Summary
Action potentials initiate in the axon initial segment (AIS), a specialized compartment enriched with Na+ and K+ channels. Recently, we found that T- and R-type Ca2+ channels are concentrated in the AIS, where they contribute to local subthreshold membrane depolarization and thereby influence action potential initiation. While periods of high-frequency activity can alter availability of AIS voltage-gated channels, mechanisms for long-term modulation of AIS channel function remain unknown. Here, we examined the regulatory pathways that control AIS Ca2+ channel activity in brainstem interneurons. T-type Ca2+ channels were downregulated by dopamine receptor activation acting via protein kinase C, which in turn reduced neuronal output. These effects occurred without altering AIS Na+ or somatodendritic T-type channel activity and could be mediated by endogenous dopamine sources present in the auditory brainstem. This pathway represents a new mechanism to inhibit neurons by specifically regulating Ca2+ channels directly involved in action potential initiation.
Keywords: action potential, axon initial segment, calcium channel, dopamine, neuromodulation
Introduction
The axon initial segment (AIS) has the lowest threshold for action potential (AP) initiation due to its high Na+ channel density (Kole and Stuart, 2008), and it is therefore the site of AP initiation in most neurons (Coombs et al., 1957; Khaliq and Raman, 2006; Kress et al., 2008; Martina et al., 2000; Palmer and Stuart, 2006; Schmidt-Hieber et al., 2008; Shu et al., 2007; Stuart et al., 1997). Classically, the AIS was thought to be enriched only in Na+ and K+ channels (Bean, 2007; Kress and Mennerick, 2009). Using Ca2+ imaging, we recently discovered that the AIS may also contain T-type (CaV3) and R-type (CaV2.3) Ca2+ channels. AIS Ca2+ transients have been observed with Ca2+-sensitive dyes in a variety of neuronal classes, including brainstem interneurons, cortical pyramidal neurons and cerebellar Purkinje neurons (Bender and Trussell, 2009; Callewaert et al., 1996; Luscher et al., 1996; Schiller et al., 1995), indicating that AIS Ca2+ channels may be a common feature of many neuronal classes. In concert with subthreshold Na+ channel activation, AIS Ca2+ channels contribute to local subthreshold membrane depolarization and therefore determine if and when an excitatory synaptic input evokes an AP (Bender and Trussell, 2009).
The biophysical properties of voltage-gated channels in the AIS control AP initiation. Availability of Na+ and K+ channels depends on recent activity or membrane potential, leading to corresponding alterations in AP waveforms and threshold (Azouz and Gray, 2000; Goldberg et al., 2008; Hu et al., 2009; Kole et al., 2007). T-type Ca2+ channels, which are thought to contribute to the generation of AP bursts, inactivate as a neuron depolarizes, altering neuronal firing patterns (Kim and Trussell, 2007; Uebachs et al., 2006). These changes occur on the time scale of seconds; however, it remains unknown what mechanisms exist for long-term control of AIS excitability through second-messenger-dependent modification of constituent channels.
Here, we examined regulatory mechanisms that control the excitability of dorsal cochlear nucleus (DCN) cartwheel interneurons. Cartwheel cells are ideal for studying mechanisms of AP initiation because they intrinsically fire APs in a variety of ways, including bursts and single spikes, and the underlying ion channels that determine AP output in both the somatodendritic compartment and the AIS are relatively well understood (Bender and Trussell, 2009; Kim and Trussell, 2007). In these cells, we found that dopamine altered neuronal output by modification of T-type Ca2+ channels involved in AP initiation. Dopamine receptor activation, either by exogenous or endogenous agonists, activated protein kinase C (PKC), leading to inhibition of AIS T-type channels. This pathway was specific for AIS T-type channels; dopamine receptor activation had no effect on intrinsic cell properties, somatodendritic T-type Ca2+ channels, AIS Na+ influx, or whole-cell K+ and persistent Na+ currents. Similar to direct antagonist block of AIS Ca2+ channels (Bender and Trussell, 2009), activation of this pathway ultimately reduced the AP output of these auditory interneurons. Thus, these data are the first direct evidence of ion channel modulation in the AIS, and suggest that dopaminergic signaling mediates fine-scale adjustments of neuronal output by controlling the activity of AIS Ca2+ channels.
