Abstract
Objective
Pazopanib, a tyrosine kinase inhibitor that blocks the receptors for vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and stem cell factor (SCF), was investigated for its effect on choroidal neovascularization (CNV).
Methods
We investigated pharmacokinetics and the ability of oral pazopanib to prevent or cause regression of CNV in mice.
Results
A single oral dose of 4 or 100 mg/kg of pazopanib resulted in an AUC(0-t) of 129.6 and 753.0 μg·hr/ml, respectively. After gavage twice daily for 7 days of 4, 20, or 100 mg/kg, plasma levels were 1.3, 4.9, and 5.8 μg/ml and retina/choroid levels were 4.8, 28.8, and 38.0 μg/g tissue. Starting the day of laser-induced rupture of Bruch’s membrane, 100 mg/kg of oral pazopanib twice daily reduced the area of CNV by 93% at day 14. Treatment of established CNV between 7 and 14 days with 8, 40 or 200 mg/kg/day of pazopanib resulted in reduction in area of CNV by 0%, 58% or 71%, respectively. Substantial regression of CNV (40%) was also achieved after periocular injection of pazopanib.
Conclusions and Clinical Relevance
Orally administered pazopanib has good bioavailability to retina/choroid and causes regression of CNV in mice. These data suggest pazopanib may be useful for treatment of CNV and clinical trials are ongoing in patients with neovascular AMD.
Introduction
Choroidal neovascularization (CNV) is a prevalent cause of vision loss. It is the most common cause of severe vision loss in patients with age-related macular degeneration and it is responsible for visual disability in a substantial number of young patients with pathologic myopia, ocular histoplasmosis, angioid streaks, and several other diseases. Although the pathogenesis of CNV is not completely understood, the demonstration that vascular endothelial growth factor is an important stimulator is a major advance 1, 2. Clinical trials have confirmed the importance of VEGF, because intraocular injections of ranibizumab, an Fab that binds all isoforms of VEGF-A, resulted in significant improvement in vision in 34–40% of patients with subfoveal CNV due to AMD 3, 4. Case series have suggested that bevacizumab, a full-length antibody that binds all isoforms of VEGF-A also provides benefit to patients with CNV due to AMD or other disease processes 5–11.
The major effect of antagonists of VEGF-A such as ranibizumab and bevacizumab is to reduce excessive vascular permeability from CNV, which results in rapid reduction in subretinal and intraretinal fluid, and improvement in visual acuity. Monthly injections of ranibizumab stopped growth of CNV, but did not cause existing CNV to regress 3. Perhaps there are survival factors other than VEGF-A that allow endothelial cells within CNV to survive and remain quiescent despite blockade of VEGF-A with ranibizumab and as soon as levels of ranibizumab are reduced beyond a critical level, leakage and growth of CNV resume. Likely candidates for adjunctive survival factors include other VEGF family members and platelet-derived growth factor-B (PDGF-B), which promotes survival of pericytes another source of survival factors for endothelial cells in new vessels 12.
An efficient way to target multiple VEGF family members is to block VEGF receptors (VEGFRs) with relatively selective VEGFR kinase inhibitors. Since there is high homology between VEGFRs and PDGF receptors (PDGFRs), many kinase inhibitors block both. Pazopanib is a small molecule kinase inhibitor that blocks VEGFR1, VEGFR2, and VEGFR3 with IC50s of 10, 30, and 47 nM, respectively 13. Pazopanib also has substantial activity directed against PDGFRα (IC50, 71 nM), PDGFRβ (IC50, 84 nM), c-Kit (IC50, 74 nM), fibroblast growth factor receptor-1 (FGFR1; IC50, 140 nM), FGFR3 (IC50, 130 nM), and c-fms (IC50, 146 nM). Activity is substantially less against many other kinases that were tested and thus pazopanib has an inhibitory profile that is very interesting with regards to potential effects in angiogenic diseases. Pazopanib showed strong anti-tumor and anti-angiogenic activity in mouse models 13. In this study, we investigated the effects of pazopanib in mouse models of subretinal neovascularization.
