Abstract
Isocitrate dehydrogenase 1 (IDH1) and isocitrate dehydrogenase 2 (IDH2) are enzymes which convert isocitrate to α-ketoglutarate while reducing nicotinamide adenine dinucleotide phosphate (NADP+ to NADPH). IDH1/2 were recently identified as mutated in a large percentage of progressive gliomas. These mutations occur at IDH1R132 or the homologous IDH2R172. Melanomas share some genetic features with IDH1/2-mutated gliomas, such as frequent TP53 mutation. We sought to test whether melanoma is associated with IDH1/2 mutations. 78 human melanoma samples were analyzed for IDH1R132 and IDH2R172 mutation status. A somatic, heterozygous IDH1 c.C394T (p.R132C) mutation was identified in one human melanoma metastasis to the lung. Having identified this mutation in one metastasis, we sought to test the hypothesis that certain selective pressures in the brain environment may specifically favor the cell growth or survival of tumor cells with mutations in IDH1/2, regardless of primary tumor site. To address this, we analyzed IDH1R132 and IDH2R172 mutation status 53 metastatic brain tumors, including 9 melanoma metastases. Results revealed no mutations in any samples. This lack of mutations would suggest that mutations in IDH1R132 or IDH2R172 may be necessary for the formation of tumors in a cell-lineage dependent manner, with a particularly strong selective pressure for mutations in progressive gliomas; this also suggests the lack of a particular selective pressure for growth in brain tissue in general. Studies on the cell-lineages of tumors with IDH1/2 mutations may help clarify the role of these mutations in the development of brain tumors.
Keywords: isocitrate dehydrogenase 1, isocitrate dehydrogenase 2, melanoma, brain tumors metastases
Introduction
Isocitrate dehydrogenase 1 (IDH1) and isocitrate dehydrogenase 2 (IDH2), which convert isocitrate to α-ketoglutarate while reducing nicotinamide adenine dinucleotide phosphate (NADP+ to NADPH), were identified as mutated in a large percentage of progressive gliomas and acute myeloid leukemias [1,2,3,4,5]. These mutations occur at the R132 residue in IDH1 or the homologous R172 residue in IDH2. Melanomas share some genetic features with IDH1/2-mutated gliomas, such as frequent TP53 mutation [6]. Previously, no IDH1R132 mutations were found in a group of 23 melanomas [7], and to our knowledge, melanoma has not been analyzed for IDH2R172 mutations. We sought to test whether melanoma is associated with IDH1/2 mutations by analyzing a larger group of samples. In addition, given the finding of high IDH1/2 mutation frequency (80%) in progressive gliomas, we sought to test the hypothesis that certain selective pressures in the brain environment may favor cell growth/survival of tumor cells with mutations in IDH1/2. Therefore, to address whether mutation of IDH1/2 might be required for the development of brain metastases from non-primary central nervous system (CNS) tumors, we sequenced IDH1132 and IDH2R172 in 53 metastatic brain tumors, including melanomas.
Materials and Methods
IDH1/2 gene mutation status was first analyzed in a panel of cell lines derived from human non-CNS metastatic melanoma tumor resections, paired with pheresis-collected peripheral blood mononuclear cells from 78 patients enrolled in Institutional Review Board-approved clinical trials at the Surgery Branch of the National Cancer Institute at the United States National Institutes of Health. Pathology-confirmed melanoma cell lines were derived from mechanically or enzymatically dispersed tumor cells, was subsequently analyzed in a panel of 53 tumor resections from metastases to the brain. Genomic DNA was isolated from frozen tumor tissue samples obtained from the Tissue Bank at the Preston Robert Tisch Brain Tumor Center at Duke University. In both cases, genomic DNA was isolated using the DNeasy Blood & Tissue kit (Qiagen, Valencia, CA, USA). PCR and sequencing primers were designed using Primer 3 (http://www-genome.wi.mit.edu/cgibin/primer/primer3_www.cgi) and synthesized by Invitrogen (Carlsbad, CA, USA) and Integrated DNA Technologies (Coralville, IA, USA). PCR primers were designed to amplify the selected IDH1 and IDH2 exons. PCR products were 300–500 bp in length. PCR primers were designed to amplify the region surrounding the commonly mutated codons in both IDH1 and IDH2. In one melanoma sample, all coding exons of TP53, CDKN2A, and CDKN2B, as well as hotspot exons of BRAF and NRas, were sequenced using these methods.