Results
Axon initial segment Ca2+ channels are regulated by dopamine
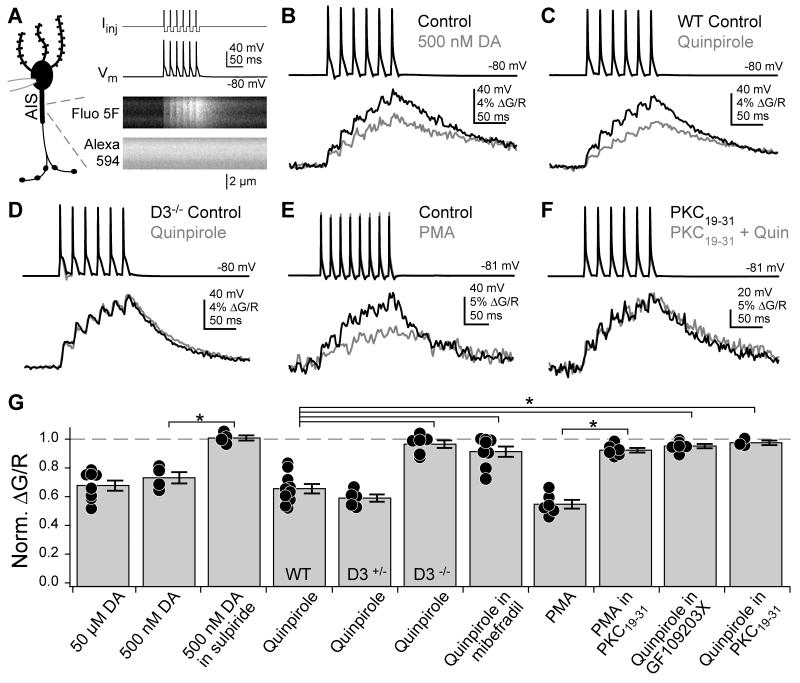
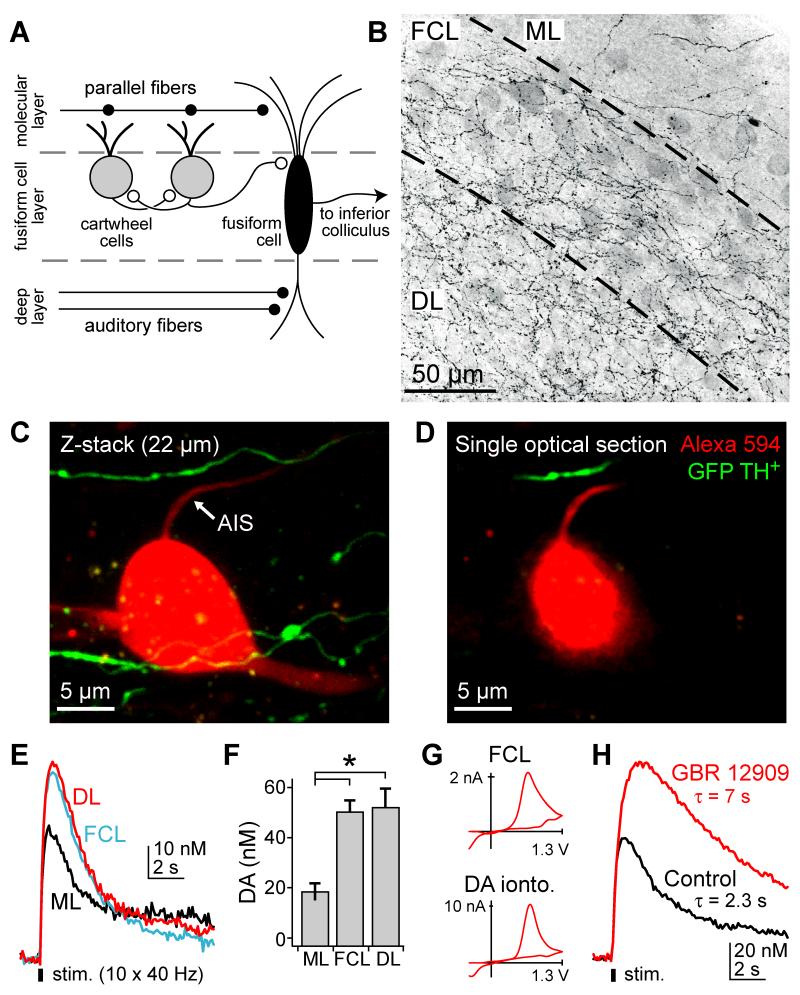
Whole-cell recordings were made from DCN cartwheel cells in acute brain slices prepared from 16-24 day old mice. Cells were filled via patch pipettes with the red volume marker Alexa 594 and the green Ca2+-sensitive fluorophore Fluo-5F. Morphology and Ca2+ activity were visualized with a 2-photon microscope. To examine AIS Ca2+ channel modulation, we evoked AP trains with somatic current injection and imaged concomitant Ca2+ transients 14-18 μm from the axon hillock (Fig. 1A). Using these techniques, we showed previously that Ca2+ transients imaged at these locations were blocked by the T- and R-type Ca2+ channel antagonists mibefradil and SNX-482 (Bender and Trussell, 2009). AIS Ca2+ transients were also reduced by 50 μM Ni2+, suggesting that the AIS contains the Ni2+-sensitive CaV3.2 T-channel subunit (Lee et al., 1999). These channels are common targets for modulation via dopaminergic pathways (Chemin et al., 2006; Perez-Reyes, 2003) and indeed we found that dopamine (500 nM – 50 μM) decreased AIS Ca2+ transients by 27 ± 4 and 32 ± 4% (n = 4 and 8, respectively; Fig. 1B, G).
