Abstract
Neuronal differentiation requires exquisitely timed cell cycle arrest for progenitors to acquire an appropriate neuronal cell fate and is achieved by communication between soluble signals, such as growth factors and extracellular matrix molecules. Here we report that the expression of TIMP-2, a matrix metalloproteinase inhibitor, is up-regulated by signals that control proliferation (bFGF and EGF) and differentiation (retinoic acid and NGF) in neural progenitor and neuroblastoma cell lines. TIMP-2 expression coincides with the appearance of neurofilament-positive neurons, indicating that TIMP-2 may play a role in neurogenesis. The up-regulation of TIMP-2 expression by proliferative signals suggests a role in the transition from proliferation to neuronal differentiation. Live labeling experiments demonstrate TIMP-2 expression only on α3 integrin-positive cells. Thus, TIMP-2 function may be mediated via interaction with integrin receptor(s). We propose that TIMP-2 represents a component of the neurogenic signaling cascade induced by mitogenic stimuli that may withdraw progenitor cells from the cell cycle permitting their terminal neuronal differentiation.
Keywords: integrin, MT1-MMP, neurogenesis, Neuro-2a, N1E-115, P19, PC12
Introduction
Neurogenesis is regulated by cell intrinsic as well as extrinsic factors. Progenitor cell proliferation and cell fate determination are dependent upon growth factors and cell adhesion to the extracellular matrix (ECM) (Ferri et al. 1996; Chipperfield et al. 2002). Microinjection of basic fibroblast growth factor (bFGF) into embryonic brains prolongs cortical progenitor proliferation (Vaccarino et al. 1999). However, cortical progenitors treated with bFGF in the presence of collagen type IV undergo neuronal differentiation (Ali et al. 1998). Moreover, cell fate determination is governed by similar growth factor-ECM cross talk (Ferri and Levitt 1995). These studies indicate that progenitors respond differently to local environmental signals. In addition to regulating cell cycle arrest, ECM molecules play a role in later phases of neuronal differentiation, including migration, neurite outgrowth and synaptogenesis. While it is well accepted that ECM molecules participate in neurogenesis, the mechanisms by which they regulate neuronal differentiation are currently poorly understood. Proteolysis of extracellular molecules as a mechanism of regulating neuronal proliferation and differentiation has recently received considerable attention.
The metzincin family of proteases includes matrixin/matrix metalloproteinase (MMP), ADAM (A Disintegrin And Metalloproteinase domain) and ADAM-TS (ADAM proteases containing a thrombospondin [TS] domain) members (reviewed in Visse and Nagase 2003; Huovila et al. 2005; Porter et al. 2005). The MMPs are products of a multigene family of endopeptidases that are the main physiologically relevant mediators of ECM degradation. In addition to ECM proteolysis, MMPs process a variety of growth factors, including proNGF and proBDNF (Lee et al. 2001; Hojilla et al. 2003) and receptors, including trkA, trkC and p75 (Diaz-Rodriguez et al. 1999; Jung et al. 2003; Mateos et al. 2003); thus, regulating proliferation, differentiation and survival. Kuzbanian (ADAM-10) mediated proteolysis of the Notch receptor inhibits neuronal differentiation (Pan and Rubin 1997), indicating that neuronal cell fate can be controlled by metzincin proteases. MMPs also regulate neuronal migration and neurite outgrowth by processing inhibitory molecules (e.g., chondroitin sulfate proteoglycans) and chemoattractants (e.g., netrin-1 receptor DCC) (Zuo et al. 1998; Galko and Tessier-Lavigne 2000). Finally, activity-dependent synaptic plasticity may be modulated by MMP-mediated proteolysis (Shiosaka and Yoshida 2000; Kaczmarek et al. 2002; Tomimatsu et al. 2002). Given the broad nature of metzincin substrates (McCawley and Matrisian 2001), their activity is tightly regulated by interaction with tissue inhibitor of metalloproteinases (TIMPs). TIMPs are 20–30 kDa secreted proteins that form tight, but relatively low selectivity 1:1 complexes with the active forms of metzincin proteases (reviewed in Edwards 1999; Baker et al. 2002).
Of the four known TIMPs, TIMP-2 and TIMP-4 are highly expressed in brain (Fager and Jaworski 2000; Young et al. 2002). In contrast to TIMP-4, whose expression is largely restricted to the cerebellum, TIMP-2 is expressed in divergent regions of the nervous system (e.g., cerebral cortical and hippocampal pyramidal cells, cerebellar Purkinje cells, spinal motor neurons) (Fager and Jaworski 2000). TIMP-2 expression in vivo is restricted to post-mitotic neurons, increases throughout development, and is maintained at high levels into adulthood. Thus, TIMP-2 likely participates in the acquisition of general features of a neuronal phenotype.
The present report details TIMP-2 expression in four cell lines (PC12, Neuro-2a, N1E-115 and P19), each which differentiates into a neuronal-like phenotype by different neurogenic signals. TIMP-2 expression was up-regulated in response to bFGF, nerve growth factor (NGF), epidermal growth factor (EGF), retinoic acid (RA) and serum withdrawal, signals which control the proliferation and differentiation of neural progenitor cells. More strikingly, cell surface expression of TIMP-2 in response to the various neurogenic signals correlated with the expression of α3 integrin, suggesting that TIMP-2 may exert its effect on neurogenesis via integrins.
Materials and Methods
Reagents
All tissue culture media was from Mediatech (Herndon, VA) and serum from Hyclone (Logan, UT). Growth factors: NGF (BD Biosciences; San Jose, CA), EGF (Austral Biologicals; San Ramon, CA), all trans-retinoic acid (Sigma; St. Louis, MO). Primary antibodies: rabbit anti-human TIMP-2 for western blots (1:1500; Chemicon; Temecula, CA), sheep anti-human TIMP-2 for immunocytochemistry (1:200; Biogenesis; Kingston, NH), mouse anti-Flag M2 (1:1500 for blots, 1:150 for cytochemistry; Stratagene; La Jolla, CA), rabbit anti-rat α1 integrin (1:200; Chemicon), goat anti-rat α3 integrin Ralph 3.2 (1:100; Santa Cruz Biotechnology; Santa Cruz, CA), rabbit anti-human α5 integrin (1:200; Chemicon), rat anti-mouse β1 integrin (1:250; Chemicon), rabbit anti-human MT1-MMP (MMP-14) (1:2500 blots, 1:500 immuno; Triple Point Biologics, Portland, OR), goat anti-human actin (1:5000; Santa Cruz). Secondary antibodies (all from Jackson ImmunoResearch; West Grove, PA) except FITC-conjugated mouse anti-rat IgG2a (1:100; Southern Biotechnology; Birmingham, AL): Cy3-conjugated donkey antibodies (1:500), HRP-conjugated donkey anti-sheep, anti-rabbit (1:3000), and donkey anti-goat (1:5000).
Cell Culture
Rat pheochromocytoma PC12 cells (American Type Culture Collection [ATCC] #CRL-1721) were maintained in RPMI medium supplemented with 10% FBS, 5% horse serum and penicillin/streptomycin (pen/strep). Cells (1.5 × 103/cm2 for immunocytochemistry and 1.4 × 104/cm2 for western blot analysis) were plated on poly-L-lysine/laminin coated tissue culture dishes in RPMI medium containing N2 supplement (Invitrogen; Carlsbad, CA) for 12 hrs prior to growth factor addition. Cultures were then maintained for 7 days in vitro (DIV) without media being replenished.
Mouse Neuro-2a (ATCC #CRL-131) and N1E-115 (ATCC #CRL-2263) neuroblastoma cells were maintained in DMEM supplemented with 10% FBS and pen/strep. Cells (0.6 × 103/cm2 for immunocytochemistry and 0.5 × 104/cm2 for western blot analysis) were plated on poly-L-lysine/laminin coated tissue culture dishes in growth medium for 12 hrs prior to the induction of differentiation by serum reduction to 0.5%. Media was replenished after 3, 4, and 6 DIV.
Mouse embryonal carcinoma P19 cells (ATCC #CRL-1825) were maintained in α-MEM with ribonucleosides and deoxyribonucleosides supplemented with 7.5% newborn calf serum, 2.5% FBS and pen/strep. To induce differentiation, 0.5 × 106 cells were plated on a non-adherent petri dish (Kord-Valmark; Ontario, Canada) in the presence of 1 μM RA to allow cell aggregation. To replenish medium after 2 DIV, embryoid bodies were collected by brief centrifugation and gentle dispersion with a wide bore pipet. After 4 DIV, embryoid bodies were collected by brief centrifugation and gently dissociated with trypsin-EDTA (0.025%). Cells (1.4 × 104 cells/cm2 for both immunocytochemistry and western blot analysis) were plated on poly-L-lysine alone coated tissue culture dishes in α-MEM with 10% serum in the absence of RA. Twenty-four hrs after plating, cells were treated with 20 μM cytosine arabinoside. Media was replenished after 3, 4, and 6 DIV.
Sample preparation and Western blot Analysis
Cells were lysed in lysis buffer (25 mM Hepes, pH 7.7, 150 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, O.5% Triton X-100, 0.5 mM DTT, 20 mM β-glycerophosphate, 1 mM Na3VO4, 5 mM NaF, 4 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin) for 30 min at 4°C. Lysates were spun at 14,000 rpm for 10 min at 4°C, and the supernatant used for western blot analysis.
