Abstract
Epidemiological studies support a link between melanoma risk and UV exposure early in life, yet the molecular targets of UV's mutagenic actions are not known. By using well characterized murine models of melanoma, we provide genetic and molecular evidence that identifies components of the Rb pathway as the principal targets of UV mutagenesis in murine melanoma development. In a melanoma model driven by H-RAS activation and loss of p19ARF function, UV exposure resulted in a marked acceleration in melanoma genesis, with nearly half of these tumors harboring amplification of cyclin-dependent kinase (cdk) 6, whereas none of the melanomas arising in the absence of UV treatment possessed cdk6 amplification. Moreover, UV-induced melanomas showed a strict reciprocal relationship between cdk6 amplification and p16INK4a loss, which is consistent with the actions of UV along the Rb pathway. Most significantly, UV exposure had no impact on the kinetics of melanoma driven by H-RAS activation and p16INK4a deficiency. Together, these molecular and genetic data identify components of the Rb pathway as critical biological targets of UV-induced mutagenesis in the development of murine melanoma in vivo.
Keywords: p16INK4a‖p19ARF‖UVB‖cdk6
Melanoma, the most lethal human skin cancer, shows an alarming rate of increase worldwide and causes >7,000 deaths annually in the United States alone (1–3). Epidemiological evidence has established that a history of sunburn and intermittent exposure to UV light, particularly early in life, promotes melanoma development (4, 5). This epidemiological association is strong and is causally linked by investigations using human skin grafts (6, 7). However, the specific molecular targets, if any, of this environmental carcinogen are not known. Although the finding of C>T point mutations (“UV-signature”) of p16INK4a in human melanoma suggested its targeting by this carcinogen in humans (8–10), the observation of a similar C>T mutation bias in glioma (reviewed in ref. 11), a non-UV induced tumor, has called this conclusion into question. Alternatively, the relationship between p16INK4a and melanoma has been explained by the observation that UV light can induce p16INK4a expression in human melanocytes (12, 13), thereby implying a role for p16INK4a in the repair of UV-induced lesions. Finally, several groups have suggested that UV functions in a noncell autonomous manner to facilitate melanoma either by inducing immune suppression (14) or by eliciting the elaboration of tumor-promoting paracrine factors (15). Thus, a definitive UV-p16INK4a link, and the nature of this interaction, has yet to be clarified on either the molecular, physiological, or genetic levels.
In view of the presence of activating B-RAF mutation, a direct signaling surrogate of RAS activation, in ≈70% of human melanomas (16), and RAS mutation in an additional 20% (17), RAS-pathway activation appears to represent a rite of passage for human melanoma. We have previously generated transgenic mice expressing an activated form of human H-Ras (Tyr-RAS) in melanocytes that are highly melanoma-prone when introduced onto Ink4a/Arf−/− or p53−/− backgrounds (18, 19). Importantly, we have shown that RAS-induced melanomas from these models harbor secondary genomic changes detectable by conventional and array-based comparative genomic hybridization (CGH) that are syntenic to known hotspots of human melanomas (ref. 19; L.C. and B. Bastian, unpublished observations), further validating the use of this model in the study of the human disease.