Fig. 1. Dopamine reduces AIS Ca2+ transients in cartwheel cells.
(A) Left: Schematic of recording/imaging configuration. Whole-cell recordings were made from cartwheel cell somata, and Ca2+ transients were imaged in the AIS. Top right: AP trains were evoked by somatic current injection followed by negative current steps to ensure that only 1 AP was evoked per step. Bottom right: corresponding Fluo-5F (Ca2+) and Alexa 594 (morphology) signals in AIS. (B-F) AP train-evoked Ca2+ influx in the AIS. Shades correspond to drug conditions to right of AP trains. All Ca2+ transients were computed as the change in green fluorescence (G, Fluo-5F) over red fluorescence (R, Alexa). DA: dopamine. WT: wild type.
(G) Summary of pharmacological effects on AIS Ca2+. Values normalized to baseline ΔG/R amplitudes. For conditions expressed as “Drug X in Drug Y”, normalized ΔG/R amplitudes reflect any changes mediated by Drug X relative to a baseline period in Drug Y. Dots are single cells. Error bars are SEM. Asterisk: p < 0.0001.
Dopamine can act through a variety of receptors, broadly grouped into dopamine receptor type 1 (D1R and D5R) and type 2 families (D2R, D3R, and D4R). Dopamine-mediated effects on AIS Ca2+ transients were blocked by the D2-family specific antagonist sulpiride (Fig. 1G; 200 nM, normalized ΔG/R: 1.01 ± 0.02, n = 4, p < 0.0001) and mimicked by the D2-family agonist quinpirole (Fig. 1C, G; 1 μM, normalized ΔG/R: 0.66 ± 0.03, n = 10), suggesting that AIS Ca2+ channels were regulated by D2, D3, or D4 receptors. In situ hybridization labeling for D2-family mRNA shows D3R mRNA in the DCN, but not D2R or D4R mRNA (Bouthenet et al., 1991; Heintz, 2004; Lein et al., 2007). Consistent with these results, quinpirole did not reduce AIS Ca2+ transients in D3R knockout mice (Fig. 1D, G; normalized ΔG/R: 0.96 ± 0.03, n = 6 cells, 3 animals, p < 0.001 vs wild type). D3R heterozygotes displayed a wild type phenotype (normalized ΔG/R: 0.59 ± 0.03, n = 5, p = 0.2 vs. wild type).
Cartwheel cells express both T- and R-type Ca2+ channels in the AIS. These channels can be blocked by mibefradil, which acts on T- and R-type channels, and SNX-482, which acts on R-type channels. We observed previously that SNX-482-sensitive AIS Ca2+ transients persist in the presence of mibefradil, suggesting that mibefradil largely blocks T-type channels in the AIS (Bender and Trussell, 2009). Therefore, to determine whether T- or R-type channels were modulated by dopamine, we isolated AIS Ca2+ influx through R-type channels by imaging in the presence of 3 μM mibefradil. Consistent with previous results, AIS Ca2+ transients were 50.2% smaller in mibefradil compared to Ca2+ transients imaged in other cells in control conditions (n = 8 in mibefradil, 28 control, p < 0.0001, unpaired t-test). Quinpirole had a small effect on these Ca2+ transients (Fig. 1G; normalized ΔG/R: 0.91 ± 0.04, p < 0.0001 vs. quinpirole without mibefradil). This could be due to modulation of either R-type channels or a fraction of T-type channels that was not blocked by 3 μM mibefradil (McDonough and Bean, 1998). Overall, these results suggest that dopamine primarily affected T-type channels.
D3R signaling may decrease AIS Ca2+ influx by activating protein kinase pathways that phosphorylate AIS T-type Ca2+ channels (Perez-Reyes, 2003; Ron et al., 1999; Schroeder et al., 1990). We found that the effects of quinpirole were mimicked by the PKC activator phorbol 12-myristate 13-acetate (PMA, 10 μM, normalized ΔG/R: 0.55 ± 0.03, n = 6, Fig. 1E, G), but not by the PKA activator forskolin (50 μM, normalized ΔG/R: 1.04 ± 0.06, n = 2). Further, quinpirole-mediated effects on AIS Ca2+ were blocked by the PKC inhibitor GF109203X (1 μM) and the PKC inhibitor peptide PKC19-31 (5 μM, in pipette) (Fig. 1F, G; quin. + GF: 0.95 ± 0.02, n = 6; quin. + PKC19-31: 0.98 ± 0.02, n = 3; PMA +PKC19-31: 0.92 ± 0.02, n = 6; p < 0.0001 for each blocker vs. quinpirole). Thus, AIS Ca2+ channels were modulated by dopamine signaling via D3R-dependent activation of PKC.