Conditioned media (containing 0.5% serum) was collected, centrifuged to remove cells and concentrated by trichloroacetic acid (TCA) precipitation. Briefly, medium (100 μl) was adjusted to 0.1% deoxycholate and total protein precipitated with 10% TCA at room temp for 30 min. After centrifugation at 14,000 rpm for 20 min at 4°C, the pellet was resuspended in ether:absolute ethanol (50:50) and incubated on ice for 30 min. After centrifugation as before, the pellet was resuspended in 2X sample buffer with 5% β-mercaptoethanol and adjusted to pH 7.4 with 1M Tris-HCl, pH 10.
TIMP-2 was immunoprecipitated from conditioned media of TIMP-2 transfected cells using anti-Flag antibody (1 μg/sample) overnight at 4°C. Immune complexes were harvested with protein A/G-Sepharose for 1 hr on ice and washed as previously described (Pérez-Martínez and Jaworski 2005). Immunoprecipitated proteins were resolved by SDS-PAGE and subjected to immunoblotting.
For western blot analysis, proteins (20 μg) were fractionated by SDS-PAGE and transferred to Immobilon-P membranes (Schleicher & Schuell) as previously described (Jaworski and Fager 2000). Membranes were blocked with 5% nonfat milk in Tris-buffer saline (TBS, 10 mM Tris, pH 7.5; 150 mM NaCl) containing 0.4% Tween-20 (TBST) followed by overnight incubation at 4°C with primary antibody diluted in TBST containing either 3% BSA (for TIMP-2 and MT1-MMP) or 5% nonfat milk (for actin). After three washes with TBST, membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody diluted in 5% nonfat dry milk in TBST. After washing as before, immunocomplexes were visualized by enhanced chemiluminescence according to manufacturer’s instructions (Perkin-Elmer Life Sciences; Boston, MA). Densitometry was performed using Quantity One Image Analysis software (Bio-Rad; Hercules, CA).
Immunocytochemistry
For immunofluorescence, cells were fixed for 30 min at room temp with 2% paraformaldehyde (Polysciences; Warrington, PA). After two washes with PBS for 10 min each, cells were incubated for 1 hr at room temp with blocking buffer (DMEM with 5% FBS, 0.1% glycine, 0.1% lysine and 0.2% Triton X-100). Primary antibody was diluted in blocking buffer and incubated overnight at 4 °C. After three washes with PBS for 10 min each, cells were incubated for 1 hr at room temp with the appropriate secondary antibody diluted in blocking buffer without Triton. Samples were then washed three times with PBS and fluorescence detected using a Nikon inverted epifluorescence microscope (Micro Video Instruments; Avon, MA) and digital images captured with a Spot RT camera (Research Diagnostics; Sterling Heights, MI). Figures were prepared using Adobe Photoshop (Adobe Systems Inc., San Jose, CA).
For cell surface live labeling, cells were incubated with primary antibody for 10 min at 37°C. After two washes with PBS, cells were fixed with 4% formaldehyde for 10 min. Cells were washed as before and then incubated with secondary antibody diluted in blocking buffer lacking Triton for 30 min. After two washes with PBS, cells were immediately examined.
Plasmid Construction and Transfection
PCR of full length TIMP-2 cDNA from adult rat brain and cloning into the pIRES-hrGFP-1a vector (Stratagene; La Jolla, CA) was performed as described (Pérez-Martínez and Jaworski 2005).
For transfection experiments, PC12 cells (2 × 105 cells) or Neuro-2a or N1E-115 cells (1 × 105 cells) were plated on a poly-L-lysine/laminin coated 24 well plate. Twelve hrs after seeding, cells were rinsed briefly with OptiMEM (Invitrogen) and transfected with 800 ng DNA using Lipofectamine 2000 (Invitrogen) in growth medium containing serum. Fresh medium was added after 8 hrs.
Results
In the developing nervous system, TIMP-2 is expressed coincident with the onset of neuronal differentiation and its expression is maintained at high levels into adulthood (Blavier and DeClerck 1997; Vaillant et al. 1999; Fager and Jaworski 2000), suggesting TIMP-2 plays a role in the acquisition and maintenance of a differentiated neuronal phenotype. Previously, we demonstrated that TIMP-2 induces cell cycle arrest and neurite outgrowth in PC12 cells independent of its role in MMP inhibition (Pérez-Martínez and Jaworski 2005). Furthermore, immunoprecipitation studies revealed that TIMP-2 interacts with α3β1 integrin, suggesting TIMP-2 might exert its MMP-independent activities via integrins. To determine whether these observations are unique to PC12 cells and/or NGF-mediated differentiation, the regulation of TIMP-2 expression was examined in cell lines responsive to various neurogenic signals. Here, we demonstrate that TIMP-2 expression is up-regulated coincident with neuronal differentiation in all lines examined, but that cell surface TIMP-2 expression is primarily detected on PC12 cells, cells that express α3β1 integrin.
TIMP-2 expression is up-regulated during neuronal differentiation in a variety of cell lines
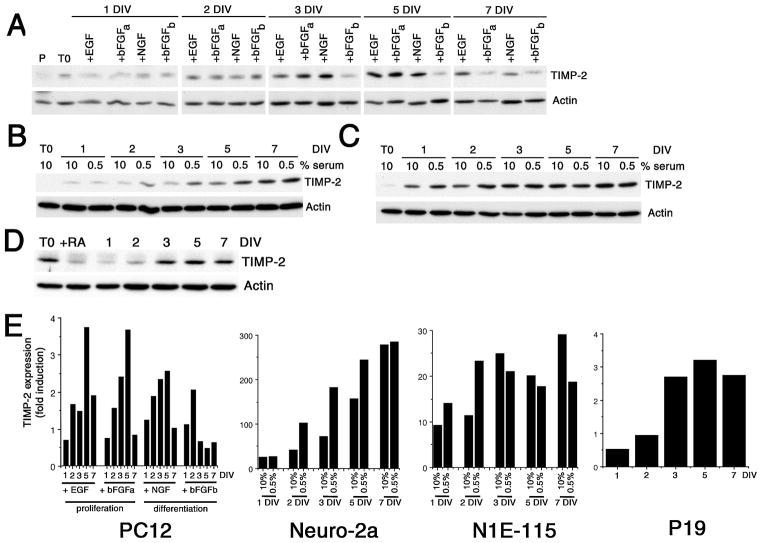
The most widely used model system for investigating the molecular mechanism underlying neuronal differentiation is the PC12 pheochromocytoma cell line. Exposure to NGF induces differentiation into sympathetic-like neurons (Greene and Tischler 1976). In contrast, proliferation is stimulated in the presence of EGF (Huff et al. 1981). bFGF induces proliferation (Kawamata et al. 2001) or differentiation (Rydel and Greene 1987) in a concentration dependent manner. Log phase growth PC12 cells were serum-starved for 12 hours prior to growth factor addition. To induce differentiation, cells were treated with either NGF or bFGF at 100 ng/ml. Since glycosaminoglycans can potentiate the ability of bFGF to induce neurite outgrowth (Damon et al. 1988), heparin (1 μg/ml) was included in bFGF treated cultures. To induce proliferation, medium containing either EGF (100 ng/ml) or bFGF (10 ng/ml) was added. To determine basal levels of TIMP-2, proliferating PC12 cells maintained in 15% serum were harvested 12 hours after plating. TIMP-2 expression was up-regulated in response to all growth factor treatments (Figs. 1A, E). Overnight serum starvation induced a 2.7-fold increase in TIMP-2 expression from proliferating conditions. Thus, growth factor deprivation itself regulates TIMP-2 expression. For NGF treated cells, TIMP-2 expression gradually increased to peak at 5 DIV. bFGF/heparin (bFGFb) treatment initially (1 and 2 DIV) resulted in increased TIMP-2 levels comparable to that observed with NGF. However, after 3 DIV TIMP-2 expression dramatically declined. In contrast to the transient TIMP-2 increase by bFGF/heparin, bFGF alone (bFGFa), at concentrations that promote proliferation, resulted in sustained TIMP-2 expression to 5 DIV. Moreover, both the time course and extent of TIMP-2 up-regulation in response to bFGF was similar to that with EGF. These data demonstrate that TIMP-2 expression in PC12 cells is regulated by multiple growth factors.
Figure 1. TIMP-2 expression is up-regulated by multiple neurogenic signals.
Western blot analysis of cell lysates for TIMP-2 expression in PC12 (A), Neuro-2a (B), N1E-115 (C) and P19 (D) cells. TIMP-2 expression in PC12 cells was up-regulated by overnight serum starvation (T0) relative to proliferating cells maintained in 15% serum (P). While EGF promotes proliferation and NGF induces differentiation, bFGF regulates both proliferation (+bFGFa, 10 ng/ml) and differentiation (+bFGFb, 100 ng/ml) of PC12 cells in a dose-dependent manner. Neuro-2a and N1E-115 cells were induced to differentiate by serum depletion (to 0.5%). P19 cells were maintained in suspension for 4 days in the presence of RA (+RA). Neuronal differentiation proceeds upon re-plating in the absence of RA. E) Densitometric analysis of TIMP-2 expression. Expression was first normalized to actin at each time point, then the extent of TIMP-2 up-regulation was determined relative to basal TIMP-2 levels prior to growth factor addition (PC12, P19) or depletion (Neuro-2a, N1E-115) (T0). Since proliferating P19 cells possessed a high basal level of TIMP-2 and expression was down-regulated by RA, the extent of TIMP-2 up-regulation in P19 cells was determined relative to RA-treated cells. Results are representative of at least two independent experiments.
