Abstract
Small intestine allotransplantation in humans is not yet feasible due to the failure of the current methods of immunosuppression. FK-506, a powerful new immunosuppressive agent that is synergistic with cyclosporine, allows long-term survival of recipients of cardiac, renal, and hepatic allografts. This study compares the effects of FK-506 and cyclosporine on host survival, graft rejection, and graft-versus-host-disease in a rat small intestine transplantation model. Transplants between strongly histoincompatible ACI and Lewis (LEW) strain rats, and their F1 progeny are performed so that graft rejection alone is genetically permitted (F1→LEW) or GVHD alone permitted (LEW→F1) or that both immunologic processes are allowed to occur simultaneously (ACI→LEW). Specific doses of FK-506 result in prolonged graft and host survival in all genetic combinations tested. Furthermore, graft rejection is prevented (ACI→LEW model) or inhibited (rejection only model) and lethal acute GVHD is eliminated. Even at very high doses, cyclosporine did not prevent graft rejection or lethal GVHD, nor did it allow long-term survival of the intestinal graft or the host. Animals receiving low doses of cyclosporine have outcomes similar to the untreated control groups. No toxicity specific to FK-506 is noted, but earlier studies by other investigators suggest otherwise.
Small intestine allotransplantation (SIA)* that results in the establishment of grafts capable of absorbing nutrients and supporting human life has not yet been achieved, despite the demonstration of its technical feasibility 30 years ago (1). The limiting obstacle in all SIA models examined to date remains the failure of traditional methods of immunosuppression, including cyclosporine, to prevent rejection. Since the introduction of CsA, some progress in long-term survival following SIA in rats and larger animals has been reported (2–6). Recently, 6-month survival has been achieved following human small intestine allotransplantation (7) as well as multivisceral organ transplantation, which included the entire small intestine and most of the colon (8). However, failures are more common than temporary successes. This is particularly distressing in light of the fact that the number of patients who would potentially benefit from SIA and who have been otherwise relegated to total parenteral nutrition or worse is significant (9).
It is clear that future improvements of SIA will depend upon the development of more-specific and less-toxic forms of immunosuppression. FK -506 is a powerful new immunosuppressant that has recently been demonstrated to successfully inhibit hepatic (10), renal (11), and cardiac (12) rejection in animal models.
The purpose of the present study was to compare the effects of FK-506 and CsA on host survival, graft rejection, and graft-versus-host disease in a rat small intestine transplantation model.
MATERIALS AND METHODS
Animals
Male Lewis (LEW) (RTl1), ACI (RT1a) strains, and (ACI×LEW)F1 hybrid rats weighing 200–300 g were purchased from Harlan Sprague Dawley (Indianapolis, IN). The rats were housed in a conventional animal facility, fed rat chow (Wayne Lab Blox F -6, Chicago, IL) and tap water ad libitum. Rats are allowed to acclimatize for at least 1 week prior to investigation. Using the two parental strains and their F1 progeny, fully or semiallogeneic graft transfers were examined. These donor/recipient combinations made it possible to independently study graft rejection (F1→LEW) or donor-mediated GVHD (LEW→F1) or their simultaneous occurrence in combination (ACI→LEW) (13).
Immunosuppressive Agents
FK -506: FK -506 was supplied in powder form by the Fujisawa Pharmaceutical Company, Ltd., Osaka, Japan. The properties and immunosuppressive activity of this drug have been recently described (10–12). All concentrations of drug were made up in sterile normal saline and administered to experimental animals using deep intramuscular hind-limb injections. Dosages are listed in Table 1.
Table l.
Group classification and treatment regimens
| Group | n | Donor | Recipient | Treatment (days)a |
|---|---|---|---|---|
| I | ||||
| A | 11 | ACI | L | — |
| B | 8 | F1 | L | — |
| C | 14 | L | F1 | — |
| II | ||||
| A | 10 | ACI | L | FK 2 mg/kg/qd (0–6); 1 mg/kg/qod (8–30) |
| B | 10 | F1 | L | FK 2 mg/kg/qd (0–6); 1 mg/kg/qod (8–30) |
| C | 8 | L | F1 | FK 2 mg/kg/qd (0–6); 1 mg/kg/qod (8–30) |
| III | 8 | ACI | L | FK 2 mg/kg/qd (3–6) |
| IV | 9 | ACI | L | ALG (donor) 100 mg/ kg/qd (−2, −1); FK 2 mg/kg/qd (3–6) |
| V | 11 | ACI | L | CsA 15 mg/kg/qd, 0–6/ qod (8–30) |
| VI | 7 | ACI | L | CsA 20 mg/kg/qd, 0–6/ qod (8–30) |
| VII | 9 | ACI | L | CsA 40 mg/kg/qd (−2– 7) |
| VIII | 6 | L | F1 | CsA 20 mg/kg/qd (−2– 14) |
Qd, treatment once daily; and qod, treatment every other day.
