Abstract
Oxidation products of low-density lipoproteins have been suggested to promote inflammation during atherogenesis, and reticulocyte-type 15-lipoxygenase has been implicated to mediate this oxidation. In addition, the 5-lipoxygenase cascade leads to formation of leukotrienes, which exhibit strong proinflammatory activities in cardiovascular tissues. Here, we studied both lipoxygenase pathways in human atherosclerosis. The 5-lipoxygenase pathway was abundantly expressed in arterial walls of patients afflicted with various lesion stages of atherosclerosis of the aorta and of coronary and carotid arteries. 5-lipoxygenase localized to macrophages, dendritic cells, foam cells, mast cells, and neutrophilic granulocytes, and the number of 5-lipoxygenase expressing cells markedly increased in advanced lesions. By contrast, reticulocyte-type 15-lipoxygenase was expressed at levels that were several orders of magnitude lower than 5-lipoxygenase in both normal and diseased arteries, and its expression could not be related to lesion pathology. Our data support a model of atherogenesis in which 5-lipoxygenase cascade-dependent inflammatory circuits consisting of several leukocyte lineages and arterial wall cells evolve within the blood vessel wall during critical stages of lesion development. They raise the possibility that antileukotriene drugs may be an effective treatment regimen in late-stage disease.
Keywords: arachidonic acid cascade‖coronary heart disease
Atherosclerosis, the disease that gives rise to myocardial infarction, stroke, and vascular occlusive disease of the extremities, is the principal cause of mortality in industrialized countries (1, 2). Risk factors for atherosclerosis have been identified by epidemiological studies, but disease-initiating mechanisms largely remain elusive (1). The response to injury hypothesis (3–5) emphasizes that atherosclerosis is a chronic inflammatory fibroproliferative disease of the arterial wall that is associated with aberrant immune reactions. In addition, the lipid oxidation hypothesis (6, 7) proposes that oxidized low-density lipoproteins (LDL) may trigger arterial wall injury and facilitate foam cell formation.
The reticulocyte-type 15-lipoxygenase (15-LO), a family member of non-heme iron-containing dioxygenases, has been implicated in LDL oxidation (6–17). However, species-dependent, i.e., pro- versus antiatherogenic, roles of 15-LO family members have been observed (16). In addition, actions of the enzyme that are independent of LDL oxidation have been considered (18). However, although its animal counterparts have been studied in considerable detail, information on 15-LO expression in human atherogenesis is very limited: Analyses of atherosclerotic lesions of the aorta (AAO) indicated its expression in foam cells (17), and stereochemical analyses of oxidized cholesteryl linoleate in lesion lipids (11, 15) were consistent with its presence in diseased arterial walls. By contrast, the relevance of the 5-LO cascade (19–24) and leukotriene receptors (LT-Rs) for atherogenesis has received less attention.
To gain insight into the relation of LOs and human atherosclerosis, we initiated studies at predilection sites of the disease, i.e., coronary and carotid arteries and the aorta. We found that 5-LO was abundantly present in monocytes/macrophages, dendritic cells (DCs), mast cells, and neutrophilic granulocytes, and that the number of 5-LO+ cells markedly increased in advanced lesions. By contrast, 15-LO was barely detectable and its expression was unrelated to atherosclerosis pathology. These data are consistent with the concept that the 5-LO cascade generates circuits of inflammation during critical stages of atherogenesis.
Methods
Materials.
5-LO activating protein (FLAP) antiserum was a kind gift of J. Evans (Merck); SYBR Green and avian myeloblastosis virus reverse transcriptase was from Roche Diagnostics; platinumTaq (Thermus aquaticus) DNA polymerase was from Invitrogen; and all other reagents were from Sigma unless otherwise stated.
Patient Details.
Information on patients is reported in Supporting Text and Table 1, which are published as supporting information on the PNAS web site, www.pnas.org. Specimens from the Pathobiological Determinants of Atherosclerosis in Youth (pday) program were obtained from the Department of Pathology, Louisiana State University Medical Center, New Orleans.
Cell Culture and Assays.
Immunoblots and real-time RT-PCR analyses were determined as reported in Supporting Text (and see Table 2, which is published as supporting information on the PNAS web site). Morphometry was performed at ×200 magnification in sections that had been graded according to the American Heart Association (AHA) system (25, 26), adjacent to sections used for RT-PCR analyses. Cells were recorded positive when antigen could be associated with a nucleus (hematoxylin) by using a Sony XC-711P video camera and the Q500/W image analysis system (Leica, Bensheim, Germany). Data were analyzed using the one-sided Mann–Whitney U test.
Immunohistochemistry.
Analyses and specificity tests were performed on 8-μm cryostat sections with LO and leukocyte marker antisera as described in Supporting Text (and see Table 3, which is published as supporting information on the PNAS web site) and as reported (27–29). For localization of 5-LO in foam cells and for morphometry, alkaline phosphatase-labeled streptavidin-biotin was used. AHA classification was performed on parallel sections stained with Oil red O and hematoxylin/eosin. Three-dimensional reconstruction of 5-LO protein was done using 3D FOR LSM software (Zeiss).
Results
Expression of LO Transcripts and Protein in Human Atherosclerotic Lesions.
