Abstract
Present cancer treatment strategies are based on the assumption that a therapy may work (“response”) or not work (“no-response”). However, the existing evidence suggests that current cancer treatment modalities may also have a cancer-promoting effect in part of the patients. In this paper, some relevant data are reviewed suggesting that surgery, irradiation, chemotherapy and immunotherapy can stimulate tumor growth / metastatic spread and decrease survival of patients in certain subgroups. Thus, results of cancer treatment may be improved by detection and use of biomarkers that correlate with positive or negative therapeutic effects. Small trials based on groups with differing biomarkers rather than large phase III trials may aid the development and efficacy testing of new anticancer drugs. Moreover, ignoring biomarkers that correlate with positive or negative therapeutic effect may not be compatible anymore with the ethical principle “First Do No Harm”.
Keywords: Surgery, Radiotherapy, Chemotherapy, Immunotherapy, Cancer-promoting effects
Introduction
“…when trials were first developed for use in agriculture, researchers were presumably concerned about the effect of interventions on the overall size and quality of the crop rather than on the wellbeing of any individual plant.”
P. M. Rothwell, 2005 [1]
Survival of patients with some forms of cancer (e.g., testicular cancer, Hodgkin’s disease, acute childhood leukemia) has improved dramatically during the last decades due to advances in chemotherapeutic regimens as well as surgical and radiotherapeutic techniques. The price of cure or long term survival are acute toxic effects (e.g. radiation pneumonitis, acute renal failure, sepsis), chronic toxic effects (e.g. pulmonary fibrosis, congestive heart failure, graft versus host disease, neurological syndromes, infertility, hypothyroidism) and second malignancies [2]. As cancer treatments have become more effective, it has even been suggested to tailor therapies based on late toxicities rather than survival [3]. A question that has received far less attention is whether present cancer treatment modalities may actually have a cancer-promoting effect. The aim of the present review is to provide the evidence, suggesting that surgery, irradiation, chemotherapy and immunotherapy may stimulate tumor growth and / or metastatic spread and by these or other mechanisms decrease the survival of certain patient subgroups.
Cancer-promoting effects of surgery
Presently, surgery is the most effective primary modality of therapy against cancer and possible detrimental effects of primary tumor removal are rarely discussed. Nevertheless, most cancer surgeons have observed rapid tumor regrowth shortly after primary surgery [4, 5].
Some experimental evidence has indicated that removal of a primary tumor (and / or surgical stress) may enhance the growth of residual tumor and the development of metastases [5–9]. Appearance of growth-stimulating factor in serum after primary tumor removal [9], abrogation of immune or nonimmunologic growth inhibiting factors [8], increased angiogenesis [5] and surgical stress induced immunosuppression [10] have been implicated as possible mechanisms for this phenomenon. It has been show in an experimental mouse model, that presence of the primary tumor influences antitumor mechanisms against the secondary tumor, although the nature of these mechanisms is not completely understood [11]. Thus, the presence of a primary tumor might have a protective effect against metastases.
For obvious ethical reasons, comparative clinical trials to evaluate the influence of primary tumor surgery on metastatic spread have not been performed. Evidence that surgery provokes activation of “latent” metastases in early breast cancer was presented by Michael Retsky and his colleagues [12]. This evidence was generated by interpretation of the results of natural history databases and clinical trials using hazard rate plots. A patient presenting with a primary breast tumor along with distant metastases is uncommon (0%, 3% and 7% in stages I, II and III, respectively). However, 18 months after diagnosis and therapy distant metastases are detected in 5% stage I and in 25% stage III breast cancer patients. The explanation of this fact could be that the clinical appearance of metastases is triggered or accelerated after the primary tumor has been removed [13]. Mean survival of patients with locally advanced or metastatic breast cancer is approximately two and a half years. However, 18% of untreated patients even with locally advanced or overt metastatic disease survived 5 years and 0.8% patients survived 15 years. A small group of breast cancer patients with untreated localized disease could be identified with near 70% 5-year survival. On the other hand, only 23% of patients treated with radical mastectomy survive 10 years [13].