D3R-PKC pathway is specific for AIS T-type Ca2+ channels
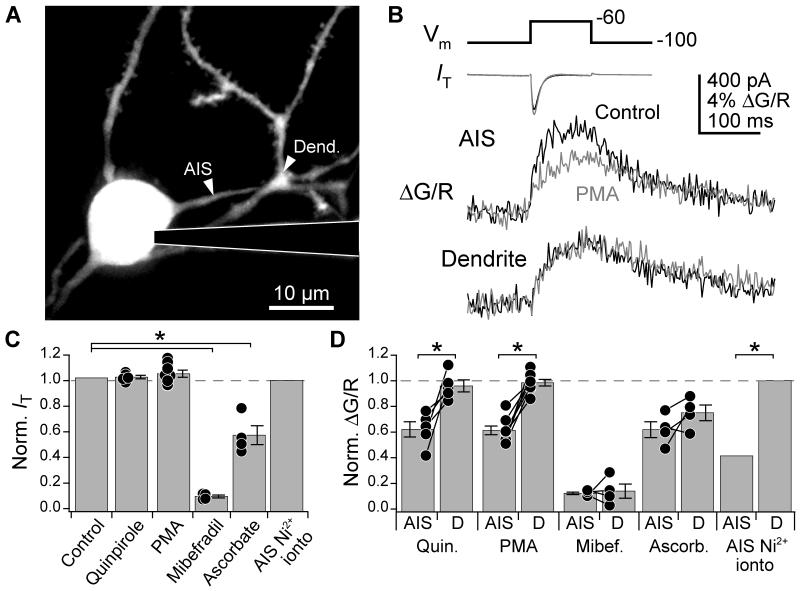
Pharmacological results suggest that D3R-PKC pathway modulates T-type channels in the AIS. To confirm these results, we isolated T-currents (IT) in voltage-clamp using a Cs+-based internal solution and an external solution containing TTX, Cs+, NBQX, strychnine, and SR95531. IT was isolated from other Ca2+ channel currents with voltage steps from −100 to −60 mV, and whole cell IT was recorded while simultaneously imaging Ca2+ transients in the AIS and a neighboring dendritic branch (Fig. 2A, B). This voltage step protocol evoked −493 ± 46 pA IT in cartwheel cells (n = 28). Mibefradil blocked IT by 90.7 ± 1.1% and AIS and dendritic Ca2+ transients by 88.2 ± 1.1% and 86.5 ± 5.5%, respectively (n = 4 cells) (10 μM, required for block from −100 mV; McDonough and Bean, 1998). Further, the CaV3.2 selective antagonist ascorbate (300 μM, Nelson et al., 2007) decreased AIS and dendritic Ca2+ transients, as well as IT (Fig. 2C, D; AIS normalized ΔG/R: 0.62 ± 0.06, dendrite: 0.75 ± 0.06; IT: 0.57 ± 0.07, n = 4). These data are consistent with previous results with 50 μM Ni2+ application (Bender and Trussell, 2009), and suggest that cartwheel cells express CaV3.2 channels in the AIS and dendrite.
Fig. 2. D3R-PKC pathway is specific for T-type channels localized to the AIS.
(A) 2-photon z-stack of cartwheel cell. Arrowheads: sites of Ca2+ transients detailed in (B). Left in AIS, right in dendrite.
(B) Voltage steps from −100 to −60 mV evoked a whole cell T-current (IT) and Ca2+ transients in the AIS and dendrite. Black: baseline, grey: in PMA.
(C-D) IT (C) and normalized ΔG/R (D) following activation of D3R-PKC pathway or block of T-type channels. Control currents were calculated as the relative IT over a time course similar to that allowed for drug wash-in (12 min). Ni2+ was iontophoresed locally to the AIS. All other drugs were added to the recording solution. Dots are single cells. Lines connecting dots in (D) link recordings made in the same cell in the AIS and dendrite. Dendritic recordings are denoted with a “D”. Bars are SEM. Asterisk: p < 0.05.
When the D3R-PKC pathway was activated with quinpirole or PMA, Ca2+ transients in the AIS were reduced, but surprisingly, Ca2+ transients in the dendrites were not (Fig. 2B, D; e.g., quinpirole AIS normalized ΔG/R: 0.62 ± 0.06, dendrite: 0.96 ± 0.05, n = 5, p < 0.02, paired t-test). IT was also unchanged (Fig. 2C; quinpirole normalized IT: 1.03 ± 0.01, n = 5, PMA: 1.05 ± 0.03, n = 8; p > 0.5 vs. control for each), probably because the contribution of dendritic T-type channels dominated the whole-cell IT; however, it was possible that the reduction in IT due to AIS Ca2+ channel block was small compared to the variance in IT observed over the course of an experiment. To test whether acute blockade of AIS Ca2+ channels could affect whole cell IT, we selectively blocked AIS Ca2+ channels with local Ni2+ iontophoresis (Bender and Trussell, 2009). In these experiments, control and Ni2+-paired currents were interleaved, eliminating time-dependent changes to IT. Local Ni2+ application reduced AIS Ca2+ transients more effectively than the D3R-PKC pathway (Fig. 2D; normalized ΔG/R: 0.41 ± 0.08, n = 3, p < 0.01 vs. quin. and PMA), but still whole cell IT was unaffected (Fig. 2C; normalized IT: 1.00 ± 0.002, p > 0.3 vs. quinpirole or PMA). Similar results were obtained when the iontophoretic pipette was moved to an isolated dendritic branch (data not shown), confirming that local block of a small fraction of a cell’s T-type channels cannot be resolved in whole-cell current recorded in the soma. Thus, dopamine regulates T-type Ca2+ channels localized to the AIS, not the dendrite.