Results and Discussion
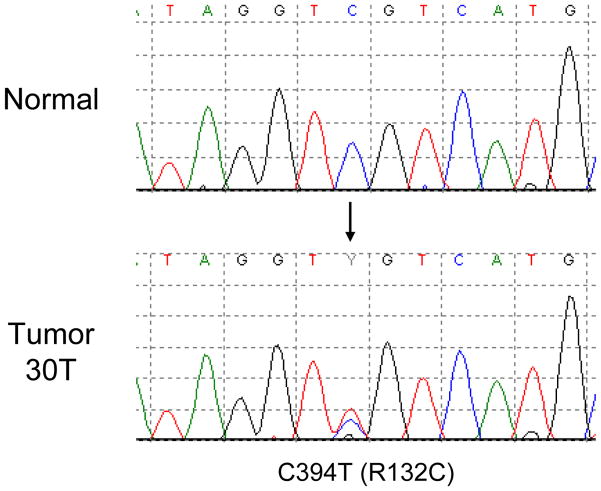
Of 78 human melanoma samples analyzed for IDH1R132 and IDH2R172 mutation status, a somatic, heterozygous IDH1 c.C394T (p.R132C) mutation was identified in tissue derived from a melanoma lung metastasis from a 53 year old female (Figure 1). No IDH2R172 mutations were detected in any of these samples. In the IDH1-mutated sample, we sequenced regions of BRAF, NRas, TP53, and CDKN2A/CDKN2B to identify any changes in genes that are commonly altered in melanoma. We found a BRAF c.T1799A (p.V600E) mutation, while NRas, TP53, and CDKN2A/CDKN2B were unaltered in this sample.
Figure 1.
Sequencing of melanoma metastases identified IDH1 R132C mutation in a melanoma cell line derived from a melanoma lung metastasis.
IDH1/2 mutations are frequent in progressive gliomas, but are very rare in other cancers besides acute myelogenous leukemia. To date, mutations in IDH1 have been identified only in one B-cell acute lymphoblastic leukemia [8], two prostate cancers [8], and one colorectal cancer [9]. Noting the presence of mutations in this small fraction of other cancers, we postulated two hypotheses. In the first hypothesis, mutations in IDH1/2 may be cell-lineage dependent, and thus only occur in the cell-of-origin for a specific subset of tumors, including progressive gliomas. In the second hypothesis, a tissue-dependent selective pressure for growth in brain tissue may select for frequent mutations in IDH1/2. If the second hypothesis is correct, we should be able to identify the presence of relatively frequent mutations in IDH1/2 in non-primary CNS metastases to the brain. Therefore, we analyzed IDH1R132 and IDH2R172 mutation status in a set of 9 melanoma metastases to the brain and an additional panel of 44 metastases of other tumor types to the brain, including lung, breast, renal, uterine, ovarian, esophageal, and urothelial cancer metastases. Results revealed no mutations regardless of tumor type, consistent with a previous small-scale study looking at colon cancer metastases to the brain [10].
Here, we present the first identification of a mutation in IDH1 or IDH2 in melanoma. These results have important clinical implications regarding the role of mutations in IDH1/2 in the development of tumors. Mutations in IDH1 have now been identified in gliomas, leukemias, prostate cancer, colorectal cancers, and melanomas, suggesting the possibility of a commonly altered pathway that may prove advantageous to the formation of tumors in all these cell types. The lack of mutations in a panel of non-primary CNS metastases to the brain would suggest that mutations in IDH1R132 or IDH2R712 are not necessarily required specifically for growth in brain tissue. The low frequency of mutations in IDH1R132 and IDH2R172 in primary glioblastomas, which grow extremely aggressively in brain tissue, further supports this. This leads to the conclusion that mutations in these genes may be necessary for formation of tumors in a cell-lineage dependent manner, with a particularly strong selective pressure for mutations in progressive gliomas and acute myelogenous leukemias. Studies focusing on the cell-lineages from which tumors with IDH1 or IDH2 mutations develop may help to elucidate the role of these mutations in cancer pathogenesis.
Research Highlights.
An IDH1R132C mutation was identified in a human melanoma metastasis to the lung.
