Abstract
Staphylococcus aureus (S. aureus) represents a major threat to a broad range of healthcare and community associated infections. This bacterium has rapidly evolved resistance to multiple drugs throughout its antibiotic history and thus it is imperative to develop novel antimicrobial strategies to enrich the currently shrinking therapeutic options against S. aureus. This study evaluated the antimicrobial activity and therapeutic efficacy of oleic acid (OA) in a liposomal formulation as an innate bactericide against methicillin-resistant S. aureus (MRSA). In vitro studies showed that these OA-loaded liposomes (LipoOA) could rapidly fuse into the bacterial membranes, thereby significantly improving the potency of OA to kill MRSA compared with the use of free OA. Further in vivo tests demonstrated that LipoOA were highly effective in curing skin infections caused by MRSA bacteria and preserving the integrity of the infected skin using a mouse skin model. Moreover, a preliminary skin toxicity study proved high biocompatibility of LipoOA to normal skin tissues. These findings suggest that LipoOA hold great potential to become a new, effective, and safe antimicrobial agent for the treatment of MRSA infections.
Keywords: MRSA infection, Antimicrobial drug delivery, Free fatty acid, Oleic acid, Liposome
1. Introduction
Staphylococcus aureus (S. aureus) has been one of the major causes of fatal nosocomial infections as well as community-associated infections. Approximately 89.4 million people in the United States are colonized with S. aureus [1]. Over the past a few decades S. aureus has experienced several waves of antibiotic resistance and is now resistant to the entire β-lactam class of antibiotics including penicillins, cephalosporins and carbapenems [2]. These β-lactam antibiotic resistant S. aureus are also called methicillin resistant S. aureus (MRSA) or superbug. The proportion of healthcare-associated S. aureus infections due to MRSA has increased from 2% in 1974 to 64% in 2004 [3]. Increased burden of MRSA infections has led to prevalent use of vancomycin, the last remaining antibiotic to which MRSA is reliably susceptible. Note that as an inhibitory antibiotic against MRSA infections, vancomycin is incapable of eradicating the MRSA bacteria but inhibits their growth. Moreover, the extensive use of vancomycin has resulted in the emergence of vancomycin intermediate and resistant S. aureus strains since late 1990s [2,4,5]. All these facts underscore the undisputed and urgent need to develop new effective therapeutic approaches for MRSA treatment.
Recent efforts in new antimicrobial development against MRSA include new synthetic compounds from old antibiotic classes [6–9], immune globulins targeting virulence factors of MRSA [10–13], and natural antimicrobials such as cationic antimicrobial peptides, lipids, and other endogenous products [14–16]. Despite great success of clinical trials with the new synthetic compounds, their similarities in structure and antimicrobial mechanisms with the old classes of antibiotics have raised the concern that MRSA bacteria may rapidly develop resistance to these synthetic compounds after they are widely used in clinic [17]. Passive neutralization of bacterial virulence factors by injecting anti-toxin globulins and antibody fragments may be beneficial in ameliorating acute infections, but this approach does not possess any capability to inhibit or eradicate the bacteria, which, in a long run, could lead to recurrent infections. Natural antimicrobial products, on the other hand, represent a new promising solution against this hard-to-treat bacterium. Free fatty acids (FFAs) are a series of lipids that naturally exist in human skin, breast milk, and bloodstream and are important components of the innate immune system. A number of FFAs and their esters are known to possess antibacterial and antiviral activity, especially against Gram-positive bacteria [18–20]. Although the mechanism by which FFAs exert their antibacterial activity has not been fully understood, disrupting bacterial membranes and increasing membrane permeability have been suggested [21,22]. These endogenous bactericides are largely nonspecific and hold great promise to minimize bacterial drug resistance. For example, it has been reported that linoleic acid and dehydrocrepenynic acid, two unsaturated FFAs, can inhibit bacterial drug resistance by decreasing the transfer frequency of the conjugal DNA [23]. Therefore, FFAs provide great potential for the development of new antimicrobials against MRSA.
Nanoparticles such as solid nanospheres, liposomes, micelles, and nanoemulsions are novel drug delivery vehicles that have been widely employed to deliver therapeutic agents for the treatment of cancer and other notorious diseases [24–27]. Especially, nanoparticles consisting of natural lipids or biodegradable polymers provide unique advantages over other delivery vehicles for topical or systemic antimicrobial delivery [28]. In addition to tackling extracellular pathogens, antimicrobial nanoparticles can enter host cells through endocytosis and then release drug payloads to treat intracellular pathogens that have evolved ways of evading intracellular killing to survive within the cell [29,30]. For instance, liposomes are spherical lipid vesicles consisting of phospholipid bilayers that can not only improve the solubility of otherwise nonsoluble or partially soluble compounds but also fuse with biological membranes, and subsequently release their entrapped payloads into the cells or bacteria [24,31–33]. Therefore, unlike monoclonal antibody therapies, nanoparticles can reach bacteria that are inside the host cells, thereby treating intracellular infections. Moreover, nanoparticles carrying concentrated antimicrobials can increase the local intracellular concentration of antimicrobials after membrane fusion and thus decrease the high-dose requirement of free antimicrobials, resulting in less selective pressure for generation of bacterial drug resistance. Our group has previously demonstrated the synthesis and use of phospholipid liposomes to deliver lauric acid to the bacterial membranes of Propionibacterium acnes (P. acnes), a Gram-positive bacterium, for effective killing of the bacteria [33]. Herein, we report the antimicrobial activity of liposomal oleic acids (LipoOA) against MRSA infections. The synthesis and characterization of LipoOA and their fusion activity with MRSA bacteria are demonstrated. A mouse skin infection model is employed to evaluate the antimicrobial activity of LipoOA against MRSA infections and to test their toxicity to normal skin tissues.