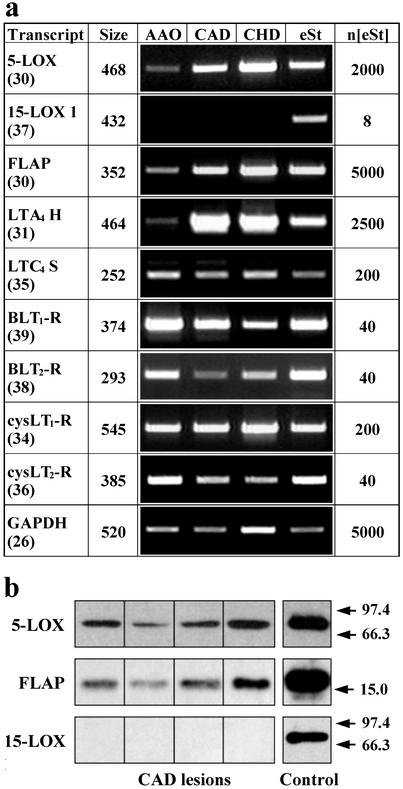
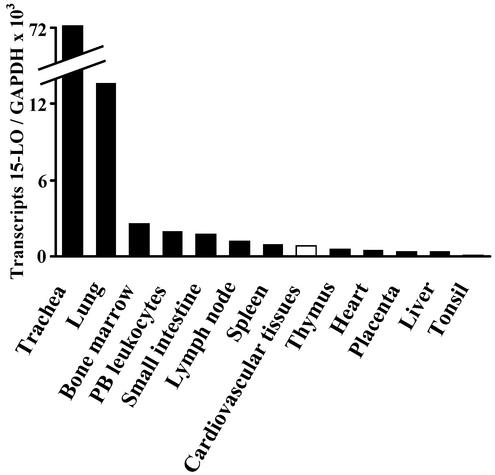
We determined absolute transcript levels of both LOs at three predilection sites of the disease, i.e., AAO, carotid artery disease (CAD), and coronary heart disease (CHD), by using real-time RT-PCR analyses. We also graded lesion stages according to the AHA system (25, 26) to determine expression levels of LOs in atherosclerotic plaques of individual patients. However, we excluded type VI lesions that are associated with plaque rupture, hemorrhage, and thrombosis (26), because these transcripts may originate from trapped leukocytes rather than from leukocytes that had infiltrated the arterial wall during bona fide atherogenesis. 5-LO transcripts were readily observed, whereas, surprisingly, reticulocyte-type 15-LO transcripts were either undetectable or found at low levels (Fig. 1a; see below). Real-time PCR analyses provided quantitative information on LO transcripts in CAD lesions. Because we found 18,900 ± 12,300 5-LO transcripts per 105 GAPDH transcripts (n = 7), and because the lowest point of the linear portion of the LO standard curve represented eight cDNA standard molecules (see Fig. 5, which is published as supporting information on the PNAS web site), 5-LO mRNA may constitute an abundant mRNA in advanced CAD lesions. Although below detection limits or barely detectable in most of our RT-PCR assays of 40 specimens (38 patients), 15-LO transcripts were readily found in human bronchial epithelium (27), trachea, human lung (Fig. 2), and IL-4-stimulated monocytes and DCs (28, 29). 5-LO transcripts in CAD lesions (type V lesions; n = 7) exceeded those of 15-LO transcripts by more than three orders of magnitude (Fig. 1a; and see Fig. 5). When 5- and 15-LO transcripts were compared across all lesions (type 0–V) of all specimens (n = 40), 5-LO transcripts exceeded 15-LO transcripts by a factor of ≈600. Transcripts of additional constituents of the 5-LO cascade (19), i.e., LTA4 hydrolase, FLAP, LTC4 synthase, and four LT-Rs (30–33), were identified by RT-PCR in AAO, CAD, and CHD (Fig. 1a). In view of this unexpected divergence between expression levels of both LOs, a detailed evaluation of RT-PCR was performed. This included sequencing of PCR products of all 5-LO cascade constituents from RNA extracts of AAO, CAD, and CHD, as well as the PCR product of 15-LO from human lung; similar sensitivities of both LO real-time RT-PCR assays were established. We excluded the possibility of inhibitors in tissue RNA extracts of the 15-LO amplification reaction and determined integrities of RNAs and correlated RNA integrity to the number of 15- and 5-LO transcripts. Each of these analyses supported the conclusion of undetectable or low expression of the 15-LO gene throughout (lesion stages 0–V) human atherogenesis. Moreover, when we determined 5- and 15-LO mRNA degradation kinetics in human lung (see Fig. 6, which is published as supporting information on the PNAS web site), there was no evidence for differential 5- and 15-LO mRNA degradation. When the mean of 15-LO transcripts across all lesion stages of atherosclerosis were compared with 15-LO transcripts in human tissue RNA extracts, 15-LO expression in several tissues other than trachea and lung exceeded that in cardiovascular tissues (Fig. 2).
Figure 1.
Expression of LO in atherosclerotic lesions. (a) LO transcripts in AAO, CAD, and CHD lesions. First column, transcript species; cycle numbers of samples in third through sixth columns are indicated in brackets; second row, sizes of PCR products in bp; third through sixth columns, ethidium bromide-stained PCR products of AAO, CAD, CHD lesions, and amplified external standards (eSt); seventh column, numbers of standard cDNA molecules added to PCR buffer before amplification (n[eSt]). (b) LO and FLAP immunoblots of CAD type V lesions. For controls, 2 ng of each 5- and 15-LO proteins was applied; for FLAP, an unspecified amount of leukocyte membrane protein was applied. Positions of MW standards are indicated by arrows at right.
