Abstract
Objective
To determine whether lutein supplementation will slow visual function decline in patients with retinitis pigmentosa receiving vitamin A.
Design
Randomized, controlled, double-masked trial of 225 non-smoking patients, age 18-60 years, evaluated over a 4-year interval. Patients received lutein 12 mg or a control tablet daily. All were given vitamin A palmitate 15,000 IU/day. Randomization took into account genetic type and baseline serum lutein.
Main Outcome Measures
The primary outcome was the total point score for the Humphrey Field Analyzer (HFA) 30-2 program; pre-specified secondary outcomes were the total point scores for the 60-4 program and for the 30-2 and 60-4 combined, 30-Hz electroretinogram amplitude, and ETDRS acuity.
Results
No significant difference in rate of decline was found between the lutein + A and control + A groups over a 4-year interval for the HFA 30-2 program. For the HFA 60-4 program a decrease in mean rate of sensitivity loss was observed in the lutein + A group (p=0.05). Mean decline with the 60-4 program was slower among those with the highest serum lutein or with the highest increase in macular pigment optical density (MPOD) at follow-up (p= 0.01 and p=0.006 respectively). Those with the highest increase in MPOD also had the slowest decline in 30-2 and 60-4 combined field sensitivity (p=0.005). No significant toxic side effects of lutein supplementation were observed.
Conclusion
Lutein supplementation 12 mg/d slowed loss of midperipheral visual field on average among nonsmoking adults with retinitis pigmentosa taking vitamin A.
Application to Clinical Practice
Data are presented that support use of lutein 12 mg/day to slow visual field loss among non-smoking adults with retinitis pigmentosa on vitamin A.
Trial Registry
Randomized Clinical Trial for Retinitis Pigmentosa, NCT00346333, www.ClinicalTrial.gov
INTRODUCTION
Retinitis pigmentosa has a prevalence of about 1:4000; an estimated 50,000-100,000 people are affected in the United States. 1 This condition is usually inherited by an autosomal dominant, autosomal recessive, or X-linked mode; almost half are isolates (i.e. simplex cases) with no family history of this disease. Affected patients typically report night deficiency in adolescence and loss of mid-peripheral and then far peripheral field in adulthood with development of tunnel vision. Patients usually lose central vision after age 60. Clinical findings include elevated final dark adaptation thresholds, attenuated retinal vessels, intra-retinal bone spicule pigment around the mid-periphery in most cases, and reduced and delayed electroretinograms (ERGs). Histologic studies of autopsy eyes have shown that visual loss is due to degeneration of rod and cone photoreceptors. 1,2
In a randomized trial of vitamin A and vitamin E supplementation for adults with retinitis pigmentosa, we reported that the rate of progression is slowed, on average, among thoses on 15,000 IU/day of vitamin A palmitate and appears to be hastened among those on 400 IU/day of vitamin E. 3 Subsequent to this vitamin A and E trial, we performed a risk factor analysis on those in the vitamin A group combining patients with all genetic types (n=79) to see if rates of loss of retinal function were related to intake of specific foods and nutrients. We found that those on vitamin A in the highest quintile of lutein intake (3.5-13 mg/day, roughly equivalent to as much as 1/2 cup of cooked spinach per day) had a slower rate of decline in visual field area compared with those in the lower 4 quintiles (p=0.05). A beneficial trend was also seen when relating 30-Hz ERG amplitude to quintile of lutein intake (p = 0.07). These findings suggested increased lutein intake further slowed disease progression among patients on vitamin A.
Lutein is a carotenoid found in dark green leafy vegetables and, along with its isomer zeaxanthin, is the only carotenoid in the human retina. 4 Lutein, being fat-soluble, follows the same intestinal absorption path as dietary fat; it is packaged into triacylglycerol-rich chylomicrons 5 and transported in the plasma by lipoproteins. 6 The mechanism by which lutein is transported from the plasma to photoreceptors is unknown; there is evidence for the existence of a specific binding protein(s) in solubilized membranes derived from human retina. 7 Although concentrated in and around the foveal depression in photoreceptor axons as macular pigment, 8,9 lutein has also been found in rod outer segments throughout the human retina. 10-13 Smoking and high alcohol intake have been associated with lower serum lutein and zeaxanthin levels. 14 Lutein (as yellow macular pigment) is thought to screen the foveal cone photoreceptors from short-wavelength light to minimize chromatic aberration and enhance visual acuity. 15 In rod outer segments lutein may serve as an antioxidant to quench free radicals produced by high-energy short-wavelength illumination and thereby minimize light-induced retinal damage. 16,17 There is no established Dietary Recommended Intake for lutein; however, 6 mg/day has been associated with a reduced risk of cataracts and age-related macular degeneration. 18-20 Most Americans only ingest 1-2 mg/day in their diet. 21-24
Our preliminary data showing a relationship between increased lutein intake and slowing of loss of visual function in adults with retinitis pigmentosa on vitamin A as well as the known presence of lutein in photoreceptors, possibly serving as an antioxidant, 10,11,16 provided the rationale for this randomized trial of the effect of lutein supplementation on visual function in adults with retinitis pigmentosa taking vitamin A.
METHODS
Protocol
We first conducted a randomized, controlled, double-masked phase I/II study from May 2000 through January 2001 to evaluate ocular and systemic safety as a function of lutein supplementation and to evaluate different doses of lutein supplementation in 41 patients (age 18 to 56 years) with retinitis pigmentosa. After receiving a baseline ocular examination, patients were randomly assigned to placebo or one of three doses of lutein (3.3 mg/day, 6.6 mg/day, and 13 mg/day) and were examined after 2 and 4 months of supplementation; after stopping the supplement, they were reevaluated at 5 months (i.e. 1-month washout). All patients were given 15,000 IU/day of vitamin A palmitate. In accord with a Data and Safety Monitoring Committee, we concluded that short-term lutein supplementation raises lutein in the serum and retina in adults with retinitis pigmentosa, that the serum increase returns to baseline after 1 month of wash-out, that 12mg of lutein/day is the minimum commercially-available dose needed to achieve measurable elevations in both serum and retina, that lutein supplementation is not associated with a decrease in serum retinol, and that this supplement in the doses under study is safe for this population in the short term.
In July 2003 we began a phase III trial to evaluate the effect of lutein supplementation on the course of retinitis pigmentosa. We screened patients for eligibility according to ocular, dietary, and medical criteria (Table 1). We performed a baseline examination on eligible patients within 8 weeks of the screening examination with the protocol used in the phase I/II study. At baseline patients were randomly assigned to one of two groups: those receiving one tablet per day of lutein 12 mg and those receiving a corn starch control (supplied by Roche Vitamins, Inc., Parsippany, NJ which became DSM Nutritional Products, Inc., Parsippany, NJ in 2007). All were given vitamin A as 15,000 IU of retinyl palmitate (initially obtained from Akorn, Lake Forest, IL July 2003-January 2005 and then from J.R. Carlson Laboratories, Inc. Arlington Heights, IL February 2005-2009) and were instructed to take one study tablet and the vitamin A supplement daily with breakfast. Patients completed the Willett food frequency questionnaire 25 and a medical questionnaire at each visit with the aid of a clinical coordinator. They were also followed annually over 4 years with blood tests and ocular examinations (Table 2). Serum lutein levels were monitored as a measure of compliance, 26 and serum retinol and retinyl ester levels 27 as well as serum liver function results were evaluated to detect any possible toxic effects of vitamin A. Change in macular pigment optical density as a measure of change in intra-retinal lutein was assessed in the fovea in one eye at each visit with heterochromatic flicker photometry. 28
Table 1.
