Abstract
Purpose
To test a linear model relating the regional loss in retinal nerve fiber (RNFL) thickness to the corresponding regional loss in sensitivity with data from patients with previous anterior ischemic optic neuropathy (AION).
Design
Case–control study.
Participants
Twenty-four individuals with AION and 20 with normal vision were tested. The time since the AION attack ranged from 5.2 months to more than 20.3 years (median, 2.95 years).
Methods
Eyes were tested with standard automated perimetry (SAP) and with optical coherence tomography (OCT), both RNFL thickness scans. The average RNFL thickness of the inferior and superior disc sectors was plotted against the average total deviations (linear units) of the corresponding superior and inferior arcuate field regions, and a linear model was fitted. According to the model, the RNFL thickness R = soT + b, (1), where T is the relative SAP sensitivity loss (on a linear scale; e.g., for −3 dB, T = 0.5), so is the RNFL thickness attributable to axons in the healthy or normal state (T = 1.0), and b is the residual RNFL measured when all sensitivity and axons are lost.
Main Outcome Measures
Optical coherence tomography RNFL thickness and SAP sensitivity.
Results
The data from the AION patients resembled the data from glaucoma patients previously tested and were described by the linear model. For patients with SAP losses of more than −10 dB in the arcuate region, the RNFL thickness provided an estimate of residual RNFL thickness, b. The median value of b (45.5 µm) was similar to the value for patients with glaucoma. It varied among individuals (range, 30.4–63.3 µm), showing a very weak correlation with patient’s age (r = 0.30) and the time since the AION episode (r = 0.26), but an excellent correlation (r2 = 0.94; P<0.01) with the value of so, estimated from the unaffected eyes.
Conclusions
The relationship between a structure (OCT RNFL thickness) and function (SAP sensitivity loss) is the same for patients with AION and glaucoma and can be approximated by a simple linear model. The model may provide a framework for identifying those patients with ganglion cell axons that are malfunctioning but are alive.
Glaucoma leads to the atrophy of the axons of the retinal ganglion cells (RGCs) and to changes in visual function as measured with behavioral techniques. Although the diagnosis of glaucoma depends on measures of both structural and functional changes, the relationship between structural and functional measures of glaucomatous damage is incompletely understood.
Numerous studies of patients with glaucoma have measured functional losses with standard automated perimetry (SAP) and structural losses with one or more of a variety of techniques. Many of these studies fitted a straight line to scatterplots of structural loss (in linear units) versus losses on SAP (in decibels, log units). However, the weight of the evidence suggests that the function relating structural loss to SAP loss is nonlinear when SAP loss is expressed in log(dB) units.1 Further, Garway-Heath1 and Garway-Heath et al2 argued that the data relating structure (e.g., neural rim area) versus function (i.e., SAP loss) are more closely approximated by a straight line if plotted on linear-linear coordinates, rather than on linear-log coordinates. However, there is considerable variability in these data, which do not rule out other mathematical functions.3 Further, until recently there was little theoretical justification for a linear relationship on either a linear-log or log-log plot.
The sophisticated model of Swanson et al4 relates SAP loss to underlying RGC loss. In particular, their model predicted that under some conditions (e.g., beyond 5° to 10°), the loss in field sensitivity is related linearly to RGC loss. This is consistent with electrophysiologic2,5,6 and imaging2,7 studies that proposed that the loss of SAP sensitivity (linear units) is linearly related to RGC loss. However, although the model of Swanson et al4 represents an important step forward in our understanding of the relationship between the losses of SAP sensitivity and RGC number, it does not provide a quantitative model for relating clinical measures of structure and function. Recently, 2 models have been suggested to predict the relationship between structural and functional measures of glaucomatous damage.8–10 Both models relate changes in retinal nerve fiber layer (RNFL) thickness measured with optical coherence tomography (OCT) to changes in sensitivity measured with SAP. In one case,8,9 human data were considered, and in the other,10 data from monkeys with experimental glaucoma were analyzed.
The human model8,10 was based on an earlier model5,6 shown to predict the relationship between the amplitudes of the multifocal visual evoked potential and glaucomatous loss demonstrated on SAP. This model, which assumes that SAP sensitivity loss (in linear units) is linearly related to local loss in RGC,2,4–7 provides a good description of the data from patients with glaucoma.9
However, this linear model contains several assumptions that need testing. In particular, unlike the model proposed for the monkey, the human model of glaucoma assumes that a complete loss of axons does not yield an RNFL thickness of 0. Rather, there is some minimum value beyond which the measure of RNFL thickness cannot be reduced. This residual RNFL thickness is at least in part the result of glial cells and blood vessels. This assumption raises several questions: What is the minimum RNFL thickness present when almost all function has been lost and, presumably, almost all RGC axons have degenerated? Does it vary among individuals? Does it change with age? Does it change with time since axon death? It is difficult to answer these questions with data from patients with glaucoma. Glaucomatous damage progresses slowly and it is not clear at any given moment how many of the RGCs are alive but functioning abnormally. Thus, it is of interest to compare glaucomatous damage with a condition in which the RGCs are almost certainly dead in the damaged area and their axons almost certainly are missing.