Materials and Methods
Mouse model of choroidal neovascularization
Mice were treated in accordance with the Association for Research in Vision and Ophthalmology guidelines for the use of animals in research. CNV was induced by laser photocoagulation-induced rupture of Bruch’s membrane as previously described 14. Briefly, 5 to 6 week old female C57BL/6J mice were anesthetized with ketamine hydrochloride (100 mg/kg body weight), and pupils were dilated with 1% tropicamide. Three burns of 532 nm diode laser photocoagulation (75 μm spot size, 0.1 seconds duration, 120 mW) were delivered to each retina with the slit lamp delivery system of an OcuLight GL diode laser (Iridex, Mountain View, CA) using a handheld cover slip as a contact lens to view the retina. Burns were performed in the 9, 12, and 3 o’clock positions of the posterior pole of the retina. Production of a bubble at the time of laser, which indicates rupture of Bruch’s membrane, is an important factor in obtaining choroidal neovascularization, and therefore, only burns in which a bubble was produced were included in the study. In the initial study, mice were treated twice a day by oral gavage with 100 mg/kg of pazopanib or vehicle for 14 days and then the area of CNV at Bruch’s membrane rupture sites was measured as described below.
Measurement of the area of CNV
Mice were perfused with 1 ml of PBS containing 50 mg/ml of fluorescein-labeled dextran (2 × 106 average molecular weight, Sigma-Aldrich, St. Louis, MO) and choroidal flat mounts were prepared as previously described 15. Briefly, eyes were removed and fixed for 1 hour in 10% phosphate-buffered formalin. The cornea, lens, and retina were removed and four radial cuts were made in the eyecup allowing it to be flat mounted in aqueous mounting medium. Flat mounts were examined by fluorescence microscopy and images were digitized using a three-color CCD video camera and a frame grabber. Image analysis software (Image-Pro Plus, Media Cybernetics, Silver Spring, MD) was used to measure the total area of choroidal neovascularization at each rupture site.
Treatment of established CNV
Mice had laser-induced rupture of Bruch’s membrane at 3 locations in each eye and after 7 days, a group of the mice was perfused with fluorescein-labeled dextran and the baseline area of CNV was measured. The remaining mice were treated twice a day by oral gavage with vehicle or 4, 20, or 100 mg/kg of pazopanib. In other experiments, mice were treated with daily periocular injections of 100 μg of pazopanib or vehicle in one eye. After 7 days, the mice were perfused with fluorescein-labeled dextran and the area of CNV at Bruch’s membrane rupture sites was measured.
Pharmacokinetic studies
Female C57BL/6 mice (n=3 for each time point) weighing 18–22 g were given 4 or 100 mg/kg of pazopanib by oral gavage and plasma was collected at 0, 30, 60, 120, 240, 480, and 1440 minutes post dosing. Mice were euthanized by CO2 asphyxiation and exsanguination by cardiac puncture placing the blood in sodium EDTA-containing tubes on ice. Within 30 minutes of collection, the anticoagulated blood samples were centrifuged at 4500 rpm for 10 minutes at 4°C and the resultant plasma samples were immediately frozen on dry ice and stored at − 80°C until analysis.
Mice (n=3 per dose) were given 4, 20 or 100 mg/kg of pazopanib twice a day by gavage for 7 days and16 hours after the last dose they were euthanized by CO2 asphyxiation and blood was collection for plasma. Eyes were removed, snap frozen in liquid nitrogen, and stored at − 80°C until dissection. The right eye of each mouse was used to prepare eye cups by removing the anterior segment and vitreous leaving the sclera, choroid and retina. For the left eyes, the anterior segment and vitreous were removed and then the retina and choroid were removed together to provide retina/choroid and the remaining sclera which were analyzed separately.
Extraction of pazopanib from tissues
Isolated tissues (eyecup, sclera, and choroid/retina) were weighed, snap frozen in liquid nitrogen, and placed in a liquid nitrogen primed BioPulverizer (Biospec Products Inc., Bartlesville, OK). The tissue was pulverized and tissue powder was transferred into a polypropylene tubes. Extraction buffer (50% methanol/50% 0.5M HCl) was added to tissue powder followed by two cycles of sonication, centrifugation, and supernatant collection. Tissue homogenate supernatant was pooled, frozen on dry ice, and stored at − 80°C until analysis. The extraction efficiency of this methodology was assumed to be 100% for calculation purposes.
Measurement of pazopanib
Plasma samples and eye tissue extracts were analyzed for pazopanib using an analytical method based on protein precipitation followed by HPLC/MS/MS analysis. Using a 20μL aliquot of mouse plasma the lower limit of quantification (LLQ) for pazopanib was 100 ng/mL and the upper higher limit of quantification (HLQ) was 50,000 ng/mL. For eye tissue extracts, using a 50μL aliquot, the LLQ was 10 ng/mL and the HLQ was 5,000 ng/mL.