Cancer-promoting effects of radiotherapy
Radiation therapy has been in use as a cancer treatment for more than a century, with its earliest roots traced from the discovery of x-rays in 1895 by Wilhelm Röntgen. Two-thirds of all cancer patients will be treated with radiotherapy at some point in their life [14]. Ionizing radiation causes DNA damage through induction of breaks in one or both of DNA strands. Since cancer cells display deregulated cell cycle control and increased proliferation they are more vulnerable to irradiation than normal cells which usually are able to repair the DNA damage or to induce cell death in those cells in which DNA damage cannot be repaired.
The effect of radiation on cancer cell or normal cell does not always take place immediately after treatment. Some of the cells will stay unchanged for weeks or months after treatment. This is due to the fact that radiation damaged cells can stay alive as long as all DNA is present. However, proper cell division is impossible after DNA is damaged. These radiation damaged cells can initiate a second cancer somewhere else in the body.
Numerous studies confirmed that radiotherapy increases the risk of second malignancies. Radiation-induced bone cancers and soft tissue sarcomas have been observed in long-term survivors of hereditary retinoblastoma. Most deaths from these secondary malignancies occurred within 30 years after retinoblastoma diagnosis [15]. In patients with Hodgkin’s lymphoma, causes other than primary disease contribute most to excess mortality 10 years after radiotherapy. Solid tumors, especially from the digestive and respiratory tract, contribute most to this excess mortality, followed by cardiovascular disease [16]. Statistically significantly increased risks of solid cancers (lung, colon, bladder, pancreas, pleura and esophagus) are observed among long-term survivors of testicular cancer treated with radiotherapy alone [17]. Men who receive radiotherapy for localized prostate cancer have an increased risk of bladder cancer compared to patients undergoing radical prostatectomy and compared to the general population. The risk of rectal cancer is increased in patients who receive external beam radiotherapy compared to radical prostatectomy [18].
The incidence of second malignancies caused by radiotherapy is relatively low. Second solid malignancies are rarely seen before 10 years, with increasing incidence thereafter. From this point of view, benefits of radiotherapy clearly outweigh its potential harm and the widespread use of this form of cancer treatment seems justified [19]. On the other hand, radiotherapy-induced second cancers illustrate the cancer-promoting effect of this treatment modality.
Similarly to surgical eradication of a primary tumor, radiation therapy may also induce the explosive growth of previously dormant metastases in experimental models [20, 21]. This phenomenon has been explained by the decrease of the angiostatin production which is regulated by the primary tumor [21].
Cancer-promoting effects of chemotherapy
Chemotherapy has provided curative treatments for some forms of cancer (acute childhood leukemia, testicular cancer, and Hodgkin’s disease) that were previously fatal. However, the role of chemotherapy in other more common cancers is quite modest, with survival improvement measuring few months or only few weeks [22].
One of the ironies of chemotherapeutic successes has been the recognition that many cytotoxic drugs are themselves carcinogenic. It is worth mentioning, that death receptors, once thought to primarily induce cytotoxic signaling cascades, recently have been shown to initiate multiple signaling pathways, including regulation of cell proliferation and tumor-promoting activities [23]. The carcinogenic risk is especially great with alkylating agents and the epipodophyllotoxins, but carcinogenicity has also been described for antimetabolites, anthracyclines, cisplatin and others. Most often, these chemotherapeutic agents cause the development of acute myeloid leukemia, high-grade non-Hodgkin’s lymphoma, and myelodysplastic syndrome. The risk of secondary leukemia peaks at around 5 years after treatment [24].
It has been observed that if patients receive suboptimal doses of chemotherapeutic drugs, they are not cured, and their survival is shorter compared to patients who receive optimal doses (although is not always clear what doses should be considered “optimal”) [25, 26]. Even with optimal doses, chemotherapy is not likely to be effective for all patients. A number of molecular markers have been identified that have predictive value for the outcome of treatment with chemotherapeutic drugs of non-small cell lung cancer [27], pancreas [28], breast [29], colorectal cancer [30], diffuse large B-cell lymphoma [31] and other cancers. The efficacy and toxicity of current chemotherapeutic regimens may depend on genetic factors, like cytochrome P450 polymorphisms [32]. It has been shown that the risk of breast cancer mortality is increased in tamoxifen users with decreased CYP2D6 activity [33].