Herein the authors focus on a second type of optic nerve damage, anterior ischemic optic neuropathy (AION), which has some similarities to glaucomatous optic neuropathy. However, AION has the advantage of being a static optic neuropathy, if measured after the acute stage when atrophy of RGCs has taken place. One purpose of the current study was to see if the same model that fitted the data from patients with glaucomatous field damage would fit the data from patients with AION. A second purpose was to use the data from these patients to learn more about the residual RNFL thickness remaining after extensive RGC damage.
Patients and Methods
Twenty-four patients (mean age±standard deviation, 59.2±9.8 years) and 20 control subjects (mean age±standard deviation, 54.8±10.7 years) were tested with both SAP and OCT. The control subjects had normal fundus examination results and 20/20 visual acuity. The patients had at least 1 eye with AION that was tested with SAP and OCT at least 5 months after the ischemic attack. The 5-month cutoff was chosen to allow sufficient time to minimize the effects of optic disc swelling and to allow the RGC axons to degenerate. Eighteen of the patients had asymmetrical AION, that is, only 1 eye had a documented attack of AION. For the 6 patients with bilateral AION, only 1 eye was included in the analysis. For 5 of these 6 patients, the eye with the longest time since the AION attack was chosen. For the sixth patient, the eye with the longest time since the attack had a visual acuity of 20/400, and therefore, the other eye was included. For the 24 eyes with AION included herein, the time since the last AION attack ranged from 5.2 months to 20.6 years (median, 2.95 years). For the 42 eyes included in the analysis, all but 2 had visual acuities of 20/60 or better; 1 eye had visual acuity of 20/80 and another had visual acuity of 20/100. In sum, the analysis was performed on both eyes of the 18 unilateral AION patients, on both eyes of the 20 control subjects, and on 1 eye of the 6 bilateral AION patients. Procedures followed the tenets of the Declaration of Helsinki and the protocol was approved by the committee of the Institutional Board of Research Associates of Columbia University and the University of Iowa Institutional Review Board (IRB) for Human Use.
Static Automated Perimetry
The Swedish interactive threshold algorithm standard SAP was performed with a Humphrey Field Analyzer (Zeiss Meditech, Dublin, CA) using programs 24-2 or 30-2 on all but 2 of the eyes; 1 affected eye was tested with a full-threshold strategy and 1 unaffected eye was tested with a Swedish interactive threshold algorithm fast technique. All but 1 eye had reliable visual fields, defined as fewer than 33% fixation losses, false-positive results, and false-negative results; 1 eye had too many fixation losses, but the SAP field replicated on retest. Of the 18 patients whose better eye did not have a history of AION, 14 of these better eyes had normal 24-2 SAP results (i.e., glaucoma hemifield test, mean deviation [MD], and pattern standard deviation within normal limits). One patient’s better eye showed abnormal results on the glaucoma hemifield test, abnormal MD (<2%), and abnormal pattern standard deviation (P<0.005). The other 3 had fields with either the glaucoma hemifield test (n = 1), MD (n = 1), pattern standard deviation (n = 2), or a combination thereof falling outside the 95% limits. The MDs for these 4 eyes were: −3.44 db (<2%), −1.74 db, −1.96 db, and −3.49 db (<2%). For all patients, only 1 affected eye was included in the analysis as described above. The MD of these 24 eyes was −15.04±8.44 dB (range, −3.46 to −31.97 dB).
Optical Coherence Tomography
The RNFL thickness was measured using OCT (OCT3, Stratus, fast RNFL circular scan; Zeiss Meditech, Dublin, CA). The RNFL data consisted of the average of 3 circular scans in the set, and only sets with a signal strength of more than 6 were used.