Eligibility Criteria
| Ocular Criteria |
| Retinitis pigmentosa, typical forms* |
| Best corrected visual acuity 20/100 or better |
| HFA 30-2 total point score ≥ 250 dB† |
| No confounding ocular disease‡ |
| Dietary Criteria |
| Fruit and vegetable intake < 10 servings per day |
| Spinach or kale intake < 1 serving per day (i.e. < ½ cup of cooked spinach or kale/day) |
| Dietary lutein intake ≤ 5.4 mg/day∥ |
| No intake of cod liver oil or omega-3 capsules |
| Dietary preformed vitamin A intake ≤ 10,000 IU/day |
| Supplement intake ≤ 5000 IU/day of vitamin A and ≤ 30 IU/day of vitamin E |
| Consumption ≤ 3 alcoholic beverages/day |
| Medical and Other Criteria |
| Age 18-60 yrs |
| BMI < 40 and weight ≥ 5th percentile for age, sex, and height |
| Serum retinol level ≤ 100 μg/dL and serum retinyl ester level ≤ 380 nmol/L |
| Serum cholesterol level < 300 mg/dL and serum triglyceride level < 400 mg/dL |
| No clinically significant abnormality on blood count, glucose, BUN, lipid panel, or serum liver function profile |
| Not pregnant or planning to become pregnant |
| Not smoking currently |
| Agree not to know study tablet content or course of condition until the end of the trail |
| No other disease that might affect absorption or metabolism of lutein or vitamin A |
Abbreviations: HFA, Humphrey Field Analyzer; BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters).
SI conversion factors; to convert cholesterol to millimoles per liter multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.01129.
Elevated final dark adaptation threshold, retinal arteriolar narrowing, and reduced and delayed full-field electroretinograms (ERGs); patients with atypical forms such as paravenous retinitis pigmentosa (RP), pericentral RP, sector RP, unilateral RP, Refsum disease, Bardet-Biedl syndrome, and retinitis punctata albescens were excluded. Patients with RP and profound congenital deafness were also excluded.
A size V white test light was used.
Glaucoma, uveitis, diabetic retinopathy, posterior subcapsular cataract > 11% of total lens area (i.e. equivalent to P3 on Lens Opacity Classification System III), pupil diameter after dilation < 6mm.
Estimated from food frequency questionnaire.
Table 2.
Data Collected at Each Visit
| Screening | Baseline | Year 1 | Year 2 | Year 3 | Year 4 | |
|---|---|---|---|---|---|---|
| Medical history | Yes | Yes | Yes | Yes | Yes | Yes |
| Dietary intake | Yes | No | Yes | Yes | Yes | Yes |
| Ophthalmological findings | Yes | Yes | Yes | Yes | Yes | Yes |
| Standard lab tests* | Yes | No | Yes | Yes | Yes | Yes |
| Serum retinol and retinyl esters | Yes | No | Yes | Yes | Yes | Yes |
| Serum lutein | Yes | Yes | Yes | Yes | Yes | Yes |
| Macular pigment optical density | Yes | Yes | Yes | Yes | Yes | Yes |
| Compliance† | No | No | Yes | Yes | Yes | Yes |
| Family history form | Yes | No | No | No | No | No |
These include the following: hemoglobin, hematocrit, white blood count, blood urea nitrogen, glucose, liver function profile, serum cholesterol (total, high density lipoprotein, and low density lipoprotein) levels, and serum triglyceride level.
Completed at each annual follow-up visit and at 3-month intervals between visits by telephone.
We used the measurement of static perimetric sensitivities (i.e. total point score) with the 30-2 program size V target in the Humphrey Field Analyzer (HFA) II (Carl Zeiss Meditech, Inc., Pleasanton, CA) as the primary outcome measure. The size V target was used to minimize the number of locations with floor effects (i.e. sensitivity ≤ 0 dB). The full-field 30-Hz cone ERG amplitude was followed as a secondary outcome measure among those with ≥ 0.68 μV amplitude pre-treatment. Visual acuity (Early Treatment Diabetic Retinopathy Study [ETDRS]), 29 the total point score to a size V target with the 60-4, and total point score to a size V target with the HFA 30-2 and 60-4 programs combined were also followed as secondary outcome measures. The FASTPAC test strategy was used to test both central (30-2) and mid-peripheral (60-4) visual fields in as short a time as possible. 30-32
We estimated that 240 patients were needed to provide sufficient power (i.e. alpha = 0.05, beta = 0.10) to observe a statistically significant difference between mean change in the lutein + A group and control + A groups with respect to HFA 30-2 total point score over a 4 year interval. The project was approved by the Institutional Review Boards of the Massachusetts Eye and Ear Infirmary and Harvard Medical School, Boston, and the study conformed to the Declaration of Helsinki. All patients signed a consent form prior to the screening examination and, if eligible, prior to the baseline examination as well. A Data and Safety Monitoring Committee selected by the National Eye Institute approved the protocol and met with us annually to review the results for both patient safety and efficacy. The study was planned to allow 4 years of follow-up for each patient. The Lan-DeMets alpha spending approach with an O’Brien-Fleming boundary with 5 looks was pre-specified as the stopping-rule guideline. 33
Randomization and Masking
The procedure for randomization took into account genetic type (dominant, recessive, X-linked, isolate, or undetermined [e.g. adopted]) and serum level of lutein at the screening evaluation (i.e. serum lutein level ≤ 6.4 or > 6.4 μg/dL derived from analyses of serum lutein levels in our previous trial of docosahexaenoic acid (DHA) supplementation);34 therefore there were 5 × 2 or 10 strata. All members of the staff in contact with the patients including the Principal Investigator (ELB) were masked with respect to treatment group assignments. Only the Data Manager (CWD) and the Statistician (BR) had access to the code that listed group assignment. Each ocular examination was performed without review of previous records. Patients agreed not to know their group assignment.