CsA: CsA was supplied in powder form by Sandoz Pharmaceutical Corporation (East Hanover, NJ). The drug was dissolved in Mygliol (Dynamit Nobel, Witten/Ruhr, West Germany), an oil-based carrier, and administered as described for FK -506. Dosages are listed in Table 1.
ALG: Goat antirat lymphocyte globulin (ALG) was a gift from Dr. Richard Condie (University of Minnesota Hospitals, Minneapolis, MN). The final protein concentration was 42.7 mg/ml. A dose of 100 mg/kg/day was injected i.p. on days −2 and −1 to group IV donors.
Operative techniques
No preoperative manipulation of food and water was necessary. Donor anesthesia was induced with 3.6% chloral hydrate by intraperitoneal injection at 1 ml/100 g of body weight. Heterotopic jejunoileal allotransplantation was performed as described previously.3 Briefly, the intestinal segment was removed from the donor based upon a pedicle of the superior mesenteric artery and vein. The vascular and alimentary lumina were flushed with 3 ml of lactated Ringer’s solution containing 150 U of porcine heparin and 30 ml of 1 % neomycin solution, respectively. All solutions were maintained at 4°C. The recipients were anesthetized as described for the donors, and 20 mg/kg of Cefoxitin (Merck, Sharp and Dohme, West Point, PA) was injected intraperitoneally to inhibit enteric bacterial contamination. Donor superior mesenteric artery to host aorta and donor superior mesenteric vein to host infrarenal vena caval anastomoses were performed with continuous 9–0 Novafil. A Thiry-Vella loop was fashioned with the oral segment emanating from the right lower abdominal quadrant and the aboral segment projecting from the right upper abdominal quadrant. Animals surviving less than 5 days were considered technical failures and were excluded from the data analysis. The technical success rate was approximately 80%.
Experimental groups
Eight groups were assessed for graft and host survival as well as clinical and histologic evidence of graft rejection and/or GVHD. The specifics of the treatment protocol are listed in Table 1. FK-506 was found in a screening study to be most effective at the doses used in group II. The dose and schedule used in group III were similar to that used to prevent graft rejection and to prolong host survival following cardiac allotransplantation in the rat (12). Treatment with 10 ml/kg of rabbit antirat ALG (protein concentration unknown) for 2 days prior to transplantation has been previously demonstrated to prevent GVHD in a semiallogeneic parent to F1 rat small intestine transplantation model (14). CsA at 15 mg/kg for days 0–6 and every other day for days 8–30 has been reported to prolong graft and host survival following Brown-Norway (BN) → LEW small bowel transplants (4). Higher doses of CsA were used to increase the effectiveness of the immunosuppression in the ACI→ LEW model of small intestine transplantation.
Clinical monitoring
The recipients were evaluated daily for graft failure, and the rats were weighed every 2–3 days. The transplanted bowel was gently decompressed daily, and acute rejection was manifested as progressive stomal ischemia and death of the animal once the initial 4-day period had elapsed. Chronic rejection was diagnosed as the onset of a right-sided, firm abdominal mass that occurred more than 30 days following transplantation. GVHD was established when 4 of the 6 clinical signs were present: diffuse erythema, hyperkeratosis of the foot pads, dermatitis, unkempt appearance, diarrhea, weight loss (15).
Histologic monitoring
Ear and intestinal stoma biopsies were taken at random in a limited number of animals. All recipients were necropsied following euthanasia or at death. Ear, tongue, donor and recipient small intestine, liver, and pancreas were removed and processed routinely for light microscopy. All sections were reviewed by a surgeon (ALH) and pathologist (BB) blinded to the drug therapy.
The following histologic features were assessed: mucosal integrity, villous architecture, and type and location of inflammatory cells. “Apoptosis” was defined as single-cell necrosis within the epithelium. “Cryptitis” was defined as single or multiple necrotic cells limited to the crypts. “Endothelialitis” was defined as lymphocyte adherence to endothelium that invariably showed evidence of injury.
A diagnosis of acute rejection was made if there was multifocal cryptitis (>3 crypts involved per high-power field) with or without a mononuclear cell inflammatory infiltrate in the lamina propria, and/or endothelial or perivascular inflammation. Mucosal necrosis, ulceration, and sloughing were considered consistent with rejection and were diagnosed as such when the surrounding intact bowel displayed cryptitis or a mononuclear cell infiltrate. Chronic rejection was diagnosed when there was obliteration of the bowel architecture secondary to fibrosis without other lesions consistent with ischemia or infection.