Data Analysis
Statistical comparisons of the area of CNV in different treatment groups were made with a linear mixed model 16 using SAS software (SAS Institute, Inc. Cary, NC). The analysis included up to 3 CNV area measurements per eye. All measurements from either eye of a mouse were assumed to be exchangeable when modeling correlation structure, and were assumed to be subject to non-error variability due only to treatment for experiments in which different treatment were administered to each eye of a mouse. An overall test for treatment effect was first performed in all models, and if the overall test indicated a significant treatment effect, individual treatments were compared with the vehicle treatment using linear contrasts. The Dunnett procedure was used to adjust for multiple comparisons. Analyst Version 1.4.1 and SMS2000 Version 1.6 were used for the pharmacokinetic calculations. Pharmacokinetic analysis of the plasma concentration-time data was performed using non-compartmental methods to obtain estimates of pharmacokinetic parameters. Tissue concentrations of pazopanib were determined by the following formula: pazopanib (ng/g of tissue) = (concentration in supernatant in ng/mL x extract volume in mL)/tissue weight in g.
Results
Oral Administration of pazopanib causes strong suppression of CNV
Mice had rupture of Bruch’s membrane and were treated with 100 mg/kg of pazopanib by oral gavage twice a day. This resulted in a 93% decrease in the mean area of CNV at Bruch’s membrane rupture sites (Figure 1). This is almost complete suppression of the development of CNV.
Figure 1. Oral Administration of pazopanib causes strong suppression of choroidal neovascularization (CNV).
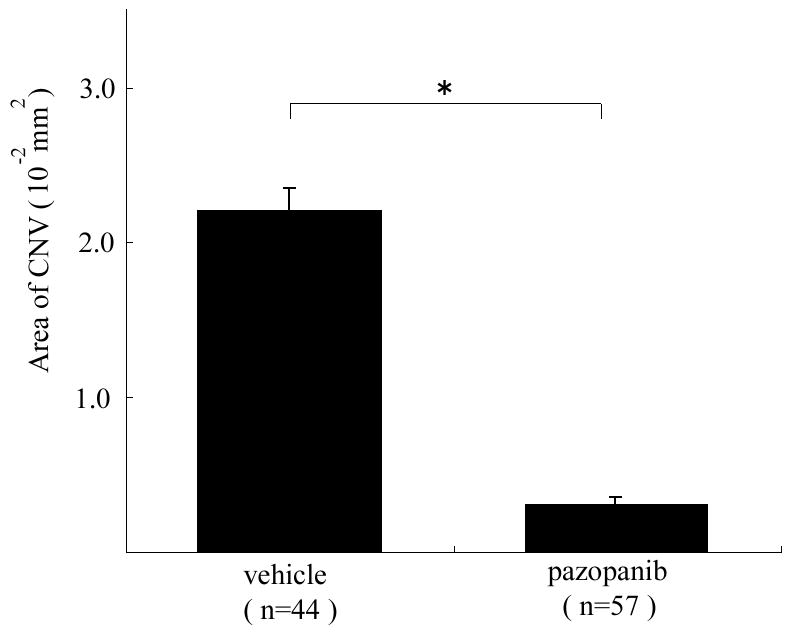
Four to six week old C57BL/6 mice had laser photocoagulation-induced rupture of Bruch’s membrane at 3 locations in each eye and were treated by gavage with 100 mg/kg or pazopanib (10 mice) or vehicle (7 mice) twice a day. After 14 days, the mice were perfused with fluorescein-labeled dextran and the area of CNV at Bruch’s membrane rupture sites was measured by image analysis at laser burns where rupture of Bruch’s membrane had been achieved. The bars represent the mean (±SEM) area of CNV and show a marked suppression of CNV in eyes of mice treated with pazopanib compared to those treated with vehicle.
*p<0.0001 by linear mixed model
Oral administration of pazopanib causes regression of CNV
To determine the effect of pazopanib on established CNV, mice had rupture of Bruch’s membrane and after 7 days a cohort of the mice were used to measure the baseline area of CNV present at 7 days. The remaining mice were treated twice a day by oral gavage with 4, 20, or 100 mg/kg of pazopanib or vehicle alone. After 7 days of treatment with 8 mg/kg/day, the area of CNV was significantly less than that seen in mice treated with vehicle, and no different from the baseline amount of CNV indicating that this dose essentially stopped further growth of CNV (Figure 2). After 7 days of treatment with 40 or 200 mg/kg/day of pazopanib, the area of CNV was significantly less than the baseline amount indicating that the CNV had regressed. The regression was very substantial, consisting of a 58% reduction from baseline area of CNV in mice treated with 40 mg/kg/day of pazopanib and a 71% in mice treated with 200 mg/kg/day.