2. Materials and Methods
2.1 Materials
Hydrogenated L-a-phosphatidylcholine (Egg PC), cholesterol and 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-lissamine rhodamine B sulfonyl (DMPE-RhB) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Oleic acid (OA), tryptic soy broth (TSB), phosphate buffered saline (PBS), trifluoroacetic acid (TFA), acetonitrile, and Sephadex G-75 were obtained from Sigma Aldrich (St Louis, MO). Agar was purchased from BD (Sparks, MD).
2.2 Preparation of MRSA bacteria
MRSA252 strain was cultured in TSB medium at 37 °C overnight in a rotary shaker. Then the culture was refreshed in TSB medium at 1:100 dilution at 37 °C under shaking for another two and a half hours till OD600 of the culture medium reached approximately 0.7 (logarithmic growth phase). The bacteria were harvested by centrifugation at 13,500 rpm for 10 minutes, and then washed with sterile PBS twice. After removal of PBS by centrifugation, the obtained bacteria pallet was then suspended in an appropriate amount of sterile PBS for future use.
2.3 Preparation and characterization of LipoOA
Liposomes were prepared by an extrusion method following a previously published protocol [33]. Briefly, LipoOA and bare liopsomes were prepared using 1.5 mg of Egg PC, cholesterol and OA at a weight ratio of 5:1:4 and 9:1:0, respectively. The lipid mixture was dissolved in 1 mL of chloroform and then dried with nitrogen gas for 10 minutes to remove all solvents. The dried lipid film was rehydrated with 3 mL of water or sterile PBS buffer (1X, pH = 7.4). The resulting lipid suspension was vortexed for 1 minute and then sonicated (Fisher Scientific FS30D) for 3 minutes to produce multilamellar vesicles (MLVs). Then a Ti-probe (Branson 450 sonifier) was used to sonicate the MLVs for 30 seconds at 20 W to produce large unilamellar vesicles (LUVs). The final small unilamellar vesicles (SUVs) were obtained by extruding LUVs through a polycarbonate membrane with 100 nm-sized pores for 11 times. The excess free OA was separated from LipoOA using a Sephadex G75 column equilibrated with PBS buffer (1X, pH = 7.4).
The hydrodynamic size (diameter, nm) and surface zeta potential (mV) of LipoOA and bare liposomes were measured using the Malvern Zetasizer ZS (Malvern Instruments, UK). The mean diameters of LipoOA were determined through dynamic light scattering (DLS) and the zeta potentials were determined from electrophoretic mobility measurements. The zeta potentials of liposomes were measured in water to eliminate the ionic strength effect from PBS. All characterization measurements were repeated three times at 25°C.
The encapsulation efficiency of OA in the synthesized LipoOA was determined using an Agilent 1100 series LC-MSD-Trap-SL system (Agilent Technologies, Santa Clara, CA) equipped with an electrospray ionization source. A fixed amount of LipoOA was dried by rotavapor (Buchi, Model R-124, Switzerland) and then dissolved in methanol for LC-MS measurements. Briefly, 30 μL samples were injected through a Supelco Discovery HS C18 (3 μm, 2.1 mm ID x 18.5 cm) column (Sigma Aldrich, St Louis, MO, USA). The mobile phase composed of 85 v% acetonitrile and 15 v% water/TFA (99.9:0.1, v/v) at a flow rate of 0.2 mL/min. The molecular mass of the effluent from the column was measured using negative ionization. The acquisition parameters were: N2 drying gas temperature of 350 °C, N2 drying gas flow rate of 10 L/min, and nebulizer of 25 psi. The acquired LC/MS chromatogram of OA was compared with a linear standard curve of OA at different concentrations to calculate the amount of OA encapsulated in LipoOA.
2.4 LipoOA-MRSA252 fusion studies
Fluorescent method was used to study the fusion of LipoOA with MRSA252. In this study, DMPE-RhB with a molar concentration of 0.5 mol% was mixed with Egg PC, cholesterol and OA for the preparation of fluorescently labeled LipoOA (LipoOA-RhB). Two sets of experiments will be conducted. First, LipoOA-RhB at different concentrations ranging from 6.25 μg/mL to 400 μg/mL was incubated with a fixed amount of MRSA252 (1.6x108 CFU/mL) with a total incubation volume of 1 mL at room temperature for 10 minutes. After incubation, the samples were centrifuged at 13,500 rpm for 10 minutes to separate the excess LipoOA-RhB from MRSA252. The obtained MRSA252 pallets were collected and resuspended in 1 mL PBS. Consequently, the fluorescence emission intensity of the bacteria at 590 nm were quantified by a fluorescent spectrophotometer (Infinite M200, TECAN, Switzerland) with an excitation wavelength of 550 nm. The background signal was subtracted from the measured fluorescence intensity of each sample to obtain the absolute intensity. Secondly, LipoOA-RhB at the concentration of 250 μg/mL was incubated with MRSA252 at different concentrations (1.6x108, 2.4x108, 3.2x108, and 6.4x108 CFU/mL, respectively) with a total incubation volume of 1 mL at room temperature for 10 minutes. Similar operations as above were conducted to evaluate the fusion activity between LipoOA and MRSA252.