GVHD in the tongue, ear, and liver was recognized as multifocal apoptosis with lymphocyte infiltration. In host intestine, a diagnosis of GVHD was made if multifocal cryptitis was present with or without inflammation in lamina propria.
The histologic similarity of rejection and GVHD, in some cases, made it necessary to know the origin of the small intestine prior to establishing a definitive diagnosis.
Statistical analysis
Host survival data were evaluated using Kaplan-Meier survival plots. Generalized savage (Mantel-Cox) statistical analysis of the plots were performed. Statistical comparisons of acute or chronic rejection or GVHD between groups was performed using Chi-square analysis with Yates correction. A value of P<0.05 was considered to be statistically significant.
RESULTS
General
The results of our experiments clearly demonstrate that FK-506 is more effective than CsA in preventing acute rejection and lethal GVHD in this model of SIA in the rat. The survival of all groups studied are listed in Tables 2–4. No toxic effects specific to FK-506 or CsA were demonstrated in this experimental protocol.
Table 2.
Host survival, clinical rejection, and GVHD in a bidirectional fully allogeneic model (ACI→LEW) of small intestine transplantation
| Group | n | Treatmenta | Survival times (days) | Mean survival ±SD (days) |
P valueb | Acute rejection |
Chronic rejection |
GVHD |
|---|---|---|---|---|---|---|---|---|
| IA | 11 | — | 9, 9, 8, 7,7,7,7,7, 7, 6, 6 |
7.3±1.0 | — | 11/11 | 0/11 | 0/11 |
| IIA | 10 | FK long course |
131, 118, 96, 38, 36, 33, 25, 13, 9, 7 |
50.6±46.5 | 0.0001 | 0/10c | 5/10c | 0/10 |
| III | 8 | FK short course |
76, 73, 63,31, 11, 10, 8, 7 |
34.9±30.8 | 0.0008 | 3/8 | 3/8 | 0/8 |
| IV | 9 | ALG (do- nor), FK short course |
79, 40, 25, 21, 11, 8, 7, 5, 5 |
22.3±24.2 | 0.0324 | 5/9 | 2/9 | 0/9 |
| V | 11 | CsA 15 long course |
24, 9, 9, 8, 8, 7, 7, 6, 6, 5, 5 |
8.5±5.3 | 0.7441 | 9/11 | 0/11 | 0/11 |
| VI | 7 | CsA 20 long course |
23, 13, 11, 10, 9, 7, 5 | 11.1±5.8 | 0.0150 | 7/7 | 0/7 | 0/7 |
| VII | 9 | CsA 40 short course |
>114, 69, 28, 27, 26, 16, 15, 8, 6 |
34.3±35.2 | 0.0007 | 4/9 | 1/9 | 0/9 |
Refer to Table 1.
Generalized savage (Mantel-Cox) value comparing survival times to that of untreated controls.
Chi-square, P value <0.05.
Table 4.
Host survival and clinical GVHD in a unidirectional, semiallogeneic model (LEW→[ACI×LEW] F1) of small intestine transplantation in the rata
| Group | n | Treatmentb | Survival times (days) | Mean survival ±SD (days) |
P valuec | GVHD |
|---|---|---|---|---|---|---|
| IC | 14 | — | 25, 24, 21, 17, 17, 16, 12, 11, 11, 10, 9, 8, 7, 7 |
13.9±6.1 | — | 6/14 d |
| IIC | 8 | FK long course | >214, >214, >213, >213, >212, >206, >202, 7 |
188.0±72.1 | 0.0002 | 1/8e,f |
| VIII | 6 | CsA 20 inter- mediate course |
>104, 53, 50, 8, 7, 7 | 38.3±38.6 | 0.1035 | 2/6 |
Genetically restricted to GVHD; graft rejection is not possible in this model.
Refer to Table 1.
Generalized savage (Mantel-Cox) value comparing survival times to that of untreated controls.
Four other animals demonstrated findings suggestive of GVHD.
Chi-square, P value <0.05.
Lone animal that developed GVHD is alive with resolution of the disease.
Bidirectional, fully allogeneic model (groups IA, IIA, III–VII)
Control animals (group IA) died from acute rejection at 7.3±1.0 days. All the other groups in these experiments, except those animals receiving low-dose CsA (group V), had statistically significant prolongation of host survival (Table 2). The longest mean survival and most effective inhibition of acute rejection, both clinically and histologically, occurred with FK-506 (group IIA). Even very high doses of CsA (group VII) were not as effective as FK-506 in prolonging survival or inhibiting acute rejection.
Although acute rejection was prevented in the ACI→LEW model by FK-506 (group IIA), chronic rejection occurred in 50% of the rats in this group. FK-506 used in low doses (data not shown), short schedules (group III), or combined with ALG donor pretreatment (group IV) was not successful in preventing acute or chronic rejection. Graft and host survival was significantly less in these groups than in group IIA. The use of equivalent doses of ALG alone did not confer prolongation of host survival relative to the untreated controls.