Chemotherapy is infamous for its side effects, encompassing virtually every system in the body [24]. Some of these side effects, like cardiotoxicity, are potentially lethal [34]. Thus, in some patients for whom chemotherapy is not curative, detrimental or even lethal effects of chemotherapeutic agents are likely to outweigh modest antitumor effect and thereby decrease survival of a patient. Our unpublished observations in a rat model have shown that docetaxel treatment of multidrug resistant prostate cancer decreases survival time of animals [JJLJ et al., manuscript in preparation]. Furthermore, patients undergoing antineoplastic treatment experience various changes in the immune system, which not only render them susceptible to infections, but also might have an overall effect on the risk of relapse [35].
The generally held view is than a placebo group in cancer clinical trials is unethical. Therefore, a cancer promoting-effect of chemotherapy may remain obscured. Experimental animal research, however, has shown that chemotherapy can even stimulate the metastatic spread of the primary tumor [36, 37].
Cancer-promoting effects of immunotherapy
In the past few decades, immunotherapy has become the fourth cancer treatment modality. Presently, intravesical immunotherapy with Bacillus Calmette-Guérin (BCG) is regarded as the most effective protection against recurrence and progression in high-risk bladder cancer patients [38]. Recombinant cytokines (interferon-α and interleukin-2) demonstrate reproducible activity in some forms of cancer, including renal cell carcinoma and melanoma. Despite complete or partial response rate with cytokines is rather low, some patients achieve long-lasting remissions [39–41]. Monoclonal antibodies, vaccination therapy and adoptive cell transfer are rapidly evolving fields of cancer immunotherapy.
The “immunostimulatory theory” of tumor growth was proposed by Prehn in the 1970s [42]. Several investigators have demonstrated in experimental models that immunotherapy against established tumors or against residual tumor cells can produce not only inhibitory or null effects, but also can stimulate tumor growth and spread of metastases [43–47].
Probably the first immunotherapy for which detrimental effects were observed was interferon-γ. Adjuvant treatment with this cytokine decreased overall survival and/or disease-free survival compared with observation arm (although not always statistically significantly) in high-risk melanoma [48], colon cancer [49] and small-cell lung cancer patients [50].
Interferon-α (IFN-α) has produced only modest benefits in unselected advanced renal carcinoma patients. Meta-analysis of 42 randomized, controlled trials of IFN-α, including 4,216 patients, revealed the overall survival advantage of 3.8 months for IFN-α treated patients [41]. Despite this benefit of IFN-α was quite modest, for considerable period of time IFN-α was the de facto standard of care of metastatic renal cell carcinoma worldwide. Regulatory agencies have supported the use of IFN-α as the control arm for randomized trials with new therapies [41]. Therefore, it was impossible to perform a study including a control group of untreated renal cell carcinoma patients. Obviously, a control group of untreated patients is necessary to determine the biomarkers of response or non-response.
In the late nineties, our group has studied the prognostic and predictive significance of immunologic markers in peripheral blood of renal cell carcinoma patients. During 1995–1999, IFN-α was just being introduced in Lithuania, and no strict guidelines towards its use for treatment of metastatic renal cell carcinoma were available. Due to considerable variability in approach to treatment of metastatic renal cell carcinoma patients in Lithuania, we were able to select subgroups of patients treated and non-treated with IFN-α. Peripheral blood lymphocyte subsets were determined using flow cytometry. Thus, we had the opportunity to analyze the predictive significance of peripheral blood lymphocyte subsets in patients with advanced renal cell carcinoma. We found remarkable differences in overall survival of advanced renal cell carcinoma patients based on peripheral blood levels of CD8highCD57+ lymphocytes [51]. In our analysis, the median survival of patients with <30% CD8highCD57+ lymphocytes in the CD8+ subset was 23.5 months (the “relatively good prognosis group”), whereas the median survival of patients with ≥ 30% CD8highCD57+ lymphocytes in the CD8+ subset was only 6 months (the “bad prognosis group”). Treatment with IFN-α significantly increased the overall survival of the “bad prognosis group” renal cell carcinoma patients (from 6 to 18.5 months). In contrast, a trend towards decreased overall survival was observed in the “relatively good prognosis group” after treatment with IFN-α (13.6 months of IFN-α-treated patients vs. 23.5 months of patients non-treated with IFN-α) (Fig. 1) [51]. Although we admit the drawbacks of our study due to its retrospective nature, the remarkable differences in patient survival related to counts of CD8highCD57+ lymphocytes cannot be ignored. The utility of CD57+ expression in T lymphocytes to measure functional immune deficiency in patients with autoimmune disease, infectious diseases, and cancers has recently been reviewed [52].