Comparing Local Static Automated Perimetry Loss with Local Retinal Nerve Fiber Layer Thickness
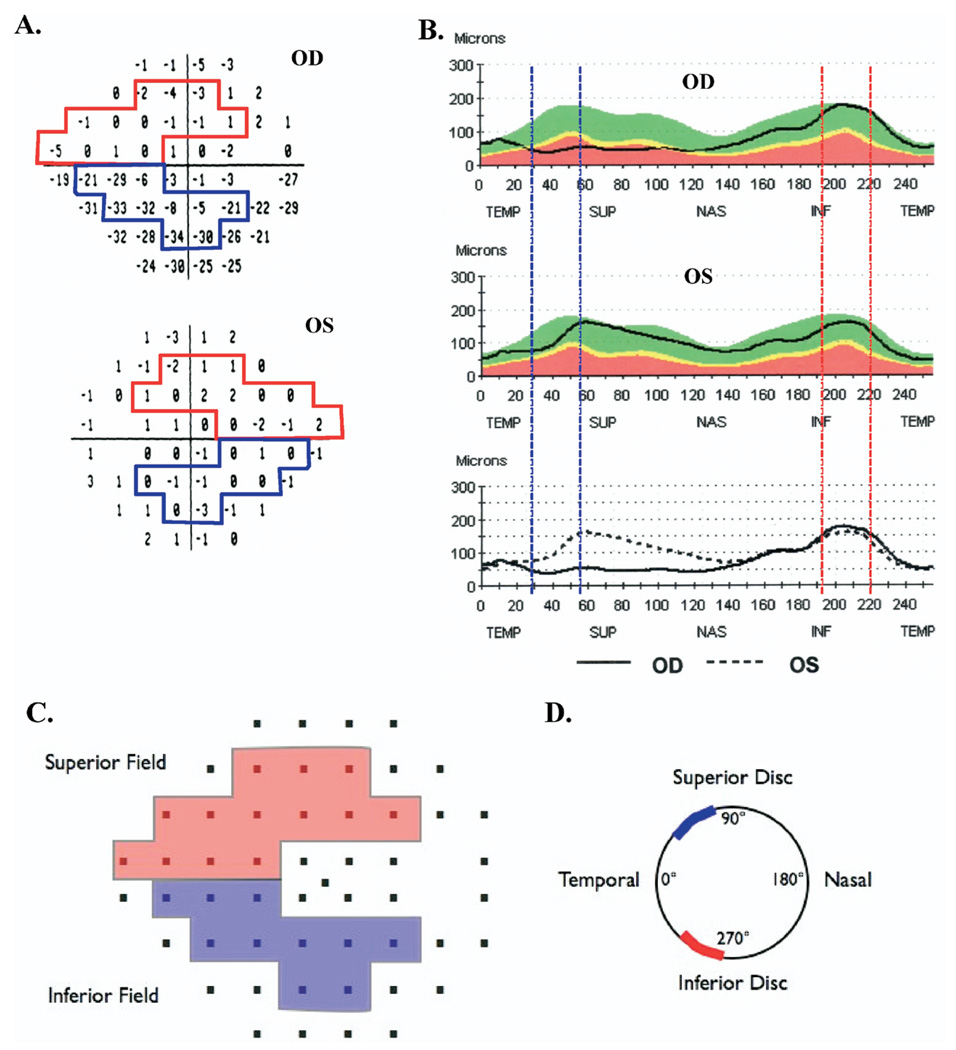
Figure 1 shows the total deviation plots (Fig 1A), as presented in the SAP field analysis, and the RNFL thickness profiles (Fig 1B), as presented in the OCT RNFL report, for one of the patients. This patient had an AION attack in the right eye approximately 8 months before this test. The left eye had no history of AION and had normal fundus examination results, 20/20 visual acuity, and normal SAP results.
Figure 1.
Static automated perimetry (SAP) (A) total deviations plots and (B) optical coherence tomography (OCT) retinal nerve fiber (RNFL) profiles for a patient with anterior ischemic optic neuropathy (AION) of the right eye (OD); the left eye (OS) was unaffected. Schematic showing the Garway-Heath et al10 map of (C) SAP regions to (D) disc sectors. The superior arcuate field region (red) maps to the inferior RNFL arcuate sector from 271° to 310°, and the inferior arcuate field region (blue) maps to the superior RNFL arcuate sector from 41° to 80°. INF = inferior disc sector; NAS = nasal disc sector; SUP = superior disc sector; TEMP = temporal disc sector.
For a quantitative comparison of local SAP field loss to local RNFL thickness, corresponding regions of the SAP field (Fig 1A) and RNFL thickness profile (Fig 1B) must be specified. The authors related superior arcuate (SA) and inferior arcuate (IA) regions of the 24-2 field to inferior temporal (IT) and superior temporal (ST) sectors of the optic disc, as defined by Garway-Heath et al11 and illustrated in Figure 1C, D. Figure 1C shows the field points associated with the SA (red) and IA (blue), and Figure 1D shows the associated sectors, IT (red) and ST (blue), of the optic disc. Note that the entire field region (i.e., SA and IA) associated with the IT and ST disc sectors falls within the 24-2 field (Fig 1A, C), which was the main reason for choosing these 2 arcuate sectors for analysis in this study.