2.5 In vitro antimicrobial activity of LipoOA against MRSA252
To determine the antimicrobial activity of LipoOA and free OA against MRSA252, a fixed amount of MRSA252 (1x106 CFU in 100 μL) was incubated with different concentrations of LipoOA (6.25–500 μg/mL in PBS buffer) and free OA (2.5–200 μg/mL in 5% dimethyl sulfoxide (DMSO) solution) at 37°C for 5 hours in a 96 well plate. After incubation, the mixtures were diluted at 1:10 to 1:106 with sterile PBS and 5 μL of the diluted suspension was spotted on TSB agar plate for overnight incubation at 37°C. Then the CFU of MRSA252 was quantified. PBS buffer, bare liposomes (without OA), and 5% DMSO were used as negative controls in the study.
2.6 In vivo antimicrobial activity of LipoOA against MRSA252 in mouse model
In order to better evaluate the anti-MRSA activity of LipoOA in the complex biological system, a proof-of-concept study was performed on a mouse skin model. The animal work was carried out following the guidelines of Institutional Animal Care and Use Committee of Veteran Affairs Medical Center. ICR mice (n = 3 for each group) were shaved to remove the back hair and the skin was disinfected with ethanol. 1×107 CFU of MRSA252 suspended in 100 μL sterile PBS were injected into the superficial skin of the mice. After 20 minutes, 100 μL LipoOA or 100 μL bare liposomes (without OA, in sterile PBS solution) were injected into the same site as MRSA252 injection. The mice were monitored for lesion development over 48 hours.
To image the infected skin tissues, the mice were euthanized 48 hours after the injection of MRSA252 and the infected skin was collected for tissue processing. The optimum cutting temperature (O.C.T.)-embedded slides were stained with hematoxylin and eosin (H&E) (Sigma diagnostics, St. Louis, MO) and viewed with an Olympus BX41 microscope (Olympus, USA). To determine the microbial burden after 48 hours of MRSA252 injection, the collected skin tissues were homogenized in 1 mL of sterile PBS with a mini-bead beater (BioSpec, Bartlesville, OK) for 2 minutes. The obtained suspensions were diluted 1:10 to 1:106 and then 5 μL of each diluted sample was spotted on a TSB agar plate for overnight culture at 37°C for quantifying the CFU of MRSA252 in the skin tissues.
2.7 Skin toxicity
ICR mice were shaved to remove the back hair and the skin was disinfected with ethanol. 100 μL of 200 μg free OA, bare liposomes (without OA), and LipoOA were injected into the superficial dorsal skin, respectively. As controls, the same volume of 5% DMSO and 100% DMSO were also injected. After 24 hours, the skin was removed from the injection sites for tissue processing. O.C.T.-embedded slides were stained with DeadEndTM Fluorometric TUNEL System (Promega Corporation, Madison, WI, USA) for apoptotic cell detection and counterstained with 4, 6-diamidino-2-phenylindole (DAPI). The pictures were taken by an Olympus BX41 microscope.
3. Results and discussion
3.1 Preparation and characterization of LipoOA
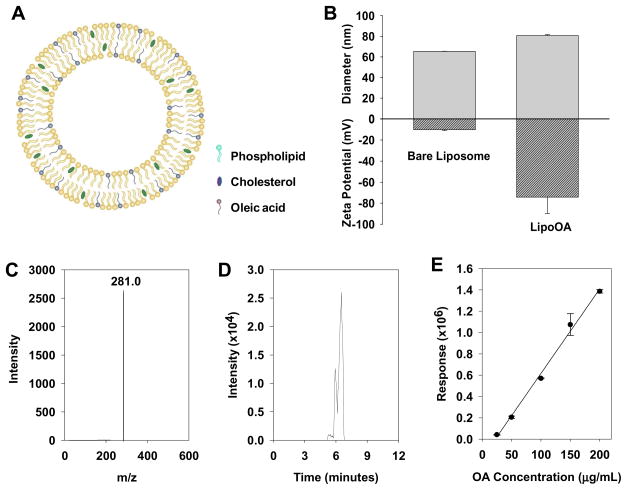
Liposomes made of egg PC and cholesterol possess a lipid bilayer structure as illustrated in Fig. 1A. This vesicular structure offers unique physicochemical properties for carrying and delivering FFAs such as oleic acid. The amphiphilic OA molecules can be readily entrapped in the hydrophobic lipid membranes by mixing OA with egg PC and cholesterol at desirable ratios prior to liposome preparation. The liposomal formulation of oleic acid (LipoOA) can improve the solubility of OA and enhance its dosing efficiency. When the liposomes fuse with bacterial membranes, the entrapped OA will be released into the bacterial membranes or the intracellular environment, resulting in higher local antimicrobial concentration and more efficient bactericidal activity.
Fig. 1.