Figure 2. Oral Administration of pazopanib causes regression of choroidal neovascularization (CNV).
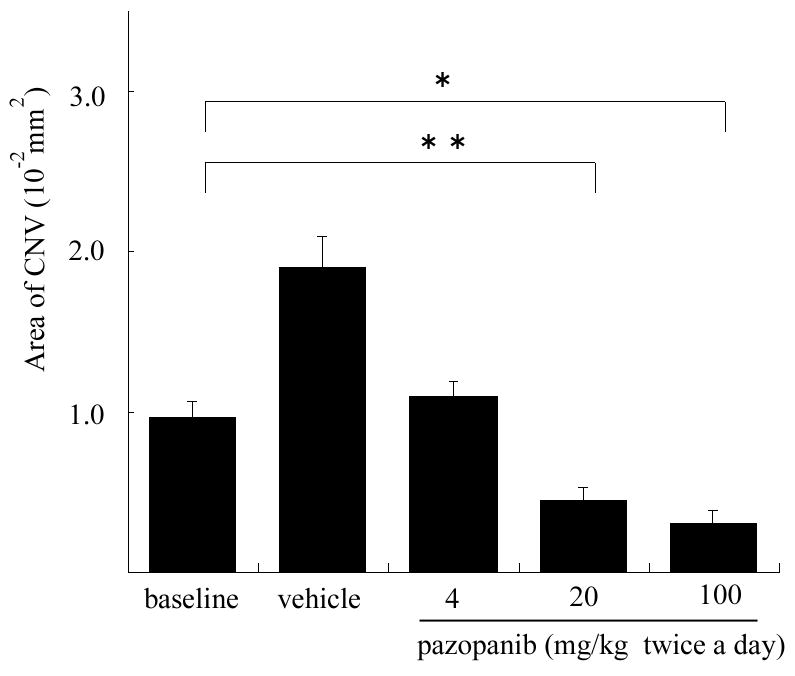
Four week old C57BL/6 mice had laser photocoagulation-induced rupture of Bruch’s membrane at 3 locations in each eye and after 7 days 5 mice were perfused with fluorescein-labeled dextran and the baseline area of CNV was measured by image analysis. The remaining mice were treated twice a day by oral gavage with vehicle (9 mice) or 4 (7 mice), 20 (7 mice), or 100 mg/kg of pazopanib (8 mice). After 7 days, the area of CNV at Bruch’s membrane rupture sites was measured. The bars represent the mean (±SEM) area of CNV at laser sites where rupture of Bruch’s membrane was achieved (n=28, 54, 40, 35, and 48 for baseline, vehicle, 4 mg, 20 mg, and 100 mg, respectively). The area of CNV in eyes treated twice a day with 4 mg/kg of pazopanib was similar to that seen at baseline and significantly less than that seen in eyes treated with vehicle, suggesting that growth of CNV had been suppressed. The area of CNV in eyes of mice treated twice a day with 20 or 100 mg/kg of pazopanib was significantly less than the baseline area indicating that regression of CNV had occurred.
*p<0.0001; **p=0.0013 by linear mixed model with Dunnett’s correction for multiple comparisons.
Pharmacokinetic studies
Single Dose Plasma Pharmacokinetics
Plasma pazopanib concentrations were measured at several time points after a single oral dose of 4 or 100 mg/kg. Peak plasma concentrations occurred at approximately 2 hours post dose for both dose groups (Table 1). The systemic exposure to pazopanib (area under the curve; AUC) for mice in the efficacy studies treated twice a day with 4 mg/kg or 100 mg/kg of pazopanib was estimated to be 259 and 1,506μg·hr/mL, respectively.
Table 1.
Pharmacokinetic Parameter Estimates Following a Single Oral Dose of 4 or 100 mg/kg of Pazopanib
| Nominal Dose (mg/kg) | Cmax (μg/mL) | Tmax (min) | AUC (0-t) (μg.hr/mL) | Estimated AUC (μg.hr/mL) (BID dose) |
|---|---|---|---|---|
| 4 | 13.5 | 123 | 129.6 | 259 (8 mg/kg/day) |
| 100 | 91.4 | 122 | 753.0 | 1,506 (200 mg/kg/day) |
AUC(0-t) refers to the area from time 0 to the last quantifiable concentration
Multiple Dose Plasma Pharmacokinetics
Plasma pazopanib concentrations were measured post oral administration of 4, 20, or 100 mg/kg twice a day for 7 days. Plasma samples were taken from individual mice 16 hours after the last dose administered. Figure 3A shows the mean (± SD) and individual mouse values for each dosing group. The average plasma pazopanib levels were 1,314, 4,893, and 5,771 ng/ml after 7 days of twice a day treatment with 4, 20, and 100 mg/kg, respectively.