Severe acute rejection was seen in histologic sections of the donor intestine from the control and CsA-treated groups but not in the FK-506–treated rats (group IIA) (Fig. 1). In the CsA treated group (group VII), multifocal cryptitis, lamina propria inflammation, and endothelialitis were prominent and progressive. Chronic rejection was detected histologically, but not clinically, in a single animal from the CsA group. Acute rejection was not present in the animals treated with FK-506 (group IIA), but histologic evidence of chronic rejection correlated with the clinical findings of an indurated intestinal graft with luminal obliteration and abscess formation resulting in death of the host within 30 days of onset of the lesion.
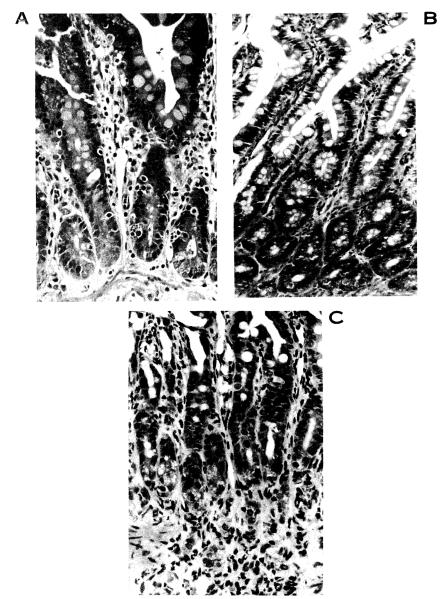
Figure 1.
Donor small intestine, fully allogeneic bidirectional model: A. control animal (group IA) at 8 days showing cryptitis and inflammation of the lamina propria (H&E; × 100); B. FK-506–treated animal (group IIA) at 22 days showing normal villi and crypts (H&E; ×63); and C. CsA-treated animals (group VII) at 26 days demonstrating severe cryptitis and inflammation in the lamina propria (H&E; ×100).
GVHD was not demonstrated clinically in the bidirectional model, although the histopathologic lesions associated with this syndrome were present in the control and all treatment groups. The host intestine, ear, tongue, and liver were affected to variable degrees (Fig. 2).
Figure 2.
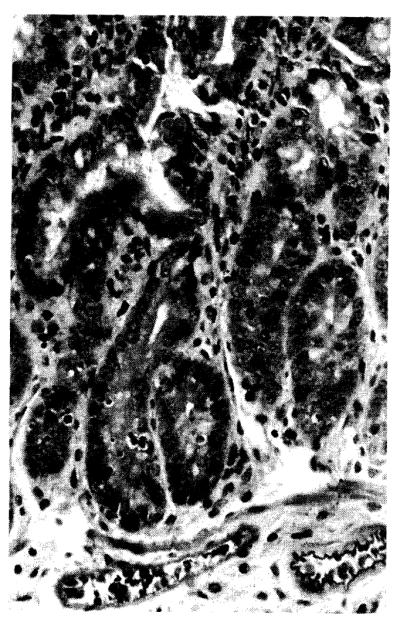
Host small intestine, control animal (group IA, fully allogeneic model) at 8 days with GVHD that is characterized by cryptitis and lamina propria inflammation (H&E; ×100).
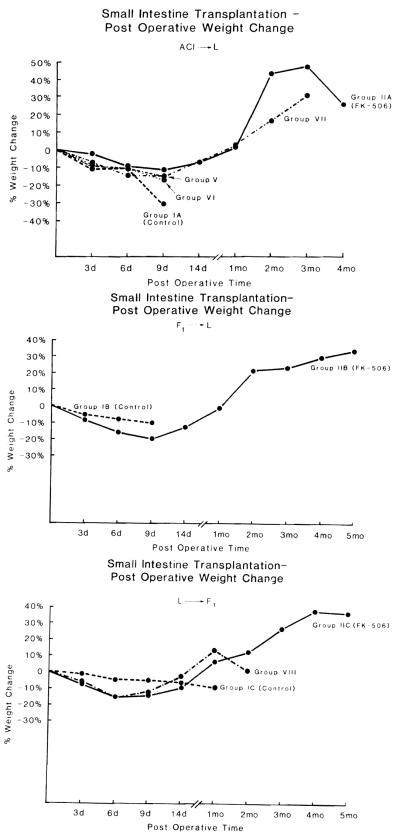
Weight gain was observed in the posttransplantation period for both FK-506 and CsA. Late weight loss correlated with the onset of chronic rejection in the FK-506 treatment group (IIA) (Fig. 3A).