Characterization of oleic acid-loaded liposomes (LipoOA). (A) Schematic structure of LipoOA consisting of phospholipid, cholesterol and OA. (B) Hydrodynamic size (diameter, nm) and surface zeta potential (mV) of LipoOA and bare liposomes (without OA) measured by dynamic light scattering (DLS). (C–E) Quantification of OA encapsulation efficiency in LipoOA by liquid chromatography-mass spectrometry (LC/MS). (C) Mass spectrum of OA. (D) LC/MS chromatogram of OA. (E) Corresponding linear calibration standard curve of OA measured by LC/MS. Data represents mean ± SD of three individual experiments.
The size of liposomes is a key parameter that determines their physicochemical properties and biological functionalities as a drug delivery vehicle. Small liposomes (diameter, < 50 nm) are very unstable and prone to fuse with one another or biological membranes due to their high surface tension. In contrast, large liposomes (diameter, >200 nm) are usually stable but may have difficulty to penetrate through skin for topical drug delivery, and thus fail to show advantages over free drugs [34,35]. Liposomes with moderate size range (50–100 nm) will have relatively prolonged stability [36], preserve the capability to fuse with bacterial or cell membranes, and possess good skin penetration ability [37]. In this study, LipoOA with a diameter of 80.6 ± 0.7 nm were prepared to assess their antimicrobial activity against MRSA252 bacteria (Fig. 1B). The quality of liposomes was characterized by polydispersity index (PDI), which was calculated from cumulant analysis of dynamics light scattering measurements. The PDI of bare liposomes and LipoOA were 0.17 ± 0.01 and 0.18 ± 0.01 respectively, indicating the relatively narrow distribution of liposome sizes. To confirm the incorporation of OA into the liposome membranes, changes in the electrostatic potential profile across the membrane and surface were determined by electrophoretic mobility measurements as summarized in Fig. 1B. The surface zeta potential of LipoOA formulated with 200 μg/mL initial OA input was −65.1 ± 1.4 mV in deionized water. In contrast, the zeta potential of the corresponding bare liposomes without OA was −10.1 ± 0.5 mV. The sharp decrease of surface zeta potential is attributed to the incorporation of OA molecules, whose carboxylic acid group is deprotonated to COO− at near physiological pH of 7.4.
Encapsulation efficiency of OA in LipoOA formulations was determined by liquid chromatography-mass spectrometry (LC/MS/MS). As shown in Fig. 1C, the mass spectrum of negative ion of OA [M-H]− at m/z 281.0 was detected and OA was eluted from chromatography column at 6 minutes (Fig. 1D). The encapsulation efficiency of OA in LipoOA was determined by comparing the measured signal of OA with a standard curve of OA ranging from 25 to 200 μg/mL (Fig. 1E). It was found that when the initial OA input concentration was 200 μg/mL, the final loading concentration of OA in the liposomes was 29.6 μg/mL, corresponding to a drug encapsulation efficiency of 14.7%. This result suggests that significant amount of OA was lost during the LipoOA preparation process including needle sonication, extrusion, and purification by Sephadex G75 column. A major source of OA loss is likely to be the Sephadex G75 column purification, where all free OA molecules and OA micelles in the LipoOA solution are removed. The possible adhesion and fusion of LipoOA with the beads in the column will also contribute to the loss of OA. Moreover, during the needle sonication and extrusion steps, some OA molecules can adhere to the needle, the glass vials, the polycarbonate membranes, and extrusion apparatus, resulting in the loss of OA.
3.2 Interaction between LipoOA and MRSA
The interaction between LipoOA and MRSA252 bacteria, a healthcare-associated MRSA strain, was investigated by monitoring the change in fluorescence intensity of MRSA252 after incubating with rhodamine B labeled LipoOA (LipoOA-RhB). If the fluorescent LipoOA can fuse with the bacterial membranes, it is expected to see increasing fluorescence intensity from the bacteria after removing excess free LipoOA. In the study, various concentrations of LipoOA-RhB were incubated with a fixed amount of MRSA252 bacteria (1.6x108 CFU/mL) for 10 minutes. As shown in Fig. 2A, the measured fluorescence intensity from the bacteria was linearly related to the concentration of LipoOA-RhB when it was in the range of 20~200 μg/mL. The fluorescence intensity reached a maximum value and remained as a plateau when the LipoOA-RhB concentration was higher than 200 μg/mL. These results suggest that each bacterium can continuously take up LipoOA till the bacterial membranes are saturated with LipoOA that no additional liposomes can fuse with the membranes. To further confirm this observation, a fixed concentration of LipoOA (250 μg/mL) was then incubated with various concentrations of MRSA252 ranging from 1.6x108 to 6.4x108 CFU/mL. As shown in Fig. 2B, the measured fluorescence intensity from the bacteria was linearly increased with increase of the MRSA252 concentrations. This result was consistent with the linear relationship observed in Fig. 2A. These fusion studies between LipoOA and MRSA252 bacteria clearly demonstrate that LipoOA can easily fuse into the bacterial membranes and the amount of fused liposomes are determined by the concentrations of both the liposomes and the bacteria.
Fig. 2.