Figure 2.
Comparison of 15-LO transcripts in human tissue RNA extracts. Multiple-tissue RNAs or tracheal and bronchial ring RNA were subjected to 15-LO and GAPDH real-time RT-PCR analyses. Data for cardiovascular tissues represent the mean of real-time RT-PCR assays performed on tissues of 38 patients (42 cardiovascular specimens) afflicted with all lesion types of atherosclerosis.
In view of abundant 5-LO and FLAP transcripts in CAD lesions, we determined whether these transcripts were translated into their corresponding proteins. When 10 μg of total CAD lesion protein extract was subjected to immunoblot analyses, each of four CAD lesions contained significant amounts of 5-LO and FLAP proteins (Fig. 1b). Although our anti-15-LO antisera detected similar amounts of 15-LO standard, this LO protein remained undetectable in immunoblots of CAD lesions (Fig. 1b). These data indicated that 5-LO and FLAP proteins, but not 15-LO protein, were present in type V CAD lesions.
Identification of 5-LO+ Leukocytes in Atherosclerotic Lesions.
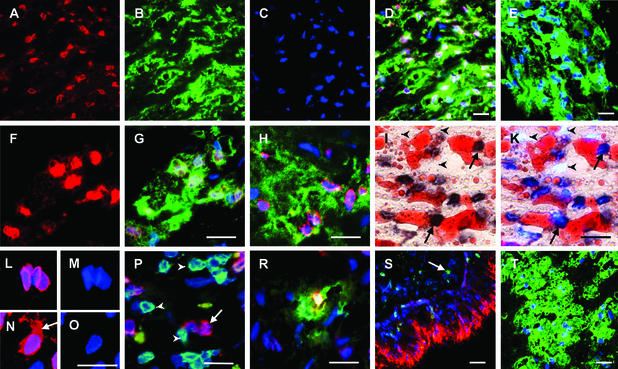
We determined the lineage(s) and phenotypes of 5-LO+ cells and searched for 15-LO+ cells. A large number of cells stained for 5-LO in advanced CAD lesions (Fig. 3 A–D). The majority of 5-LO+ cells were CD68+ indicating 5-LO expression in macrophages and/or DCs (Figs. 3B and 4). Moreover, a subpopulation of CD68+ cells also stained positive for the DC (Langerhans phenotype) marker antigen, CD1a (ref. 34; Fig. 3H). This indicated the presence of two 5-LO+/CD68+ cell populations, one resembling a macrophage phenotype and the other a DC phenotype (35). Another marker of DCs, i.e., DC lysosomal-associated membrane protein (36), was found less frequently, whereas the Langerhans cell marker, langerin (37), remained undetectable. Most 5-LO+ cells strongly stained for HLA-DR, indicating that the majority of 5-LO+ cells were activated macrophages and/or DCs capable of antigen presentation (Fig. 3G). Many 5-LO+ cells in CAD accumulated lipid droplets (Fig. 3 I and K). Because 5-LO was coexpressed with CD1a and CD68, we designate the two lipid-accumulating cell populations 5-LO+ macrophage foam cells and 5-LO+ DC foam cells. Three-dimensional construction of 5-LO immunohistochemical positivity indicated that the bulk of 5-LO protein resided in the nuclear envelope rather than in the chromatin (Fig. 3 L and M). In a subpopulation of cells, however, 5-LO was also significantly expressed in the cytosol (Fig. 3 N and O, arrow). 5-LO+ cells were often found in proximity of 5-LO−/CD3+ T lymphocytes (Fig. 3P). This cellular microenvironment of activated 5-LO+ cells raises the possibility that they interact with T cells and thereby execute a T cell-dependent immune reaction. It also became apparent that neither endothelial cells (ECs) nor smooth muscle cells (SMCs) expressed 5-LO, although both were strongly MHC-II+. Another 5-LO+ cell population consisted of mast cells (Fig. 3R) whose number was most prominent in the lamina adventitia, but mast cells were also found in the lamina intima, and a minor population of 5-LO+ cells were neutrophilic CD66b+ granulocytes (not shown). Similar results were obtained in CHD and AAO (not shown). We next attempted to identify 15-LO+ cells. Our 15-LO antisera specifically stained bronchial epithelial cells (Fig. 3S), and double LO staining of human lung revealed that alveolar and subepithelial 5-LO+ macrophages lacked 15-LO, whereas 15-LO immunostaining in human lung other than bronchial epithelium was invariably associated with eosinophil peroxidase staining (Fig. 3S; see Fig. 7, which is published as supporting information on the PNAS web site). By contrast to abundant 5-LO+ cells in all arterial wall laminae (with the exception of rare eosinophils in the lamina adventitia), no 15-LO+ cells could be detected throughout atherogenesis (Fig. 3T) in 67 patients (CHD, n = 39; AAO, n = 22; CAD, n = 6; see Table 1, which is published as supporting information on the PNAS web site). However, we identified seven cases of 15-LO+ cells in the lamina adventitia. In three patients, aortae contained 15-LO+/keratin+/vimentin+ cell tubes adjacent to a distant portion of the lamina adventitia (Fig. 7). In another aorta specimen, we found eosinophils within a leukocyte infiltrate located in the lamina adventitia and portions of the outer lamina media (AHA type V lesion) that thus was classified periarteriitis; additional specimens showed rare eosinophils in the lamina adventitia and, in exceptional cases, in the lamina intima. Because 15-LO protein remained undetectable in the lamina intima in each of 78 patients with the exception of very rare eosinophils, 15-LO could not be associated with atherosclerosis lesion pathology. These data revealed absence or very low expression of 15-LO and abundant 5-LO+ leukocytes in atherosclerotic lesions.