Macular Pigment Measurements
Macular pigment optical density (MPOD) was measured by heterochromatic flicker photometry (HFP) using a commercial tabletop instrument (Macular Metrics Corp., Rehoboth, MA) 28 in the eye with better visual acuity (or the right eye, if the two eyes were equal) after pupillary dilation to optimize sensitivity. For a given patient the same eye was followed at each visit. The standard task was for the patient to adjust the radiances of a 460 nm stimulus and an alternating 570 nm stimulus to achieve a brightness match by eliminating flicker within the central 1° where macular pigment absorbance is maximal and within a 2° diameter field at a 5° eccentric location where macular pigment absorbance is sufficiently low to serve as a reference. 35 The adjusted radiance of the 460 nm stimulus minus that of the 570 nm stimulus for the central fovea minus the same difference for the reference location, expressed in base 10 logarithms, provided an estimate of MPOD. These stimuli were centered on a 6° background of 475 nm to desensitize rods and short-wavelength sensitive cones so that they would not contribute to the patient’s judgment. Change in MPOD as measured by HFP is regarded as a measure of uptake of lutein in the retina with lutein supplementation. 36
Ability to perform this standard task was not an eligibility criterion for enrollment. A majority of the patients could not perform the standard task throughout the trial, generally because they could not consistently visualize the entire 2° field in the parafoveal location. However, since MPOD was proportional to the log radiance difference measured by HFP in the fovea alone in a subset of patients at baseline (r2 = 0.54, P <.001), change in the latter value was used to estimate lutein uptake in the retina over follow-up in the entire cohort.
Data Analysis
Outcome data for a given patient for each visit represented the test results from each eye or for a single eye if data for the other eye were not available. Visual field data (total point scores) were analyzed separately for the central field (30-2 program), for the mid-peripheral field (60-4 program), and for the 30-2 and 60-4 programs combined when both were available. Analyses of 30-Hz ERG data were limited to those who had ≥ 0.68 μV in at least one eye at baseline and data were censored when values declined to < 0.34 μV. If an eye became pseudophakic after the baseline visit, data for that eye were analyzed only for those visits prior to cataract surgery. If the total point score for a visual field in an eye became zero, the visit at which the zero score was first obtained was included in the analyses and the total point scores for all subsequent visits for that eye were set to zero. Mean change from baseline was computed for each patient by eye. Slopes were calculated using data from all available eyes by treatment group. Comparisons by assigned treatment group were also performed within genetic type and within pre-specified subgroups (e.g., above and below the median level of visual field sensitivity) at baseline. Longitudinal regression analyses 37 were performed using PROC MIXED of SAS version 9.1.3. 38 Since the distribution of change for each HFA outcome measure was skewed and non-normal, we also calculated the rate of change in visual field sensitivity using least squares regression for each eye of each patient, converted them to ranks, and used a non-parametric method (clustered Wilcoxon test) to compare the distribution of slopes in the lutein + A group versus the control + A group controlling for the correlation between the ranks of slopes for the two eyes within an individual. 39
Observational analyses were performed comparing rate of decline of HFA sensitivity over 4 years of follow-up to serum lutein level or change in MPOD using PROC MIXED of SAS. For this purpose serum lutein level and change in MPOD were expressed as dichotomous variables defined by the highest versus the lower 3 quartiles as defined above. In addition a restricted cubic spline analysis was performed 40,41 to identify a threshold effect and a maximum effect of serum lutein on change in HFA sensitivity.
Of the 240 randomized patients 225 were followed over 4 years. Of these patients 215 had measurable (i.e., greater than zero) central field sensitivities and 163 patients had measurable midperipheral field sensitivities in at least one eye in all 4 years of follow-up. The results will focus on these 2 consistent samples (i.e., those with measurable sensitivities to the 30-2 program, n=215; and those with measurable sensitivities to the 60-4 program, n=163). Patients were encouraged not to initiate new supplements. In October 2004, following publication of a trial of DHA supplementation for retinitis pigmentosa, 42 all patients were advised by letter to eat 1-2 three-ounce servings per week of oily fish of which DHA is a major constituent (e.g. salmon, tuna, herring, mackerel, or sardines), if not already doing so. They were reminded at annual visits not only to take vitamin A and the study pill but also to eat oily fish and otherwise maintain their baseline dietary pattern.
RESULTS
Participant Flow and Follow-up
From July 2003 through November 2004 we examined 412 patients from across the United States to identify 240 (one per family) with retinitis pigmentosa who met the preset list of eligibility criteria (see Table 1). Two hundred and twenty-five of these patients completed all 4 annual follow-up visits by December 2008. Baseline characteristics of these 225 patients, shown in Table 3, reveal characteristics typical of retinitis pigmentosa, balance between groups, and evidence that many variables had skewed distributions. Ninety-two percent of eligible patients had intra-retinal bone spicule pigmentation in the fundus mid-periphery. Sixty-one percent had a posterior subcapsular cataract in at least one eye. Fifteen percent reported partial hearing loss. Seven percent were minorities.
Table 3.
Baseline Demographic and Ocular Characteristics of Patients with Retinitis Pigmentosa Randomized Patients with Follow-up Visits at All 4 Years (n=225)
| Lutein + vitamin A (12 mg lutein + 15,000 IU vitamin A) (n=110) |
Control + vitamin A (control capsules + 15,000 IU vitamin A) (n=115) |
p-value* | |||
|---|---|---|---|---|---|
| Genetic Type (%) | |||||
| Dominant | 25 (23%) | 28 (24%) | |||
| Recessive | 19 (17%) | 20 (17%) | |||
| X–Linked | 7 (6%) | 8 (7%) | |||
| Isolate | 54 (49%) | 56 (49%) | |||
| Undetermined | 5 (4%) | 3(3%) | 0.95 | ||
| Dietary Intake (Mean±SE) | |||||
| Lutein (mg/day) | 2.50±0.13 | (0.81, 4.58)† | 2.41±0.13 | (0.80, 4.38)† | 0.64 |
| Lutein (mg/day) | 31 (28%) | 38 (33%) | 0.52 | ||
| Blood Values (Mean ± SE)‡ | |||||
| Serum lutein (μg/dL) | 12.1±0.61 | (5.5, 18.1) | 11.7±0.60 | (5.2, 18.7) | 0.69 |
| Serum retinol (μg/dL) | 50.4±1.04 | (38, 66) | 52.1±0.92 | (37, 66) | 0.23 |
| Serum retinyl esters (nmol/L) | 104.3±4.7 | (50, 176) | 112.5±5.30 | (55, 198) | 0.25 |
| Gender, n (%) male | 58 (53%) | 52 (47%) | 0.48 | ||
| Age in years (Mean ± SE) | 40±1.0 | (25, 53) | 38±1.00 | (22, 53) | 0.29 |
| Weight (lbs) | |||||
| Male | 186±4 | (150, 235) | 190±4 | (151, 230) | 0.37 |
| Female | 152±4 | (120, 192) | 150±5 | (117, 199) | 0.75 |
| Baseline Ocular Function§ | |||||
| HFA Total Point Scores (dB) | |||||
| HFA 30-2 Field | 831±47 | (331, 1617) | 858±46 | (316, 1625) | 0.69 |
| HFA 60-4 Field | 360±39 | (41, 877) | 393±33 | (50, 721) | 0.53 |
| HFA 30-2 and 60-4 Combined |
1280±84 | (439, 2303) | 1335±75 | (594, 2290) | 0.63 |
| ERG 30 Hz Amplitude (μV) ¶ | 1.01±0.12 (2.7 4) | (0.81, 13.0) | 1.30±0.11 (3.67) | (0.90, 16.2) | 0.08 |
| ETDRS Visual Acuity (letters) | 50.1±0.9 | (36, 59.5) | 50.3±0.8 | (36, 59) | 0.89 |
| Cataract area OD∥ | 0.59±0.07 | (0, 2.0) | 0.59±0.09 | (0, 2.0) | 0.95 |
| Cataract area OS | 0.51±0.07 | (0, 1.25) | 0.52±0.08 | (0, 2.0) | 0.91 |
p-value for t-test comparing 2 groups for continuous variables: p-value for chi-square for categorical variables.