Loss of the INK4a/ARF (CDKN2A) locus is encountered in ≈50% of human melanomas (20, 21) and, along with activating mutation of B-RAF (16), are the most common genetic lesions of this cancer type. The INK4a/ARF locus encodes two distinct proteins, p16INK4a and p14ARF (p19ARF in the mouse), both of which demonstrate tumor suppressor activity in genetically distinct anti-cancer pathways: the “Rb pathway” for p16INK4a and the “p53 pathway” for p14ARF (see below; reviewed in refs. 11 and 22). ARF and p16INK4a have different first exons (1β and 1α respectively) and 5′ regulatory units, but are spliced into a common second exon in alternate reading frames. The cyclin-dependent kinase inhibitor (CKI) p16INK4a is known to inhibit CDK4/CDK6-directed phosphorylation of RB, and loss of p16INK4a permits RB hyperphosphorylation and subsequent de-repression of RB-regulated genes. Also, a regulator of the cell cycle, p14ARF has been shown to be a principal regulator of MDM2, an E3 ubiquitin ligase important in p53 degradation. Consequently, loss of p14ARF (or p19ARF) can directly impair the stabilization of p53 in response to certain oncogenic stresses. Several lines of evidence support the view that both p16INK4a and p14ARF play roles in melanoma suppression in vivo. First, germ-line mutations of either exon 1α (affecting p16INK4a only) or exon 1β (targeting p14ARF only) have both been identified in melanoma-prone kindreds (11, 23–25). Also, animals specifically deficient for p16INK4a have a low frequency of carcinogen-induced melanoma that is augmented in the setting of p19ARF haploinsufficiency (26–28). Finally, similar to mice on Ink4a/Arf−/− (doubly null for both p16INK4a and p19ARF) background (18), Tyr-RAS mice deficient for either p16INK4a or p19ARF are susceptible to RAS-induced melanomas (unpublished observations).
Here, these genetically defined mouse models of melanoma were used to explore the role of neonatal UV exposure in promoting melanoma development, and particular emphasis was placed on whether UV-induced melanoma incidence is modulated by the status of the Rb vs. p53 pathways. We found that neonatal UV treatment accelerates melanoma formation in Tyr-RAS p19ARF−/− animals. This increase in tumorigenesis is accompanied by cdk6 amplification, which is mutually exclusive with p16INK4a loss in UV-treated animals and is not seen in tumors from non-UV-treated mice. Most significantly, UV light did not accelerate melanoma formation in Tyr-RAS mice lacking p16INK4a. These results suggest that components of the Rb pathway are the principal and rate-limiting target(s) of UV's actions in melanoma formation.
Materials and Methods
Mouse Tumor Cohorts.
Mice specifically lacking p19ARF were generated by standard techniques with Cre-mediated excision of the neomycin marker embedded in exon 1β (N.E.S. and R. DePinho, unpublished work). The phenotype of this knockout strain is similar to that of a previously published p19ARF KO (29), with the development of spontaneous tumors with a median latency of ≈60 weeks. Experimental cohorts were generated by initially crossing p19ARF−/− (or p16INK4a−/−; ref. 26) mice (both strains on FVB N2 background) onto tyrosinase enhancer-promoter-driven H-RASV12G transgenic mice (Tyr-RAS; FVB N6; ref. 18) followed by heterozygous intercrosses between Tyr-RAS p19ARF± (or p16INK4a±) animals. Cohorts were observed for melanoma development daily and moribund animals were killed for necropsy. Tumor tissues were fixed and paraffin-embedded for histopathological and also flash-frozen for subsequent analyses. Genotypes were determined by gene-specific PCR for both p16INK4a (26) and p19ARF (primers and conditions available upon request). The Tyr-RAS allele is transmitted on the Y chromosome. To minimize strain variability between these cohorts, FVB males were selected by using a marker-assisted genotyping protocol (30) to generate N3 backcrossed cohorts. In brief, “best” male founders were identified by analyzing 44 loci polymorphic between SvEv and FVB, with >14 males screened per generation. Loci were allelotyped on SYBR-stained 3% Nusieve gels by using PCR primers (The Jackson Laboratory; http://www.informatics.jax.org/, allele and primer lists available on request). Therefore, all animals analyzed in this study were >87.5% for FVB (N3). In Kaplan–Meier analyses, Tyr-RAS p16INK4a+/+ or Tyr-RAS p19ARF+/+ littermate controls (i.e., from heterozygous intercrosses) were compared with Tyr-RAS p16INK4a−/− or Tyr-RAS p19ARF−/− animals, respectively.
Tumor Analysis.