Figure 3.
Identification of 5-LO+ cells in CAD lesions. (Bars = 20 μm.) (A–D) 5-LO is expressed in macrophages and/or DCs. (A) 5-LO (Cy3 red). (B) CD68 (Cy2 green). (C) DNA stained with DAPI blue. (D) Merge of A–C. (E) Preadsorption of antiserum abolishes nuclear 5-LO staining of a foam-cell-rich area: CD68 (Cy2 green), DAPI blue. Note absence of Cy2 red 5-LO staining. (F and G) Cells containing 5-LO express high levels of HLA-DR. (F) 5-LO (Cy3 red). (G) Same as F, plus HLA-DR (Cy2 green) and DAPI blue. (H) A subpopulation of 5-LO-expressing cells has a DC phenotype; colocalization of 5-LO (Cy3 red), CD1a (Cy2 green), and DAPI blue. (I and K) Foam cells express 5-LO. (I) 5-LO (streptavidin-biotin dark brown) and lipid (Sudan IV red). (K) Same as I, plus DAPI blue. 5-LO+ cells are indicated by arrows and 5-LO− cells are indicated by arrowheads. (L–O) 5-LO is located in the nucleus and in the cytoplasm of macrophages; 3-dimensional reconstruction of 12-μm stacks of confocal images. (L) 5-LO (Cy3 red) and DAPI blue. (M) DAPI blue only of L. (N) 5-LO (Cy3 red) and DAPI blue. The cytoplasmic 5-LO+ compartment is indicated by an arrow. (O) DAPI blue only of N. (P) 5-LO-expressing cells are located in the vicinity of CD3+ lymphocytes. A 5-LO+ (Cy3 red) cell is indicated by the arrow and CD3+ (Cy2 green) cells by the arrowheads (DAPI blue). (R) 5-LO is expressed in lesion mast cells. 5-LO (Cy3 red), mast cell tryptase (Cy2 green), and DAPI blue. (S) Positive control showing 15-LO staining in human lung and 5-LO staining in lung macrophages. A normal medium-sized human bronchus from a patient afflicted with lung carcinoma was analyzed for 15-LO (Cy3 red), 5-LO (Cy2 green; indicated by the arrow), and DAPI blue. (T) Advanced CAD lesions lack 15-LO-expressing cells. A foam-cell-rich area of a CAD lesion was analyzed for CD68 (Cy2 green) and DAPI blue. Note the absence of Cy2 red 15-LO staining.
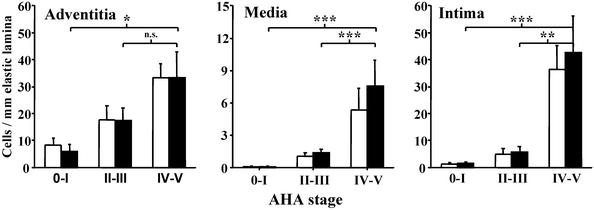
Figure 4.
5-LO+ cells increase during transition from early to advanced CHD. The numbers of CD68+ macrophages/foam cells/DCs (open bars) and 5-LO+ cells (filled bars) were determined in arterial wall laminae of type 0–V lesions and grouped for combined 0–I (n = 13), II–III (n = 9), and IV–V (n = 7) lesions. Cell numbers were determined in specimens of the left-descending circumflex coronary artery from 29 patients. A total of 7,300 CD68+ macrophages/foam cells/DCs, 7,902 5-LO+ cells, 713 mast cell tryptase+ cells, and 120 CD66b+ cells were counted. Data represent means of 29 specimens ± SEM. n.s., not significant. *, P < 0.05; **, P < 0.001; ***, P < 0.0001.
5-LO+ Cells Increase in CHD During Transition from Early to Advanced Lesions.