Fig. 1.
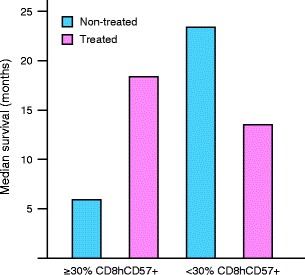
Median survival times of advanced renal cell carcinoma patients according to treatment with IFN-α and percentage of CD8highCD57+ lymphocytes in CD8+ subset (in peripheral blood) [51]
Similarly to IFN-α therapy of renal cell carcinoma, adjuvant therapy with IFN-α of high-risk melanoma is only minimally effective in a nonselected patient population [53]. In a recent systematic review and meta-analysis of 14 randomized controlled trials including a total of 8,122 patients, statistically significant improvement in overall survival of high-risk melanoma patients treated with IFN-α vs. comparator regimen or observation was shown [54]. However, the authors of this systematic review and meta-analysis admit that this improvement cannot be considered satisfying in terms of anticancer efficacy.
Several reports have suggested the involvement of CD8+CD57+ T lymphocytes in control of melanoma progression [55–57]. In our study of melanoma patients treated with adjuvant IFN-α, we observed remarkable differences in survival depending on pre-treatment levels of CD8highCD57+ lymphocytes in peripheral blood. Median survival time of patients with >23% CD8highCD57+ lymphocytes in CD8+ subset was 14.2 months, whereas median survival time of patients with <23% CD8highCD57+ lymphocytes was not reached at the time of analysis (median follow-up 24.6 months). The cut-off level of 23% in the present study was found by separating patients with increasing values of CD8highCD57+ lymphocytes during the first few months of treatment with IFN-α from those with decreasing values. This means that lower (<23%) pre-treatment values of these lymphocytes tend to increase, whereas higher values (>23%) tend to decrease during therapy. Thus, IFN-α seems to induce opposite changes in peripheral blood CD8highCD57+ lymphocyte levels (i.e. increase or decrease) depending on their pre-treatment values [58]. The opposite action of adjuvant IFN-α on immune parameters in different subgroups of patients may explain the null or minimal effect of this treatment on the whole population of unselected melanoma patients.
Probably the most recent examples of detrimental effects of cancer immunotherapy include the failure of several vaccine trials. The survival of stage III and stage IV melanoma patients receiving the allogeneic cancer vaccine Canvaxin™ was shorter compared with untreated patients. The large phase III EORTC 18961 trial of adjuvant ganglioside vaccine GMK in 1,314 patients with stage II melanoma was stopped earlier because of inferior survival in the vaccine arm [59].
Conclusions and future perspectives
In this paper, the data have been reviewed suggesting that current cancer treatment modalities may also have a cancer-promoting effect (Fig. 2). These data do not mean that current cancer therapies are not beneficial. In some cases, like radiotherapy of localized cancer, benefits of treatment clearly outweigh the risk of second cancers developing after long latent period. In other cases, like cytokine therapy of renal cell carcinoma or melanoma the benefit / risk ratio seems much more uncertain. A cancer promoting-effect of many therapies may remain obscured due to the generally held view that an untreated group in cancer clinical trials is unethical.
Fig. 2.