Methylation-specific PCR and LOH analysis in exon 1α of p16INK4a were performed as described (19, 26). RNA was isolated from tumor specimens immediately after surgical removal by using Trizol reagents (GIBCO/BRL). For the detection of point mutations, 2 μg of DNase-treated RNA was used for cDNA synthesis (Superscript, Invitrogen). To determine the sequence of cdk4 and p16INK4a, primers spanning the entire ORFs were used to amplify tumor cDNA. All mutations were confirmed by sequencing at least twice. For sequence-tagged site (STS)-PCR and quantitative RT-PCR, primers from the 3′UTR of cdk6 and gapdh were used; STS marker D6mit104 was used as a normal copy number control. PCR was performed by using 100 ng of genomic DNA or 1 μl of RT reaction mix with marker or gene-specific primers for 17 cycles of 95°C for 30 s, 56°C for 1 min, and 72°C for 45 s. PCR products were run on a 1% agarose TBE gel (100 mM Tris/100 mM boric acid/2.0 mM EDTA, pH 8.3) and transferred to Hybond N+ (Amersham Pharmacia). Probes were generated by PCR of pooled normal genomic DNA from the mice of origin for each tumor by using the same primers as for the quantitative PCR analysis. Randomly primed P32-labeled probes were hybridized for 2 h at 65°C in RapidHyb (Amersham Pharmacia). Quantification was performed by using a PhosphorImager. Rb and ink4a Southern blots were performed as described (refs. 19 and 31; Rb probe courtesy of T. Jacks, Boston). Cdk6 Southern analysis was performed by using an exonic probe generated by PCR (primers available upon request) and was normalized by using a nonamplified genomic DNA probe (32). Total protein lysates were prepared by briefly sonicating the tumor tissues in the presence of RIPA buffer with protease and phosphatase inhibitors and analyzed for p16INK4a as described (26). For cdk4 Western blots and immunoprecipitation, antibody C22 (Santa Cruz Biotechnology) was used. For immunoprecipitation analyses, 1 mg of protein extract was precleared by incubation with protein A Sepharose (Sigma) and preimmune serum and incubated for 2 h with anti-cdk4 antibody. Extracts were incubated for 1 h after the addition of protein A Sepharose. Immunoprecipitated complexes were fractionated by using SDS/PAGE and transferred to poly(vinylidene difluoride) membrane, and probed with anti-p16INK4a (M156, Santa Cruz Biotechnology) or anti-cdk4 antibody.
Results and Discussion
We examined the impact of p19ARF status in UV-mediated melanoma genesis by subjecting littermate Tyr-RAS p19ARF−/− and Tyr-RAS p19ARF+/+ animals to a single neonatal erythrogenic dose of UVB irradiation as described (33) and followed them for melanoma development. For controls, alternating litters from the same colony were withheld from UVB treatment and observed for spontaneous melanoma development. Although none of the Tyr-RAS p19ARF+/+ mice, with or without UVB exposure, developed melanoma during a 50-week period of observation, UV-treated Tyr-RAS p19ARF−/− animals demonstrated numerous melanomas arising with significantly shorter latency (Fig. 1a) relative to untreated Tyr-RAS p19ARF−/− controls. Furthermore, in the UV-treated cohort, the multiplicity of tumors was markedly increased from an average of 1.1 tumors per animal in the untreated group to 3.0 melanomas per animal in the UV-treated group (P < 0.001). These data demonstrate potent cooperation between UVB and the genetic alterations of activated H-RAS and p19ARF deficiency in melanoma formation.
Figure 1.
Loss of p19ARF can cooperate with UV exposure to facilitate melanoma formation. FVB (N3) neonatal mice (1- to 3-day-old pups) were treated with a single dose of total body UV irradiation (9 kJ/M2) by using an FS20T12 UV lamp (peak emittance in the UVB range, 310 nm). (a) Tyr-RAS p19ARF−/− mice with (n = 31) or without (n = 22) neonatal UV exposure were observed for tumor formation. No UV-treated Tyr-RAS p19ARF+/+ mice developed melanoma (n = 18). Melanoma-free survival is shown, and nonmelanoma tumors (e.g., lymphoma) were censored in this analysis. The survival curves were compared with the log-rank test. (b) Western blot analysis of p16INK4a expression in UV-treated Tyr-RAS p19ARF−/− melanomas.