Fluorescent studies to measure the fusion activity of LipoOA with MRSA252 bacteria. Rhodamine B labeled LipoOA (LipoOA-RhB) were incubated with MRSA252 bacteria for 10 minutes and then the excess LipoOA-RhB were removed. The fluorescence emission intensity at 590 nm of the treated MRSA252 bacteria was quantified with an excitation wavelength of 550 nm. (A) Fluorescence intensity of MRSA252 (1.6x108 CFU/mL) incubated with different concentrations of LipoOA-RhB, ranging from 6.25 μg/mL to 400 μg/mL. The fluorescence intensity of MRSA252 linearly increased and reached a plateau at the LipoOA-RhB concentration of 200 μg/mL. (B) Fluorescence intensity of MRSA252 at various bacteria concentrations (1.6x108, 2.4x108, 3.2x108, and 6.4x108 CFU/mL respectively) incubated with LipoOA-RhB at a constant concentration of 250 μg/mL. The fluorescence intensity of MRSA252 linearly increased with the concentration of the bacteria. Data represents mean ± SD of three individual experiments.
3.3 In vitro antimicrobial activity of LipoOA against MRSA
In vitro antimicrobial activity of LipoOA and free OA against MRSA252 was determined by measuring their minimal bactericidal concentration (MBC), respectively. Here the MBC was defined as the minimal concentration of an antimicrobial that kills 99.9% of the target bacteria. MRSA252 (1x106 CFU) was incubated with various concentrations of LipoOA and free OA for 5 hours and then subcultured on a TSB agar plate at 37 °C overnight. LipoOA killed all the MRSA252 at 12.5 μg/mL (Fig. 3B). Notably, the initial OA input to prepare LipoOA was 40 wt% of the total liposome weight and the LC/MS measured OA encapsulation efficiency was about 15 %. Therefore, the total OA concentration was about 0.8 μg/mL at the MBC value of the LipoOA, which was about 12 fold more effective than free OA, which had a MBC value of 10 μg/mL against MRSA252 (Fig. 3B). This enhanced antimicrobial activity of LipoOA can be explained by the fast fusion process between LipoOA with MRSA252 bacteria, which takes place within 10 minutes. By rapidly fusing into the bacterial membranes, LipoOA can induce a “burst” release of a lethal dose of OA to the bacterial membranes, thereby killing the bacteria even before they develop any possible resistance mechanisms. In contrast to the fast fusion process of LipoOA, free OA needs to diffuse into the bacterial membranes on an individual molecule base. The accumulation of OA in the bacterial membranes is time consuming and highly dependent on the OA concentration.
Fig. 3.
In vitro antimicrobial activity of LipoOA and free OA against MRSA252. LipoOA and free OA at various concentrations were incubated with MRSA252 (1x106 CFU/mL) for 5 hours in PBS and then the CFU of the bacteria was quantified through agar plate culture (A) The incubated LipoOA concentrations were 6.25, 12.5, 25, 50, 125, 250, and 500 μg/mL, respectively, dissolved in PBS buffer. The results showed that LipoOA completely killed the bacteria when its concentration was 12.5 μg/mL (corresponding to a OA concentration of 0.8 μg/mL) or higher. PBS and bare liposomes (without OA) served as negative controls. (B) The incubated OA concentrations were 2.5, 5, 10, 50, 100, and 200 μg/mL, respectively, dissolved in 5% DMSO solution. The results showed that OA completely killed the bacteria when its concentration was 10 μg/mL or higher. 5% DMSO was used as negative control. Data represents mean ± SD of three individual experiments. UD: undetectable.
3.4 In vivo antimicrobial activity of LipoOA against MRSA
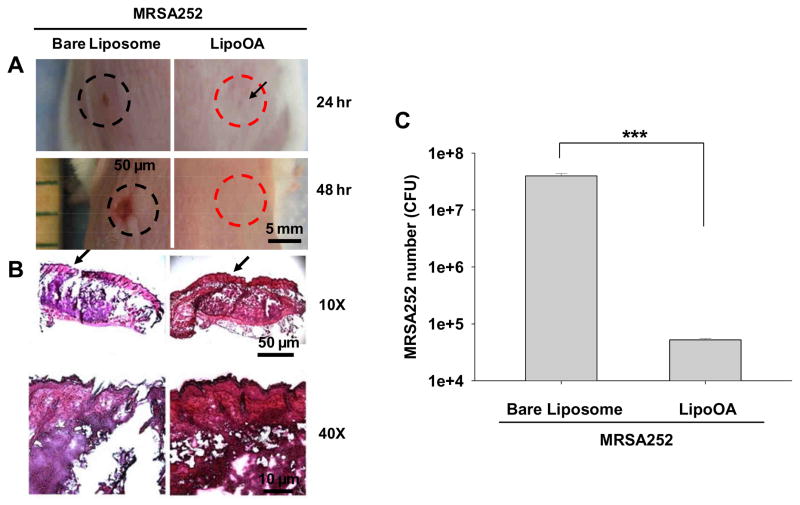
The antimicrobial activity and therapeutic efficacy of LipoOA against MRSA252 infections were further evaluated in ICR mouse skin. A proof-of-concept study was carried out by intradermally injecting MRSA252 followed by LipoOA injection into the dorsal skin of the mice. Bare liposomes (without OA) suspended in sterile PBS were included as a negative control. As expected, a pus-containing lesion started to appear 24 hours post injection on the back of the mice that were treated with bare liposomes whereas no lesion was observed in the mice that were treated with LipoOA (Fig. 4A). After 48 hours the lesion became more evident in the mice treated with bare liposomes, indicating the proliferation of the infection in these mice over time. In contrast, the mice treated with LipoOA did not present any apparent lesion (Fig. 4A). Histology analysis was conducted after 48 hours of MRSA252 injection to evaluate skin integrity. The skin tissues at the injection sites were collected for H&E staining. As shown in Fig. 4B, examination of the skin of the bare liposome-treated mice showed epidermal rupture upon MRSA252 injection. On the contrary, the skin of the LipoOA-treated mice preserved intact epidermis structure. Severe suppurative inflammatory infiltration was also observed in bare liposome-treated mouse skin while LipoOA-treated mice only showed minimal inflammation (Fig. 4B). Furthermore, to confirm the killing capability of LipoOA, the microbial burden was determined 48 hours post injection of MRSA252 and LipoOA. The skin tissues were homogenized in PBS and cultured on TSB agar plate overnight. The results showed that the amount of MRSA252 bacteria remaining in the skin of LipoOA-treated mice was approximately 500 times lower than those remaining in bare liposome-treated mice, with a p-value less than 0.001 from a Student’s t-test (Fig. 4C). These in vivo results further confirmed the bactericidal effect of LipoOA against MRSA252.