Because TIMP-2 expression was also up-regulated in response to growth factor depletion, TIMP-2 expression was examined in two cells lines that differentiate upon serum withdrawal. Murine Neuro-2a neuroblastoma cells (Klebe and Ruddle 1969) differentiate into cholinergic neuron-like cells following retinoic acid treatment (Shea et al. 1985) or serum removal that is associated with a rapid and persistent increase in the cellular levels of ceramide (Tsuji et al. 1988; Riboni et al. 1995). Similar to its regulation in PC12 cells, TIMP-2 expression increased in Neuro-2a cells in a time dependent manner (Figs. 1B, E). This temporal increase was observed even in cells maintained in 10% serum. At both 1 and 7 DIV, TIMP-2 expression in serum reduced cells was not different from cells in 10% serum. However, serum depletion resulted in a 2.4-fold increase of TIMP-2 at 2 DIV, a 2.5-fold increase at 3 DIV, and a 1.6-fold increase at 5 DIV relative to cells in 10% serum. The murine N1E-115 neuronal cell line (Amano et al. 1972) exhibits neurite outgrowth in response to serum deprivation, presumably due to removal of lipophosphatidic acid in serum (Jalink et al. 1994; Kozma et al. 1997). Serum depletion of N1E-115 cells resulted in a 1.5-fold increase of TIMP-2 at 1 DIV and a 2-fold increase at 2 DIV (Figs. 1C, E). Afterwards, TIMP-2 expression was greater in cells maintained in 10% serum than in serum reduced cells. Thus, TIMP-2 expression in N1E-115 cells is regulated transiently, similar to TIMP-2 regulation observed in PC12 cells.
To determine the ontogenic expression of TIMP-2 during neurogenesis, pluripotent murine P19 embryonal carcinoma cells were examined. In contrast to the other cell lines examined, a significant basal level of TIMP-2 expression was detected in undifferentiated cells (Fig. 1D). P19 cells can be induced to differentiate in neurons/glia, myocytes, and fibroblast-like cells by retinoic acid (RA) (McBurney et al. 1982), with the cell type generated depending on the concentration of RA used (Jones-Villeneuve et al. 1982; Edwards and McBurney 1983). RA-induced P19 differentiation requires two phases. In the induction phase, cells are allowed to aggregate with RA for 4 days, during which time the cells are determined into neuronal progenitors. Interestingly, TIMP-2 expression was down-regulated during this phase (Fig. 1D, +RA lane). Neuronal differentiation is then initiated upon plating cells in the absence of RA. TIMP-2 expression was up-regulated in differentiated P19 cells in a time course similar to that of NGF-differentiated PC12 cells (Figs. 1D, E). Expression peaked at 5 DIV and then declined. Taken together, these data demonstrate that TIMP-2 expression is modulated by changes in growth factor conditions and signaling pathways that regulate neuronal differentiation.
Cell surface expression of TIMP-2 is differentially regulated in neuronal cell lines
To more closely examine the regulation of TIMP-2 expression during neuronal differentiation, its spatial distribution was determined immunocytochemically. TIMP-2 is secreted into the extracellular milieu were it functions as a soluble molecule. In addition, TIMP-2 acts at the cell surface via specific, saturable, high-affinity receptors (Hayakawa et al. 1994; Hoegy et al. 2001). To determine whether TIMP-2 serves as a soluble regulator or acts at the cell surface, both live labeling and Triton-permeabilized cells were subjected to immunolabeling. In contrast to the intracellular TIMP-2 expression detected in all cell lines examined, cell surface TIMP-2 expression was only observed in PC12 cells.
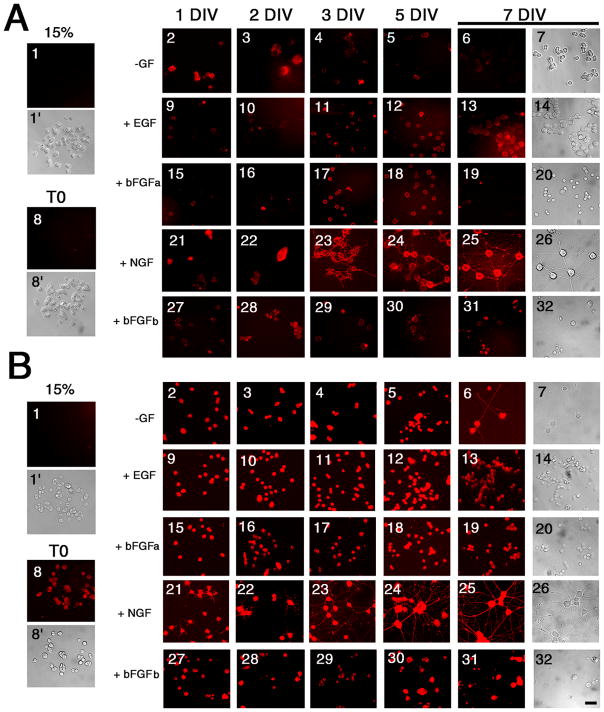
In contrast to proliferating cells (15% serum), cell surface (Fig. 2A) and intracellular (Fig. 2B) TIMP-2 expression was present in PC12 cells under all treatment conditions (Figs 2A1, 2B1). Serum deprived PC12 cells prior to growth factor addition (T0) did not show cell surface labeling (Fig. 2A8). With an additional day of growth factor deprivation, cell surface TIMP-2 was detectable (Fig. 2A2), but the labeling declined with time (Figs. 2A3–7). Cells maintained under proliferating conditions, either with EGF (Figs. 2A9–14) or bFGF (Figs. 2A15–20) showed cell surface TIMP-2 expression. In NGF differentiated cells, cell surface TIMP-2 was present on the cell soma and neuritic processes (Figs. 2A21–26). Cells differentiated with bFGF/heparin (bFGFb) had TIMP-2 on the soma, but none detectable on processes (Figs. 2A27–32). Intracellular TIMP-2 expression, in Triton-permeabilized cells, was much greater than cell surface expression (Fig. 2B). A low level of intracellular TIMP-2 was present in overnight serum deprived PC12 cells (Fig. 2B8). As observed for cell surface TIMP-2, an additional day of growth factor deprivation increased intracellular TIMP-2 expression (Fig. 2B2). However, unlike cell surface labeling which declined, intracellular labeling was maintained (Figs. 2B3–7). Even in the absence of exogenous growth factor, PC12 cells spontaneously differentiated and expressed TIMP-2 in the soma and processes (Figs. 2B6,7). Intracellular TIMP-2 expression in EGF (Figs. 2B9–14) and bFGF (Figs. 2B15–20) treated cells was much more intense than cell surface expression. Expression of TIMP-2 in neuritic processes of NGF differentiated cells was detectable as early as 1 DIV (Fig. 2B21) as opposed to 3 DIV for cell surface TIMP-2 (Fig. 2A23). TIMP-2 expression in bFGF/heparin differentiated cells (Figs. 2B27–32) was not as intense as NGF differentiated cells. These data demonstrate that TIMP-2 expression is up-regulated by both proliferative and neurogenic signals. Most importantly, cell surface TIMP-2 expression coincides with PC12 cell differentiation indicative of the interaction of TIMP-2 with specific PC12 cell surface receptor(s).
Figure 2. Regulation of TIMP-2 expression in PC12 cells.
A) Live-labeling immunocytochemically was performed to detect cell surface TIMP-2 expression. TIMP-2 was not detected on the cell surface of proliferating cells maintained in 15% serum (1) or after 12 hours of serum deprivation (8). Otherwise, cell surface TIMP-2 expression was present in response to all growth factor treatments. TIMP-2 expression was even up-regulated in the absence of exogenous growth factors (2–7). B) Cells were permeabilized with Triton X-100 to detect intracellular TIMP-2. Although no TIMP-2 was detected in proliferating cells (1), TIMP-2 expression was up-regulated by serum depletion (8). Expression was further up-regulated by growth factor treatment. In all cases, TIMP-2 expression was greater intracellularly than on the cell surface. TIMP-2 expression is particularly enriched in the soma and neuritic processes of NGF differentiated cells. Scale bar = 25 μm.