Dopamine activation reduces neuronal output
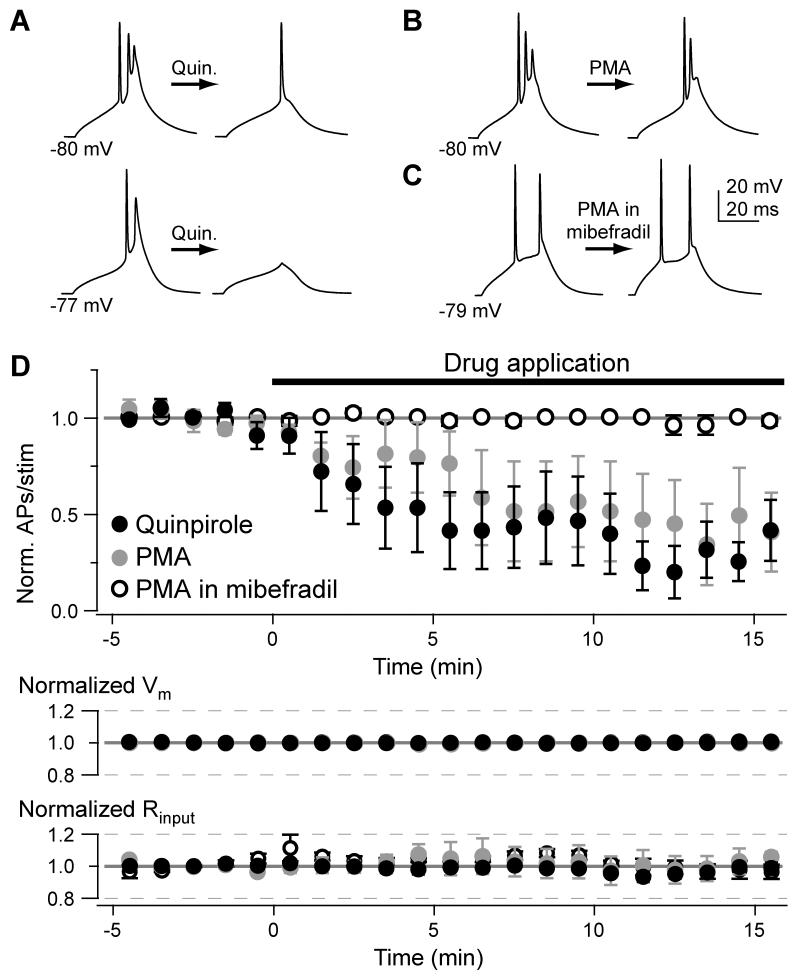
T-type channels contribute to neuronal excitation and are activated at relatively negative membrane potentials. As a result, partial blockade of T-type channels with local mibefradil or Ni2+ application results in elevation of AP thresholds. Indeed, APs evoked by a given synaptic input are either delayed or never initiated under these conditions (Bender and Trussell, 2009). Since the D3R-PKC pathway reduces AIS Ca2+ influx, its activation should have similar effects on neuronal output. To test this, we evoked APs with 30-ms current pulses just suprathreshold for bursts of 2-3 APs. Cartwheel cells reliably evoked bursts throughout whole-cell recordings (control recordings: 2.6 ± 0.3 APs/stim, 0-5 min; 2.5 ± 0.4 APs/stim, 15-20 min, n = 5), but when the D3R-PKC pathway was activated with either quinpirole or PMA, the number of APs evoked per stimulus was reduced by at least 50% (Fig. 3; PMA: normalized APs/stim: 0.50 ± 0.18, n = 5; quinpirole: 0.35 ± 0.17, n = 5, p < 0.02 vs. control for both).
Fig. 3. Dopamine reduces action potential output.
(A) AP bursts evoked with somatic current injection during a baseline period (left) and after quinpirole application (right). Current injection was not altered over the course of an experiment.
(B) Same as (A), but with PMA.
(C) Same as (B), but in the presence of mibefradil throughout the recording.
(D) Time course of AP inhibition by quinpirole and PMA. Data were normalized to the baseline number of APs evoked per stimulus. Bars are SEM.