According to the scheme in Figure 1D, the IT and ST disc sectors extend from 271° to 310° (IT) and 41° to 80° (ST), where the 9-o’clock point on the right optic disc is 0°. To obtain an RNFL thickness measure for these regions, the 256 RNFL thickness measures for each eye (Fig 1B) were exported. The RNFL thickness associated with each region was obtained by averaging the points corresponding to the IT and ST sectors, shown as the regions between the dashed vertical lines in Figure 1B. To obtain a measure of field loss for the SA and IA regions, we used the values in the 24-2 total deviation plot (i.e., values within the regions marked with the color borders in Fig 1A). In particular, these values were converted to a linear scale (e.g., 0 dB becomes 1.0 and −6 dB becomes 0.25) and then were averaged for the SA and IA regions of the field. These averaged values were converted back to a decibel scale for graphical presentation of the data. This procedure is consistent with the simple linear model, whereas the more common practice of averaging the decibel values is not.5,6,12
Simple Linear Model
The simple linear model, proposed to predict the results from patients with glaucoma8,9 predicts the following linear relationship between RNFL thickness (R) and relative field loss (T): R = soT + b (1), where T is on a scale from 0 to 1.0, so is the RNFL thickness in the healthy or normal state, and b, the base level, is the smallest response thickness, R, obtainable when a patient has lost all sensitivity to light and all RGC axons. For relative field sensitivity expressed in decibels, equation 1 becomes R = so100.1D + b (2), where D is the deviation from normal sensitivity in decibels and the 0.1 is the exponent term that converts from decibels to log units. (Note that when D is 0 dB, then T is 1.0, and when D is −20 dB, then T is 0.01.)
Results
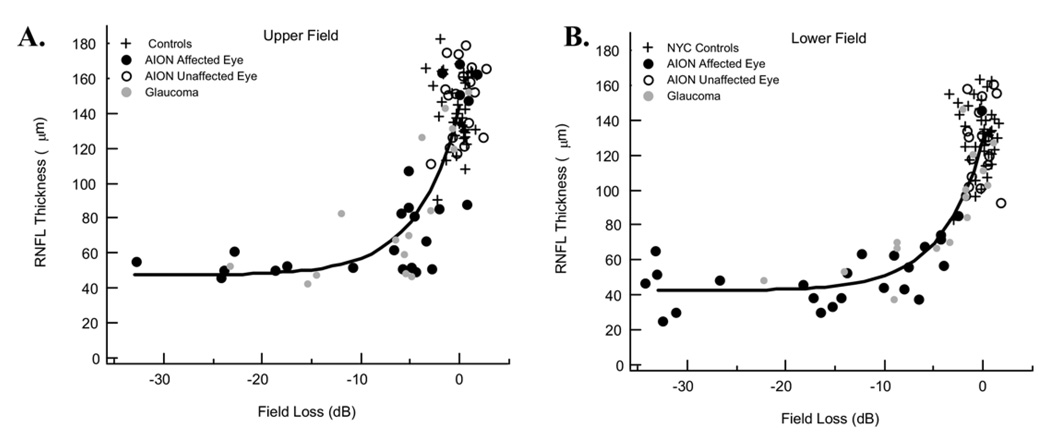
Figure 2 shows the comparison of RNFL thickness to field loss for the upper (SA field/IT disc; Fig 2A) and lower (IA field/ST disc; Fig 2B) visual fields. Each point is the result for a single eye. The control eyes are shown as the pluses (+). The black circles represent the results for the 24 eyes with AION, whereas the open circles represent the 18 companion eyes without a history of AION. The latter fall within the range of the control values. In fact, the mean for the unaffected eyes of the patients for the upper (lower) visual field was 147.1 µm (126.7 µm), similar to the mean for the controls, 143.1 µm (131.2 µm). As previously shown for patients with glaucoma, the RNFL thickness of the affected eyes decreases exponentially with early SAP field loss on a log-linear plot and approaches an asymptotic loss of RNFL thickness for field losses of −10 dB or more.8,9
Figure 2.
A, Graph demonstrating the retinal nerve fiber (RNFL) thickness of the inferior disc sector as a function of superior visual field loss for the 24 affected eyes and 19 unaffected eyes of the patients with anterior ischemic optic neuropathy (AION), as well as for 20 control eyes and 15 eyes with glaucoma.8 B, Graph demonstrating the RNFL thickness of the superior disc sector as a function of inferior visual field loss for the same groups in A. In both panels, the curve is the fit of the linear model. See text for details. dB = decibels; NYC = New York City.
One of the objectives here was to see if the results from patients with AION followed a similar pattern to those obtained from patients with glaucomatous loss. The gray circles are the results from 15 eyes with glaucomatous damage previously published.9 The results for the AION and glaucoma patients show considerable overlap and seem to follow a similar course. The second objective was to examine the residual RNFL thickness after extreme losses resulting from AION. Before turning to this topic, the fit of the linear model and the curves in Figure 2 must be considered.