10th, 90th percentiles for continuous variables.
Normal range for serum retinol = 38-100 μg/dL, for serum retinyl esters = 34-380 nmol/L ; sample sizes for the lutein and control groups respectively were lutein: 110 and 115; serum retinol: 110 and 115; retinyl esters: 110 and 115.
Lower norm for HFA 30-2 field (size V target) = 2500 dB; HFA 60-4 (size V target) = 1580 dB; HFA 30-2 and 60-4 combined field (size V target) = 4200 dB. Lower norm for 30 Hz ERG = 50 μV . Lower norm for ETDRS visual acuity = 60 letters; 51 letters = Snellen acuity of 20/30; sample sizes for lutein and control groups respectively were: HFA 30-2: 110 and 115; HFA 60-4: 90 and 95; HFA 30-2 and 60-4 combined: 88 and 94, 30 Hz ERG : 84 and 87; ETDRS visual acuity: 110 and 115; cataract area: 103 and 110.
Values designated as mean loge±SE. Geometric means for ERG amplitudes in μV in parentheses; 10th, 90th percentiles of ERGs in μV on the raw scale.
Values expressed as percent of total lens area; patients without cataract are included in the analyses with area of zero.
At baseline 52% of the 225 patients reported eating 1-2 servings of oily fish per week (49% in the lutein + A group and 54% in the control + A group). In response to our advice to eat oily fish between year 1 and year 2 of follow-up, 92% of these patients reported that they were following this instruction at year 4 (94% in the lutein + A group and 90% in the control + A group).
Safety and Compliance
Capsule counts indicated that 92% of the lutein tablets, 93% of the control tablets, and 95% of the vitamin A tablets were consumed over all 4 years. Similar results were seen with returned monthly calendars. One patient in the control + A group died in a motorcycle accident after year 3 of follow-up. Two patients in the lutein + A group showed slight elevations of serum liver function levels (SGOT and SGPT) of unknown etiology at year 4 and stopped vitamin A and the study tablet as a precaution. No patient experienced a complete loss of vision in an eye over the course of this trial. Furthermore, there was no evidence of systemic illness or toxicity attributable to the study tablet or vitamin A based on blood studies, serum liver function assessments, serum retinol and serum retinyl ester values, and responses to a symptom questionnaire.
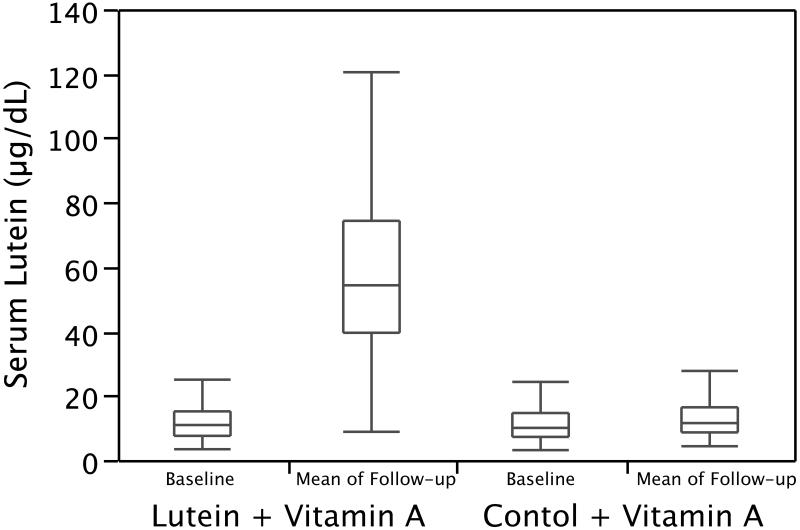
At follow-up, mean serum lutein level (mean of all follow-up measures) was significantly higher in the lutein group compared with the control group (p < 0.001) (Figure 1); this difference was detectable by year 1 and maintained over 4 years. The lutein + A group (n=75) showed a significantly greater increase in MPOD over 4 years of follow-up compared to the control + A group (n=88) (p<0.0001). The mean annual rate of change in percent cataract area was 0.05 ± 0.03 in the lutein + A group and 0.10 ± 0.03 in the control + A group; these rates of change were not significantly different from one another. Serum retinol increased slightly but comparably in both the lutein and control groups.
Figure 1.
Box plots of serum lutein at baseline and follow-up. Horizontal lines designate the median, 25th, and 75th percentiles; vertical lines define the upper quartile plus 1.5 times the inter-quartile range and the lower quartile minus 1.5 times the inter-quartile range.
Analysis of Outcome Measures
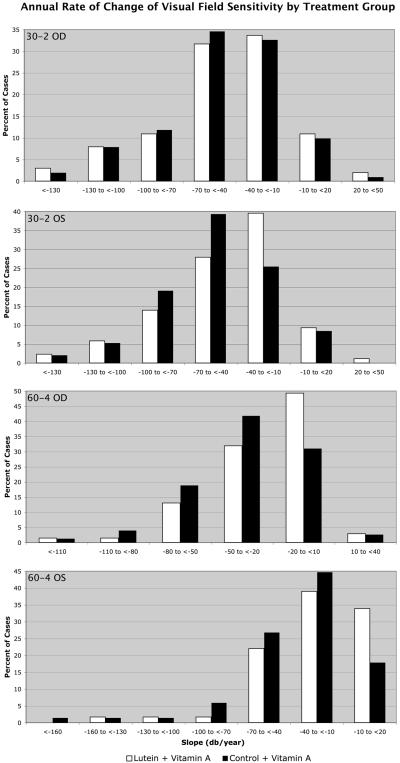
Table 4 shows no significant difference in the primary outcome measure of central visual field sensitivity with the 30-2 program for the randomized comparison between the lutein and control groups. However, Table 4 does show a significant difference (p=0.05) in the secondary outcome measure of mid-peripheral visual field sensitivity with the 60-4 program; the lutein group lost, on average, 27 dB per year while the control group lost, on average, 34 dB per year. Comparisons of treatment groups stratified by initial median values did not reveal significant interactions according to baseline levels. Because rates of change of visual field sensitivity were skewed, particularly with the HFA 60-4 program (see Figure 2), we performed additional analyses with the clustered Wilcoxon rank sum test for (A) the consistent samples and (B) for the total available samples. Table 5 shows the results of these non-parametric analyses. Rates of change for the HFA 60-4 program showed a significant beneficial effect of lutein for both the consistent sample and the sample including all available data (p=0.03 in each case).