There is little information on the kinetics of leukocyte infiltration of arterial wall laminae during human atherogenesis (38–40). To determine these kinetics and those of 5-LO+ cells, we performed morphometric analyses of patients afflicted with CHD AHA stages 0–V. We first determined cell numbers of CD68+, mast cell tryptase+, and CD66b+, and 5-LO+ cells per length (mm) of internal elastic lamina in a defined area of the left descending circumflex coronary artery of 29 patients. In preliminary analyses, we found that ≈98% of all 5-LO+ cells were CD68+ macrophages/foam cells/DCs in both laminae media and intima of stage IV-V CAD lesions (n = 7; Fig. 4). However, another sizable population of 5-LO+ cells in CHD were adventitial mast cells (9.3% tryptase+ mast cells, 89.4% CD68+ cells, 1.3% CD66b+ neutrophils, and negligible numbers of eosinophils). Mast cells (40) were also found in both laminae intima and media, although to a much lesser extent (<2%), and CD66b+ neutrophilic granulocytes represented a still smaller population (<1.5%) in these compartments. To examine the possibility that 5-LO expression was related to disease development, we compared 5-LO+ cells of AHA lesion stages 0–I with those of AHA stages II–III and IV–V. Most 5-LO+ cells in subclinical stages (0–I and II–III) resided in the lamina adventitia (Fig. 4), although in each of the lesions, smaller numbers of 5-LO+ macrophages were found in the lamina intima. However, as atherogenesis progresses, marked increases were observed in both laminae intima and media (Fig. 4). When 5-LO+ cells in individual laminae in stages 0–III (clinically silent) were correlated with lesion stages IV–V (clinically overt), significant differences became apparent in each case, i.e., 5-LO+ cells increased ≈13.5-, 13-, and 3-fold in laminae intima, media, and adventitia, respectively. These data indicated that the net 5-LO+ cell influx into arterial walls increases during the time window when clinically silent lesions develop into clinically apparent lesions, and that, unlike early lesions, the majority of 5-LO+ leukocytes per total arterial wall resides in the lamina intima in late-stage disease. The data also revealed that clinically significant atherosclerosis resembles panarteriitis. Major constituents of atherosclerotic plaques are SMCs, T cells, collagen, extracellular lipid, and calcified as well as necrotic tissue (3–5), none of which harbor 5-LO. In view of this heterogeneity, we determined whether the density of 5-LO+ cells (calculated per mm2 lesion area) increased during atherogenesis. We found that this density increased 19-fold in the lamina media (P < 0.001, n = 7) and 3-fold in laminae intima and adventitia (P < 0.01, n = 22) when subclinical (0–III) and clinical (IV–V) stages were compared. These numbers represent averages of 5-LO+ cells per tissue volume that incompletely reflect the precise cell distribution within the arterial wall. Thus, 5-LO+ leukocytes are not distributed uniformly but accumulate at distinct sites such as the shoulder region below the fibrous cap, thereby forming foci leaving other areas largely devoid of 5-LO+ cells. Indeed, inspection of advanced lesions revealed that the density of 5-LO+ cells was found to be very high, sometimes occupying the majority of the total surface area of a given focus (Fig. 3 D, G, H, and K). The morphology of atherosclerotic lesions of CHD and extracardial lesions of muscular arteries are similar; we found that absolute densities of 5-LO+ cells in laminae intimae of type V CHD and CAD lesions were not very different: CAD, 191 ± 37, n = 6; CHD, 74 ± 23, n = 6 (P = 0.026; means ± SEM; two-sided Mann–Whitney U test).
Discussion
Mechanisms of arterial wall inflammation in atherogenesis are poorly understood. Our data reveal expression of the entire 5-LO cascade and of four LT-Rs in AAO, CAD, and CHD. By contrast, reticulocyte-type 15-LO remains undetectable or low with no apparent association to atherosclerosis pathology. That 5-LO+ cells sharply increase during transition from silent to advanced disease is consistent with formation of increasing numbers of circuits of inflammation consisting of 5-LO cascade-expressing leukocytes and LT-R-expressing ECs, SMCs, macrophages, mast cells, and T cells during clinically significant stages of lesion development. This proposition is supported by the many activities that LTs can exert in intact cardiovascular tissues (see below), and by our unpublished data that reveal that each of the cell types that form atherosclerotic lesions show a distinctive LT-R expression pattern as evidenced by both LT-R transcript determination and pharmacological profiles using LT-R antagonists (K.L., R.S., M.H., R. Heller, E. Bretschneider, H. Galczenski, J. F. Evans, and A.J.R.H.).
When considering our observation of low 15-LO expression in human lesions, several caveats deserve attention: (i) Human atherosclerosis requires years to decades to develop into clinically acute disease; expression of 15-LO during a narrow time window (not represented in the lesions studied here) may have escaped detection. (ii) 15-LO expression does not necessarily coincide with predominance of 13(S)-hydroxyoctadecanoic acid (HODE) in lesion lipids (11, 15); thus, 13(S)-HODE may persist longer than 15-LO mRNA or protein. (iii) The lack of selective LO mRNA stability in human lung can only provide presumptive evidence for LO stability in lesion macrophages. (iv) Lesion macrophages represent heterogeneous cell populations (35, 38, 39); 15-LO may be expressed in a small subtype of macrophages. (v) The absolute levels of 15-LO transcripts per total arterial wall RNA may be misleadingly low, because RNA derived from 15-LO-negative cells may be a dilution factor. (vi) Atherosclerosis is an ill defined combination of various chronic inflammatory arterial wall pathologies (3–7); hence, 15-LO may be expressed in some but not all forms of atherosclerosis. Also, 12/15-LO/apo-E double-deficient mice, 12/15-LO/LDL receptor double-deficient mice, and 12/15-LO/apolipoprotein B mRNA editing catalytic polypeptide-1/LDL receptor triple-deficient mice have been shown to develop fewer aorta lesions than apoE-deficient control mice (9, 10, 18). This may be due to reduced LDL oxidation, but also to effects of the enzyme on targets yet to be defined (18). When taken together, it cannot be excluded that there is a role of 15-LO in human atherogenesis.