The curves in Figure 2 are the fit of equation 2. Fitting the equation required estimating 2 parameters, b and so. The parameter b, the residual RNFL thickness for extreme losses in SAP sensitivity, was estimated by taking the median of the 15 eyes with arcuate region field losses of more than −10 dB. (For the 6 eyes in which both superior and inferior fields met the −10-dB criterion, the 2 values were averaged so that there was only 1 estimate per eye.) The resulting value was 45.5 µm. This value was taken as the estimate of b for both the upper and lower arcuate disc sectors; there were too few data points to obtain reliable estimates separately for each sector. In principle, the values of b for the upper and lower sectors should be very close.
The value of so+b is the average RNFL thickness when the field sensitivity is normal. This value was estimated separately for the upper and lower fields by taking the mean for the RNFL thickness for the control group. The estimates of so+b were 143.1 and 131.2 µm, and thus, given the values of b, the estimates of so were 97.6 and 85.7 µm. The theoretical curves in Figure 2 are the fits of equation 2 using these values. The data from both the AION and glaucoma patients fall around these curves.
Residual Retinal Nerve Fiber Layer Thickness
The residual RNFL thickness shows a range of values, 30.4 to 63.3 µm, for the 15 AION patients with at least 1 arcuate field with a loss of more than −10 dB. (The range was 24.8–65.3 µm for 21 hemifields with a loss of more than −10 dB.) Various factors may contribute to this range of values. First, of course, some of this variability among individuals may be the result of measurement error (see “Discussion”).
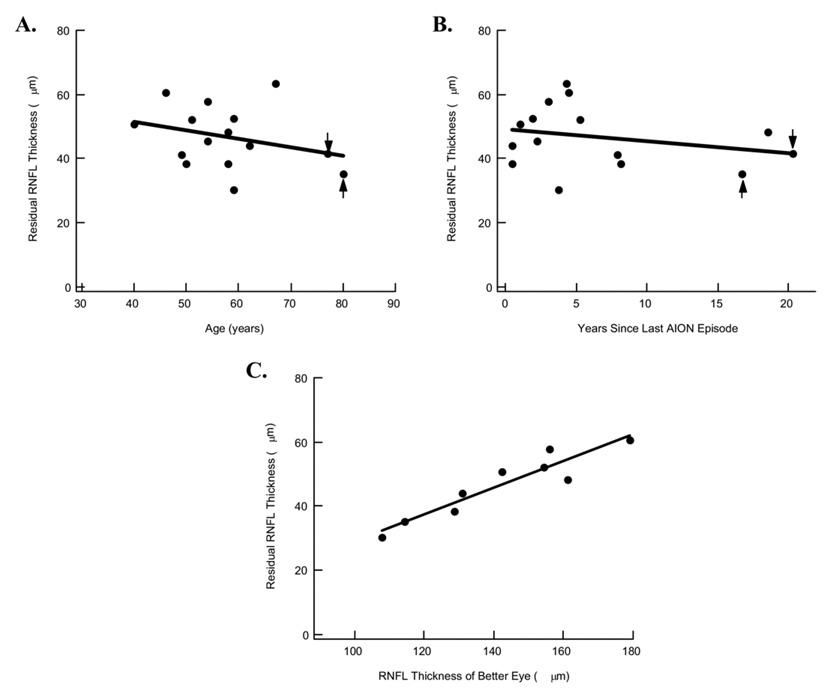
Second, it is well documented that the RNFL thickness decreases with age.13–16 Perhaps the nonaxonal contribution to RNFL thickness also varies with age. To examine the association between residual RNFL thickness and age, we plotted the RNFL thickness for the 15 eyes with arcuate losses of more than −10 dB against the individual’s age in Figure 3A. (For the 6 eyes in which both superior and inferior fields met the −10-dB criterion, the 2 values were averaged so that there was only 1 estimate per eye.) The residual RNFL thickness showed a weak negative, and nonsignificant, association with age (r2 = 0.09). The slope of the best-fitting line was −0.26 µm per year, or only −2.6 µm per decade. Further, there was essentially no association (r2 of 0.0; slope of −0.03 µm per year) when the data for the 2 oldest patients (Fig 3A, B) were removed from the analysis. These patients were among the 3 with the longest time since the last episode of AION.
Figure 3.
A, Graph demonstrating residual retinal nerve fiber layer (RNFL) thickness, estimated from eyes with arcuate field losses resulting from anterior ischemic optic neuropathy (AION) of more than −15 dB in Figure 2, plotted against the age of the individual. Arrows, 2 oldest patients. B, Graph demonstrating residual RNFL thickness, as in A, plotted against the time since the last AION episode. Arrows, 2 oldest patients. C, Graph demonstrating residual RNFL thickness, as in A, plotted against the RNFL thickness of the unaffected eye.