Figure 3. Pazopanib levels in plasma and posterior eye tissues following twice a day oral administration of 4, 20, or 100 mg/kg for 7 days.
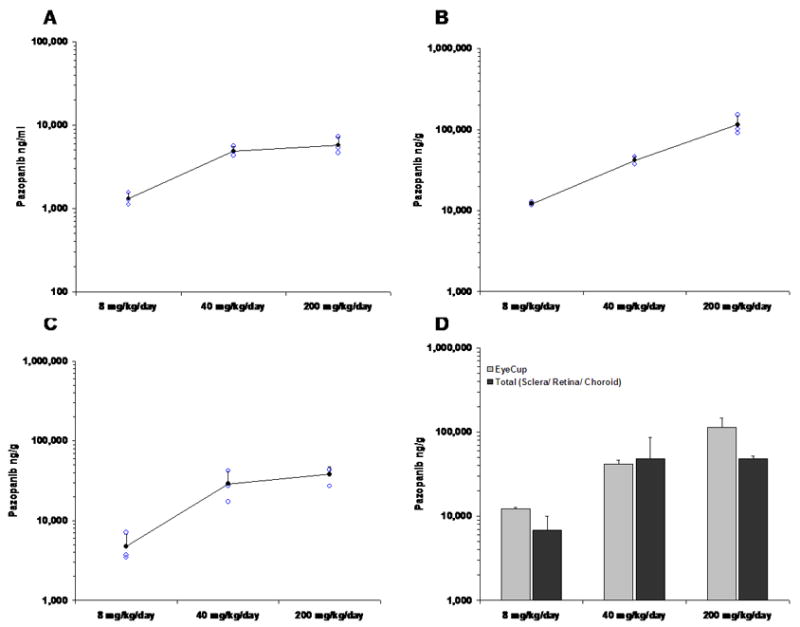
Solid circles show the mean (±SD) level of pazopanib and the open circles show level from each mouse. (A) Plasma levels of pazopanib after multiple administrations; (B) pazopanib in eyecups (posterior sclera + choroid + retina) (C) pazopanib in choroid/retina (choroid + retina); (D) Open bars show the mean (±SD) level of pazopanib in eyecups (posterior sclera + choroid + retina) and the solid bars show the mean (±SD) level of the sum of levels measured in isolated choroid/choroid + levels measured in isolated sclera. Statistical comparisons of the levels obtained with the two different techniques showed no significant differences for any of the 3 doses.
Ocular Tissue Concentrations of Pazopanib Following Oral Administration for 7 days
Ocular tissue drug distribution was assumed to be equivalent between the two eyes. Right eyes were used to dissect eye cups (posterior sclera, choroid, and retina) and left eyes were used to dissect posterior sclera alone and retina/choroid. The mean concentration of pazopanib extracted from eye cups increased in a dose-dependent manner and measured 12.2, 41.7, and 114.5 μg/g for twice a day dosing with 4, 20, and 100 mg/kg, respectively (Figure 3B). These values were statistically different from each other by ANOVA (p ≤ 0.02).
The mean concentration of pazopanib in isolated choroid/retina was 4.8, 28.8, and 38.0μg/g after twice a day dosing for 7 days with 4, 20 and 100 mg/kg, respectively (Figure 3C). There was a significant difference between the first two values (p ≤ 0.03 by ANOVA), but not between the second and third values (p = 0.365 by ANOVA) suggesting a possible limit to the pazopanib binding capacity in retina/choroid. The mean concentration of pazopanib in isolated posterior sclera was 13.9, 78.3, and 94.5μg/g after twice a day dosing for 7 days with 4, 20 and 100 mg/kg, respectively (Figure 3C). There was substantial variability in pazopanib content extracted from the sclera among mice and the values were not statistically different by ANOVA, but they clearly demonstrate that pazopanib that enters the choroid from the circulation is able to penetrate into the sclera. If pazopanib concentrations in isolated retina/choroid and sclera are meaningful, one would anticipate that their sum should approximate values obtained in eye cups and this was found to be the case (Figure 3D).
Local administration of pazopanib by periocular injection causes regression of CNV
Since pharmacokinetic studies demonstrated that substantial amounts of pazopanib entering the choroid from the circulation was able to enter the sclera, we postulated that periocular injection of pazopanib should gain access to the choroid and have an effect on CNV. Seven days after rupture of Bruch’s membrane a cohort of mice was used to measure the baseline amount of CNV and the remaining mice were given periocular injection of 3 μl containing 100 μg of pazopanib in one eye and vehicle in the other eye. After 7 days, the area of CNV at Bruch’s membrane rupture sites was significantly less in eyes treated with pazopanib than those treated with vehicle and significantly less than the baseline area of CNV (Figure 4).