A salient feature of our data is that 5-LO-dependent inflammatory circuits expand within the arterial wall notably during late stage atherogenesis. Participants of these circuits may be LT-forming leukocytes and LT-R-expressing arterial wall cells, and possibly other hematopoietic cell lineages. Moreover, release of intermediates of the 5-LO pathway, i.e., LTA4, from macrophages/DCs/foam cells/mast cells and its subsequent uptake and conversion to LTs by neighboring cells may provide mechanisms for augmented, i.e., transcellular, LT synthesis (41–45). In this regard, it is of interest that both SMCs and ECs express LTA4 hydrolase and that ECs express LTC4 synthase and an additional microsomal glutathione S-transferase, both of which have been implicated in transcellular LT formation (45). LTs are long known to exert powerful effects when added to cardiovascular preparations. Actions of LTs in intact tissues are as varied as coronary artery contraction, chronotropic effects on heart rate, and impairment of left ventricular contractility (20), edema formation in the microcirculation (21), and mononuclear cell recruitment into tissues (21). LTs also trigger a variety of activities in cultured arterial wall cells, including induction of P-selectin, von Willebrand factor (46), and platelet-activating factor (47) in ECs, and stimulation of SMC proliferation (48–50). Beyond its established role in inflammation, the 5-LO pathway has been implicated in the regulation of immune responses: The 5-LO cascade is expressed in DCs (29, 34), LTs activate T and B cells (references in ref. 51) and affect DC migration to regional lymph nodes (52), and most cells that participate in immune reactions express LT-Rs (29–33, 53). Despite these powerful activities, the role of 5-LO in the pathogenesis of atherosclerosis has not received much attention. Because detailed information on the 5-LO pathway in atherosclerosis has not yet been available, potential mechanisms of LT formation and LT-R target cells have been difficult to conceive. Our data suggest that large numbers of 5-LO+ leukocytes may act from within the lesions rather than from the blood vessel lumen in the proximity of cells that express LT-Rs during late-stage atherogenesis. Because 5-LO+ macrophages/DCs/foam cells express MHC-II molecules and localize in the proximity of activated T cells (54), they may present antigen and thereby establish immune response circuits. In unpublished studies (K.L., R.S., M.H., R. Heller, E. Bretschneider, H. Galczenski, J. F. Evans, and A.J.R.H.), we have observed differential LT-R expression in cultured human SMCs and ECs and that cysLTs induce sustained oscillatory Ca2+ transients in ECs. When previously observed actions of LTs on ECs and SMCs are considered with our present data, LT-generating leukocytes may activate arterial wall cell and leukocyte LT-Rs and thus exert a multitude of actions on blood vessel homeostasis. CysLT-dependent coronary artery contraction was documented by Corey and colleagues more than 20 years ago (20). Recently, Allen et al. (24) reported hypercontractility toward cysLTs in diseased versus normal coronary arteries. The anatomy, frequency, and kinetics of generation of 5-LO-containing infiltrates observed here are compatible with participation of LTs to several hallmarks of clinically overt atherosclerosis, including EC dysfunction, intimal edema, leukocyte infiltration, aberrant contractility, SMC proliferation, and immune reactivity. A recent study by Mehrabian et al. (55) indicates a significant role of the 5-LO gene in atherosclerosis susceptibility of hyperlipidemic LDL receptor−/− mice providing functional evidence for an indispensable involvement of 5-LO in the pathogenesis of the disease in experimental atherosclerosis models. Although these data require confirmation in additional model systems, deficiency of only one 5-LO allele surprisingly conferred potent protection against atherosclerosis development of LDL receptor−/− mice. In addition, Aiello et al. (56) demonstrated that pharmacological BLT-R antagonism was also protective in three atherosclerosis-susceptible mouse strains. Anti-LT drugs are already in use for the treatment of bronchial asthma in man (57). Their availability represents a unique opportunity to test whether pharmacological inhibition of the 5-LO cascade will beneficially affect the clinical outcome of the disease.
Supplementary Material
Acknowledgments
B.S. and A.J.R.H. dedicate this article to the memory of Russell Ross. We thank J. Glomset (Howard Hughes Medical Institute Laboratory, University of Washington, Seattle) and D. Steinberg (Department of Medicine, University of California at San Diego, La Jolla) for critical comments, H. Kosmehl (Department of Pathology, University of Jena) for identification of 15-LO-expressing cell structures, Sem Saeland (Schering-Plough, Dardilly, France) for a gift of anti-langerin antiserum, and G. Weber, M. Voigt, C. Ströhl, and M. Franke for expert technical assistance. Antiserum against 5-LO was prepared by Dr. Y.-Y. Zhang and A. Nordberg (Karolinska Institute). This study was supported by Deutsche Forschungsgemeinschaft Grant Ha 1083/13-1/13-2, European Union Research Network Grant QLG1-CT-2001-01521, Interdisziplinäres Zentrum für Klinische Forschung Jena Grants TP4.4 and TP4.9, Swedish Research Council Grant 03X-217, and a grant from the Stiftung für Verhalten und Umwelt.