Third, it is possible that over a longer period, the glial content in a region of damage may change and the RNFL thickness may decrease. Figure 3B shows the residual RNFL thickness, as in Figure 3A, plotted against time since the last AION episode. There was a weak negative, and nonsignificant, association (r2 = 0.07) with a shallow slope of −0.38 µm per year. Further, there was essentially no association (r2 of 0.0; slope of −0.07 µm per year) when the data for the 2 oldest patients (Fig 3A, B) were removed from the analysis.
Fourth, there is a wide range of RNFL thickness in the normal population. Some of this variation is probably the result of the known variation in total number or RGC axons among normal individuals, which can vary by a factor of 2.16–21 It seems reasonable to assume that more glial cells may accompany the larger number of RGC axons. If so, then whatever portion of the residual RNFL thickness that is attributable to glial cells also may vary among individuals. To test this notion, the data from the 18 patients who had 1 normal eye were analyzed, that is, those without a previous AION episode. In 9 of these patients, the affected eye had a SAP loss of more than −15 dB in either the superior or inferior arcuate field. (If both superior and inferior arcuates met this criterion, the values were averaged.) Figure 3C shows that there was an excellent positive association (r2 = 0.88; P<0.01) between the residual RNFL thickness in the affected eye and the RNFL thickness in the unaffected eye. Thus, part of the variation in the residual RNFL thickness probably is present in the normal state.
Discussion
The relationship between a structural measure, OCT RNFL thickness, and a functional measure, SAP field loss, was examined after the acute stage of an ischemic attack of AION had subsided. The results seemed to be qualitatively similar to those previously published for patients with glaucoma9; the data from both the AION and glaucoma groups fall together in Figure 2. Further, the linear model describes the general characteristics of the AION and glaucoma data, as can be seen in Figure 2. A similar model was shown to fit the local multifocal visual evoked potential amplitude changes with loss in SAP sensitivity for both AION and glaucoma patients.5,6 Together, these findings have implication for the state of the RGCs in the glaucomatous eyes. If we assume that AION kills RGCs rather than just making them less functional, then this study, along with the multifocal visual evoked potential study,5,6 supports those who believe that nearly all the RGCs in the glaucomatous eye are either responding normally or have died. That is, there are few so-called sick cells in most patients. However, a glaucoma patient with an RNFL thickness greater than predicted by the model may have ganglion cell axons that are malfunctioning but are alive.
The second objective was to learn more about the residual RNFL thickness remaining after extensive RGC damage. This residual thickness almost certainly contains contributions from glial cells and also may have a contribution from the algorithm that determines RNFL thickness. That is, perhaps the algorithm that calculates the RNFL thickness has difficulty dealing with a RNFL thickness near 0 or one that contains very few axons. In any case, it is clear that after extensive RGC damage, there is a residual RNFL thickness, approximately 45.5 µm in this study. This is in agreement with previous work with extreme losses in patients with glaucoma. For example, Sihota et al22 reported an average overall RNFL thickness of 44.9 µm in patients who lost light perception because of glaucoma. Further, a review of 6 OCT studies22–26 concluded that there was a range of residual values from 33.7 to 54.3 µm in these 6 studies.8 In general, the data support the assumption of the present model that a complete loss of RGC axons leaves a residual OCT thickness, whereas they are in conflict with the assumption of the monkey model,10 which assumes that a complete loss of RGC reduces the thickness to 0.
In the present study, the residual thicknesses in patients with extreme field loss varied across individuals, with a range from 30.4 to 63.3 µm for the individual AION patients. The present analysis suggested that no single factor accounts for this range. Rather, a number of factors probably contribute. Of course, measurement error will play a part, because reproducibility is not perfect. Although good estimates of repeat reliability exist for a variety of OCT RNFL thickness measures,27 there is no measure that is comparable directly to residual thickness. However, from published measures27 of repeat reliability for quadrants and sectors, a standard deviation in the range of 3 to 4 µm seems reasonable for regions (e.g., clock hours 3 and 9) in which the RNFL thickness is thin. The standard deviation for the present residual thickness measurements was 9.6 µm, suggesting that other factors are involved. Further, there is some evidence here, and in previous work,9 that individual variation in residual thickness can remain even after repeated measures are obtained.
The residual thickness, on average, tended to be smaller in individuals who were older (Fig 3A), just as overall RNFL thickness becomes smaller with age,13–16 although this factor accounted for only approximately 9% of the variance and was confounded with time since the last ION episode. Similarly, although a small decrease in RNFL thickness that could still be ongoing as a function of time since RGC damage cannot be ruled out, it is unlikely that this is a significant factor. The time since the onset of AION (Fig 3B) accounted for only 7% of the variance, and part of this may be because 2 of the 3 patients with the longest times were the oldest. In short, neither age nor the time since RGC damage seems to be a significant contributor to the variation in the residual thickness, in the admittedly relatively small sample.