Fig. 4.
In vivo antimicrobial activity of LipoOA against MRSA252. The mice were injected with 1x107 CFU of MRSA252 to induce superficial skin lesion. 100 μL of LipoOA (200 μg/mL) and corresponding bare liposomes (without OA) were injected into the same sites respectively 20 minutes post injection of MRSA252. (A) Lesions at the injection sites after 24 and 48 hours of MRSA252 injection. (B) Histological analysis of H&E stained sections of the infected skin 48 hours post injection of MRSA252. Broken skin tissue was observed for the sites treated with bare liposomes while the skin remained intact for the sites treated with LipoOA. (C) Microbial burden after 48 hours of MRSA252 injection. The infected skin was removed from mice, homogenized and cultured on agar plate for counting the bacterial CFU. Data represents mean ± SD of three individual experiments. Asterisks denote p-value significance (*** p < 0.001).
3.5 Toxicity of LipoOA to normal skin tissues
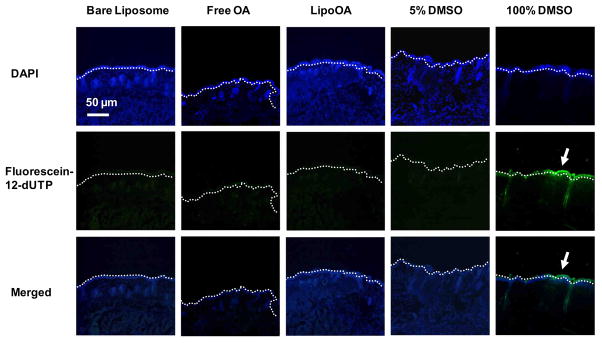
Liposomal formulations have been employed in cosmetics and pharmaceuticals to improve compound solubility and skin penetration, and have proved to be a safe and friendly cosmetic or pharmaceutical delivery system [38]. To evaluate any possible toxicity of LipoOA to normal skin tissues, the serial sections of ICR mouse skin treated with bare liposomes, free OA, LipoOA, 5% DMSO, and 100% DMSO were stained with fluorescein-12 dUTP for apoptotic cells and with DAPI for cell nuclei. Thus the apoptotic cells in the epidermal layer and dermal layer were stained green and the nuclei were counterstained blue. As shown in Fig. 5, no apparent epidermal cell apoptosis was observed in the skin treated with bare liposomes, free OA, LipoOA, or 5% DMSO demonstrating good biocompatibility of LipoOA with mouse skin. 100% DMSO is commonly known to be toxic to skin cells, which was demonstrated by the thick epidermal layer of apoptotic cells. Since human skin is generally thicker than mouse skin, it is reasonable to expect that LipoOA will not cause any toxicity to normal human epidermis.
Fig. 5.
TUNEL assay of apoptotic cells in mouse skin. Bare liposomes, 200 μg free OA, LipoOA, 5% DMSO and 100% DMSO were injected into the superficial dorsal skin of mice. 24 hours post-injection, the skin was collected for TUNEL assay and the nuclei of the cells were counterstained with DAPI, a blue fluorescent dye. The apoptotic cells were shown in green in the images. The results showed both LipoOA and free OA had undetectable toxicity to the mouse skin. All experiments were repeated three times.
3.6 General comments
While these results showed great therapeutic potential of LipoOA to benefit patients suffering from persistent skin MRSA infections, below are some future research perspectives to further explore LipoOA as a novel and robust anti-MRSA agent. First, for better patient compliance, it is worthwhile to test if LipoOA exhibits similar therapeutic effectiveness with topical application. Secondly, other regular antimicrobials can be incorporated into LipoOA for combination therapy, which may produce synergism among the different antimicrobial agents. For example, vancomycin, as a hydrophilic anti-MRSA agent, can be loaded into the aqueous compartment of LipoOA without interfering with the loading yield of OA. Lastly, LipoOA can be functionalized with a targeting ligand specific to the MRSA surface antigen and thus preferentially reach the pathogen in the circulation system. This approach can then be employed to treat systemic MRSA infections in a targeted manner. Studies in these directions are in progress and will be reported presently.