Summary of data indicating cancer-promoting effects of surgery, radiotherapy, chemotherapy and immunotherapy
The evidence reviewed in this paper challenges the assumption that in cancer patients who do not respond to therapy, the course of disease is the same as without treatment. The existing data highlight the possibility of the qualitative heterogeneity of relative treatment effect, defined as the treatment effect being in different directions in different groups of patients [1]. Thus, a subgroup of patients may have beneficial effects from a therapy, whereas another subgroup may have detrimental effects. This possibility has major implications for cancer clinical practice and for testing efficacy of new drugs.
The results of cancer treatment may be improved by using biomarkers that correlate with positive or negative therapeutic effect. Great numbers of biomarkers have been detected, but few of them are used in clinical practice. The suspicion that a given therapy has a cancer-promoting effect in a subgroup of patients might provide an urgent stimulus to introduce and to use the predictive biomarkers. Therefore, the most relevant for implementation biomarkers should be selected and applied in clinical practice.
Large clinical trials always may contain subgroups in which beneficial or detrimental effects are obtained. As a result, the total effect of the therapeutic intervention would be nullified. This would be a strong argument against large clinical phase III trials. Thus, small comparative trials based on groups of patients with different predictive biomarkers may be preferable for testing efficacy of new drugs [22, 60]. Another argument in favor of small comparative trials based on predictive biomarkers is that a lower number of patients would suffer a detrimental effect of an investigative therapy, if such a detrimental effect would exist. Given the possibility of a cancer-promoting effect of an anticancer intervention, a group of untreated patients might be acceptable from the ethical point of view.
In conclusion, the probability of a cancer-promoting effect of current cancer treatment modalities emphasizes the importance of prediction and personalized approach in oncology. Ignoring biomarkers that correlate with positive or negative therapeutic effect may not be compatible anymore with the ethical principle “First Do No Harm”.
Acknowledgments
The authors thank Professor Willem Den Otter (Department of Urology, VU University Medical Centre) for critical comments. JJLJ acknowledges a grant from SNFK, Amsterdam, the Netherlands.
Conflict of interest
The authors declare that they have no conflict or competing interests.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–86. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 2.Ganz PA. The price of anticancer intervention. Treatment-induced malignancy. Lancet Oncol. 2002;3:575–6. doi: 10.1016/S1470-2045(02)00851-3. [DOI] [PubMed] [Google Scholar]
- 3.Ganz PA. Harnessing personalised medicine to prevent late effects. Lancet Oncol. 2010;11:7–9. doi: 10.1016/S1470-2045(09)70344-4. [DOI] [PubMed] [Google Scholar]
- 4.Lundy J. Anesthesia and surgery: a double-edged sword for the cancer patient. J Surg Oncol. 1980;14:61–5. doi: 10.1002/jso.2930140109. [DOI] [PubMed] [Google Scholar]
- 5.Lee JW, Shahzad MM, Lin YG, et al. Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res. 2009;15:2695–702. doi: 10.1158/1078-0432.CCR-08-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson-Herren L, Sanford AH, Holmquist JP. Effects of surgery on the cell kinetics of residual tumor. Cancer Treat Rep. 1976;60:1749–60. [PubMed] [Google Scholar]
- 7.Gunduz N, Fisher B, Saffer EA. Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res. 1979;39:3861–5. [PubMed] [Google Scholar]