Abbreviations
- AAO
atherosclerosis of the aorta
- AHA
American Heart Association
- CAD
carotid artery disease
- CHD
coronary heart disease
- DC
dendritic cell
- ECs
endothelial cells
- LO
lipoxygenase
- FLAP
5-LO activating protein
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- LDL
low-density lipoprotein
- LT
leukotriene
- LT-R
LT receptor
- SMCs
smooth muscle cells
References
- 1.Braunwald E. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 2.Breslow J L. Nat Med. 1997;3:600–601. doi: 10.1038/nm0697-600. [DOI] [PubMed] [Google Scholar]
- 3.Ross R, Glomset J A. N Engl J Med. 1976;295:369–377. doi: 10.1056/NEJM197608122950707. , 420–425. [DOI] [PubMed] [Google Scholar]
- 4.Ross R. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 5.Lusis A J. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinberg D, Parthasarathy S, Carew T E, Khoo J C, Witztum J L. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 7.Witztum J L. Lancet. 1994;344:793–795. doi: 10.1016/s0140-6736(94)92346-9. [DOI] [PubMed] [Google Scholar]
- 8.Chisolm G M, Hazen S L, Fox P L, Cathcart M K. J Biol Chem. 1999;274:25959–25962. doi: 10.1074/jbc.274.37.25959. [DOI] [PubMed] [Google Scholar]
- 9.Cyrus T, Witztum J L, Rader D J, Tangirala R, Fazio S, Linton M F, Funk C D. J Clin Invest. 1999;103:1597–1604. doi: 10.1172/JCI5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George J, Afek A, Shaish A, Levkovitz H, Bloom N, Cyrus T, Zhao L, Funk C D, Sigal E, Harats D. Circulation. 2001;104:1646–1650. doi: 10.1161/hc3901.095772. [DOI] [PubMed] [Google Scholar]
- 11.Folcik V A, Nivar-Aristiy R A, Krajewski L P, Cathcart M K. J Clin Invest. 1995;96:504–510. doi: 10.1172/JCI118062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harats D, Shaish A, George J, Mulkins M, Kurihara H, Levkovitz H, Sigal E. Arterioscler Thromb Vasc Biol. 2000;20:2100–2105. doi: 10.1161/01.atv.20.9.2100. [DOI] [PubMed] [Google Scholar]
- 13.Hilttunen T, Luoma J, Nikkari T, Ylä-Herttula S. Circulation. 1995;92:3297–3303. doi: 10.1161/01.cir.92.11.3297. [DOI] [PubMed] [Google Scholar]
- 14.Kühn H, Belkner J, Zaiss S, Fährenklempner T, Wohlfeil S. J Exp Med. 1994;179:1903–1911. doi: 10.1084/jem.179.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kühn H, Heydeck D, Hugou I, Gniwotta C. J Clin Invest. 1997;99:888–893. doi: 10.1172/JCI119253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kühn H, Chan L. Curr Opin Lipidol. 1997;8:111–117. doi: 10.1097/00041433-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Ylä-Herttuala S, Rosenfeld M E, Parthasarathy S, Sigal E, Sarkioja T, Witztum J L, Steinberg D. J Clin Invest. 1991;87:1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao L, Cuff C A, Moss E, Wille U, Cyrus T, Klein E A, Pratico D, Rader D J, Hunter C A, Pure E, Funk C D. J Biol Chem. 2002;277:35350–35356. doi: 10.1074/jbc.M205738200. [DOI] [PubMed] [Google Scholar]
- 19.Samuelsson B, Dahlén S-E, Lindgren J A, Rouzer C A, Serhan C N. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 20.Burke J A, Levi R, Guoz G, Corey E J. J Pharmacol Exp Ther. 1981;221:235–241. [PubMed] [Google Scholar]
- 21.Dahlén S-E, Bjork J, Hedqvist P, Arfors K E, Hammarstrom S, Lindgren J A, Samuelsson B. Proc Natl Acad Sci USA. 1981;78:3887–3891. doi: 10.1073/pnas.78.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michelassi F, Landa L, Hill R D, Lowenstein E, Watkins W D, Petkau A J, Zapol W M. Science. 1982;217:841–843. doi: 10.1126/science.6808665. [DOI] [PubMed] [Google Scholar]
- 23.Smedegård G, Hedqvist P, Dahlen S E, Revenas B, Hammarstrom S, Samuelsson B. Nature. 1982;295:327–329. doi: 10.1038/295327a0. [DOI] [PubMed] [Google Scholar]
- 24.Allen S, Dashwood M, Morrison K, Yacoub M. Circulation. 1998;97:2406–2413. doi: 10.1161/01.cir.97.24.2406. [DOI] [PubMed] [Google Scholar]
- 25.Stary H C, Chandler A B, Dinsmore R E, Fuster V, Glagov S, Insull W, Jr, Rosenfeld M E, Schwartz C J, Wagner W D, Wissler R W. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 26.Wissler R W. Am J Med Sci. 1995;310, Suppl. 1:29–36. doi: 10.1097/00000441-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Sigal E, Dicharry S, Highland E, Finkbeiner W E. Am J Physiol. 1992;262:L392–L398. doi: 10.1152/ajplung.1992.262.4.L392. [DOI] [PubMed] [Google Scholar]