Figure 3.
Postoperative weight change following small intestine transplantation in the rat. A. Fully allogeneic model—progressive weight gain is noted in the FK-506–treated rats (group IIA). Following a several-month period of well-being, chronic rejection resulted in weight loss and death of the animals. Only high doses of CsA (group VII) resulted in weight gain in the recipients. The remaining survivor is alive and thriving at >3 months posttransplantation. B. Semiallogeneic model of rejection after an initial period of slight loss, the surviving FK-506–treated animals gained weight. Control animals lost weight and died in a short period of time. C. Semiallogeneic model of GVHD—the FK-506–treated animals show progressive weight gain. The CsA-treated group gained weight until the late onset of lethal GVHD resulted in weight loss and death.
Unidirectional, semiallogeneic model of graft rejection (groups IB, IIB)
Control animals (group IB) died of acute graft rejection 9.0±2.0 days posttransplantation. FK -506–treated rats (group IIB) had a mean survival of 83 days (Table 3). In this model FK-506 inhibited, but did not prevent, acute rejection (group IIB). Three of 10 rats died of acute rejection while receiving FK-506 (group IIB). The histopathologic lesions in this group were much less severe when compared to the control group (Fig. 4). The FK-506–treated animals had cryptitis alone, while the control group had cryptitis with lamina propria inflammation and, commonly, mucosal necrosis. Progressive weight gain was present in the FK-506 group (group IIB) but not in the control group (Fig. 3B).
Table 3.
Host survival and clinical rejection in a unidirectional, semiallogeneic model ([ACl×LEW] F1→LEW) of small intestine transplantation in the rata
| Group | n | Treatment | Survival times (days) | Mean survival ±SD (days) |
P valueb | Acute rejection |
Chronic rejection |
|---|---|---|---|---|---|---|---|
| IB | 8 | — | 12, 11, 10, 9, 9, 8, 7, 6 | 9.0±2.0 | — | 8/8 | 0/8 |
| IIB | 10 | FK long course | >206, >205, >139, 117, 32, 23, 12, 12, 8, 8 |
83.0±82.6 | 0.0022 | 3/10c | 1/10 |
Genetically restricted to graft rejection; GVHD is not possible in this model.
Generalized savage (Mantel-Cox) value comparing survival times to that of untreated controls.
Chi-square, P value <0.05.
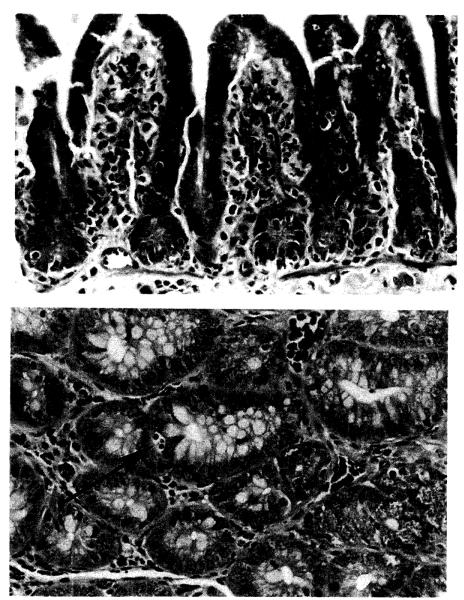
Figure 4.
Donor small intestine, semiallogeneic rejection model: A. control animal (group IB) at 8 days showing severe rejection with villous blunting, cryptitis, and inflammation (H&E; ×100); and B. FK-506–treated animal (group IIB) at 12 days demonstrating rare singlecell necrosis (arrow) and minimal inflammation (H&E; ×125).
Unidirectional, semiallogeneic model of GVHD (groups IC, IIC, VIII)
Control animals that received donor small bowel transplants in a combination that allowed for the development of GVHD without graft rejection died in 13.9±6.1 days. Six died with clinical manifestations of GVHD, and four others died with 3/6 clinical signs of GVHD. FK-506 (group IIC) prevented fatal GVHD and prolonged mean survival to 188.0±72.1 days (Table 4). Seven of eight remain alive and well, including a single rat that developed clinical GVHD at 53 days posttransplant that then resolved in 2 weeks. CsA treatment (group VIII) delayed, but did not prevent GVHD, and two animals died with the syndrome at 50 and 53 days. Three animals died of unknown causes, perhaps secondary to a subclinical form of GVHD. FK-506 treatment was effective enough to be associated with a progressive weight gain beginning 9 days posttransplantation (Fig. 3C).