In contrast to PC12 cells, cell surface TIMP-2 expression was not detected in either Neuro-2a (Fig. 3) or N1E-115 (Fig. 4) cells. TIMP-2 was not present on cells maintained in 10% serum (Figs. 3 and 4A–F) or differentiated cells in 0.5% serum (Figs. 3 and 4M-Q). However, intracellular TIMP-2 expression was abundant in both cell types. TIMP-2 expression was greater in serum depleted cells (Figs. 3 and 4R-V) than cells maintained in 10% serum (Figs. 3 and 4G-L) and resembled that of NGF differentiated PC12 cells, with intense expression in both the soma and neuritic processes. To determine whether the lack of cell surface TIMP-2 labeling in these cells was due to an absence of TIMP-2 secretion, western blot analysis was performed with conditioned media from cells grown in differentiation media (Fig. 5A). Neuro-2a and N1E-115 cells secreted considerably less TIMP-2 than PC12 cells. Only a weak TIMP-2 signal was observed in Neuro-2a cells after 7 DIV. TIMP-2 expression was first detected in N1E-115 conditioned media at 2 DIV, increased at 3 DIV, and then declined at 5 DIV. To determine whether cell surface labeling could be achieved by increasing the level of TIMP-2 released into the media, TIMP-2 was over-expressed via transient transfection using a TIMP-2 expression vector previously described (Pérez-Martínez and Jaworski 2005). A significant amount of Flag-tagged TIMP-2 was present in the conditioned media of Neuro-2a and N1E-115 cells 3 days after transfection (Fig. 5B). In contrast to the abundant cell surface Flag labeling detected on GFP-positive transfected PC12 cells (Figs. 5CA, D, G, J), neither Neuro-2a (Figs. 5CB, E, H, K) nor N1E-115 (Figs. 5CC, F, I, L) cells showed cell surface Flag labeling, suggesting that secreted TIMP-2did not bind cells in an autocrine manner. Furthermore, the extent of Flag immunolabeling on untransfected cells was no greater than in vector transfected controls (data not shown), suggesting that secreted TIMP-2 did not bind cells in a paracrine manner. Thus, TIMP-2 is secreted, but unlike PC12 cells where it acts at the cell surface, TIMP-2 likely serves as a soluble regulator of matrix proteolysis in Neuro-2a and N1E-115 cells.
Figure 3. Regulation of TIMP-2 expression in Neuro-2a cells.
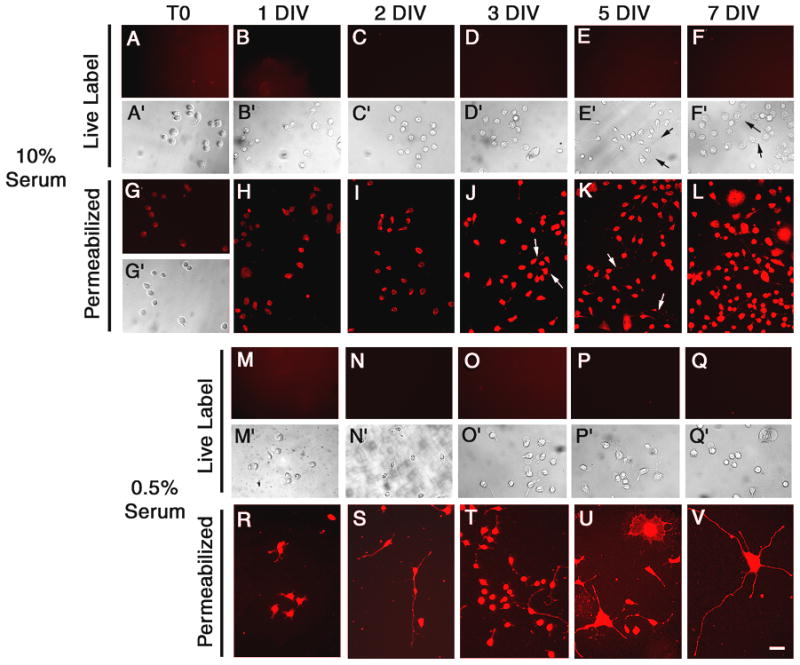
Cell surface TIMP-2 was not detected on proliferating Neuro-2a cells (10% serum, A-F) or cells differentiated by serum depletion (0.5% serum, M-Q). However, intracellular TIMP-2 was present in both proliferating (G-L) and Triton-permeabilized differentiated cells (R-V). TIMP-2 was even detected in neurites of spontaneously differentiated cells maintained in 10% serum (J, K arrows). In differentiated cells, TIMP-2 was present in both fibrous processes and lamellapodial processes (U). Scale bar = 50 μm.
Figure 4. Regulation of TIMP-2 expression in N1E-115 cells.
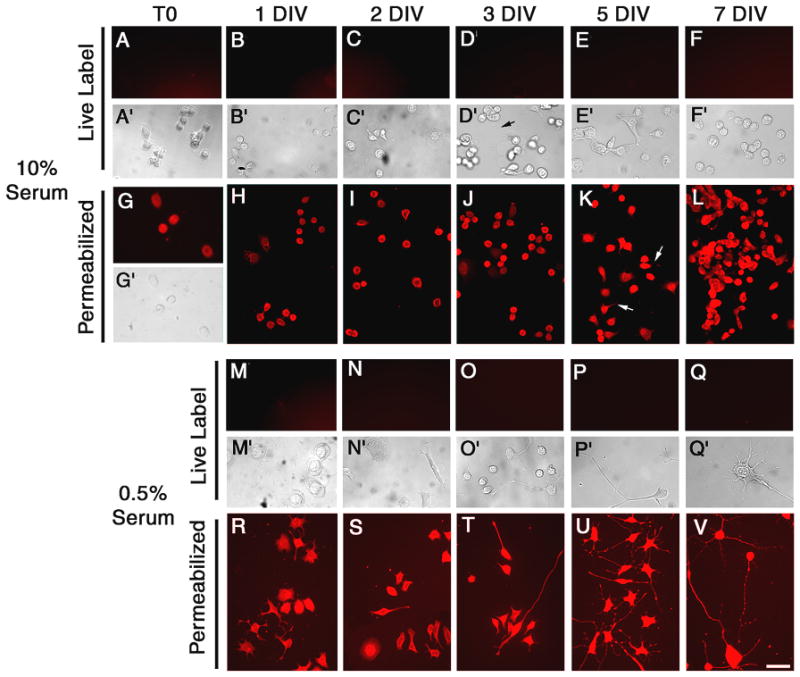
Cell surface TIMP-2 was not detected on proliferating N1E-115 cells (10% serum, A-F) or cells differentiated by serum depletion (0.5% serum, M-Q). Intracellular TIMP-2 was present in both proliferating (G-L) and Triton-permeabilized differentiated cells (R-V). TIMP-2 was present in neuritic processes of spontaneously differentiated cells in 10% serum (K arrows) and serum depleted cells (R-V). Scale bar = 50 μm.
Figure 5. TIMP-2 is secreted from Neuro-2a and N1E-115 cells, but does not bind to the cell surface.
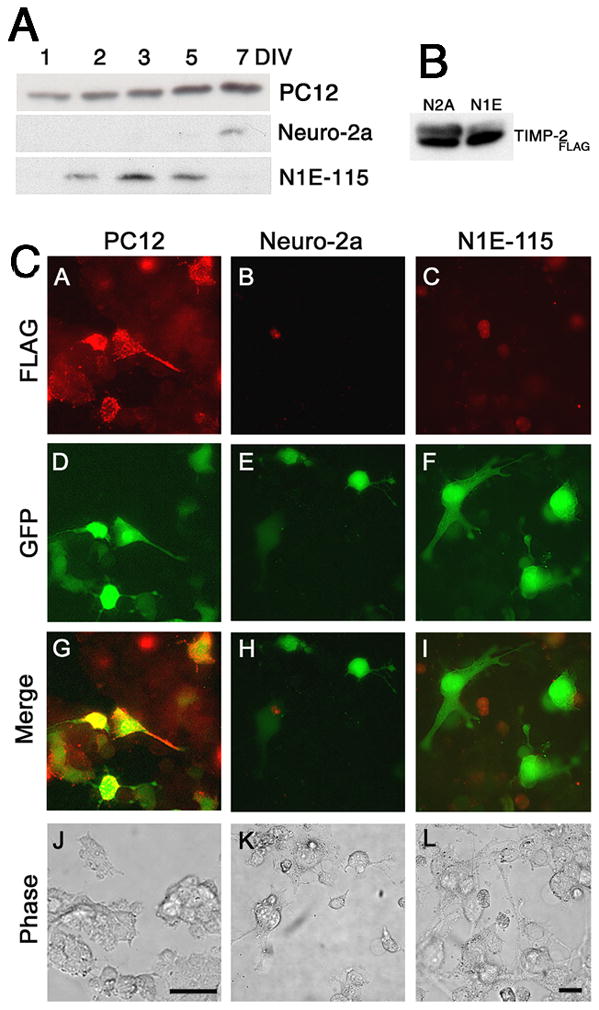
A) TIMP-2 expression in conditioned media (100 μl) from differentiated PC12, Neuro-2a and N1E-115 cells. The presence of TIMP-2 in the media of Neuro-2a and N1E-115 cells demonstrates that the lack of cell surface labeling is not due to the lack of TIMP-2 secretion, but may be due to reduced secretion relative to PC12 cells or the lack of TIMP-2 receptor(s) expression. B) Western blot analysis of conditioned media (100 μl) from Neuro-2a and N1E-115 cells 3 days after transient transfection to over-express TIMP-2. Exogenously produced TIMP-2 (TIMP-2Flag) was immunoprecipitated with Flag antibody. TIMP-2 was abundantly released from both cell lines. C) Live label immunocytochemistry with Flag (red) 3 days after TIMP-2 transfection (GFP-positive green cells) demonstrates that cell surface TIMP-2 “receptors” are expressed on PC12 cells, but not on Neuro-2a or N1E-115 cells. Scale bar = 50 μm.