Figure 4. Periocular injection of pazopanib causes regression of choroidal neovascularization (CNV).
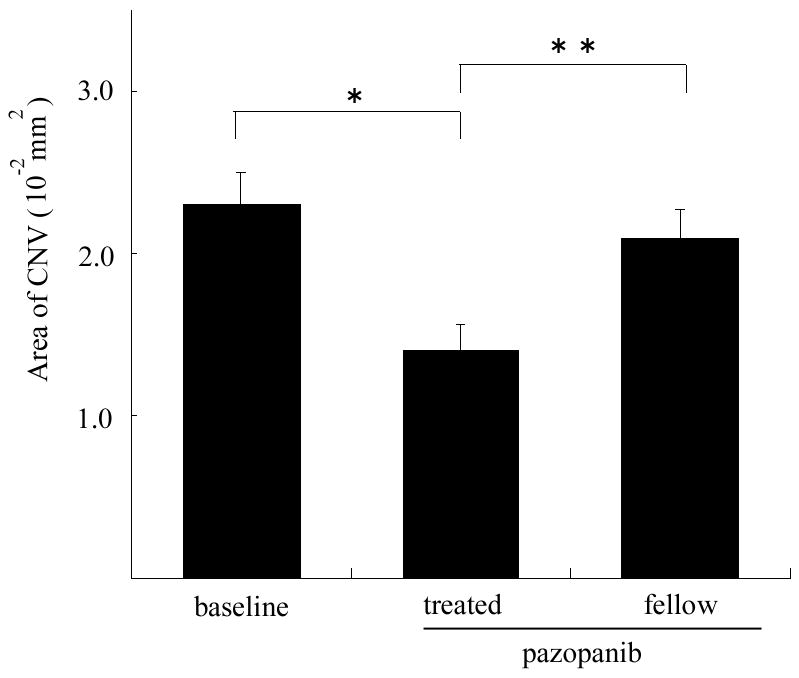
Four week old C57BL/6 mice had laser photocoagulation-induced rupture of Bruch’s membrane at 3 locations in each eye and after 7 days five mice were perfused with fluorescein-labeled dextran and the baseline area of CNV was measured by image analysis. The remaining 9 mice were treated with daily periocular injections of 100 μg of pazopanib in one eye. The bars represent the mean (±SEM) area of CNV at laser sites where rupture of Bruch’s membrane was achieved (n=30, 23, 26 for baseline, pazopanib-treated, and fellow eyes respectively). The area of CNV was significantly smaller in eyes treated with periocular injections of pazopanib compared to that in fellow eyes or the baseline area of CNV.
*p=0.0034; **p=0.0227 by linear mixed model with Dunnett’s correction for multiple comparisons
Discussion
It is very clear that VEGF-A is a critical target for treatment of patients with CNV, but the role of other VEGF family members and related proangiogenic molecules such as PDGF is uncertain. One way to inhibit the effects of all VEGF family members, PDGF, and other stimulators that activate receptors with homology to VEGF and PDGF receptors is to utilize small molecules that block activating phosphorylation signaling induced by ligand binding to these receptors.. Pazopanib is a kinase inhibitor with a unique kinase inhibitory profile; VEGFR1≈VEGFR2≈VEGFR3 >PDGFRβ≈PDGFRα≈c-Kit > FGFR1≈FGFR3≈cfms ≫ other kinases. Pazopanib has been shown to suppress tumor angiogenesis and growth in xenograft tumor models in mice 13. In this study, we found that systemic administration of pazopanib strongly suppressed the development of CNV. The level of suppression is greater than several other agents that have been tested 17–20 and in fact the only other agents that have similar efficacy in this mouse model of CNV are other kinase inhibitors that block VEGF and PDGF receptors as well as some other related molecules 1. In this study, we also found that treatment of established CNV with sufficient doses of pazopanib, either systemically or by periocular injection, resulted in regression of the CNV.