However, the residual thickness was smaller in individuals who had a relatively thin RNFL layer before damage occurred. That is, normal eyes probably differ in the number of glial cells in the RNFL, just as they differ in the number of RGC axons. Alternatively, the contribution of other elements (e.g., blood vessels) to the arcuate disc sectors may differ among individuals. In an ongoing study of the causes of variability in normal RNFL profiles, it has come to the authors’ attention that blood vessels make variable contributions to the RNFL profiles of both patients and controls. The excellent correlation (r = 0.94) between the residual RNFL thickness of the affected eye and the RNFL thickness of the unaffected eye (Fig 3C) suggests that glial cells, blood vessels, or both play an important role. Finally, as argued in a previous study,9 individual variations in the mapping of field regions to corresponding disc sectors also contributes to the range of residual values. In their mapping study, Garway-Heath et al11 estimated that the 95% confidence interval for locating a particular SAP point on the optic disc is almost 30°, and they discussed various factors that may contribute to the variability among individuals.
In summary, a simple linear model, with a single set of parameters, describes the relationship between RNFL thickness and SAP field losses resulting from AION and glaucoma. This model predicts that a complete loss of RGC leaves a residual RNFL thickness. The thickness of this residual layer varies among individuals.
Acknowledgments
Supported by the National Eye Institute, Bethesda, Maryland (grant nos. R01-EY-09076, R01-EY-02115); Veterans Administration Rehabilitation Division and Merit Review, Omaha, Nebraska; and an unrestricted grant from Research to Prevent Blindness, Inc., New York, New York.
Footnotes
The authors have no conflicts of interest related to the article.
References
- 1.Garway-Heath DF. Comparison of structural and functional methods I. In: Weinreb RN, Greve EL, editors. Glaucoma Diagnosis: Structure and Function. Reports and Consensus Statements of the 1st Global AIGS Meeting on “Structure and Function in the Management of Glaucoma.”. The Hague: Kugler Publications; 2004. pp. 135–143. [Google Scholar]
- 2.Garway-Heath DF, Holder GE, Fitzke FW, Hitchings RA. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2213–2220. [PubMed] [Google Scholar]
- 3.Bowd C, Zangwill LM, Medeiros FA, et al. Structure-function relationships using confocal scanning laser ophthalmoscopy, optical coherence tomography, and scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2006;47:2889–2895. doi: 10.1167/iovs.05-1489. [DOI] [PubMed] [Google Scholar]
- 4.Swanson WH, Felius J, Pan F. Perimetric defects and ganglion cell damage: interpreting linear relations using a two-stage neural model. Invest Ophthalmol Vis Sci. 2004;45:466–472. doi: 10.1167/iovs.03-0374. [DOI] [PubMed] [Google Scholar]
- 5.Hood DC, Greenstein VC, Odel JG, et al. Visual field defects and multifocal visual evoked potentials: evidence of a linear relationship. Arch Ophthalmol. 2002;120:1672–1681. doi: 10.1001/archopht.120.12.1672. [DOI] [PubMed] [Google Scholar]
- 6.Hood DC, Greenstein VC. The multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog Retin Eye Res. 2003;22:201–251. doi: 10.1016/s1350-9462(02)00061-7. [DOI] [PubMed] [Google Scholar]
- 7.Schlottmann PG, De Cilla S, Greenfield DS, et al. Relationship between visual field sensitivity and retinal nerve fiber layer thickness as measured by scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2004;45:1823–1829. doi: 10.1167/iovs.03-0692. [DOI] [PubMed] [Google Scholar]
- 8.Hood DC. Relating retinal nerve fiber thickness to behavioral sensitivity in patients with glaucoma: the application of a linear model. J Opt Soc Am A Opt Image Sci Vis. 2007;24:1426–1430. doi: 10.1364/josaa.24.001426. [DOI] [PubMed] [Google Scholar]
- 9.Hood DC, Anderson SC, Wall M, Kardon RH. Structure versus function in glaucoma: a test of a linear model. Invest Ophthalmol Vis Sci. 2007;48:3662–3668. doi: 10.1167/iovs.06-1401. [DOI] [PubMed] [Google Scholar]