Like PC12 cells, P19 cells express cell surface receptors for TIMP-2 (Fig. 6). However, unlike PC12 cells where cell surface labeling was not detected in undifferentiated cells (Figs. 2A1,8), cell surface labeling was only detected in pluripotent P19 cells (Fig. 6A). Cell surface labeling was lost after 4 days of RA treatment (Figs. 6B–G). Significant intracellular TIMP-2 expression was present in P19 cells. Similar to cell surface TIMP-2, intracellular levels declined as pluripotent cells (Fig. 6H) were exposed to RA (Fig. 6I). To determine whether cell surface or intracellular TIMP-2 was expressed at a greater level in embryoid bodies than adherent cells (Figs. 6B, I), immunocytochemistry was performed in suspension. The embryoid bodies showed no cell surface labeling and no greater intracellular TIMP-2 than that observed for adherent cells (data not shown). Intracellular TIMP-2 expression increased as cells differentiated into neurons (Figs. 6J–N).
Figure 6. Regulation of TIMP-2 expression in P19 cells.
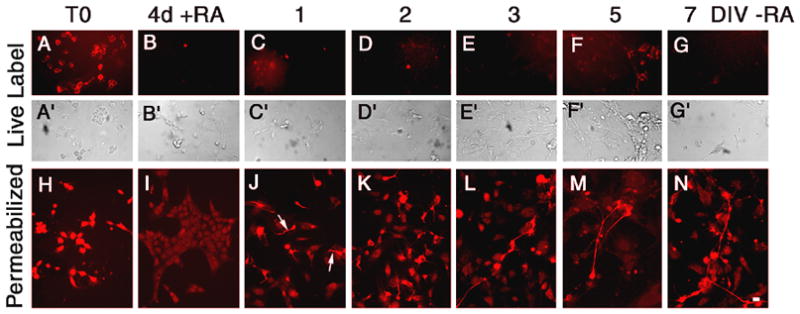
In contrast to other cells lines examined which showed little or no TIMP-2 expression in proliferating cells, TIMP-2 expression was enriched in pluripotent progenitors (T0). Expression was detected both on the cell surface (A) and intracellularly (H). Exposure to RA for 4 days abrogated cell surface TIMP-2 expression (B) and significantly reduced intracellular expression (I). After re-plating cells in the absence of RA, cell surface TIMP-2 was not detectable at any time point. Intracellular TIMP-2 expression was detected as early as 1 DIV in process bearing neurons (J, arrows). Scale bar = 20 μm.
Finally, experiments were conducted to determine the nature of the TIMP-2 receptor. Some TIMPs are tethered to the cell by membrane type MMPs (MT-MMPs). Once secreted, TIMP-2 forms a ternary complex with pro-MMP-2 and MT1-MMP (Butler et al. 1998; Zucker et al. 1998). To determine whether cells lacking surface TIMP-2 labeling possessed MT1-MMP, its expression in undifferentiated (−) and differentiated (+) PC12, Neuro-2a, N1E-115, and P19 cells was examined at 5 DIV. By western blot analysis, all cells expressed MT1-MMP (Fig. 7A). Interestingly, MT1-MMP expression was greater in proliferating PC12, Neuro-2a, and N1E-115 cells and down-regulated upon differentiation, similar to its expression pattern as myoblasts differentiate into mytotubes (Lluri and Jaworski 2005). To determine whether MT1-MMP was present at the cell surface to serve as a TIMP-2 “receptor”, live label immunohistochemistry was performed (Fig. 7B). In PC12 cells, both TIMP-2 (Fig. 7BA) and MT1-MMP (Fig. 7BE) were expressed at the cell surface (Figs. 7BI, M). Neither TIMP-2 nor MT1-MMP was detected on the cell surface of Neuro-2a (Figs. 7BB, F, J, N) or P19 (Figs. 7BD, H, L, P). However, cell surface MT1-MMP, but not TIMP-2 was present on N1E-115 cells (Figs. 7BC, G, K, O).
Figure 7. MT1-MMP expression in neuronal cell lines.
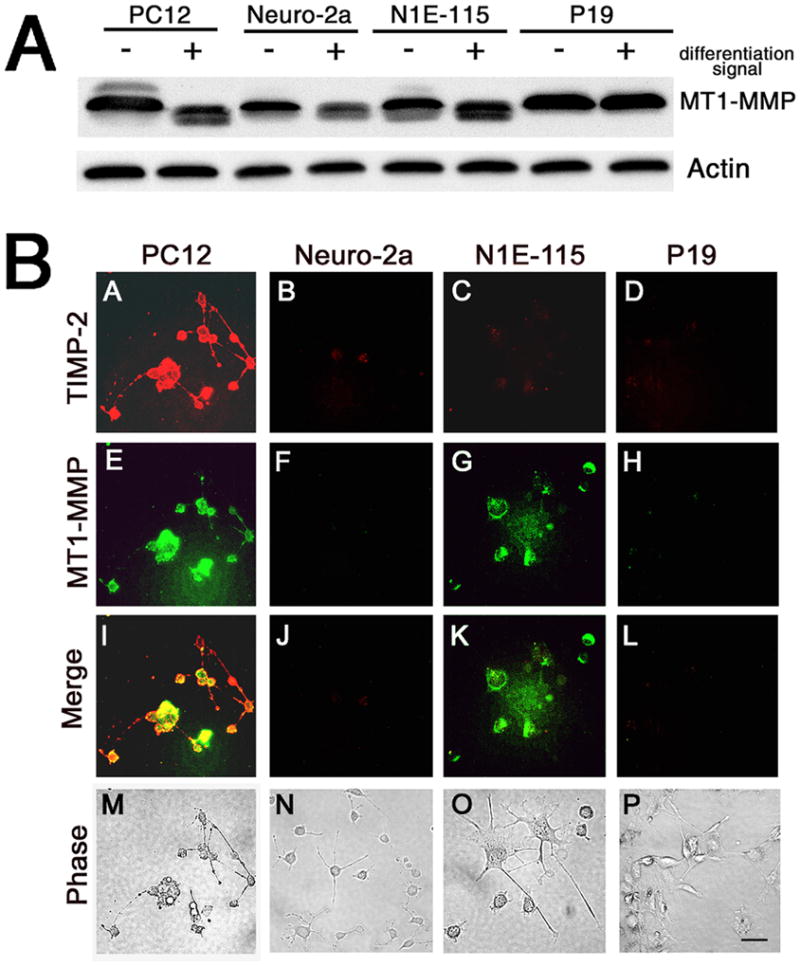
A) Western blot analysis of cell lystates for MT1-MMP expression in cells grown in the absence or presence of differentiation signal for 5 days. Although Neuro-2a, N1E-115, and P19 cells lack TIMP-2 cell surface expression, these cell lines express abundant amounts of MT1-MMP. B) Live label immunocytochemistry for TIMP-2 (red) and MT1-MMP (green) demonstrates that both PC12 cells and N1E-115 cells possess cell surface MT1-MMP, but only PC12 cells have detectable cell surface TIMP-2 expression. Scale bar = 50 μm.
TIMP-2 also binds the cell surface independent of MT-MMPs, suggesting a distinct receptor mechanism (Corcoran and Stetler-Stevenson 1995; Hoegy et al. 2001). TIMP-2 inhibits endothelial cell proliferation by binding α3β1 integrin (Seo et al. 2003). In addition, we recently demonstrated that TIMP-2 binds to α3β1 integrin in vitro and in vivo (Pérez-Martínez and Jaworski 2005). Thus, we determined whether cell surface TIMP-2 expression was associated with integrin expression during neuronal differentiation. Live label immunocytochemistry was performed for α3 integrin at 5 DIV, a time point when TIMP-2 expression was greatest in most cell lines. With a few exceptions, cell surface TIMP-2 expression correlated with α3 integrin expression (Fig. 8A). In PC12 cells, α3 integrin was expressed under all treatment conditions (Figs. 8AA–F). Although surface TIMP-2 was not detected prior to growth factor addition (Fig. 2A8), α3 integrin was observed (Fig. 8AA). Similar to TIMP-2, α3 integrin expression was not detected on Neuro-2a (Figs. 8AG–I) or N1E-115 (Figs. 8AJ–L) cells. Surface TIMP-2 was present on pluripotent P19 cells (Fig. 6A), yet no α3 integrin expression was detectable (Fig. 8AM). Because PC12 cells express α3β1 and α1β1 integrins (Tomaselli et al. 1990; Arregui et al. 1994), live label immunocytochemistry was performed to determine whether TIMP-2 co-localized with these integrins in PC12 cells. TIMP-2 co-localized with α1 (Fig. 8BI), α3 (Fig. 8BJ), and β1 (Fig. 8BL) integrins on the soma and neuritic processes. Labeling specificity is demonstrated by the lack of α5 integrin expression (Fig. 8BK). These data corroborate our previous observation that TIMP-2 binds to α3β1 in NGF treated PC12 cells (Pérez-Martínez and Jaworski 2005). In sum, TIMP-2 expression is regulated by multiple factors coincident with neuronal differentiation and its cell surface expression principally correlates with the expression of α3β1 integrin.