In histologic sections, florid GVHD was present, as expected, in the control animals (group IC) (Fig. 5A). The lesions were more extensive than the bidirectional ACI→LEW controls with frequent single-cell necrosis, cryptitis, and moderate to severe lamina propria inflammation. The FK-506–treated rats in this donor/recipient combination (group IIC) were not sacrificed, and no histopathologic comparisons could be made with the control group. However, 2 ear biopsies from group IIC animals did not display any evidence of GVHD (Fig. 5B). As expected, there was no clinical nor histologic evidence of rejection of the donor intestine in the LEW→F1, model at any time.
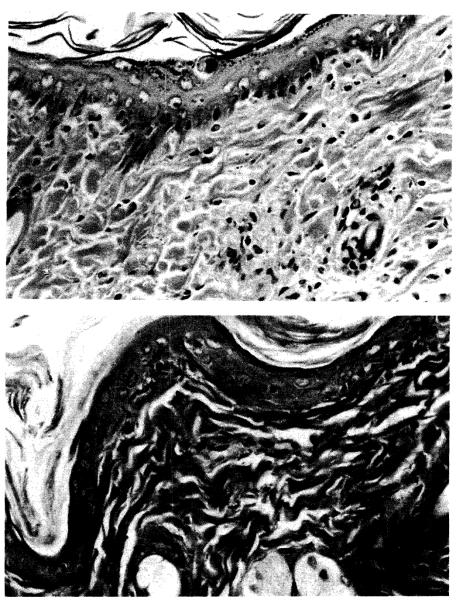
Figure 5.
Sections of ear, semiallogeneic GVHD model: A. control animal (group IC) at 25 days with apoptosis throughout the basal layer and lymphocytic infiltrate in the dermis; and B. FK-506–treated animal (group IIC) at 21 days with normal skin (H&E; ×125).
DISCUSSION
Small intestine allotransplantation has important potential for clinical application as an effective therapy modality for the many manifestations of short-bowel syndrome. The operation can be technically accomplished in a variety of species, including man, and progress has been made since the introduction of CsA (2–8, 16). However, the application of this procedure is restricted because of the failure of traditional methods of immunosuppression, including CsA, to prevent graft rejection. Multiple investigators have recently demonstrated that the use of the new immunosuppressive agent, FK-506, is an effective means of preventing the rejection of cardiac, renal, and hepatic allografts (10–12). In these studies, we have examined the effectiveness of FK-506, as compared to CsA, as an immunosuppressive agent in a rat small intestine allotransplantation model. These experiments were conducted in a highly histoincompatible ACI→LEW donor/recipient strain combination and one that allowed us to independently examine the effect of these agents on graft rejection and GVHD.
The results of our experiments demonstrate that FK -506 is a more effective single agent than CsA in the prevention of acute rejection and lethal GVHD. In the fully allogeneic and semiallogeneic rejection models, FK -506 prolonged graft and host survival, allowed a higher yield of long-term survivors and significantly inhibited or prevented (bidirectional model only) clinical and histologic manifestations of acute rejection when compared to CsA-treated or control animals (Tables 2–3). In the semiallogeneic model in which only graft-versus-host disease is genetically possible, FK -506 prevented lethal acute GVHD, decreased the incidence of clinical GVHD, and was associated with long-term disease-free survival in 7/8 animals (Table 4). CsA was capable of ablating or at least inhibiting GVHD during its administration, but a fatal syndrome ensued approximately 1 month following cessation of treatment. Though early studies by Tutschka et al. (17) had been promising, many investigators have demonstrated that CsA is not completely effective in the prevention of GVHD following bone marrow (18, 19) or small bowel transplantation (20). The prevention of acute lethal GVHD by FK-506 represents a distinct therapeutic advantage for this agent.
The results also demonstrated a dose effect for each of the immunosuppressive agents and the variable effectiveness of CsA when tested across different histoincompatibility barriers. The dose of FK-506 used in group II was very effective as outlined above. Reduction of the dose by 75% with the same schedule and route of administration (data not shown) resulted in acute rejection and the death of 4/5 animals in 8–17 days. In this ACI→LEW model, higher doses of CsA were slightly more effective than lower doses of the drug in prolonging graft and host survival and in inhibiting acute rejection. However, in a BN → LEW SIA model (4), the regimen of CsA as used in group V was effective in securing high-yield, long-term host survival suggesting that it is easier to suppress rejection in this strain combination. Doses of CsA that were 25% larger or several times greater were unable to overcome the problem of graft rejection or allow prolonged graft or host survival in the ACI→ LEW strain combination when compared to low doses of FK-506. Hatcher et al. (21) also had low-yield, long-term survival when using oral CsA in an ACI→LEW small intestine transplantation model. Erratic uptake of intramuscular CsA that is dissolved in the lipid soluble carrier, Mygliol, has been described (22) and is also a possible explanation for this phenomenon.