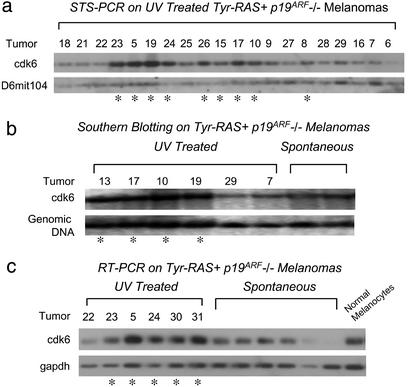
Although the histopathology of UV-induced and spontaneous tumors were indistinguishable (data not shown), molecular characterization of the p16INK4a-pRb axis by candidate gene survey in UV-induced melanomas revealed a shift in the mutational profile from that of spontaneous tumors. As was the case for spontaneous melanomas, p16INK4a methylation, Rb loss, cdk4 overexpression or point mutation, and c-myc overexpression were not detected in melanomas from UV-treated Tyr-RAS p19ARF−/− mice (data not shown). In contrast, molecular analysis of the spontaneous tumors showed that Rb pathway inactivation does occur via p16INK4a loss or point mutation in ≈50% of cases (unpublished observations). In tumors from UV-treated Tyr-RAS p19ARF−/− mice, this frequency of p16INK4a functional loss (as measured by direct sequencing, Western and IP-Western with cdk4) was decreased (8/36 tumors = 22%; Fig. 1b and data not shown). Instead, cdk6 amplification and overexpression (Fig. 2a–c) emerged as the principal Rb pathway lesion in UV-induced melanomas. A twofold or greater increase in cdk6 gene copy number was detected in 16 of 35 (46%) melanomas from UV-treated Tyr-RAS p19ARF−/− mice, compared with 0 of 22 melanomas from untreated Tyr-RAS p19ARF−/− mice (Table 1; P < 0.0001). A commensurate gene dosage increase of proximal chromosome 5 was documented by an independent method, i.e., array-based CGH profiling of the Tyr-RAS p19ARF−/− melanomas from UV-treated mice. The minimal region of this amplification was mapped to ≈1 MB, within which resides three annotated genes, one of which encodes cdk6 (R.C.O.-H., C. Brennan, and L.C., unpublished work). In accord with the result of the candidate gene survey, proximal chromosome 5 amplification was not detected in spontaneous melanomas from this cohort. Consistent with their known functional overlap in Rb pathway regulation, p16INK4a loss and cdk6 amplification were mutually exclusive (Table 1, P < 0.008) among the UV-treated Tyr-RAS p19ARF−/− melanomas. This strict reciprocal relationship, coupled with the emergence of cdk6 amplification in the UV-treated cohort, suggests that UVB's melanoma-promoting activities in this model are functionally linked to inactivation of the Rb pathway and are achieved most often by p16INK4a loss or cdk6 amplification.
Figure 2.
Cdk6 is amplified and overexpressed in a subset of UV-treated, but not spontaneous, Tyr-RAS p19ARF−/− melanomas. Melanomas that harbor cdk6 amplification do not demonstrate p16INK4a loss. (a) STS-PCR analysis of cdk6 copy number in UV-treated Tyr-RAS p19ARF−/− melanomas. Signal was normalized to a nonamplified normal copy number control marker (D6mit104). *, Greater than twofold amplification by densitometry. (b) Southern blot analysis of Tyr-RAS p19ARF−/− melanomas by using a cdk6 probe, normalized to a chromosome 19 genomic DNA probe (32) that is not amplified. *, Greater than twofold amplification by densitometry. (c) Quantitative RT-PCR analysis of cdk6 expression in Tyr-RAS p19ARF−/− melanomas. cdk6 signal was normalized to gapdh expression. Nontransformed primary cultured melanocytes isolated from Ink4a/Arf−/− animals were used as control. *, Greater than twofold overexpression by densitometry.