- 8.Nomi S, Naito K, Kahan BD, Pellis NR. Effects of concomitant and sinecomitant immunity on postsurgical metastasis in mice. Cancer Res. 1986;46:6111–5. [PubMed] [Google Scholar]
- 9.Fisher B, Gunduz N, Coyle J, et al. Presence of a growth-stimulating factor in serum following primary tumor removal in mice. Cancer Res. 1989;49:1996–2001. [PubMed] [Google Scholar]
- 10.Carter JJ, Feingold DL, Oh A, et al. Perioperative immunomodulation with Flt3 kinase ligand or a whole tumor cell vaccine is associated with a reduction in lung metastasis formation after laparotomy in mice. Surg Innov. 2006;13:41–7. doi: 10.1177/155335060601300107. [DOI] [PubMed] [Google Scholar]
- 11.Characiejus D, Dullens HF, Den Otter W. Mechanisms of tumour rejection in the murine DBA/2-SL2 concomitant immunity system. Cancer Immunol Immunother. 1990;32:179–84. doi: 10.1007/BF01771454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Retsky M, Bonadonna G, Demicheli R, et al. Hypothesis: Induced angiogenesis after surgery in premenopausal node-positive breast cancer patients is a major underlying reason why adjuvant chemotherapy works particularly well for those patients. Breast Cancer Res. 2004;6:R372–4. doi: 10.1186/bcr804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baum M, Demicheli R, Hrushesky W, Retsky M. Does surgery unfavourably perturb the “natural history” of early breast cancer by accelerating the appearance of distant metastases? Eur J Cancer. 2005;41:508–15. doi: 10.1016/j.ejca.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 14.Hogle WP. The state of the art in radiation therapy. Semin Oncol Nurs. 2006;22:212–20. doi: 10.1016/j.soncn.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Marees T, van Leeuwen FE, de Boer MR, et al. Cancer mortality in long-term survivors of retinoblastoma. Eur J Cancer. 2009;45:3245–53. doi: 10.1016/j.ejca.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol. 2003;21:3431–9. doi: 10.1200/JCO.2003.07.131. [DOI] [PubMed] [Google Scholar]
- 17.Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40, 576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–65. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 18.Nieder AM, Porter MP, Soloway MS. Radiation therapy for prostate cancer increases subsequent risk of bladder and rectal cancer: a population based cohort study. J Urol. 2008;180:2005–9. doi: 10.1016/j.juro.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 19.Hoskin P. The price of anticancer intervention. Secondary malignancies after radiotherapy. Lancet Oncol. 2002;3:577–8. [PubMed] [Google Scholar]
- 20.von Essen CF. Radiation enhancement of metastasis: a review. Clin Exp Metastasis. 1991;9:77–104. doi: 10.1007/BF01756381. [DOI] [PubMed] [Google Scholar]
- 21.Camphausen K, Moses MA, Beecken WD, et al. Radiation therapy to a primary tumor accelerates metastatic growth in mice. Cancer Res. 2001;61:2207–11. [PubMed] [Google Scholar]
- 22.Stewart DJ, Kurzrock R. Cancer: the road to Amiens. J Clin Oncol. 2009;27:328–33. doi: 10.1200/JCO.2008.18.9621. [DOI] [PubMed] [Google Scholar]
- 23.Guicciardi ME, Gores GJ. Life and death by death receptors. FASEB J. 2009;23:1625–37. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowenthal RM, Eaton K. Toxicity of chemotherapy. Hematol/Oncol Clin North Am. 1996;10:967–90. doi: 10.1016/S0889-8588(05)70378-6. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman D, Longo DL. Hodgkin’s disease. Crit Rev Oncol Hematol. 1992;13:135–87. doi: 10.1016/1040-8428(92)90021-H. [DOI] [PubMed] [Google Scholar]
- 26.Carmo-Pereira J, Costa FO, Henriques E, et al. A comparison of two doses of adriamycin in the primary chemotherapy of disseminated breast carcinoma. Br J Cancer. 1987;56:471–3. doi: 10.1038/bjc.1987.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West H, Lilenbaum R, Harpole D, et al. Molecular analysis-based treatment strategies for the management of non-small cell lung cancer. J Thorac Oncol. 2009;4:S1029–39. doi: 10.1097/JTO.0b013e3181b27170. [DOI] [PubMed] [Google Scholar]