IDH1/2 mutations were not identified in non-CNS tumor metastases to the brain.
Acknowledgments
We thank Kristin E. Yates for technical assistance. This work is supported by the Intramural Research Programs of the National Human Genome Research Institute and the National Cancer Institute, National Institutes of Health, USA.
Abbreviations
- CNS
central nervous system
- IDH1
isocitrate dehydrogenase 1
- IDH2
isocitrate dehydrogenase 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Giselle Y. Lopez, Email: giselle.lopez@duke.edu.
Zachary J. Reitman, Email: zachary.reitman@duke.edu.
David Solomon, Email: das67@georgetown.edu.
Todd Waldman, Email: waldmant@georgetown.edu.
Darell D. Bigner, Email: bigne001@mc.duke.edu.
Roger E. McLendon, Email: roger.mclendon@duke.edu.
Yardena Samuels, Email: samuelsy@mail.nih.gov.
Hai Yan, Email: yan00002@mc.duke.edu.
References
- 1.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins G, Friedman HS, Friedman AH, Reardon DA, Herndon JE, Kinzler KW, Velculescu VE, Vogelstein B, Bigner D. IDH1 and IDH2 Mutations in Gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross S, Cairns RA, Minden MD, Driggers EM, Bittinger MA, Jang HG, Sasaki M, Jin S, Schenkein DP, Su SM, Dang L, Fantin VR, Mak TW. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med. 2010;207:339–344. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green A, Beer P. Somatic mutations of IDH1 and IDH2 in the leukemic transformation of myeloproliferative neoplasms. N Engl J Med. 2010;362:369–370. doi: 10.1056/NEJMc0910063. [DOI] [PubMed] [Google Scholar]
- 4.Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, Cross JR, Fantin VR, Hedvat CV, Perl AE, Rabinowitz JD, Carroll M, Su SM, Sharp KA, Levine RL, Thompson CB. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, Koboldt DC, Fulton RS, Delehaunty KD, McGrath SD, Fulton LA, Locke DP, Magrini VJ, Abbott RM, Vickery TL, Reed JS, Robinson JS, Wylie T, Smith SM, Carmichael L, Eldred JM, Harris CC, Walker J, Peck JB, Du F, Dukes AF, Sanderson GE, Brummett AM, Clark E, McMichael JF, Meyer RJ, Schindler JK, Pohl CS, Wallis JW, Shi X, Lin L, Schmidt H, Tang Y, Haipek C, Wiechert ME, Ivy JV, Kalicki J, Elliott G, Ries RE, Payton JE, Westervelt P, Tomasson MH, Watson MA, Baty J, Heath S, Shannon WD, Nagarajan R, Link DC, Walter MJ, Graubert TA, DiPersio JF, Wilson RK, Ley TJ. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibrahim N, Haluska FG. Molecular pathogenesis of cutaneous melanocytic neoplasms. Annu Rev Pathol. 2009;4:551–579. doi: 10.1146/annurev.pathol.3.121806.151541. [DOI] [PubMed] [Google Scholar]
- 7.Bleeker FE, Lamba S, Leenstra S, Troost D, Hulsebos T, Vandertop WP, Frattini M, Molinari F, Knowles M, Cerrato A, Rodolfo M, Scarpa A, Felicioni L, Buttitta F, Malatesta S, Marchetti A, Bardelli A. IDH1 mutations at residue p.R132 (IDH1(R132)) occur frequently in high-grade gliomas but not in other solid tumors. Hum Mutat. 2009;30:7–11. doi: 10.1002/humu.20937. [DOI] [PubMed] [Google Scholar]
- 8.Kang MR, Kim MS, Oh JE, Kim YR, Song SY, Seo SI, Lee JY, Yoo NJ, Lee SH. Mutational analysis of IDH1 codon 132 in glioblastomas and other common cancers. Int J Cancer. 2009;125:353–355. doi: 10.1002/ijc.24379. [DOI] [PubMed] [Google Scholar]
- 9.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 10.Holdhoff M, Parsons DW, Diaz LA., Jr Mutations of IDH1 and IDH2 are not detected in brain metastases of colorectal cancer. J Neurooncol. 2009;94:297. doi: 10.1007/s11060-009-9855-y. [DOI] [PubMed] [Google Scholar]