- 10.Harwerth RS, Vilupuru AS, Rangaswamy NV, Smith EL., III The relationship between nerve fiber layer and perimetry measurements. Invest Ophthalmol Vis Sci. 2007;48:763–773. doi: 10.1167/iovs.06-0688. [DOI] [PubMed] [Google Scholar]
- 11.Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology. 2000;107:1809–1815. doi: 10.1016/s0161-6420(00)00284-0. [DOI] [PubMed] [Google Scholar]
- 12.Hood DC, Zhang X. Multifocal ERG and VEP responses and visual fields: comparing disease-related changes. Doc Ophthalmol. 2000;100:115–137. doi: 10.1023/a:1002727602212. [DOI] [PubMed] [Google Scholar]
- 13.Guedes V, Schuman JS, Hertzmerk E, et al. Optical coherence tomography measurement of macular and nerve fiber layer thickness in normal and glaucomatous human eyes. Ophthalmology. 2003;110:177–189. doi: 10.1016/s0161-6420(02)01564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpineto P, Ciancaglini M, Zuppardi E, et al. Reliability of retinal nerve fiber layer thickness measurements using optical coherence tomography in normal and glaucomatous eyes. Ophthalmology. 2003;110:190–195. doi: 10.1016/s0161-6420(02)01296-4. [DOI] [PubMed] [Google Scholar]
- 15.Varma R, Bazzaz S, Lai M. Optical tomography-measured retinal nerve fiber layer thickness in normal Latinos. Invest Ophthalmol Vis Sci. 2003;44:3369–3373. doi: 10.1167/iovs.02-0975. [DOI] [PubMed] [Google Scholar]
- 16.Sony P, Sihota R, Tewari HK, et al. Quantification of the retinal nerve fibre layer thickness in normal Indian eyes with optical coherence tomography. Indian J Ophthalmol. 2004;52:303–309. [PubMed] [Google Scholar]
- 17.Kupfer C, Chumbley L, Downer JC. Quantitative histology of optic nerve, optic tract and lateral geniculate nucleus of man. J Anat. 1967;101:393–401. [PMC free article] [PubMed] [Google Scholar]
- 18.Balazsi AG, Rootman J, Drance SM, et al. The effect of age on the nerve fiber population of the human optic nerve. Am J Ophthalmol. 1984;97:760–766. doi: 10.1016/0002-9394(84)90509-9. [DOI] [PubMed] [Google Scholar]
- 19.Mikelberg FS, Drance SM, Schulzer M, et al. The normal human optic nerve: axon count and axon diameter distribution. Ophthalmology. 1989;96:1325–1328. doi: 10.1016/s0161-6420(89)32718-7. [DOI] [PubMed] [Google Scholar]
- 20.Repka MX, Quigley HA. The effect of age on normal human optic nerve fiber number and diameter. Ophthalmology. 1989;96:26–32. doi: 10.1016/s0161-6420(89)32928-9. [DOI] [PubMed] [Google Scholar]
- 21.Jonas JB, Muller-Bergh JA, Schlotzer-Schrehardt UM, Naumann GO. Histomorphometry of the human optic nerve. Invest Ophthalmol Vis Sci. 1990;31:736–744. [PubMed] [Google Scholar]
- 22.Sihota R, Sony P, Gupta V, et al. Diagnostic capability of optical coherence tomography in evaluating the degree of glaucomatous retinal nerve fiber damage. Invest Ophthalmol Vis Sci. 2006;47:2006–2010. doi: 10.1167/iovs.05-1102. [DOI] [PubMed] [Google Scholar]
- 23.Zangwill LM, Williams J, Berry CC, et al. A comparison of optical coherence tomography and retinal nerve fiber layer photography for detection of nerve fiber layer damage in glaucoma. Ophthalmology. 2000;107:1309–1315. doi: 10.1016/s0161-6420(00)00168-8. [DOI] [PubMed] [Google Scholar]
- 24.Kanamori A, Nakamira M, Escano MF, et al. Evaluation of the glaucomatous damage on retinal nerve fiber layer thickness measured by optical coherence tomography. Am J Ophthalmol. 2003;135:513–520. doi: 10.1016/s0002-9394(02)02003-2. [DOI] [PubMed] [Google Scholar]
- 25.Leung CK, Yung WH, Ng AC, et al. Evaluation of scanning resolution on retinal nerve fiber layer measurement using optical coherence tomography in normal and glaucomatous eyes. J Glaucoma. 2004;13:479–485. doi: 10.1097/01.ijg.0000138205.99424.24. [DOI] [PubMed] [Google Scholar]
- 26.El Beltagi TA, Bowd C, Boden C, et al. Retinal nerve fiber layer thickness measured with optical coherence tomography is related to visual function in glaucomatous eyes. Ophthalmology. 2003;110:2185–2191. doi: 10.1016/S0161-6420(03)00860-1. [DOI] [PubMed] [Google Scholar]
- 27.Paunescu LA, Schuman JS, Price LL, et al. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using StratusOCT. Invest Ophthalmol Vis Sci. 2004;45:1716–1724. doi: 10.1167/iovs.03-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]