These effects were not due to changes in intrinsic membrane properties—resting membrane potential and input resistance were unaffected by PMA or quinpirole (Fig. 3; e.g., baseline Vm: −80.1 ± 0.6 mV, PMA: −80.0 ± 0.6, p = 0.6; baseline Rin: 55.7 ± 8.3 MΩ, PMA: 54.1 ± 7.5, p = 0.36, paired t-test). Rather, the reduced excitability was mediated by inhibition of T-type Ca2+ channels. When T-type channels were blocked with mibefradil (3 μM), cartwheel cells did not fire characteristic AP bursts (Kim and Trussell, 2007). When the stimulus intensity was then increased, the AIS could be depolarized enough to evoke 2 APs with a 30-ms current injection (Fig. 3C; stim. intensity: PMA: 181 ± 21 pA; PMA + mibefradil: 327 ± 38 pA). Under these conditions, PMA did not affect AP output (normalized APs/stim: 0.92 ± 0.07, n = 4, p = 0.86 vs. control), indicating that PMA reduced AP initiation through its actions on T-type channels.
To quantify the elevation of spike threshold by the D3R-PKC pathway, we increased the stimulation intensity by 20 pA to ensure that the first AP in a burst would always be present, even in PMA or quinpirole. Relative to control experiments, AP threshold, as detected in phase plane plots, depolarized by 1.8 mV in quinpirole and by 1.5 mV in PMA (Supplemental Fig. 1; p < 0.05 for both). PMA did not affect threshold after blockade of T-type channels with mibefradil (relative depolarization: 0.2 ± 0.4 mV, p > 0.7 vs. control), confirming that these effects were mediated by changes to T-type channel activity.
D3R-PKC pathway does not affect Na+ or K+ channels
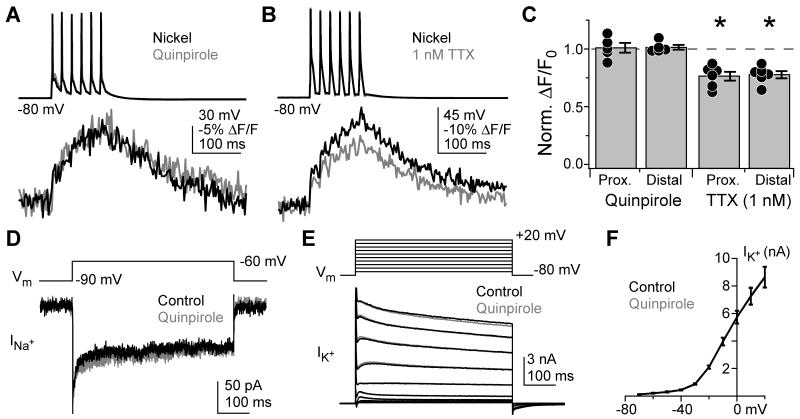
These results indicate that AIS T-type channels are modulated by the D3R-PKC pathway; however, they do not exclude actions on other AIS ion channels. For example, dopaminergic suppression of Na+ channel function might have led to reduced activation of voltage-gated Ca2+ channels in the AIS. Indeed, dopaminergic signaling is reported to alter Na+ channel activity, although it is unclear if this modulation extends to AIS Na+ channels (Cantrell and Catterall, 2001; Dai et al., 2009; Maurice et al., 2004). Therefore, we determined whether quinpirole altered AIS Na+ channel activity by imaging Na+ with SBFI (1 mM). In these experiments, AIS Ca2+ channels were blocked with 100 μM Ni2+ to avoid indirect effects of Ca2+ channel modulation on Na+ influx. In many neurons, Na+ channel subtype expression varies with distance from the axon hillock. Typically, NaV1.1 or 1.2 channels are expressed in the proximal AIS whereas NaV1.6 channels are more distal (Hu et al., 2009; Lorincz and Nusser, 2008). As these channels may be subject to different regulatory pathways, we imaged AP train-evoked Na+ transients at two locations (proximal: 9.7 ± 1.4 μm from the hillock; distal: 24.2 ± 2.2 μm, n = 5). Na+ transients from individual APs were clearly resolved with SBFI, and were not altered by 1 μM quinpirole (Fig. 4A, C; proximal normalized ΔF/F0: 1.01 ± 0.04, distal: 1.02 ± 0.02, n = 5, p > 0.5 for each location, one sample t-test).
Fig. 4. D3R signaling does not affect Na+ or K+.
(A) AP train-evoked Na+ influx in the AIS, imaged with SBFI, before (black) and after 1 μM quinpirole (grey). Na+ transients were computed as the change in SBFI fluorescence over baseline.
(B) AP train-evoked Na+ influx in the AIS before (black) and after 1 nM TTX (grey).
(C) Summary of pharmacological effects on AIS Na+. Values normalized to baseline ΔF/F0 amplitudes. Dots are single cells. Error bars are SEM. Asterisk: p < 0.01.