- 28.Eguchi H, Ishikawa O, Ohigashi H, et al. Serum REG4 level is a predictive biomarker for the response to preoperative chemoradiotherapy in patients with pancreatic cancer. Pancreas. 2009;38:791–8. doi: 10.1097/MPA.0b013e3181ac5337. [DOI] [PubMed] [Google Scholar]
- 29.Derenzini M, Brighenti E, Donati G, et al. The p53-mediated sensitivity of cancer cells to chemotherapeutic agents is conditioned by the status of the retinoblastoma protein. J Pathol. 2009;219:373–82. doi: 10.1002/path.2612. [DOI] [PubMed] [Google Scholar]
- 30.Godai TI, Suda T, Sugano N, et al. Identification of colorectal cancer patients with tumors carrying the TP53 mutation on the codon 72 proline allele that benefited most from 5-fluorouracil (5-FU) based postoperative chemotherapy. BMC Cancer. 2009;9:420. doi: 10.1186/1471-2407-9-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart DA, Bahlis N, Mansoor A. pY-STAT3 and p53 expression predict outcome for poor prognosis diffuse large B-cell lymphoma treated with high dose chemotherapy and autologous stem cell transplantation. Leuk Lymphoma. 2009;50:1276–82. doi: 10.1080/10428190903015628. [DOI] [PubMed] [Google Scholar]
- 32.van Schaik RH. CYP450 pharmacogenetics for personalizing cancer therapy. Drug Resist Updat. 2008;11:77–98. doi: 10.1016/j.drup.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Bijl MJ, van Schaik RH, Lammers LA, et al. The CYP2D6*4 polymorphism affects breast cancer survival in tamoxifen users. Breast Cancer Res Treat. 2009;118:125–30. doi: 10.1007/s10549-008-0272-2. [DOI] [PubMed] [Google Scholar]
- 34.Viale PH, Yamamoto DS. Cardiovascular toxicity associated with cancer treatment. Clin J Oncol Nurs. 2008;12:627–38. doi: 10.1188/08.CJON.627-638. [DOI] [PubMed] [Google Scholar]
- 35.Lehrnbecher T, Koehl U, Wittekindt B, et al. Changes in host defence induced by malignancies and antineoplastic treatment: implication for immunotherapeutic strategies. Lancet Oncol. 2008;9:269–78. doi: 10.1016/S1470-2045(08)70071-8. [DOI] [PubMed] [Google Scholar]
- 36.Geldof AA, Rao BR. Doxorubicin treatment increases metastasis of prostate tumor (R3327-MatLyLu) Anticancer Res. 1988;8:1335–9. [PubMed] [Google Scholar]
- 37.De Larco JE, Wuertz BR, Manivel JC, et al. Progression and enhancement of metastatic potential after exposure of tumor cells to chemotherapeutic agents. Cancer Res. 2001;61:2857–61. [PubMed] [Google Scholar]
- 38.Böhle A. Bladder cancer: meta-analysis of BCG versus mitomycin C—a deeper insight? Nat Rev Urol. 2010;7:8–10. doi: 10.1038/nrurol.2009.253. [DOI] [PubMed] [Google Scholar]
- 39.Den Otter W, Jacobs JJ, Battermann JJ, et al. Local therapy of cancer with free IL-2. Cancer Immunol Immunother. 2008;57:931–50. doi: 10.1007/s00262-008-0455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Margolin K. Cytokine therapy in cancer. Expert Opin Biol Ther. 2008;8:1495–505. doi: 10.1517/14712598.8.10.1495. [DOI] [PubMed] [Google Scholar]
- 41.McDermott DF, Atkins MB. Immunotherapy of metastatic renal cell carcinoma. Cancer J. 2008;14:320–4. doi: 10.1097/PPO.0b013e31818675c4. [DOI] [PubMed] [Google Scholar]
- 42.Prehn RT. An immune reaction may be necessary for cancer development. Theor Biol Med Model. 2006;3:6. doi: 10.1186/1742-4682-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly SA, Gschmeissner S, East N, et al. Enhancement of metastatic potential by gamma-interferon. Cancer Res. 1991;51:4020–7. [PubMed] [Google Scholar]
- 44.Lollini PL, Bosco MC, Cavallo F, et al. Inhibition of tumor growth and enhancement of metastasis after transfection of the gamma-interferon gene. Int J Cancer. 1993;55:320–9. doi: 10.1002/ijc.2910550224. [DOI] [PubMed] [Google Scholar]