4. Conclusions
In this study, we demonstrated the therapeutic potential of liposomal oleic acids (LipoOA) to treat MRSA252 bacterial infections. The synthesized LipoOA with a size of about 80 nm were prone to fuse with bacterial membranes, and subsequently release the entrapped OA to the bacteria. Given the high OA dose of each LipoOA and its rapid fusion activity, the use of liposomes resulted in about 12-fold increase in the antimicrobial activity of OA against MRSA252. In vivo tests further proved that LipoOA were able to maintain an intact structure of the skin infected by MRSA252 by killing most of the bacteria within 48 hours. Finally, a skin toxicity test demonstrated good biocompatibility of LipoOA to normal mouse skin. Overall, this study highlights the promising possibility of using LipoOA as a new therapeutic option to the current antimicrobial strategies against MRSA infections.
Acknowledgments
LZ acknowledges a financial support from the University of California-San Diego (faculty startup funds and a faculty career development award). CH acknowledges financial support from NIH (R01-AI067395-01, R21-R022754-01, 1R41AR056169-01 and 5R21AI088147-02). We thank Sage Olsen for assistance in drawing cartoons.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, et al. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J Infect Dis. 2006;193:172–9. doi: 10.1086/499632. [DOI] [PubMed] [Google Scholar]
- 2.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–41. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klevens RM, Edwards JR, Tenover FC, McDonald LC, Horan T, Gaynes R. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992–2003. Clin Infect Dis. 2006;42:389–91. doi: 10.1086/499367. [DOI] [PubMed] [Google Scholar]
- 4.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–73. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 5.Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, Flannagan SE, et al. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science. 2003;302:1569–71. doi: 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- 6.Anderson SD, Gums JG. Ceftobiprole: an extended-spectrum anti-methicillin-resistant Staphylococcus aureus cephalosporin. Ann Pharmacother. 2008;42:806–16. doi: 10.1345/aph.1L016. [DOI] [PubMed] [Google Scholar]
- 7.Bergallo C, Jasovich A, Teglia O, Oliva ME, Lentnek A, de Wouters L, et al. Safety and efficacy of intravenous tigecycline in treatment of community-acquired pneumonia: results from a double-blind randomized phase 3 comparison study with levofloxacin. Diagn Microbiol Infect Dis. 2009;63:52–61. doi: 10.1016/j.diagmicrobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Florescu I, Beuran M, Dimov R, Razbadauskas A, Bochan M, Fichev G, et al. Efficacy and safety of tigecycline compared with vancomycin or linezolid for treatment of serious infections with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci: a Phase 3, multicentre, double-blind, randomized study. J Antimicrob Chemother. 2008;62 (Suppl 1):i17–28. doi: 10.1093/jac/dkn250. [DOI] [PubMed] [Google Scholar]
- 9.Nannini EC, Stryjewski ME. A new lipoglycopeptide: telavancin. Expert Opin Pharmacother. 2008;9:2197–207. doi: 10.1517/14656566.9.12.2197. [DOI] [PubMed] [Google Scholar]
- 10.Burnie JP, Matthews RC, Carter T, Beaulieu E, Donohoe M, Chapman C, et al. Identification of an immunodominant ABC transporter in methicillin-resistant Staphylococcus aureus infections. Infect Immun. 2000;68:3200–9. doi: 10.1128/iai.68.6.3200-3209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall AE, Domanski PJ, Patel PR, Vernachio JH, Syribeys PJ, Gorovits EL, et al. Characterization of a protective monoclonal antibody recognizing Staphylococcus aureus MSCRAMM protein clumping factor A. Infect Immun. 2003;71:6864–70. doi: 10.1128/IAI.71.12.6864-6870.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holden MTG, Feil EJ, Lindsay JA, Peacock SJ, Day NPJ, Enright MC, et al. Complete genomes of two clinical Staphylococcus aureus strains: Evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci USA. 2004;101:9786–91. doi: 10.1073/pnas.0402521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanagisawa C, Hanaki H, Natae T, Sunakawa K. Neutralization of staphylococcal exotoxins in vitro by human-origin intravenous immunoglobulin. J Infect Chemother. 2007;13:368–72. doi: 10.1007/s10156-007-0551-6. [DOI] [PubMed] [Google Scholar]
- 14.Cha JD, Moon SE, Kim JY, Jung EK, Lee YS. Antibacterial activity of sophoraflavanone G isolated from the roots of Sophora flavescens against methicillin-resistant Staphylococcus aureus. Phytother Res. 2009;23:1326–31. doi: 10.1002/ptr.2540. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich CL, Moyles D, Beveridge TJ, Hancock RE. Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria. Antimicrob Agents Chemother. 2000;44:2086–92. doi: 10.1128/aac.44.8.2086-2092.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madhuri, Shireen T, Venugopal SK, Ghosh D, Gadepalli R, Dhawan B, et al. In vitro antimicrobial activity of alpha-melanocyte stimulating hormone against major human pathogen Staphylococcus aureus. Peptides. 2009;30:1627–35. doi: 10.1016/j.peptides.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Aarestrup FM. Association between decreased susceptibility to a new antibiotic for treatment of human diseases, everninomicin (SCH 27899), and resistance to an antibiotic used for growth promotion in animals, avilamycin. Microb Drug Resist. 1998;4:137–41. doi: 10.1089/mdr.1998.4.137. [DOI] [PubMed] [Google Scholar]