The immunosuppressive properties of FK-506 and GVHD (23, 24) are well documented. Based on the histologic findings, GVHD and FK-506 may be additive or synergistic in the suppression of acute graft rejection. In the fully allogeneic control animals, rejection predominates. When appropriate doses of FK-506 are administered, rejection is not present though histologic evidence of GVHD persists. At these same doses of FK-506, if GVHD is genetically restricted, as in the F1→LEW model, rejection can be seen clinically and histologically. Furthermore, donor pretreatment with ALG, which prevents GVHD induced by small intestine transplantation (14), when used in conjunction with low-dose FK-506 does not prevent rejection and is associated with short survival times. Using only low-dose FK-506 without ALG, survival times are prolonged. A balance between graft rejection and GVHD has been suggested previously (25, 26). The relative predominance of either of these immunologic reactions is related to the length of the intestinal graft (number of immunocompetent cells transplanted) (25), the effectiveness of the immunosuppression (26), and other less well-defined factors, which may include species variation and immune responsiveness.
These experiments demonstrate that FK-506 is a better single agent than CsA for prolonging graft and host survival, preventing acute graft rejection, and ablating lethal GVHD following rat small intestine allotransplantation. FK-506 is not however a pharmacologic panacea for the prevention of allograft rejection although it represents a powerful addition to the immunosuppressive armentarium. Chronic rejection occurred in 50% of the recipients in the clinically relevant bidirectional model following cessation of FK-506, but protracted treatment may have prevented this complication. Though concerns about deleterious toxic side effects (27–29) are primarily based on the early work with this powerful immunosuppressive agent, more extensive and controlled toxicity studies involving FK-506’s effects on various organs and species are clearly indicated. The possibility that FK-506 and CsA may be synergistic in their actions as immunosuppressive agents could prove to be clinically important since the complimentary therapeutic effects may avoid much of the toxicity associated with the use of each agent independently.
Acknowledgments
We appreciate the excellent technical assistance provided by Judy Wargo, M.S., and Jo Harnaha, B.S. Special thanks also go to Ms. Donna Ross for the preparation of this manuscript.
Footnotes
This work was supported by Research Grants from the Veterans Administration and Project Grant No. DK-29961 from the National Institutes of Health, Bethesda, MD.
- ALG
- antirat lymphocyte globulin
- SIA
- small intestine allotransplantation.
Hoffman AL, Makowka L, Cai X, et al. Near-total heterotopic small intestine transplantation in the rat (submitted for publication).
REFERENCES
- 1.Lillehei RC, Goott B, Miller FA. The physiologic response of the small bowel of the dog to ischemia including prolonged in vitro preservation of the bowel with successful replacement and survival. Ann Surg. 1959;150:543. doi: 10.1097/00000658-195910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reznick RK, Craddock GN, Langer B, et al. Structure and function of small bowel allografts in the dog: immunosuppression with cyclosporin A. Can J Surg. 1982;25:51. [PubMed] [Google Scholar]
- 3.Grant P, Duff J, Zhong R, et al. Successful intestinal transplantation in pigs treated with cyclosporine. Transplantation. 1988;45:279. doi: 10.1097/00007890-198802000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Lee KKW, Schraut WH. Structure and function of orthotopic small-bowel allografts in rats with cyclosporin A. Am J Surg. 1986;151:55. doi: 10.1016/0002-9610(86)90011-5. [DOI] [PubMed] [Google Scholar]
- 5.Harmel RP, Stanley M. Improved survival after allogeneic small intestinal transplantation in the rat using cyclosporine immunosuppression. J Pediatr Surg. 1986;21:214. doi: 10.1016/s0022-3468(86)80836-3. [DOI] [PubMed] [Google Scholar]
- 6.Kimura K, LaRosa CA, Money SR, et al. Segmental intestinal transplantation in rats with resected entire small bowel, ileocecal valve and cecum. J Surg Res. 1988;45:349. doi: 10.1016/0022-4804(88)90130-8. [DOI] [PubMed] [Google Scholar]
- 7.Goulet OJ, Reveillon Y, Cerf-Bensussann, et al. Small intestinal transplantation in a child using cyclosporine. Transplant Proc. 1988;20:288. [PubMed] [Google Scholar]