Table 1.
Rb pathway status in Tyr-RAS p19ARF−/− melanomas
| Rb pathway status | Spontaneous | UV-treated |
|---|---|---|
| p16INK4a Loss | 7 of 18 | 8 of 36* |
| cdk6 Amplification | 0 of 20† | 16 of 35*† |
| Both | 0 of 15 | 0 of 35* |
P < 0.008 for lack of association between p16INK4a loss and cdk6 amplification.
P < 0.0001 for frequency of cdk6 amplification in spontaneous vs. UV-treated tumors.
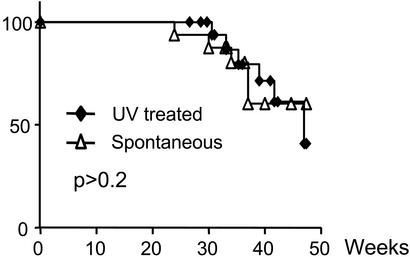
To validate this UV-pRB pathway hypothesis by genetic means, we repeated the above UV study by employing Tyr-RAS p16INK4a−/− animals to reveal whether Rb pathway inactivation, via p16INK4a loss, is functionally equivalent to UVB exposure (Fig. 3). In sharp contrast to Tyr-RAS p19ARF−/− mice, no cooperation was seen between UVB exposure and p16INK4a loss in this model. Consistent with this lack of cooperation, the mutational profile of the p19ARF-p53 axis in UV-induced melanomas from Tyr-RAS p16INK4a−/− mice was similar to that of spontaneous melanomas from the same mice (not shown). Therefore, germ-line inactivation of p16INK4a eliminated the melanoma-promoting effect of UVB exposure.
Figure 3.
UV treatment does not accelerate melanoma formation in Tyr-RAS p16INK4a−/− mice. Tyr-RAS p16INK4a−/− were randomized to UV exposure as in Fig. 1a and observed for tumor formation (n = 16 for non-UV-treated, n = 23 for UV-treated, P > 0.2).
The finding that UV exposure cooperates with p19ARF deficiency, but not p16INK4a deficiency, in melanoma formation was unanticipated, given the presumed broad mutagenic action of UV, and suggests several possible explanations. First, this result might reflect a role of p19ARF in the repair of UV-induced damage. Although, when compared with p53−/− cells, p19ARF−/− cells are considerably less resistant to agents that induce double-strand breaks (34, 35); p19ARF−/− mouse embryo fibroblasts do demonstrate a modest resistance to G1 arrest after IR exposure and increased polyploidy after nocodazole treatment (36). If an impairment of DNA damage checkpoints were to underlie the increase in tumorigenesis of Tyr-RAS p19ARF−/− mice upon UVB exposure, we would expect to see increased cytogenetic complexity in UV-induced tumors. However, an analysis of these tumors by array-based CGH reveals exactly the opposite: UV-treated tumors are far less complex cytogenetically than spontaneously emerged melanomas (R.C.O.-H., C. Brennan, and L.C., unpublished work). Therefore, UV-light seems to be targeting a specific oncogenic pathway rather than engendering broad, genome-wide DNA damage in UV-treated Tyr-RAS p19ARF−/− mice. A second interpretation would be that this dose of UVB does not accelerate tumorigenesis by inducing DNA damage, but rather serves as a mitogenic stimulus for melanocytes, as suggested (3), and that p19ARF perhaps plays a role in limiting this mitogenic response. When measured at an age near the time of melanoma emergence, however, melanocyte numbers are not altered in the skin of UV-treated Tyr-RAS p19ARF−/−mice relative to untreated littermate controls, arguing against such an effect (data not shown). Moreover, neither of these explanations would explain the lack of cooperation between UV exposure and p16INK4a loss, nor the mutually exclusive loss of p16INK4a or amplification of cdk6 seen in UV-treated Tyr-RAS p19ARF−/− tumors. Similarly, the inability of UV to accelerate melanoma development in p16INK4a−/− animals would argue against a general immune suppressive role of UV as an explanation for this observation. For these reasons, we favor an alternative possibility: that UV exposure enhances melanoma risk by inactivating the Rb pathway, primarily via p16INK4a loss or cdk6 amplification in our model system. However, it is important to note that, because Rb loss was not seen in any tumor, our data do not rigorously exclude an Rb-independent role for p16INK4a and CDK6 in melanoma formation as has been suggested (37).