Table 4.
Annual Rate of Decline Over 4 Years for Measures of Ocular Function by Treatment Group
| Lutein + vitamin A Group (n = 110)* |
Control + vitamin A Group (n = 115)* |
p-value† | |
|---|---|---|---|
| HFA 30-2 field (dB/y) | 49.6±3.3‡ (n = 105) |
51.5±3.2 (n= 110) |
0.66 |
| HFA 60-4 field (dB/y) | 26.6±3.1 (n= 81) |
34.1±3.0 (n= 82) |
0.05 |
| HFA total field (dB/y) § | 83.1±6.2 (n=79) |
92.9±5.7 (n=78) |
0.24 |
| 30 Hz ERG amplitude, loge % decline per year¶ |
0.09±0.01 (8.4) (n=79) |
0.08±0.12 (7.7) (n=77) |
0.59 |
| ETDRS visual acuity, number of letters/y |
0.53±0.12 (n= 110) |
0.49±0.12 (n= 111) |
0.80 |
Differing sample sizes reflect occasional instances where test results were not available at one or more visits for a given outcome variable.
p-value for PROC MIXED analysis.
Values designated as mean ±SE.
Total field sensitivity= 30-2 and 60-4 total point scores combined when both are available.
Derived from 100 X [1-exp (mean log change)]; geometric means for ERG amplitudes are in parentheses.
Figure 2.
Percent of cases of patients with retinitis pigmentosa by annual rate of change of HFA 30-2 sensitivity and HFA 60-4 sensitivity by treatment group for each eye.
Table 5.
Non-parametric Analyses of Annual Rate of Decline in HFA Over 4 Years
| Lutein + A Group |
Control + A Group |
Θ* (± SE) |
p-value† | |
|---|---|---|---|---|
| A. Consistent samples with all 4 years of follow-up | ||||
| HFA 30-2 field total patients (eyes) ‡ |
105 (187) | 110 (195) | 0.52±0.04 | 0.52 |
| HFA 60-4 field total patients (eyes) |
80 (128) | 83 (141) | 0.59±0.04 | 0.03 |
| HFA 30-2 and 60-4 combined total patients (eyes) |
78 (120) | 79 (134) | 0.56±0.04 | 0.20 |
| B. Total available samples§ | ||||
| HFA 30-2 field total patients (eyes) |
119 (227) | 119 (223) | 0.52±0.04 | 0.54 |
| HFA 60-4 field total patients (eyes) |
97 (174) | 97 (178) | 0.58±0.04 | 0.03 |
| HFA 30-2 and 60-4 combined total patients (eyes) |
97 (174) | 97 (178) | 0.55±0.04 | 0.18 |
Estimated probability that an eye from a random patient in the lutein + vitamin A group will have a slower decline (i.e. less negative slope) than an eye from a random patient in the control + vitamin A group. If treatment was totally ineffective, Θ would be 0.50; Θ > 0.5 indicates benefit for lutein and Θ <0.5 would indicate lutein adversity.
p-value based on clustered Wilcoxon test
Number of patients contributing one or two eyes to the analyses. A single eye can be unreliable (and data not used) while the fellow eye is reliable and included in these analyses.
Total available samples consist of eyes with usable screening or baseline data and at least one usable value at a follow-up visit. For the 30-2 condition 238 out of a possible 240 (99% ) patients are represented and for the 60-4 and for the 30-2 and 60-4 conditions combined 194 out of a possible 199 patients (97%) are represented.
Among the observational analyses (Table 6), the rate of HFA 60-4 sensitivity loss was significantly different (p=0.01) among patients in the highest quartile of serum lutein compared with those in the lower 3 quartiles. The mean rate of decline of HFA 60-4 sensitivity was 21 dB/year for those in the highest quartile of serum lutein versus 33 dB/year in the lower 3 quartiles. Table 6 also shows that the rate of decline of HFA 60-4 sensitivity was significantly slower among those in the highest quartile of change in MPOD at all follow-up visits combined (i.e., 18 dB/year) versus the rate (i.e., 34 dB/year) among those in the lower 3 quartiles (p=0.006). Similarly, those in the highest quartile of change in MPOD had a significantly slower rate of decline in central plus mid-peripheral field sensitivity combined (i.e. 30-2 plus 60-4 program combined) versus those in the lower 3 quartiles (−62 dB/year for the highest quartile and −92 dB/year for the lower 3 quartiles, p=0.005).
Table 6.
Loss of Mid-peripheral Visual Field Sensitivity as a Function of Serum Lutein Level or Macular Pigment Optical Density
| A. Change in HFA by Serum Lutein Level Mean serum lutein (mean of years 1-4 of follow-up) | ||||
|---|---|---|---|---|
| Mean serum lutein level (μg/dL) | 0 - <53 Mean ± SE (n) |
53+ Mean ± SE (n) |
t | p-value |
| Mean change* in HFA 30-2 | ||||
| OD | −211.5±12.7 (148) |
−177.6±20.2 (51) |
−1.38 | 0.17 |
| OS | −220.9±12.4 (133) |
−170.0±21.5 (44) |
−2.04 | 0.04 |
| Estimate of slope per year (dB)† | −52.6±2.8 | −45.5±4.7 | 0.17 | |
| Mean change* in HFA 60-4 | ||||
| OD | −139.6±10.6 (107) |
−98.5±13.8 (35) |
−2.03 | 0.04 |
| OS | −137.8±12.7 (100) |
−112.7±21.4 (25) |
−0.91 | 0.37 |
| Estimate of slope per year (dB)† | −33.1±2.6 | −21.3±4.2 | 0.01 | |
| B. Change in HFA by Change in Macular Pigment Optical Density‡ Mean macular pigment optical density (mean of years 1-4 of follow-up) | ||||
|---|---|---|---|---|
| Mean macular pigment | ||||
| optical density change | < 0.109 | 0.109+ | t | p-value |
| Mean change* in HFA 30-2 | −219.6±14.6 (114) |
−179.6±22.8 (38) |
−1.40 | 0.16 |
| Estimate of slope per year (dB)† | −51.0±3.2 | −43.0±5.6 | 0.22 | |
| Mean change* in HFA 60-4 | −139.6±13.9 (79) |
−94.9±13.7 (31) |
−2.29 | 0.02 |
| Estimate of slope per year (dB)† | −33.8±3.0 | −18.4±4.7 | 0.006 | |
| Mean change* in HFA total | −399.7±27.5 | −288.0±32.8 | −2.33 | 0.02 |
| (ie. 30-2 and 60-4 combined) | (76) | (31) | ||
| Estimate of slope per year (dB)† | −92.3±5.8 | −61.7±9.0 | 0.005 | |
Mean of year 4 values minus mean of screening and baseline values
Slope based on PROC MIXED of SAS
Macular pigment optical density was tested in one eye only; HFA values in this analysis were only from the eyes used in macular pigment optical density testing.