(D) Persistent Na+ currents before (black) and after quinpirole (grey).
(E) K+ currents before (black) and after quinpirole (grey).
(F) K+ current vs. step voltage. Black: baseline. Grey: quinpirole. Data from each condition superimpose. Bars are SEM.
To ensure that our imaging system could detect changes in Na+ influx that might underlie D3R-PKC pathway-mediated reductions in neuronal output, we first mimicked the effects of quinpirole and PMA by directly blocking Na+ channels with tetrodotoxin (TTX). We found that partial block of Na+ channels with 1 nM TTX was sufficient to reduce AP output to 47.1 ± 12.8% of baseline conditions (Supplemental Fig. 2; n = 7, p > 0.7 vs. either quinpirole or PMA). Then, we again imaged Na+ transients in the proximal and distal AIS with SBFI. In contrast to quinpirole, 1 nM TTX reduced AP-evoked Na+ transients in both regions (Fig. 4B, C; proximal normalized ΔF/F0: 0.76 ± 0.04, distal: 0.78 ± 0.03, n = 6, p < 0.01 for both, one sample t-test). This reduction in SBFI signal was comparable to the block of Na+ currents observed by voltage clamping NaV1.1, 1.2 and 1.6 channels in heterologous expression systems (Rosker et al., 2007), and indicate that quinpirole had no effect on AIS Na+ influx.
While transient Na+ currents cannot be clamped well in whole-cell recordings from dendritic neurons, sub-threshold, persistent Na+ currents can. Both persistent and transient Na+ currents may originate from the same Na+ channel source in the AIS (Astman et al., 2006; Fleidervish et al., 2010; Taddese and Bean, 2002). Therefore, whole-cell persistent Na+ currents provide a second, independent measure of whether quinpirole affects AIS Na+ channels. TTX-sensitive currents were isolated with voltage steps from −90 to −60 mV in the presence of K+ channel, Ca2+ channel, and ionotropic blockers (Fig. 4D; see Methods). Consistent with results obtained with SBFI, quinpirole did not alter persistent Na+ currents (normalized current: 0.99 ± 0.01, n = 6; p > 0.4, one sample t-test).
Dopaminergic signaling did not alter Vm or Rin in current clamp recordings (Fig. 3). This suggests that cartwheel cells lack dopamine-sensitive inward-rectifying K+ conductances; however, it was still unclear whether dopamine affected other K+ conductances. To address this, we examined K+ currents with voltage steps from −80 to +20 mV (10 mV increments), activating K+ conductances through the full AP voltage range (Fig. 4E, F). As with Na+ currents, quinpirole had no effect (e.g., normalized current at −40 mV: 1.03 ± 0.06; at 0 mV: 1.00 ± 0.01; n = 8, p > 0.9, repeated measures ANOVA). Thus, the D3R-PKC pathway selectively modulates AIS Ca2+ channels without affecting AIS Na+ channels, persistent Na+ currents, or K+ currents.
Dopamine signaling in the DCN
While results described above show that dopamine can alter the output of cartwheel cells, it is unclear whether dopaminergic signaling occurs in the DCN, and if so, whether endogenous dopamine could alter AIS Ca2+ channels. To examine these questions, we first determined whether axons capable of dopamine release were present in the DCN. Coronal sections were obtained from mice expressing GFP in all neurons expressing the dopamine synthetic enzyme tyrosine hyroxylase (TH). Consistent with previous reports (Klepper and Herbert, 1991), TH+ axons were most dense in the fusiform and deep layers, with comparably less innervation in the molecular layer (Fig. 5B). This localization could position TH+ fibers close to cartwheel cell initial segments, since cartwheel somata are often located near the molecular/fusiform cell layer border (Fig. 5A). To determine whether TH+ fibers appose cartwheel axons, we made acute slices from TH-GFP animals and filled cartwheel cells with Alexa 594. GFP and Alexa 594 were visualized simultaneously with 2-photon microscopy (excitation wavelength: 880 nm). TH+ fibers often passed near cartwheel cell initial segments but did not overlap in 10 of 11 cells (Fig. 5C-D; shortest distance between process centers: 2.6 ± 0.6 μm, range: 0.25-6.52 μm). Given their proximity to the cartwheel AIS, these fibers are a potential source of dopamine.
Fig. 5. Monoamine anatomy and release in the DCN.
(A) Schematic DCN circuit. Excitatory synapses are represented by filled circles, inhibitory synapses by open circles. Cartwheel cell somata are typically located near molecular/fusiform cell layer border.
(B) TH+ axonal fibers in the DCN. Image is a z-stack of a 50 μm confocal series. Greyscale inverted for clarity. Dashed lines denote layer borders. ML: molecular layer, FCL: fusiform cell layer, DL: deep layer.
(C) Z-stack of cartwheel cell filled with Alexa 594 in a slice from a TH-GFP animal. Recording pipette exits cell on left. GFP-TH+ axonal fibers are in green, cartwheel cell is filled with Alexa 594 (red).