- 18.Drake DR, Brogden KA, Dawson DV, Wertz PW. Thematic review series: Skin lipids - Antimicrobial lipids at the skin surface. J Lipid Res. 2008;49:4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Georgel P, Crozat K, Lauth X, Makrantonaki E, Seltmann H, Sovath S, et al. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect Immun. 2005;73:4512–21. doi: 10.1128/IAI.73.8.4512-4521.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skrivanova E, Marounek M, Dlouha G, Kanka J. Susceptibility of Clostridium perfringens to C-C fatty acids. Lett Appl Microbiol. 2005;41:77–81. doi: 10.1111/j.1472-765X.2005.01709.x. [DOI] [PubMed] [Google Scholar]
- 21.Chamberlain NR, Mehrtens BG, Xiong Z, Kapral FA, Boardman JL, Rearick JI. Correlation of carotenoid production, decreased membrane fluidity, and resistance to oleic acid killing in Staphylococcus aureus 18Z. Infect Immun. 1991;59:4332–7. doi: 10.1128/iai.59.12.4332-4337.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dye ES, Kapral FA. Characterization of a bactericidal lipid developing within staphylococcal abscesses. Infect Immun. 1981;32:98–104. doi: 10.1128/iai.32.1.98-104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith PA, Romesberg FE. Combating bacteria and drug resistance by inhibiting mechanisms of persistence and adaptation. Nat Chem Biol. 2007;3:549–56. doi: 10.1038/nchembio.2007.27. [DOI] [PubMed] [Google Scholar]
- 24.Castro GA, Ferreira LA. Novel vesicular and particulate drug delivery systems for topical treatment of acne. Expert Opin Drug Deliv. 2008;5:665–79. doi: 10.1517/17425247.5.6.665. [DOI] [PubMed] [Google Scholar]
- 25.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–82. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 26.Tong R, Cheng JJ. Anticancer polymeric nanomedicines. Polymer Rev. 2007;47:345–81. [Google Scholar]
- 27.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: Therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–69. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Pornpattananangku D, Hu CM, Huang CM. Development of nanoparticles for antimicrobial drug delivery. Curr Med Chem. 2010;17:585–94. doi: 10.2174/092986710790416290. [DOI] [PubMed] [Google Scholar]
- 29.Drulis-Kawa Z, Dorotkiewicz-Jach A. Liposomes as delivery systems for antibiotics. Int J Pharm. 2010;387:187–98. doi: 10.1016/j.ijpharm.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 30.Kisich KO, Gelperina S, Higgins MP, Wilson S, Shipulo E, Oganesyan E, et al. Encapsulation of moxifloxacin within poly(butyl cyanoacrylate) nanoparticles enhances efficacy against intracellular Mycobacterium tuberculosis. Int J Pharm. 2007;345:154–62. doi: 10.1016/j.ijpharm.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 31.Lieb LM, Ramachandran C, Egbaria K, Weiner N. Topical delivery enhancement with multilamellar liposomes into pilosebaceous units: I. In vitro evaluation using fluorescent techniques with the hamster ear model. J Invest Dermatol. 1992;99:108–13. doi: 10.1111/1523-1747.ep12611886. [DOI] [PubMed] [Google Scholar]
- 32.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 33.Yang DR, Pornpattananangkul D, Nakatsuji T, Chan M, Carson D, Huang CM, et al. The antimicrobial activity of liposomal lauric acids against Propionibacterium acnes. Biomaterials. 2009;30:6035–40. doi: 10.1016/j.biomaterials.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Natsuki R, Morita Y, Osawa S, Takeda Y. Effects of liposome size on penetration of dl-tocopherol acetate into skin. Biol Pharm Bull. 1996;19:758–61. doi: 10.1248/bpb.19.758. [DOI] [PubMed] [Google Scholar]
- 35.Pornpattananangkul D, Olson S, Aryal S, Sartor M, Huang CM, Vecchio K, et al. Stimuli-responsive liposome fusion mediated by gold nanoparticles. ACS Nano. 4:1935–42. doi: 10.1021/nn9018587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dragicevic-Curic N, Winter S, Krajisnik D, Stupar M, Milic J, Graefe S, et al. Stability evaluation of temoporfin-loaded liposomal gels for topical application. J Liposome Res. 2010;20:38–48. doi: 10.3109/08982100903030263. [DOI] [PubMed] [Google Scholar]
- 37.Dragicevic-Curic N, Grafe S, Gitter B, Winter S, Fahr A. Surface charged temoporfin-loaded flexible vesicles: In vitro skin penetration studies and stability. Int J Pharm. 2010;384:100–08. doi: 10.1016/j.ijpharm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 38.de Leeuw J, de Vijlder HC, Bjerring P, Neumann HA. Liposomes in dermatology today. J Eur Acad Dermatol Venereol. 2009;23:505–16. doi: 10.1111/j.1468-3083.2009.03100.x. [DOI] [PubMed] [Google Scholar]