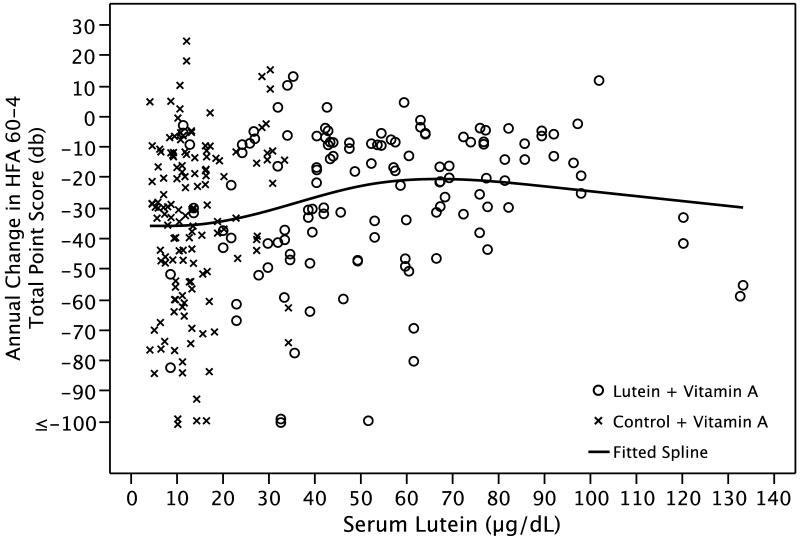
Figure 3 shows a spline regression of annual change in HFA 60-4 total point score by serum lutein based on all patients. The fitted curve shows that the change in HFA 60-4 sensitivity starts to decrease (i.e. disease progression is slowed) at a serum lutein level of 20 μg/dL and stops decreasing at 60-70 μg/dL.
Figure 3.
Spline regression of the annual change in HFA 60-4 total point score by serum lutein based on 269 eyes of 163 patients with retinitis pigmentosa.
No significant differences by treatment group assignment were observed for either the primary or secondary outcome measures within the dominant, recessive, X-linked or isolate forms of retinitis pigmentosa or within category of baseline serum lutein level (data not shown).
COMMENTS
The present trial among adults with retinitis pigmentosa showed no significant treatment effect on the course of retinal degeneration in central field sensitivity as monitored by the HFA 30-2 program (the primary outcome measure) or in the central macula as monitored by ETDRS acuity (a secondary outcome measure). The trial did, however, show a significant beneficial effect of lutein 12 mg/day on preserving mid-peripheral visual field sensitivity as monitored by the HFA 60-4 program, a secondary outcome measure; the lutein + A group lost on average 27 dB per year versus 34 dB per year in the control + A group (see Table 4). The effect of treatment was the same for patients with initial HFA sensitivities above and below the median, indicating that the observed benefit of lutein on slowing mid-peripheral field loss was not simply limited to those patients with milder disease who retained more sensitivity in this region. No effect of lutein could be detected with respect to preserving the full-field 30-Hz cone ERG (a secondary outcome measure); a possible explanation for the difference between the mid-peripheral HFA findings and the ERG results is that the latter is generated not only by central plus mid-peripheral cones but also by far peripheral cones. Since all the outcome measures were specified a priori and are correlated, no statistical adjustments for multiple comparisons were performed. The detectable benefit of lutein supplementation on preserving mid-peripheral function but not central function in retinitis pigmentosa may reflect an increased requirement for antioxidants in photoreceptor outer segments in a region of the retina where the photoreceptors are most impaired.
The effect of lutein on slowing mid-peripheral field decline was consistent with the observation that the annual rate of decline of mid-peripheral field sensitivity was significantly slower among those in the upper quartile of serum lutein at follow-up versus those in the lower 3 quartiles. Most patients taking lutein 12 mg/day had a serum lutein level above 20 μg/dL which was associated with a decrease in decline of HFA 60-4 sensitivity (see Figure 3). Serum lutein levels above 60-70 μg/dL were not associated with greater benefit. The finding that serum lutein levels vary widely among patients taking the same dose of lutein, described by others, 43 was also observed in this study (see Figure 1). In addition, patients in the highest quartile of MPOD elevation at follow-up — as a measure of increase in intra-retinal lutein — had a significantly slower rate of decline not only of mid-peripheral field but also of central and mid-peripheral field combined compared with the rate among those in the lower 3 quartiles.
The randomized comparisons (Table 4 and Table 5) demonstrating a beneficial effect of lutein on slowing mid-peripheral field sensitivity loss, and the observational data that maximal slowing occurred among those with the highest serum lutein and greatest increase in MPOD (Table 6), provide evidence to support the use of lutein supplementation 12 mg/day among adults with typical retinitis pigmentosa also taking vitamin A palmitate 15,000 IU/day and eating 1-2 servings of oily fish per week. It should be noted that no significant adverse effects were found with use of lutein with respect to both general health and lowering serum retinol over the 4 year duration of this trial.
The short-term safety of lutein has been reported in two other studies of retinitis pigmentosa in which patients were given this supplement in higher doses for up to 6 months. 35,44 However, some concern has been raised that long-term lutein supplementation is associated with an increased risk of lung cancer among smokers over age 50 in the general population. 45 The present trial was conducted in current non-smokers and therefore the recommendation for lutein supplementation 12 mg/day is limited to adult patients with typical retinitis pigmentosa who do not smoke. The long-term safety of lutein even in non-smokers remains to be established. Because the highest serum lutein levels were not associated with greater benefit in this study (see Figure 3) and because the long-term safety of higher dose lutein supplementation is unknown, patients should not exceed 12 mg/day based on current knowledge.
Patients on vitamin A palmitate 15,000 IU/day, 1-2 three-ounce servings of oily fish per week, and lutein 12 mg/day should be reminded to have a fasting serum vitamin A and liver function profile annually as a precaution. Women who are pregnant or planning to become pregnant should not take high-dose vitamin A supplements because of an increased risk of birth defects. Patients age 49 and over should monitor their bone health because of the slight (0.5 – 1.0%) increased risk of hip fracture among patients on long-term high-dose vitamin A supplementation. 46,47
The benefit of lutein supplementation on the long-term course of mid-peripheral visual field loss among patients also on vitamin A and an oily fish diet can only be estimated. Based on the randomized comparison (see Table 4), a patient age 40 would be expected to lose 27 dB/year of total point score, on average, in the lutein group versus 34 dB/year in the control group (i.e. 7 dB saved per year) over the 4-year interval of this trial. Assuming 60 measurable test locations within the HFA 60-4 program and recognizing that 1 dB=0.1 log10 unit, we calculate that lutein supplementation saved, on average, 2.7% per test location per year of mid-peripheral field sensitivity (i.e. 100 × [10(7dB × 0.1/60)−1] = 2.7%) that is equivalent to 0.12 dB per test location per year (i.e., 0.12 dB = 7 dB/60 test locations). With respect to change in serum lutein (see Table 6), we calculate a saving of 12 dB/year or 4.7% per year (i.e. 100 × [10(12 dB × 0.1/60) −1] = 4.7%) that is equivalent to 0.2 dB per test location per year. With respect to change in MPOD we calculate a saving of 16 dB/year or 6.3% per year (i.e. 100 × [10(16 dB × 0.1/60) −1] = 6.3%) or 0.27 dB per test location per year. Therefore, depending on the analysis, the average yearly saving of mid-peripheral visual field sensitivity during the trial ranged from 2.7% to 6.3% per test location per year.