Figure 8. Cell surface TIMP-2 expression correlates with α3 integrin expression.
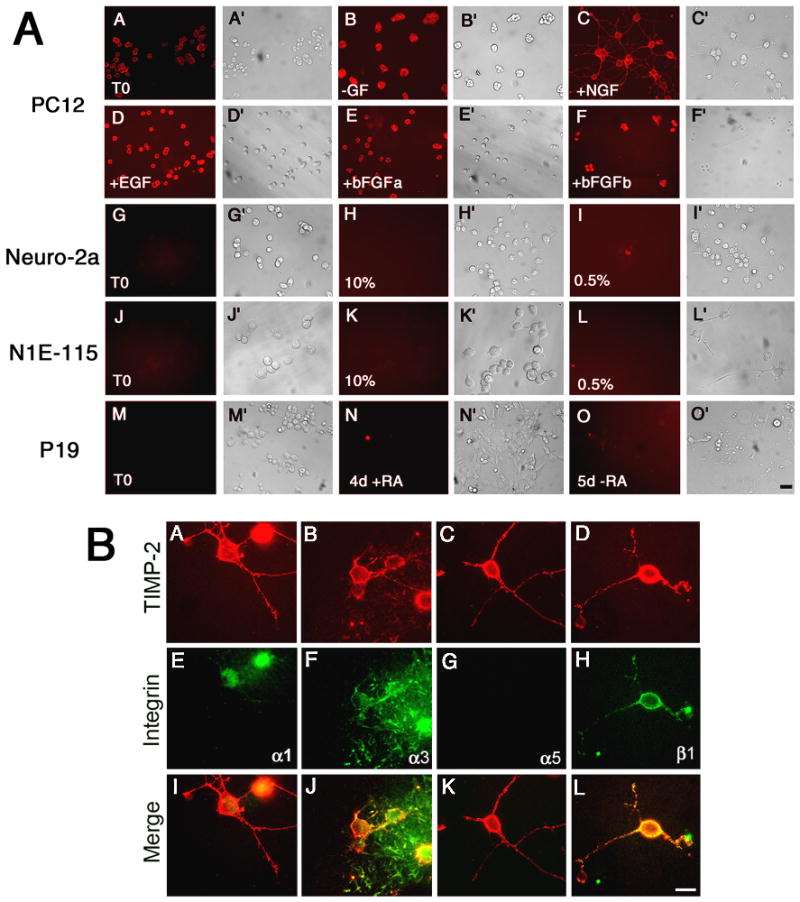
A) Live label immunocytochemistry with α3 integrin at 5 DIV revealed that, like TIMP-2, cell surface labeling for α3 integrin was only present on PC12 cells. α3 integrin expression (A) was greater than cell surface TIMP-2 (Fig. 2A8) in serum starved PC12 cells. Like TIMP-2, α3 integrin expression was up-regulated by overnight growth factor deprivation (B) and further increased by growth factor addition (C, D, E and F). In NGF differentiated PC12 cells, α3 integrin was expressed on the soma and neuritic processes (C). Neuro-2a, N1E-115 and P19 cells lack detectable α3 integrin expression. B) Live label immunocytochemistry for TIMP-2 (A-D) and integrin (E-H) in PC12 cells treated with NGF for 5 days. TIMP-2 co-localizes with α1 (I), α3 (J), and β1 (L) integrin. Scale bar = 50 μm (A), 25 μm (B).
Discussion
The regulation of TIMP-2 expression in a variety of cell lines induced to differentiate into neurons by different neurogenic signals was described. TIMP-2 expression was up-regulated in response to growth factor treatments, as well as serum deprivation. The time course of TIMP-2 expression suggests that TIMP-2 may play a role in neurogenesis. The most notable observation was that cell surface TIMP-2 expression principally correlated with α3β1 integrin expression, suggesting the interaction of these two molecules during neuronal differentiation.
In three of the four cell lines examined here (PC12, Neuro-2a, and N1E-115), TIMP-2 expression was present at low levels in proliferating cells, but was significantly up-regulated upon neuronal differentiation. This confirms our in vivo observation that TIMP-2 is expressed by post-mitotic neurons (Fager and Jaworski 2000). The abundant TIMP-2 expression in pluripotent P19 cells is somewhat unexpected given that TIMP-2 is not highly expressed in the neuroepithelium. P19 cells can be induced to differentiate into a neuronal/glial lineage or muscle lineage depending on the RA concentration (Edwards and McBurney 1983). Since TIMP-2 is expressed in muscle (Young et al. 2002; Jaworski et al. 2006), its presence in undifferentiated P19 cells may reflect expression by myoblasts. This hypothesis is substantiated by the fact that TIMP-2 is expressed in proliferating C2C12 myoblasts (Lluri and Jaworski 2005). Given that TIMP-2 exerts growth inhibitory properties (Hoegy et al. 2001; Seo et al. 2003), its expression in pluripotent P19 cells could also reflect a more specific role in cell cycle control early in development. Accordingly, TIMP-2 is expressed in human embryonic CNS stem cells (Frölichsthal-Schoeller et al. 1999). However, unlike P19 cells, its expression in stem cells is not regulated by neuronal differentiation and suggests multiple functions for TIMP-2 during neuronal differentiation.
The up-regulation of TIMP-2 expression in PC12 cells in response to EGF and bFGF, at a proliferating concentration, might seem in conflict with a growth-inhibitory role. However, a number of reports have suggested that negative regulatory signals might function as a feedback loop to regulate mitogenic signaling pathways. In Drosophila, EGF up-regulates the expression of Argos, an inhibitor of EGF receptor activity, which is required for ventral ectodermal pattering (Golembo et al. 1996). Similarly, REN, whose expression is up-regulated by retinoic acid, EGF, and NGF, induces growth arrest and neuronal differentiation (Gallo et al. 2002). As inhibition of cell proliferation is a prerequisite for neuronal differentiation, we propose that TIMP-2 expression in response to EGF and bFGF might function in a similar feedback loop to induce cell cycle arrest; thus, allowing neurons to acquire and maintain a fully differentiated state.
Given that both the growth promoting (Hayakawa et al. 1994) and growth inhibiting (Hoegy et al. 2001; Seo et al. 2003) effects of TIMP-2 are independent of its MMP-inhibitory activity, the putative “TIMP-2 receptor” must recognize an epitope distinct from that involved in MMP inhibition. Seo and colleagues (2003) identified α3β1 integrin as a TIMP-2 receptor in human microvascular endothelial cells and we have detected α3β1 interaction with TIMP-2 in PC12 cells and the mouse cerebral cortex (Pérez-Martínez and Jaworski 2005). Herein, we demonstrated that cell surface TIMP-2 expression during neuronal differentiation correlated with α3β1 integrin expression, raising the possibility that TIMP-2 exerts its actions during neurogenesis via integrins.
Several possible explanations exist for the lack of cell surface TIMP-2 expression in Neuro-2a, N1E-115 and P19 cells. First, the amount of TIMP-2 secreted by these lines is considerably less than that by PC12 cells and may be of sufficiently low abundance to be undetectable by the immunocytochemical methods used. Second, the classical TIMP-2 receptor MT1-MMP is not expressed on the cell surface of Neuro-2a or P19 cells. N1E-115 cells possess surface MT1-MMP expression at 5 DIV, but not TIMP-2. This could be due to reduced TIMP-2 secretion at 5 DIV relative to 3 DIV (Fig. 5A). Third, the integrin expression profile might explain the lack of TIMP-2 cell surface staining in these cell lines. For example, P19 cells express αvβ1 and αvβ3 integrins (Dedhar et al. 1991) and β1 integrin signaling is required for neuritogenesis in serum-starved N1E-115 cells (Sarner et al. 2000). Thus, if TIMP-2 primarily binds to α3β1 integrin, cell surface expression would not be detected on cells other integrin isoforms. Finally, if TIMP-2 serves to maintain a differentiated neuronal phenotype, the lack of cell surface TIMP-2 expression in Neuro-2a and N1E-115 cells might be related to the lack of permanent cell cycle arrest in these cell lines (discussed below). Altogether the data suggests that TIMP-2 is expressed on the cell surface in a cell type specific manner, indicative of a specific TIMP-2-receptor interaction. Based on our observations, we propose a TIMP-2-α3 integrin interaction responsible for mediating neuronal differentiation.
While transformed cell lines serve as useful models of neurogenesis, they are not without shortcomings. For example, in contrast to neuronal cell cycle arrest in vivo, which is irreversible, serum starved “differentiated” Neuro-2a and N1E-115 cells retract neurites, become round, and divide upon application of agonists, including serum (Roisen et al. 1981; Sarner et al. 2000). We acknowledge that caution must be taken not to over interpret data obtained from transformed cell lines; however, several in vivo observations substantiate our proposed role for TIMP-2 in neurogenesis. The presence of increased nestin-positive progenitors and reduced neurite outgrowth in TIMP-2−/− cerebral (Pérez-Martínez and Jaworski 2005) and cerebellar (Jaworski et al. 2006) cortical neurons suggests that TIMP-2 plays a role in both cell cycle arrest and terminal neuronal differentiation. In addition, fear-potentiated startle, a model of synaptic plasticity in the amygdala, is deficient in TIMP-2−/− mice, suggesting that TIMP-2 may play a role in activity-dependent plasticity (Jaworski et al. 2005).