The most studied types of UV-induced DNA damage are C→T base substitutions at dipyrimidine sites leading to formation of pyrimidine dimers. The findings of such C→T mutation in human melanomas (8–10) has implicated p16INK4a as a target of UV-induced mutagenesis in human. However, UV radiation is known to induce a wide range of other DNA damages, including protein–DNA crosslinks, oxidative base damage, single-strand breaks (38), as well as chromosomal aberrations classically associated with double-strand breaks such as deletion (39). Although point mutations of p16INK4a were not detected in our model system, the emergence of cdk6 amplification in UV-treated murine melanomas, in light of the biochemical relationship between these two proteins (Fig. 4), is consistent with the observation of UV-signature mutation in human melanoma and provides further molecular support for the p16INK4a-pRB pathway as a target of UV's mutagenic action. In addition, CDK6 overexpression has been described in ≈40% of primary human tumors and cell lines (40), and large amplifications of 7q (including Cdk6 at 7q21–22) have been described in ≈50% of primary tumors (41). Importantly, this cross-species concordance of CDK6 involvement lends support to the view that data derived from genetically defined mouse models of human melanoma could guide the analysis of UV exposure history and CDK6 interactions in human populations.
Figure 4.
The Rb pathway is targeted in melanoma by UV light. The INK4a/ARF locus encodes two potent regulators of the cell cycle that function in genetically distinct pathways (p16INK4a–RB and p14ARF–p53). Although inactivation of both pathways seems crucial in melanoma formation, the data in this paper and the human genetics of melanoma have, to date, only identified targeting of components (underlined) of the Rb pathway by UV radiation. These results suggest that RB pathway inactivation by UV light is a major and rate-limiting step in melanoma formation.
In summary, this genetic model of melanoma points to Rb pathway inactivation as a major rate-limiting step in UV's melanoma-promoting actions and provides impetus for its comprehensive molecular examination in human cancer. Clearly, our data suggest that such an analysis should consider the possibility of a variety of Rb pathway lesions, including point mutations, genomic amplifications and deletions, and aberrations of expression. Likewise, several known components of the RB pathway (e.g., p16INK4a, CDK4, and CDK6, RB) warrant analysis in a well annotated collection of clinical samples with detailed UV exposure history and outcome. As UV exposure likely occurs decades before tumor development, our data would predict that the molecular targeting of RB pathway components might be detectable in dysplatic nevi years before their conversion to frank melanoma. Thus, the observation revealed in this work suggests a rational method of risk stratification in the large and clinically heterogeneous cohort of individuals with significant prior history of sunburn that is at increased risk of melanoma.
Acknowledgments
We thank G. Merlino and F. Noonan for advice on UV-induced tumorigenesis and N. Bardeesy, G. Merlino, and R. DePinho for critical reading of the manuscript. N.E.S. and M.B. were supported by grants from the National Institutes of Health and the Howard Hughes Medical Institute. This work was supported in part by grants from the National Institutes of Health, the Claudia Adams Barr Program in Cancer Research, and the Rockefeller Brothers Fund (to L.C.). L.C. is a Charles E. Culpeper Medical Scholar.