Over the longer term, taking into account that a patient age 40 has, on average, 375 dB of mid-peripheral sensitivity based on total point scores (see Table 3), the estimated benefit in preserving mid-peripheral field sensitivity based on the randomized comparison would be 3 additional years (i.e., 375/27 = 14 versus 375/34 = 11), based on the serum lutein observational results would be 6 additional years (i.e., 375/21 = 18 versus 375/33 = 12), and based on the MPOD observational results would be 10 additional years (i.e., 375/18 = 21 versus 375/34 = 11). In the latter case an average patient on vitamin A who starts lutein at age 40 could expect to lose mid-peripheral field by age 61 (i.e., 40 + 21), while a patient not on lutein would be expected to lose mid-peripheral field by age 51 (i.e., 40 + 11). Follow-up of patients taking lutein and vitamin A with an oily fish diet for at least 10 years would be needed to confirm the above estimates with respect to preserving mid-peripheral visual field.
ACKNOWLEDGMENTS
This research was supported by U10EY13945 from the National Eye Institute, Bethesda, MD and in part by the Foundation Fighting Blindness, Owings Mills, MD.
The authors thank the study patients for participating in this research.
Members of the Data and Safety Monitoring Committee for the current phase III trial were: Janet Wittes, Ph.D. (Chair), Michael B. Gorin, M.D., Ph.D., Susan Taylor Mayne, Ph.D., Cynthia S. McCarthy, DHCE, MA, Paul Sternberg, M.D., Michael Wall, M.D., and Maryann Redford, DDS, MPH (ad hoc).
Members of the Data and Safety Monitoring Committee for the phase I/II study were: Britain W. Nicholson, M.D., (Chair), Lawrence I. Rand, M.D., Robert J. Glynn, Ph.D., and Donald Everett, M.A. (ad hoc).
We wish to thank Roche Pharmaceuticals and their successor DSM Pharmaceuticals (Parsippany, NJ) for providing the study pills. We also thank Marion McPhee, BEd, for her assistance in data analysis.
The Principal Investigator (ELB) had full access to the data upon completion of the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Footnotes
Authors’ Financial Disclosure: None
REFERENCES
- 1.Berson EL. Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1993;34:1659–1676. [PubMed] [Google Scholar]
- 2.Harton DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet, Seminar Series. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 3.Berson EL, Rosner B, Sandberg MA, Hayes KC, Nicholson BW, Weigel-DiFranco C, Willett W. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993;111:761–772. doi: 10.1001/archopht.1993.01090060049022. [DOI] [PubMed] [Google Scholar]
- 4.Bone RA, Landrum JT, Tarsis SE. Preliminary identification of the human macular pigment. Vision Res. 1985;25:1531–1535. doi: 10.1016/0042-6989(85)90123-3. [DOI] [PubMed] [Google Scholar]
- 5.Parker R. Absorption, metabolism and transport of carotenoids. FASEB J. 1996;10:542–551. [PubMed] [Google Scholar]
- 6.Clevidence BA, Bieri JG. Associations of carotenoids with human lipoproteins. Methods in Enzymology. 1993;214:33–46. doi: 10.1016/0076-6879(93)14051-j. [DOI] [PubMed] [Google Scholar]
- 7.Yemelyanov AY, Katz NB, Bernstein PS. Ligand-binding characterization of xanthophyll carotenoids to solubilized membrane proteins derived from human retina. Invest Ophthalmol Vis Sci. 2001;42(4):S359. doi: 10.1006/exer.2000.0965. [DOI] [PubMed] [Google Scholar]
- 8.Snodderly DM, Brown PK, Delori FC, Auran JD. The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:660–673. [PubMed] [Google Scholar]
- 9.Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:674–684. [PubMed] [Google Scholar]
- 10.Sommerburg O, Siems WG, Hurst JS, Lewis JW, Kliger DS, van Kuijk JGM. Lutein and zeaxanthin are associated with photoreceptors in the human retina. Current Eye Res. 1999;19:491–495. doi: 10.1076/ceyr.19.6.491.5276. [DOI] [PubMed] [Google Scholar]
- 11.Rapp LM, Maple SS, Choi JH. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest Ophthalmol Vis Sci. 2000;41:1200–1209. [PubMed] [Google Scholar]
- 12.Handelman GJ, Dratz EA, Reay CC, van Kuijk FJGM. Carotenoids in the human macula and whole retina. Invest Ophthalmol Vis Sci. 1988;29:850–855. [PubMed] [Google Scholar]
- 13.Bone RA, Landrum JT, Friedes LM, et al. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res. 1997;64:211–218. doi: 10.1006/exer.1996.0210. [DOI] [PubMed] [Google Scholar]
- 14.Gruber M, Chappell R, Millen A, LaRowe T, Moeller SM, Iannaccone A, Kritchevsky SB, Mares J. Correlates of serum lutein + zeaxanthin: Findings from the Third National Health and Nutrition Examination Survey. J Nutr. 2004;134:2387–2394. doi: 10.1093/jn/134.9.2387. [DOI] [PubMed] [Google Scholar]
- 15.Ham WT., Jr Ocular hazards of light sources: Review of current knowledge. J Occup Med. 1983;25:101–103. [PubMed] [Google Scholar]
- 16.Khachik F, Bernstein PS, Garland PL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci. 1997;38:1802–1811. [PubMed] [Google Scholar]
- 17.Schalch W. Carotenoids in the retina: A review of their possible role in preventing or limiting damage caused by light and oxygen. In: Emerit I, Chase B, editors. Free Radicals and Aging. Birkhauser; Basel: 1992. [DOI] [PubMed] [Google Scholar]
- 18.Brown L, Rimm EB, Seddon JM, et al. A prospective study of carotenoid intake and risk of cataract extraction in US men. Am J Clin Nutr. 1999;70:517–524. doi: 10.1093/ajcn/70.4.517. [DOI] [PubMed] [Google Scholar]
- 19.Chasan-Taber L, Willett WC, Seddon JM, Stamper MJ, Rosner B, Colditz GA. A prospective study on vitamin supplement intake and cataract extraction among US women. Epidemiology. 1999;10:679–684. [PubMed] [Google Scholar]
- 20.Seddon JM, Ajani UA, Sperduto RD, et al. Eye Disease Case-Control Study Group Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA. 1994;272:1413–20. [PubMed] [Google Scholar]
- 21.Mohammedshah FDJS, Amann MM, Heimbach JM. Dietary intakes of lutein and zeaxanthin and total carotenoids among Americans age 50 and above. FASEB J. 1999;13 [Google Scholar]