Although traditionally recognized for their MMP-inhibitory activity, it is now well accepted that TIMPs exert diverse biological functions distinct from MMP inhibition (reviewed in Baker et al. 2002; Crocker et al. 2004). By binding to α3β1 integrin TIMP-2 could influence migration (Milner and Campbell 2002; Schmid and Anton 2003), neurite outgrowth (Ivins et al. 2000), and synaptic plasticity (Rohrbough et al. 2000; Wildering et al. 2002; Chan et al. 2003) in an MMP-independent manner. Therefore, further experiments are required to determine whether TIMP-2 exerts it activities on neurogenesis in vivo via MMP-dependent or -independent mechanisms.
The study of TIMPs during nervous system development is at its infancy. A paucity of information exists regarding the function of TIMPs, the substrates to which they bind, and their mechanism of action within the nervous system. Further studies are necessary to determine whether TIMP-2 subserves its functions via interaction with integrins in vivo. These studies will further our understanding of the role of extracellular matrix proteins in developmental tissue remodeling and the role of the MMP/TIMP axis in mature nervous system function.
Acknowledgments
This work was supported by Grant NS045225 co-funded by NINDS and NCRR, NS35874 and American Heart Association grant 9950039N (to DMJ), and the University of Vermont College of Medicine. Densitometric analysis was performed in the VT Cancer Center DNA Analysis Facility and was supported, in part, by grant P30CA22435 from the NCI. We thank Dr. Felix Eckenstein, Univ. of Vermont Dept. of Neurology, for kindly providing the bFGF and heparin used in these studies.
Abbreviations
- BDNF
brain derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- BSA
bovine serum albumin
- DIV
days in vitro
- DMEM
Dulbecco’s Modified Eagle’s Media
- DRG
dorsal root ganglion
- ECM
extracellular matrix
- EGF
epidermal growth factor
- FBS
fetal bovine serum
- HRP
horseradish peroxidase
- MEM
minimal essential media
- MMP
matrix metalloproteinase
- NGF
nerve growth factor
- PBS
phosphate buffered saline
- RA
retinoic acid
- TBS
Tris buffered saline
- TBST
Tris buffered saline with Tween
- TIMP
tissue inhibitor of metalloproteinase
References
- Ali SA, Pappas IS, Parnavelas JG. Collagen type IV promotes the differentiation of neuronal progenitors and inhibits astroglial differentiation in cortical cell cultures. Dev Brain Res. 1998;110:31–38. doi: 10.1016/s0165-3806(98)00091-1. [DOI] [PubMed] [Google Scholar]
- Amano T, Richelson E, Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones. Proc Natl Acad Sci. 1972;69:258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arregui CO, Carbonetto S, McKerracher L. Characterization of neural cell adhesion sites: point contacts are the sites of interaction between integrins and the cytoskeleton in PC12 cells. J Neurosci. 1994;14:6967–6977. doi: 10.1523/JNEUROSCI.14-11-06967.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- Blavier L, DeClerck YA. Tissue inhibitor of metalloproteinases-2 is expressed in the interstitial matrix in adult mouse organs and during embryonic development. Mol Biol Cell. 1997;8:1513–1527. doi: 10.1091/mbc.8.8.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, van Westrum SS, Crabbe T, Clements J, d’Ortho MP, Murphy G. The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A. J Biol Chem. 1998;273:871–880. doi: 10.1074/jbc.273.2.871. [DOI] [PubMed] [Google Scholar]
- Chan CS, Weeber EJ, Kurup S, Sweatt JD, Davis RL. Integrin requirement for hippocampal synaptic plasticity and spatial memory. J Neurosci. 2003;23:7107–7116. doi: 10.1523/JNEUROSCI.23-18-07107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield H, Bedi KS, Cool SM, Nurcombe V. Heparan sulfates isolated from adult neural progenitor cells can direct phenotypic maturation. Int J Dev Biol. 2002;46:661–670. [PubMed] [Google Scholar]
- Corcoran ML, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinase-2 stimulates fibroblast proliferation via a cAMP-dependent mechanism. J Biol Chem. 1995;270:13453–13459. doi: 10.1074/jbc.270.22.13453. [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Pagenstecher A, Campbell IL. The TIMPs tango with MMPs and more in the central nervous system. J Neurosci Res. 2004;75:1–11. doi: 10.1002/jnr.10836. [DOI] [PubMed] [Google Scholar]
- Damon DH, D’Amore PA, Wagner JA. Sulfated glycosaminoglycans modify growth factor-induced neurite outgrowth in PC12 cells. J Cell Physiol. 1988;135:293–300. doi: 10.1002/jcp.1041350217. [DOI] [PubMed] [Google Scholar]
- Dedhar S, Robertson K, Gray V. Induction of expression of the αvβ1 and αvβ3 integrin heterodimers during retinoic acid-induced neuronal differentiation of murine embryonal carcinoma cells. J Biol Chem. 1991;266:21846–21852. [PubMed] [Google Scholar]
- Diaz-Rodriguez E, Cabrera N, Esparis-Ogando A, Montero JC, Pandiella A. Cleavage of the TrkA neurotrophin receptor by multiple metalloproteases generates signalling-competent truncated forms. Eur J Neurosci. 1999;11:1421–1430. doi: 10.1046/j.1460-9568.1999.00552.x. [DOI] [PubMed] [Google Scholar]
- Edwards DR. The tissue inhibitors of metalloproteinases (TIMPs). Biology and regulation. In: Clendennin NJ, Appelt K, editors. Matrix metalloproteinase inhibitors in cancer therapy. Humana Press Inc; Totawa, NJ: 1999. [Google Scholar]
- Edwards MK, McBurney MW. The concentration of retinoic acid determines the differentiated cell types formed by a teratocarcinoma cell line. Dev Biol. 1983;98:187–191. doi: 10.1016/0012-1606(83)90348-2. [DOI] [PubMed] [Google Scholar]
- Fager N, Jaworski DM. Differential spatial distribution and temporal regulation of tissue inhibitor of metalloproteinase mRNA expression during rat central nervous system development. Mech Dev. 2000;98:105–109. doi: 10.1016/s0925-4773(00)00437-8. [DOI] [PubMed] [Google Scholar]
- Ferri RT, Levitt P. Regulation of regional differences in the differentiation of cerebral cortical neurons by EGF family-matrix interactions. Development. 1995;121:1151–1160. doi: 10.1242/dev.121.4.1151. [DOI] [PubMed] [Google Scholar]
- Ferri RT, Eagleson KL, Levitt P. Environmental signals influence expression of a cortical areal phenotype in vitro independent of effects on progenitor cell proliferation. Dev Biol. 1996;175:184–190. doi: 10.1006/dbio.1996.0106. [DOI] [PubMed] [Google Scholar]
- Frölichsthal-Schoeller P, Vescovi AL, Krekoski CA, Murphy G, Edwards DR, Forsyth P. Expression and modulation of matrix metalloproteinase-2 and tissue inhibitors of metalloproteinases in human embryonic CNS stem cells. Neuroreport. 1999;10:345–351. doi: 10.1097/00001756-199902050-00025. [DOI] [PubMed] [Google Scholar]
- Galko MJ, Tessier-Lavigne M. Function of an axonal chemoattractant modulated by metalloprotease activity. Science. 2000;289:1365–1367. doi: 10.1126/science.289.5483.1365. [DOI] [PubMed] [Google Scholar]
- Gallo R, Zazzeroni F, Alesse E, Mincione C, Borello U, Buanne P, D’Eugenio R, Mackay AR, Argenti B, Gradini R, Russo MA, Maroder M, Cossu G, Frati L, Screpanti I, Gulino A. REN: a novel, developmentally regulated gene that promotes neural cell differentiation. J Cell Biol. 2002;158:731–740. doi: 10.1083/jcb.200202024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golembo M, Schweitzer R, Freeman M, Shilo BZ. Argos transcription is induced by the Drosophila EGF receptor pathway to form an inhibitory feedback loop. Development. 1996;122:223–230. doi: 10.1242/dev.122.1.223. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Yamashita K, Ohuchi E, Shinagawa A. Cell growth-promoting activity of tissue inhibitor of metalloproteinase-2 (TIMP-2) J Cell Sci. 1994;107:2373–2379. doi: 10.1242/jcs.107.9.2373. [DOI] [PubMed] [Google Scholar]
- Hoegy SE, Oh HR, Corcoran ML, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinases-2 (TIMP-2) suppresses TKR-growth factor signaling independent of metalloproteinase inhibition. J Biol Chem. 2001;276:3203–3214. doi: 10.1074/jbc.M008157200. [DOI] [PubMed] [Google Scholar]