- 8.Starzl TE, Rowe MI, Todo S, et al. Transplantation of multiple abdominal viscera. JAMA. 1989;261:1449. [PMC free article] [PubMed] [Google Scholar]
- 9.Talsma SE, Marks WH, Marks C, et al. Potential recipients for small-bowel transplantation in the United States and United Kingdom. In: Deltz E, Thiede A, Hamelmann H, editors. Small-bowel transplantation: experimental and clinical fundamentals. Springer; New York: 1986. [Google Scholar]
- 10.Todo S, Podesta L, Chapchap P, et al. Orthotopic liver transplantation in dogs receiving FK-506. Transplant Proc. 1987;19:64. [PMC free article] [PubMed] [Google Scholar]
- 11.Todo S, Demetris AJ, Ueda Y, et al. Canine kidney transplantation with FK -506 alone or in combination with cyclosporine and steroids. Transplant Proc. 1987;19:57. [PMC free article] [PubMed] [Google Scholar]
- 12.Murase N, Todo S, Lee PH, et al. Heterotopic heart transplantation in the rat receiving FK-506 alone or with cyclosporine. Transplant Proc. 1987;19:71. [PMC free article] [PubMed] [Google Scholar]
- 13.Monchik GJ, Russell PS. Transplantation of small bowel in the rat: technical and immunologic considerations. Surgery. 1971;70:693. [PubMed] [Google Scholar]
- 14.Shaffer D, Maki T, DeMichele SJ, et al. Studies in small bowel transplantation: prevention of graft-versus-host disease with preservation of allograft function by donor pretreatment with antilymphocyte serum. Transplantation. 1988;45:262. [PubMed] [Google Scholar]
- 15.Hoffman AL, Makowka L, Cramer DV, et al. Induction of stable chimerism and elimination of graft-versus-host disease by depletion of T lymphocytes from bone marrow using immunomagnetic beads. Surgery. 1989;106:354. [PMC free article] [PubMed] [Google Scholar]
- 16.Lillehei RC, Idezuki Y, Feemster JA, et al. Transplantation of stomach, intestine, pancreas: experimental and clinical observations. Surgery. 1967;62:721. [PubMed] [Google Scholar]
- 17.Tutschka PJ, Beschorner WE, Allison AC, et al. Use of cyclosporin A in allogeneic bone marrow transplantation in the rat. Nature. 1979;280:148. doi: 10.1038/280148a0. [DOI] [PubMed] [Google Scholar]
- 18.Deeg HJ, Storb R, Weiden PL, et al. Cyclosporin A and methotrexate in canine marrow transplantation: engraftment, graft versus host disease and induction of tolerance. Transplantation. 1982;34:30. doi: 10.1097/00007890-198207000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Powles RL, Clink HM, Spence D, et al. Cyclosporin A to prevent graft-versus-host disease in man after allogeneic bone-marrow transplantation. Lancet. 1980;1:327. doi: 10.1016/s0140-6736(80)90881-8. [DOI] [PubMed] [Google Scholar]
- 20.Kirkman RL, Lear PA, Madara JL, et al. Small intestine transplantation in the rat: immunology and function. Surgery. 1984;96:280. [PubMed] [Google Scholar]
- 21.Hatcher PA, Deaton DH, Bollinger RR. Transplantation of the entire small bowel in inbred rats using cyclosporine. Transplantation. 1987;43:478. doi: 10.1097/00007890-198704000-00004. [DOI] [PubMed] [Google Scholar]
- 22.D’Souza MJG. Effect of immunosuppressants cyclosporine and prednisolone on drug disposition [Dissertation] University of Pittsburgh; 1987. p. 69. [Google Scholar]
- 23.Cerottini JC, Nordin AA, Brunner KT. Cellular and humoral response to transplantation antigens: I. Development of alloantibody forming cells and cytotoxic lymphocytes in the graft-versus-host reaction. J Exp Med. 1971;134:553. doi: 10.1084/jem.134.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant D, Zhong R, Gunn H, et al. Graft-versus-host reactions induced by intestinal transplantation in the rat produce host immunosuppression. Transplant Proc. 1988;20:1279. [PubMed] [Google Scholar]
- 25.Lillehei RC, Longerbeam JK, Goott B, et al. Gastrointestinal transplantation. Surg Clin North Am. 1962;42:1191. doi: 10.1016/s0039-6109(16)36786-x. [DOI] [PubMed] [Google Scholar]
- 26.Cohen Z, MacGregor AB, Moore KTH, et al. Canine small bowel transplantation—a study of immunologic responses. Arch Surg. 1976;248 doi: 10.1001/archsurg.1976.01360210042008. [DOI] [PubMed] [Google Scholar]
- 27.Nalesnik MA, Todo S, Murase N, et al. Toxicology of FK-506 in the Lewis rat. Transplant Proc. 1987;29:89. [PMC free article] [PubMed] [Google Scholar]
- 28.Ochiai T, Hamaguchi K, Isono K. Histopathologic studies in renal transplant recipient dogs receiving treatment with FK-506. Transplant Proc. 1987;19:93. [PubMed] [Google Scholar]
- 29.Thiru S, Collier D St. J, Calne R. Pathologic studies in canine and baboon renal allograft recipients immunosuppressed with FK-506. Transplant Proc. 1987;19:98. [PubMed] [Google Scholar]