The ability to cause regression of CNV may be useful. Monthly injections of ranibizumab suppress leakage and growth of CNV in patients with neovascular AMD, but do not cause regression 3. In most patients, when ranibizumab injections are stopped, there is recurrent leakage and growth of CNV and many patients require frequent injections to maintain stability. It is reasonable to speculate that complete or even partial regression of CNV would substantially set back the clock and possibly allow for less frequent treatments. In a phase I study investigating the effect of VEGF Trap-Eye, a fusion protein consisting of binding domains from VEGFR1 and VEGFR2, a single intraocular injection of 2 or 4 mg resulted in partial regression of CNV and 4 of 6 patients did not require any additional treatment of any kind at 12 weeks, the end of the study (Presented at the Association of Research in Vision and Ophthalmology Meeting, Fort Lauderdale Florida, April 28, 2008). Since VEGF Trap binds other members of the VEGF family in addition to VEGF-A, this suggests that some or all of them may contribute to endothelial cell survival in CNV and by blocking all of them, endothelial cells of CNV are compromised and partial regression occurs. Recently, we found that intraocular expression of soluble VEGFR1 was not sufficient to cause regression of CNV21. VEGF family members bound by VEGF Trap-Eye, but not soluble VEGFR1, are VEGF-C, -D, and –E 22; one or more of these may promote survival of endothelial cells in the absence of VEGF-A and –B and placental growth factor.
It is unclear if the other inhibitory activities of pazopanib contribute to its high level of efficacy, but there is some evidence to suggest that combined blockade of PDGF and VEGF-A may be superior to either alone. Increased expression of PDGF-B in the retina causes severe proliferative retinopathy and retinal detachment like the most advanced stages of proliferative diabetic retinopathy 23. Endothelial cells produce PDGF-B which promotes the recruitment, proliferation and survival of pericytes. PDGF-B also recruits glial cells and retinal pigmented epithelial (RPE) cells 24 which promotes scarring, a complication of ocular neovascularization that is the major cause of permanent loss of vision. Antagonists of PDGFs may help to reduce scarring, but may also synergize with VEGF antagonists to reduce neovascularization through their antagonism of pericytes, which provide survival signals for endothelial cells of new vessels 25. Blockade of VEGF and PDGF-B also appear additive in models of ocular neovascularization 26.
There is also evidence that stem cell factor and its receptor c-Kit contribute to angiogenesis by increasing nuclear localization of HIF-1α resulting in elevation of VEGF, PDGF-B, angiopoietin 2, and the products of other genes containing a hypoxia response element within their promoter 27. It is not known whether blocking c-Kit provides added benefit in the treatment of ocular neovascularization to blocking the receptors for VEGF and PDGF, but it is a reasonable supposition, because angiopoietin 2 is an important stimulator of angiogenesis 28–30. Thus, pazopanib inhibits several proangiogenic signaling pathways and many of these inhibitory activities may contribute to its strong suppression and ability to cause regression of CNV which sets it apart from VEGF-A-specific antagonists.
The oral doses of pazopanib, 20 or 100 mg/kg twice a day for 7 days that caused substantial regression of CNV, were associated with pazopanib levels ranging from 28.8 to 38.0 μg/g in the retina/choroid tissue. This is useful information, because it provides a target tissue concentration range. The high levels achieved in the sclera after oral dosing suggests that pazopanib from the circulation is able to penetrate sclera and suggested that pazopanib deposited along the outside surface of the sclera should have good access to CNV. This proved to be the case, because daily periocular injection of 100 μg of pazopanib caused significant regression of CNV suggesting that local administration may be worth considering.
In conclusion, we have shown that either systemic or local administration of pazopanib causes regression of already established CNV making it a good candidate for clinical trials in patients with neovascular AMD. Such clinical trials are underway.
Acknowledgments
Supported by the National Neuroscience Research Institute, Owings Mills, MD and GlaxoSmithKline. PAC is the George S. and Dolores Dore Eccles Professor of Ophthalmology.