- Hojilla CV, Mohammed FF, Khokha R. Matrix metalloproteinases and their tissue inhibitors direct cell fate during cancer development. Br J Cancer. 2003;89:1817–1821. doi: 10.1038/sj.bjc.6601327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff K, End D, Guroff G. Nerve growth factor-induced alteration in the response of PC12 pheochromocytoma cells to epidermal growth factor. J Cell Biol. 1981;88:189–198. doi: 10.1083/jcb.88.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huovila AP, Turner AJ, Pelto-Huikko M, Karkkainen I, Ortiz RM. Shedding light on ADAM metalloproteinases. Trends Biochem Sci. 2005;30:413–422. doi: 10.1016/j.tibs.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Ivins JK, Yurchenco PD, Lander AD. Regulation of neurite outgrowth by integrin activation. J Neurosci. 2000;20:6551–6560. doi: 10.1523/JNEUROSCI.20-17-06551.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski DM, Fager N. Regulation of tissue inhibitor of metalloproteinase-3 (TIMP-3) mRNA expression during rat CNS development. J Neurosci Res. 2000;61:396–408. doi: 10.1002/1097-4547(20000815)61:4<396::AID-JNR6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Jaworski DM, Soloway P, Caterina J, Falls WA. Tissue inhibitor of metalloproteinase-2 (TIMP-2)-deficient mice display motor deficits. J Neurobiol. 2006;66:82–94. doi: 10.1002/neu.20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski DM, Boone J, Caterina J, Soloway P, Falls WA. Prepulse inhibition and fear-potentiated startle are altered in tissue inhibitor of metalloproteinase-2 (TIMP-2) knockout mice. Brain Res. 2005;1051:81–89. doi: 10.1016/j.brainres.2005.05.057. [DOI] [PubMed] [Google Scholar]
- Jones-Villeneuve EMV, McBurney MW, Rogers KA, Kalnins VI. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol. 1982;94:253–262. doi: 10.1083/jcb.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KM, Tan S, Landman N, Petrova K, Murray S, Lewis R, Kim PK, Kim DS, Ryu SH, Chao MV, Kim TW. Regulated intramembrane proteolysis of the p75 neurotrophin receptor modulates its association with the TrkA receptor. J Biol Chem. 2003;278:42161–42169. doi: 10.1074/jbc.M306028200. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L, Lapinska-Dzwonek J, Szymczak S. Matrix metalloproteinases in the adult brain physiology: a link between c-fos, AP-1 and remodeling of neuronal connections? EMBO J. 2002;21:6643–6648. doi: 10.1093/emboj/cdf676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata T, Yamaguchi T, Shin-ya K, Hori T. Time courses of increased expression of signaling transduction molecules induced by basic fibroblast growth factor in PC12 cells. Neurol Res. 2001;23:327–330. doi: 10.1179/016164101101198695. [DOI] [PubMed] [Google Scholar]
- Klebe RJ, Ruddle FH. Neuroblastoma: cell culture analysis of a differentiating stem cell system. J Cell Biol. 1969;43:69A. [Google Scholar]
- Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lluri G, Jaworski DM. Regulation of TIMP-2, MT1-MMP, and MMP-2 expression during C2C12 differentiation. Muscle Nerve. 2005;32:492–499. doi: 10.1002/mus.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos S, Calothy G, Lamballe F. The noncatalytic TrkCNC2 receptor is cleaved by metalloproteases upon neurotrophin-3 stimulation. Oncogene. 2003;22:740–745. doi: 10.1038/sj.onc.1206213. [DOI] [PubMed] [Google Scholar]
- McBurney MW, Jones-Villeneuve EM, Edwards MK, Anderson PJ. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature. 1982;299:165–167. doi: 10.1038/299165a0. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL. The integrin family of cell adhesion molecules has multiple functions within the CNS. J Neurosci Res. 2002;69:286–291. doi: 10.1002/jnr.10321. [DOI] [PubMed] [Google Scholar]
- Pan D, Rubin GM. Kuzbanian controls proteolytic processing of Notch and mediates lateral inhibition during Drosophila and vertebrate neurogenesis. Cell. 1997;90:271–280. doi: 10.1016/s0092-8674(00)80335-9. [DOI] [PubMed] [Google Scholar]
- Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Martínez L, Jaworski DM. Tissue inhibitor of metalloproteinase-2 promotes neuronal differentiation by acting as an anti-mitogenic signal. J Neurosci. 2005;25:4917–4929. doi: 10.1523/JNEUROSCI.5066-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riboni L, Prinetti A, Bassi R, Caminiti A, Tettamanti G. A mediator role of ceramide in the regulation of neuroblastoma Neuro2a cell differentiation. J Biol Chem. 1995;270:26868–26875. doi: 10.1074/jbc.270.45.26868. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Grotewiel MS, Davis RL, Broadie K. Integrin-mediated regulation of synaptic morphology, transmission, and plasticity. J Neurosci. 2000;20:6868–6878. doi: 10.1523/JNEUROSCI.20-18-06868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisen FJ, Bartfeld H, Nagele R, Yorke G. Ganglioside stimulation of axonal sprouting in vitro. Science. 1981;214:577–578. doi: 10.1126/science.7291999. [DOI] [PubMed] [Google Scholar]
- Rydel RE, Greene LA. Acidic and basic fibroblast growth factors promote stable neurite outgrowth and neuronal differentiation in cultures of PC12 cells. J Neurosci. 1987;7:3639–3653. doi: 10.1523/JNEUROSCI.07-11-03639.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarner S, Kozma R, Ahmed S, Lim L. Phosphatidylinositol 3-kinase, Cdc42, and Rac1 act downstream of Ras in integrin-dependent neurite outgrowth in N1E-115 neuroblastoma cells. Mol Cell Biol. 2000;20:158–172. doi: 10.1128/mcb.20.1.158-172.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid RS, Anton ES. Role of integrins in the development of the cerebral cortex. Cereb Cortex. 2003;13:219–224. doi: 10.1093/cercor/13.3.219. [DOI] [PubMed] [Google Scholar]
- Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- Shea TB, Fischer I, Sapirstein VS. Effect of retinoic acid on growth and morphological differentiation of mouse NB2a neuroblastoma cells in culture. Brain Res. 1985;353:307–314. doi: 10.1016/0165-3806(85)90220-2. [DOI] [PubMed] [Google Scholar]
- Shiosaka S, Yoshida S. Synaptic microenvironments - structural plasticity, adhesion molecules, proteases and their inhibitors. Neurosci Res. 2000;37:85–89. doi: 10.1016/s0168-0102(00)00115-2. [DOI] [PubMed] [Google Scholar]
- Tomaselli KJ, Hall DE, Flier LA, Gehlsen KR, Turner DC, Carbonetto S, Reichardt LF. A neuronal cell line (PC12) expresses two β1-class integrins-α1β1 and α3β1-that recognize different neurite outgrowth-promoting domains in laminin. Neuron. 1990;5:651–662. doi: 10.1016/0896-6273(90)90219-6. [DOI] [PubMed] [Google Scholar]
- Tomimatsu Y, Idemoto S, Moriguchi S, Watanabe S, Nakanishi H. Proteases involved in long-term potentiation. Life Sci. 2002;72:355–361. doi: 10.1016/s0024-3205(02)02285-3. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Yamashita T, Tanaka M, Nagai Y. Synthetic sialyl compounds as well as natural gangliosides induce neuritogenesis in a mouse neuroblastoma cell line (Neuro2a) J Neurochem. 1988;50:414–423. doi: 10.1111/j.1471-4159.1988.tb02928.x. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Schwartz ML, Raballo R, Nilsen J, Rhee J, Zhou M, Doetschman T, Coffin JD, Wyland JJ, Hung YT. Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat Neurosci. 1999;2:246–253. doi: 10.1038/6350. [DOI] [PubMed] [Google Scholar]
- Vaillant C, Didier-Bazès M, Hutter A, Belin MF, Thomasset N. Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum. J Neurosci. 1999;19:4994–5004. doi: 10.1523/JNEUROSCI.19-12-04994.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Wildering WC, Hermann PM, Bulloch AG. Rapid neuromodulatory actions of integrin ligands. J Neurosci. 2002;22:2419–2426. doi: 10.1523/JNEUROSCI.22-07-02419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DA, Phillips BW, Lundy C, Nuttall RK, Hogan A, Schultz GA, Leco KJ, Clark IM, Edwards DR. Identification of an initiator-like element essential for the expression of the tissue inhibitor of metalloproteinases-4 (Timp-4) gene. Biochem J. 2002;364:89–99. doi: 10.1042/bj3640089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker S, Drews M, Conner C, Foda HD, DeClerck YA, Langley KE, Bahou WF, Docherty AJP, Cao J. Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase 1 (MT1-MMP) J Biol Chem. 1998;273:1216–1222. doi: 10.1074/jbc.273.2.1216. [DOI] [PubMed] [Google Scholar]
- Zuo J, Ferguson TA, Hernandez YJ, Stetler-Stevenson WG, Muir D. Neuronal matrix metalloproteinase-2 degrades and inactivates a neurite-inhibiting chondroitin sulfate proteoglycan. J Neurosci. 1998;18:5203–5211. doi: 10.1523/JNEUROSCI.18-14-05203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]