Abbreviation
- CGH
comparative genomic hybridization
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.SEER. SEER Cancer Statistics Review, 1973–1995. Bethesda: National Cancer Institute; 1998. [Google Scholar]
- 2.Chin L, Merlino G, DePinho R A. Genes Dev. 1998;12:3467–3481. doi: 10.1101/gad.12.22.3467. [DOI] [PubMed] [Google Scholar]
- 3.Gilchrest B A, Eller M S, Geller A C, Yaar M. N Engl J Med. 1999;340:1341–1348. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- 4.Harrison S L, MacLennan R, Speare R, Wronski I. Lancet. 1994;344:1529–1532. doi: 10.1016/s0140-6736(94)90348-4. [DOI] [PubMed] [Google Scholar]
- 5.Zanetti R, Franceschi S, Rosso S, Colonna S, Bidoli E. Eur J Cancer. 1992;28A:1172–1176. doi: 10.1016/0959-8049(92)90480-p. [DOI] [PubMed] [Google Scholar]
- 6.Atillasoy E S, Seykora J T, Soballe P W, Elenitsas R, Nesbit M, Elder D E, Montone K T, Sauter E, Herlyn M. Am J Pathol. 1998;152:1179–1186. [PMC free article] [PubMed] [Google Scholar]
- 7.Berking C, Takemoto R, Binder R L, Hartman S M, Ruiter D J, Gallagher P M, Lessin S R, Herlyn M. Carcinogenesis. 2002;23:181–187. doi: 10.1093/carcin/23.1.181. [DOI] [PubMed] [Google Scholar]
- 8.Pollock P M, Pearson J V, Hayward N K. Genes Chromosomes Cancer. 1996;15:77–88. doi: 10.1002/(SICI)1098-2264(199602)15:2<77::AID-GCC1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Kamb A, Gruis N A, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian S V, Stockert E, Day R S, III, Johnson B E, Skolnick M H. Science. 1994;264:436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- 10.Peris K, Chimenti S, Fargnoli M C, Valeri P, Kerl H, Wolf P. J Invest Dermatol. 1999;112:825–826. doi: 10.1046/j.1523-1747.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 11.Ruas M, Peters G. Biochim Biophys Acta. 1998;1378:F115–F177. doi: 10.1016/s0304-419x(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 12.Pavey S, Conroy S, Russell T, Gabrielli B. Cancer Res. 1999;59:4185–4189. [PubMed] [Google Scholar]
- 13.Piepkorn M. J Am Acad Dermatol. 2000;42:741–745. doi: 10.1067/mjd.2000.103988. [DOI] [PubMed] [Google Scholar]
- 14.Donawho C K, Kripke M L. Cancer Res. 1991;51:4176–4181. [PubMed] [Google Scholar]
- 15.Jamal S, Schneider R J. J Clin Invest. 2002;110:443–452. doi: 10.1172/JCI13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies H, Bignell G R, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett M J, Bottomley W, et al. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 17.Herlyn M, Satyamoorthy K. Am J Pathol. 1996;149:739–744. [PMC free article] [PubMed] [Google Scholar]
- 18.Chin L, Pomerantz J, Polsky D, Jacobson M, Cohen C, Cordon-Cardo C, Horner J W, II, DePinho R A. Genes Dev. 1997;11:2822–2834. doi: 10.1101/gad.11.21.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bardeesy N, Bastian B C, Hezel A, Pinkel D, DePinho R A, Chin L. Mol Cell Biol. 2001;21:2144–2153. doi: 10.1128/MCB.21.6.2144-2153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flores J F, Walker G J, Glendening J M, Haluska F G, Castresana J S, Rubio M P, Pastorfide G C, Boyer L A, Kao W H, Bulyk M L, et al. Cancer Res. 1996;56:5023–5032. [PubMed] [Google Scholar]
