 2009;20(5–6):429–434. doi: 10.1016/j.cytogfr.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki T. The effects of basic fibroblast growth factor and doxorubicin on cultured human skin fibroblasts: relevance to wound healing. J Dermatol. 1992;19(11):664–666. doi: 10.1111/j.1346-8138.1992.tb03755.x. [DOI] [PubMed] [Google Scholar]
- 45.Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol. 1996;149(1):293–305. [PMC free article] [PubMed] [Google Scholar]
- 46.Goto F, Goto K, Weindel K, Folkman J. Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor on the proliferation and cord formation of bovine capillary endothelial cells within collagen gels. Lab Invest. 1993;69(5):508–517. [PubMed] [Google Scholar]
- 47.Raman R, Sasisekharan V, Sasisekharan R. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem Biol. 2005;12(3):267–277. doi: 10.1016/j.chembiol.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 48.Hodde J, Janis A, Ernst D, Zopf D, Sherman D, Johnson C. Effects of sterilization on an extracellular matrix scaffold: part I. Composition and matrix architecture. J Mater Sci Mater Med. 2007;18(4):537–543. doi: 10.1007/s10856-007-2300-x. [DOI] [PubMed] [Google Scholar]
- 49.Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol. 2000;156(5):1489–1498. doi: 10.1016/S0002-9440(10)65020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]