- 22.Nebeling LC, Forman MR, Graubard BI, Snyder RA. Changes in carotenoid intake in the United States: the 1987 and 1992 National Health Interview Surveys. J Am Diet Assoc. 1997;97:991–6. doi: 10.1016/S0002-8223(97)00239-3. [DOI] [PubMed] [Google Scholar]
- 23.Tucker KL, Chen H, Wilson PWF, Schaefer EJ, Lammi-Keefe CJ. Carotenoid intakes, assessed by dietary questionnaire, are associated with plasma carotenoid concentrations in an elderly population. 1999 doi: 10.1093/jn/129.2.438. [DOI] [PubMed] [Google Scholar]
- 24.Vandenlangenberg GM, Brady WE, Nebeling LC, et al. Influence of using different sources of carotenoid data in epidemiologic studies. J Am Diet Assoc. 1996;96:1271–1275. doi: 10.1016/S0002-8223(96)00332-X. [DOI] [PubMed] [Google Scholar]
- 25.Willett WC, Sampson L, Browne ML, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127:188–199. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 26.Neuringer M, Snodderly DM, Sandstrom M, Johnson EJ, Schalch W. Nutritional manipulation of primate retinas: I. Effects of lutein or zeaxanthin supplements on serum and macular pigment of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2004;45:3234–3243. doi: 10.1167/iovs.02-1243. [DOI] [PubMed] [Google Scholar]
- 27.Broich CR, Gerber LE, Erdman JW., Jr. Determination of lycopene alpha- and beta-carotene and retinyl esters in human serum by reversed-phase high performance chromatography. Lipids. 1983;18(3):253–258. doi: 10.1007/BF02534557. [DOI] [PubMed] [Google Scholar]
- 28.Wooten BR, Hammond BR, Land RI, Snodderly DM. A practical method for measuring macular pigment optical density. Invest Ophthalmol Vis Sci. 1999;40:2481–9. [PubMed] [Google Scholar]
- 29.Ferris FL, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–96. [PubMed] [Google Scholar]
- 30.Mills RP, Barnebey HS, Migliazzo CV, Li Y. Does saving time using FASTPAC or suprathreshold testing reduce quality of visual fields? Ophthalmology. 1994;101:1596–1603. doi: 10.1016/s0161-6420(94)31132-8. [DOI] [PubMed] [Google Scholar]
- 31.Schaumberger M, Schafer B, Lachenmayr BJ. Glaucomatous visual fields. FASTPAC versus full threshold strategy of the Humphrey Field Analyzer. Invest Ophthalmol Vis Sci. 1995;36:1390–1397. [PubMed] [Google Scholar]
- 32.Flanagan JG, Moss ID, Wild JM, Hudson C, Prokopich L, Whitaker D, O’Neill EC. Evaluation of FASTPAC: A new strategy for threshold estimation with the Humphrey Field Analyzer. Graefes Arch Clin Exp Ophthalmol. 1993;231:465–469. doi: 10.1007/BF02044233. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 34.Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Moser A, Brockhurst RJ, Hayes KC, Johnson CA, Anderson EJ, Gaudio AR, Willett WC, Schaefer EJ. Clinical trial of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment. Arch Ophthalmol. 2004;122:1297–1305. doi: 10.1001/archopht.122.9.1297. [DOI] [PubMed] [Google Scholar]
- 35.Aleman TS, Duncan JL, Bieber ML, de Castro EB, Marks DA, Gardner LM, Steinberg JD, Cideciyan AV, Maguire MG, Jacobson SG. Macular pigment and lutein supplementation in retinitis pigmentosa and Usher syndrome. Invest Ophthalmol Vis Sci. 2001;42:1873–1881. [PubMed] [Google Scholar]
- 36.Johnson EJ, Chung H-Y, Caldarella SM, Snodderly DM. The influence of supplemental lutein and docosahexaenoic acid on serum, lipoproteins, and macular pigmentation. Am J Clin Nutr. 2008;87:1521–1529. doi: 10.1093/ajcn/87.5.1521. [DOI] [PubMed] [Google Scholar]
- 37.Diggle P, Heagerty P, Liang K-Y, Zeger S, editors. Analysis of Longitudinal Data. Oxford University Press; Oxford: 2002. [Google Scholar]
- 38.SAS Institute, Inc. SAS Version 9.1.3. SAS Institute; Cary, NC: 2006. [Google Scholar]
- 39.Rosner B, Glynn RJ, Lee M-LT. Extension of the rank sum test for clustered data: Two-group comparisons with group membership defined at the subunit level. Biometrics. 2006;62:1251–1259. doi: 10.1111/j.1541-0420.2006.00582.x. [DOI] [PubMed] [Google Scholar]
- 40.Stone CJ, Koo CY. Proceedings of the Statistical Computing Section of the American Statistical Association. American Statistical Association; Alexandria, VA: 1985. Additive splines in statistics; pp. 45–48. [Google Scholar]
- 41.Harrell FJ. Regression Modeling Strategies. Springer Publishing Co.; New York, NY: 2001. [Google Scholar]
- 42.Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Moser A, Brockhurst RJ, Hayes KC, Johnson CA, Anderson EJ, Gaudio AR, Willett WC, Schaefer EJ. Further evaluation of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment: Subgroup analyses. Arch Ophthalmol. 2004;122:1306–1314. doi: 10.1001/archopht.122.9.1306. [DOI] [PubMed] [Google Scholar]
- 43.Hammond BR, Johnson EJ, Russell RM, Krinsky NI, Yeum KJ, Edwards RB, Snodderly DM. Dietary modification of human macular pigment density. Invest Ophthalmol Vis Sci. 1997;38:1795–1801. [PubMed] [Google Scholar]
- 44.Bahrami H, Melia M, Dagnelie G. Lutein supplementation in retinitis pigmentosa: PC-based vision assessment in a randomized double-masked placebo-controlled clinical trial [ NCT00029289] BMC Ophthalmol. 2006;6:23. doi: 10.1186/1471-2415-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satia JA, Littman A, Slatore CG, Galanko JA, White E. Long-term use of BETA-carotene, retinol, lycopene, and lutein supplements and lung cancer risk: Results from the VITamins and Lifestyle (VITAL) Study. Am J Epidemiology. 2009;169:815–828. doi: 10.1093/aje/kwn409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feskanich D, Singh V, Willett WC, Colditz GA. Vitamin A intake and hip fractures among postmenopausal women. JAMA. 2002;287:47–54. doi: 10.1001/jama.287.1.47. [DOI] [PubMed] [Google Scholar]
- 47.Michaelsson K, Lithell H, Vessby B, Melhus H. Serum retinol levels and the risk of fracture. N Engl J Med. 2003;348:287–294. doi: 10.1056/NEJMoa021171. [DOI] [PubMed] [Google Scholar]