- 28.Conrad D J, Kühn H, Mulkins M, Highland E, Sigal E. Proc Natl Acad Sci USA. 1992;89:217–221. doi: 10.1073/pnas.89.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spanbroek R, Hildner M, Köhler A, Müller A, Zintl F, Kühn H, Rådmark O, Samuelsson B, Habenicht A J R. Proc Natl Acad Sci USA. 2001;98:5152–5157. doi: 10.1073/pnas.091076998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynch K R, O'Neill G P, Liu Q, Im D S, Sawyer N, Metters K M, Coulombe N, Abramovitz M, Figueroa D J, Zeng Z, et al. Nature. 1999;399:789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 31.Heise E C, O'Dowd B F, Figueroa D J, Sawyer N, Nguyen T, Im D S, Stocco R, Bellefeuille J N, Abramovitz M, Cheng R, et al. J Biol Chem. 2000;275:30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- 32.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 33.Yokomizo T, Kato T, Terawaki K, Izumi T, Shimizu T. J Exp Med. 2000;192:421–432. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spanbroek R, Stark H J, Janssen-Timmen U, Kraft S, Hildner M, Andl T, Bosch F X, Fusenig N E, Bieber T, Rådmark O, et al. Proc Natl Acad Sci USA. 1998;95:663–668. doi: 10.1073/pnas.95.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bobryshev Y V, Ikezawa T, Watanabe T. Atherosclerosis. 1997;133:193–202. doi: 10.1016/s0021-9150(97)00129-9. [DOI] [PubMed] [Google Scholar]
- 36.De Saint-Vis B, Vincent J, Vandenabeele S, Vanbervliet B, Pin J J, Ait-Yahia S, Patel S, Mattei M G, Banchereau J, Zurawski S, et al. Immunity. 1998;9:325–336. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 37.Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, Duvert-Frances V, Vincent C, Schmitt D, Davoust J, et al. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 38.Gown A M, Tsukada T, Ross R. Am J Pathol. 1986;125:191–207. [PMC free article] [PubMed] [Google Scholar]
- 39.Tsukada S, Coltrera M D, Ross R, Gown A M. Am J Pathol. 1993;142:1787–1796. [PMC free article] [PubMed] [Google Scholar]
- 40.Atkinson J B, Harlan C W, Harlan G C, Virmani R. Hum Pathol. 1994;25:154–159. doi: 10.1016/0046-8177(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 41.Datta Y H, Romano M, Jacobson B C, Golan D E, Serhan C N, Ewenstein B M. Circulation. 1995;92:3304–3311. doi: 10.1161/01.cir.92.11.3304. [DOI] [PubMed] [Google Scholar]
- 42.Heimbürger M, Palmblad J E W. Clin Exp Immunol. 1996;103:454–460. doi: 10.1111/j.1365-2249.1996.tb08302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maclouf J, Murphy R C, Henson P M. Blood. 1989;74:703–707. [PubMed] [Google Scholar]
- 44.Serhan C N, Haeggström J Z, Leslie C C. FASEB J. 1996;10:1147–1158. doi: 10.1096/fasebj.10.10.8751717. [DOI] [PubMed] [Google Scholar]
- 45.Sjöström M, Jakobsson P-J, Heimburger M, Palmblad J, Haeggström J Z. Eur J Biochem. 2001;268:1–10. doi: 10.1046/j.1432-1327.2001.02142.x. [DOI] [PubMed] [Google Scholar]
- 46.Huang A J, Manning J E, Bandak T M, Ratau M C, Hanser K R, Silverstein S C. J Cell Biol. 1993;120:1371–1380. doi: 10.1083/jcb.120.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McIntyre T M, Zimmerman G A, Prescott S M. Proc Natl Acad Sci USA. 1986;83:2204–2208. doi: 10.1073/pnas.83.7.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamohara M, Takasaki J, Matsumoto M, Matsumoto Si, Saito T, Soga T, Matsushime H, Furuichi K. Biochem Biophys Res Commun. 2001;287:1088–1092. doi: 10.1006/bbrc.2001.5695. [DOI] [PubMed] [Google Scholar]
- 49.Nomoto A, Mutoh S, Haghara H, Yamaguchi I. Atherosclerosis. 1988;72:213–219. doi: 10.1016/0021-9150(88)90083-4. [DOI] [PubMed] [Google Scholar]
- 50.Palmberg L, Claesson H-E, Thyberg J. J Cell Sci. 1987;88:151–159. doi: 10.1242/jcs.88.2.151. [DOI] [PubMed] [Google Scholar]
- 51.Spanbroek R, Hildner M, Steinhilber D, Fusenig N, Yoneda K, Rådmark O, Samuelsson B, Habenicht A J R. Blood. 2000;96:3857–3861. [PubMed] [Google Scholar]
- 52.Robbiani D F, Finch R A, Jager D, Muller W A, Sartorelli A C, Randolph G J. Cell. 2000;103:757–768. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 53.Yokomizo T, Izumi T, Shimizu T. Life Sci. 2001;68:2207–2212. doi: 10.1016/s0024-3205(01)01007-4. [DOI] [PubMed] [Google Scholar]
- 54.Hansson G K. Curr Opin Lipidol. 1997;8:301–311. doi: 10.1097/00041433-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Mehrabian M, Allayee H, Wong J, Shih W, Wang X P, Shaposhnik Z, Funk C D, Lusis A J. Circ Res. 2002;91:120–126. doi: 10.1161/01.res.0000028008.99774.7f. [DOI] [PubMed] [Google Scholar]
- 56.Aiello R J, Bourassa P-A, Lindsey S, Weng W, Freeman A, Showell H J. Arterioscler Thromb Vasc Biol. 2002;22:443–449. doi: 10.1161/hq0302.105593. [DOI] [PubMed] [Google Scholar]
- 57.Drazen J M, Israel E, O'Byrne P M. N Engl J Med. 1999;340:197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.