- 21.Walker G J, Flores J F, Glendening J M, Lin A H, Markl I D, Fountain J W. Genes Chromosomes Cancer. 1998;22:157–163. doi: 10.1002/(sici)1098-2264(199806)22:2<157::aid-gcc11>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 22.Sharpless N E, DePinho R A. Curr Opin Genet Dev. 1999;9:22–30. doi: 10.1016/s0959-437x(99)80004-5. [DOI] [PubMed] [Google Scholar]
- 23.FitzGerald M G, Harkin D P, Silva-Arrieta S, MacDonald D J, Lucchina L C, Unsal H, O'Neill E, Koh J, Finkelstein D M, Isselbacher K J, et al. Proc Natl Acad Sci USA. 1996;93:8541–8545. doi: 10.1073/pnas.93.16.8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randerson-Moor J A, Harland M, Williams S, Cuthbert-Heavens D, Sheridan E, Aveyard J, Sibley K, Whitaker L, Knowles M, Newton Bishop J, Bishop D T. Hum Mol Genet. 2001;10:55–62. doi: 10.1093/hmg/10.1.55. [DOI] [PubMed] [Google Scholar]
- 25.Rizos H, Puig S, Badenas C, Malvehy J, Darmanian A P, Jimenez L, Mila M, Kefford R F. Oncogene. 2001;20:5543–5547. doi: 10.1038/sj.onc.1204728. [DOI] [PubMed] [Google Scholar]
- 26.Sharpless N E, Bardeesy N, Lee K H, Carrasco D, Castrillon D H, Aguirre A J, Wu E A, Horner J W, DePinho R A. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- 27.Sharpless N E, Alson S, Chan S, Silver D P, Castrillon D H, DePinho R A. Cancer Res. 2002;62:2761–2765. [PubMed] [Google Scholar]
- 28.Krimpenfort P, Quon K C, Mooi W J, Loonstra A, Berns A. Nature. 2001;413:83–86. doi: 10.1038/35092584. [DOI] [PubMed] [Google Scholar]
- 29.Kamijo T, Bodner S, van de Kamp E, Randle D H, Sherr C J. Cancer Res. 1999;59:2217–2222. [PubMed] [Google Scholar]
- 30.Markel P, Shu P, Ebeling C, Carlson G A, Nagle D L, Smutko J S, Moore K J. Nat Genet. 1997;17:280–284. doi: 10.1038/ng1197-280. [DOI] [PubMed] [Google Scholar]
- 31.Williams B O, Remington L, Albert D M, Mukai S, Bronson R T, Jacks T. Nat Genet. 1994;7:480–484. doi: 10.1038/ng0894-480. [DOI] [PubMed] [Google Scholar]
- 32.Sharpless N E, Ferguson D O, O'Hagan R C, Castrillon D H, Lee C, Farazi P A, Alson S, Fleming J, Morton C C, Frank K, et al. Mol Cell. 2001;8:1187–1196. doi: 10.1016/s1097-2765(01)00425-7. [DOI] [PubMed] [Google Scholar]
- 33.Noonan F P, Recio J A, Takayama H, Duray P, Anver M R, Rush W L, De Fabo E C, Merlino G. Nature. 2001;413:271–272. doi: 10.1038/35095108. [DOI] [PubMed] [Google Scholar]
- 34.Kamijo T, Zindy F, Roussel M F, Quelle D E, Downing J R, Ashmun R A, Grosveld G, Sherr C J. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 35.Stott F J, Bates S, James M C, McConnell B B, Starborg M, Brookes S, Palmero I, Ryan K, Hara E, Vousden K H, Peters G. EMBO J. 1998;17:5001–5014. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan S H, Moritsugu J, Wahl G M. Proc Natl Acad Sci USA. 2000;97:3266–3271. doi: 10.1073/pnas.050560997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fahraeus R, Lane D P. EMBO J. 1999;18:2106–2118. doi: 10.1093/emboj/18.8.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Gruijl F R, van Kranen H J, Mullenders L H. J Photochem Photobiol B. 2001;63:19–27. doi: 10.1016/s1011-1344(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 39.Horiguchi M, Masumura K I, Ikehata H, Ono T, Kanke Y, Nohmi T. Cancer Res. 2001;61:3913–3918. [PubMed] [Google Scholar]
- 40.Tang L, Li G, Tron V A, Trotter M J, Ho V C. Melanoma Res. 1999;9:148–154. doi: 10.1097/00008390-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Bastian B C, LeBoit P E, Hamm H, Brocker E B, Pinkel D. Cancer Res. 1998;58:2170–2175. [PubMed] [Google Scholar]