References
- 1.Kwak N, Okamoto N, Wood JM, Campochiaro PA. VEGF is an important stimulator in a model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41:3158–3164. [PubMed] [Google Scholar]
- 2.Kryzstolik MG, Afshari MA, Adamis AP, et al. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002;120:338–346. doi: 10.1001/archopht.120.3.338. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Eng J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 4.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Eng J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 5.Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman MS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration. Ophthalmology. 2005;112:1035–1047. doi: 10.1016/j.ophtha.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen QD, Shah SM, Tatlipinar S, Do DV, Van Anden E, Campochiaro PA. Bevacizumab suppresses choroidal neovascularization due to pathologic myopia. Br J Ophthalmol. 2005;89:1368–1370. [PMC free article] [PubMed] [Google Scholar]
- 7.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–372. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Laud K, Spaide RF, Freund KB, Slakter J, Klancnik JM., Jr Treatment of choroidal neovascularization in pathologic myopia with intravitreal bevacizumab. Retina. 2006;26:960–963. doi: 10.1097/01.iae.0000240121.28034.c3. [DOI] [PubMed] [Google Scholar]
- 9.Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006;26:383–390. doi: 10.1097/01.iae.0000238561.99283.0e. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen QD, Shah SM, Hafiz G, et al. Intravenous bevacizumab causes regression of choroidal neovascularization secondary to diseases other than age-related macular degeneration. Am J Ophthalmol. 2007 Dec 3;2007 doi: 10.1016/j.ajo.2007.09.025. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakaguchi H, Ikuno Y, Gomi F, et al. Intrvitreal injection of bevacizumab for choroidal neovascularisation associated with pathological myopia. Br J Ophthalmol. 2007;91:161–165. doi: 10.1136/bjo.2006.099887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindblom P, Gerhardt H, Liebner S, et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes & Dev. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar R, Knick VB, Rudolph SK, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Canc Res. 2007;6:2012–2021. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- 14.Tobe T, Ortega S, Luna JD, et al. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori K, Ando A, Gehlbach P, et al. Inhibition of choroidal neovascularization by intravenous injection of adenoviral vectors expressing secretable endostatin. Am J Pathol. 2001;159:313–20. doi: 10.1016/S0002-9440(10)61697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer-Verlag, Inc; 2000. pp. 93–120. [Google Scholar]
- 17.Nambu H, Nambu R, Melia M, Campochiaro PA. Combretastatin A-4 Phosphate Suppresses Development and Induces Regression of Choroidal Neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3650–3655. doi: 10.1167/iovs.02-0985. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Saishin Y, Saishin Y, et al. Topical nepafenac inhibits ocular neovascularization. Invest Ophthalmol Vis Sci. 2003;44:409–415. doi: 10.1167/iovs.02-0346. [DOI] [PubMed] [Google Scholar]
- 19.Saishin Y, Saishin Y, Takahashi K, et al. VEGF-TRAPR1R2 suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195:241–248. doi: 10.1002/jcp.10246. [DOI] [PubMed] [Google Scholar]
- 20.Lima e Silva R, Saishin Y, Saishin Y, et al. Suppression and regression of choroidal neovascularization by polyamine analogs. Invest Ophthalmol Vis Sci. 2005;46:3323–3330. doi: 10.1167/iovs.04-1210. [DOI] [PubMed] [Google Scholar]
- 21.Ueno S, Pease ME, Wersinger DMB, et al. Prolonged blockade of VEGF family members does not cause identifiable damage to retinal neurons or vessels. J Cell Physiol. 2008 doi: 10.1002/jcp.21445. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holash J, Davis S, Papadoupoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo M-S, Okamoto N, Vinores MA, et al. Photoreceptor-specific expression of PDGF-B results in traction retinal detachment. Am J Pathol. 2000;157:995–1005. doi: 10.1016/S0002-9440(10)64612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campochiaro PA, Glaser BM. Platelet-derived growth factor is chemotactic for human retinal pigment epithelial cells. Arch Ophthalmol. 1985;103:576–579. doi: 10.1001/archopht.1985.01050040118034. [DOI] [PubMed] [Google Scholar]
- 25.Bergers G, Song S, Meyer-Morse N, Bersland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jo N, Mailhos C, Ju M, et al. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol. 2006;168:2036–2053. doi: 10.2353/ajpath.2006.050588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Litz J, Krystal GW. Imatinib inhibits c-Kit-induced hypoxia-inducible factor-1-alpha activity and vascular endothelial growth factor expression in small cell lung cancer cells. Mol Cancer Ther. 2006;5:1415–1422. doi: 10.1158/1535-7163.MCT-05-0503. [DOI] [PubMed] [Google Scholar]
- 28.Hackett SF, Ozaki H, Strauss RW, et al. Angiopoietin 2 expression in the retina: upregulation during physiologic and pathologic neovascularization. J Cell Physiol. 2000;184:275–284. doi: 10.1002/1097-4652(200009)184:3<275::AID-JCP1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Hackett SF, Wiegand SJ, Yancopoulos G, Campochiaro P. Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol. 2002;192:182–187. doi: 10.1002/jcp.10128. [DOI] [PubMed] [Google Scholar]
- 30.Oshima Y, Deering T, Oshima S, et al. Angiopoietin-2 enhances retinal vessel sensitivity to vascular endothelial growth factor. J Cell Physiol. 2004;199:412–417. doi: 10.1002/jcp.10442. [DOI] [PubMed] [Google Scholar]