- 45.Siegel CT, Schreiber K, Meredith SC, et al. Enhanced growth of primary tumors in cancer-prone mice after immunization against the mutant region of an inherited oncoprotein. J Exp Med. 2000;191:1945–56. doi: 10.1084/jem.191.11.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiarella P, Reffo V, Bruzzo J, et al. Therapeutic anti-tumor vaccines: from tumor inhibition to enhancement. Clin Med Oncol. 2008;2:237–45. doi: 10.4137/cmo.s538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fifis T, Lam I, Lin D, et al. Vaccination with in vitro grown whole tumor cells induces strong immune responses and retards tumor growth in a murine model of colorectal liver metastases. Vaccine. 2008;26:241–9. doi: 10.1016/j.vaccine.2007.10.068. [DOI] [PubMed] [Google Scholar]
- 48.Meyskens FL, Jr, Kopecky KJ, Taylor CW, et al. Randomized trial of adjuvant human interferon gamma versus observation in high-risk cutaneous melanoma: a Southwest Oncology Group study. J Natl Cancer Inst. 1995;87:1710–3. doi: 10.1093/jnci/87.22.1710. [DOI] [PubMed] [Google Scholar]
- 49.Wiesenfeld M, O’Connell MJ, Wieand HS, et al. Controlled clinical trial of interferon-gamma as postoperative surgical adjuvant therapy for colon cancer. J Clin Oncol. 1995;13:2324–9. doi: 10.1200/JCO.1995.13.9.2324. [DOI] [PubMed] [Google Scholar]
- 50.Jett JR, Maksymiuk AW, Su JQ, et al. Phase III trial of recombinant interferon gamma in complete responders with small-cell lung cancer. J Clin Oncol. 1994;12:2321–6. doi: 10.1200/JCO.1994.12.11.2321. [DOI] [PubMed] [Google Scholar]
- 51.Characiejus D, Pasukoniene V, Kazlauskaite N, et al. Predictive value of CD8highCD57+ lymphocyte subset in interferon therapy of patients with renal cell carcinoma. Anticancer Res. 2002;22:3679–83. [PubMed] [Google Scholar]
- 52.Focosi D, Bestagno M, Burrone O, et al. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol. 2010;87:107–16. doi: 10.1189/jlb.0809566. [DOI] [PubMed] [Google Scholar]
- 53.Janku F, Kurzrock R. Adjuvant interferon in high-risk melanoma: end of the era? J Clin Oncol. 2010;28:e15–6. doi: 10.1200/JCO.2009.24.9326. [DOI] [PubMed] [Google Scholar]
- 54.Mocellin S, Pasquali S, Rossi CR, et al. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2010;102:493–501. doi: 10.1093/jnci/djq009. [DOI] [PubMed] [Google Scholar]
- 55.Cartei G, Sala PG, Sanzari M, et al. Reduced lymphocyte subpopulations in patients with advanced or disseminated melanoma. J Am Acad Dermatol. 1993;28:738–44. doi: 10.1016/0190-9622(93)70103-Z. [DOI] [PubMed] [Google Scholar]
- 56.Elliott GT, McLeod RA, Perez J, Von Eschen KB. Interim results of a phase II multicenter clinical trial evaluating the activity of a therapeutic allogeneic melanoma vaccine (theraccine) in the treatment of disseminated malignant melanoma. Semin Surg Oncol. 1993;9:264–72. [PubMed] [Google Scholar]
- 57.Batliwalla FM, Bateman BA, Serrano D, et al. A 15-year follow-up of AJCC stage III malignant melanoma patients treated postsurgically with Newcastle disease virus (NDV) oncolysate and determination of alterations in the CD8 T cell repertoire. Mol Med. 1998;4:783–94. [PMC free article] [PubMed] [Google Scholar]
- 58.Characiejus D, Pasukoniene V, Jonusauskaite R, et al. Peripheral blood CD8highCD57+ lymphocyte levels may predict outcome in melanoma patients treated with adjuvant interferon-alpha. Anticancer Res. 2008;28:1139–42. [PubMed] [Google Scholar]
- 59.Eggermont AM. Therapeutic vaccines in solid tumours: can they be harmful? Eur J Cancer. 2009;45:2087–90. doi: 10.1016/j.ejca.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Jacobs JJL, Characiejus D, Scheper RJ, et al. The Amiens strategy: small phase III trials for clinically relevant progress in the war against cancer. J Clin Oncol. 2009;27:3062–3. doi: 10.1200/JCO.2009.22.5359. [DOI] [PubMed] [Google Scholar]