(D) Single optical section of closest apposition of TH+ fiber and cartwheel cell axon.
(E) Fast-scan cyclic voltammetric recordings vs. time in DCN layers in response to local electrical stimulation in the same layer. Data are single traces all from one slice. Onset and offset of stimulation are indicated by the black bar. Layer abbreviations are as in (B).
(F) Summary of peak dopamine amplitudes in cochlear nucleus layers. Bars are SEM. Asterisk: p < 0.001. n = 12 slices. Layer abbreviations are as in (B).
(G) Top: Representative voltammagram recorded in the fusiform layer. Bottom: voltammagram in response to exogenous dopamine delivered via iontophoresis.
(H) Effect of GBR 12909 on voltammetric recordings in the fusiform layer. Data are single traces.
We next used fast-scan cyclic voltammetry to assess the release of monoamines from the TH+ fibers in the DCN following stimulation (Heien et al., 2004). A carbon fiber recording electrode was placed serially in the center of the molecular, fusiform, and deep layers, parallel to layer borders. Release was evoked using a monopolar glass electrode placed in the same layer, with trains of 10 stimuli delivered at 40 Hz. Voltammograms recorded in the fusiform cell layer following stimulation were similar in profile to those of exogenous dopamine applied via iontophoresis (Fig. 5G). Voltammetric transients were largest in the fusiform and deep layers of the DCN and relatively small in the molecular layer (Fig. 5E, F). When reuptake was blocked with the specific dopamine transport blocker GBR 12909 (300 nM), voltammetric current increased to 158 ± 15% of baseline (Fig. 5H; n = 12, p < 0.01, paired t-test) and their decay slowed by 202 ± 29% (p < 0.01, paired t-test; baseline τ: 1.7 ± 0.2 s, range: 0.8-2.7 s, n = 12). These effects were comparable to those observed in the striatum (Chen and Rice, 2001) and they indicate that dopamine is released from TH+ fibers in the DCN.
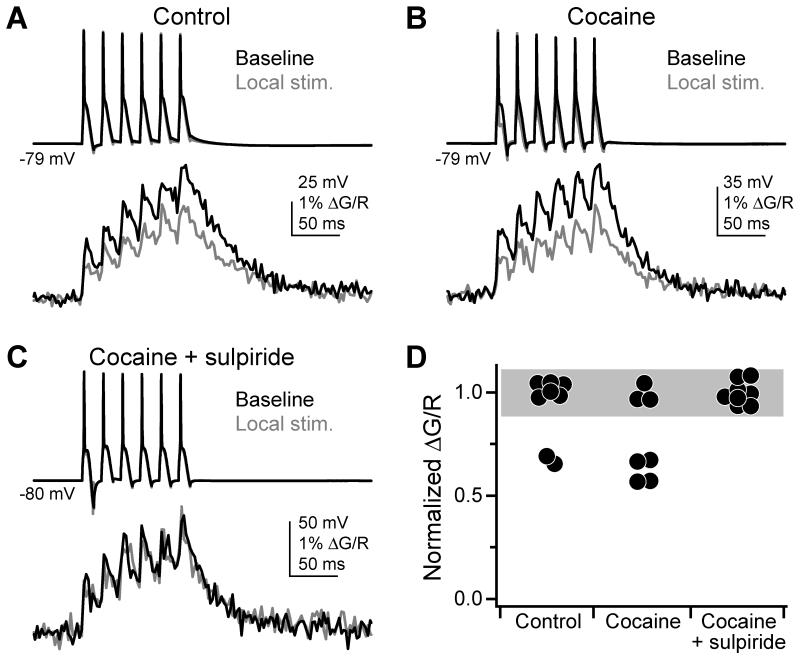
To determine if this endogenous dopamine was sufficient to alter AIS Ca2+, we assayed AP-evoked AIS Ca2+ transients before and after local stimulation of dopaminergic fibers. In these experiments, extracellular Ca2+ was maintained at 2.4 mM to match voltammetry experiments, and Ca2+ was imaged with Fluo-4FF to prevent dye saturation. Ionotropic, GABAB, and mGluR transmission was blocked throughout. Release was evoked using the same stimulation train as in voltammetry experiments (see Methods). Stimulating electrodes were placed within 30 μm of visualized cartwheel cell axons, but unfortunately we had no positive control to know whether this placement recruited TH+ fibers that were in close proximity to the AIS of the recorded cell. As a result, our findings were mixed: AIS Ca2+ transients were decreased following stimulation in 2 cells, but in 6 others, no change was observed (Fig. 6A).
Fig. 6. Electrical stimulation of dopaminergic fibers affects AIS Ca2+.
(A) AP train-evoked (top) Ca2+ transients (bottom) in the AIS, imaged with Fluo-4FF. Ca2+ transients were imaged before (black) and after (grey) dopamine release evoked with local electrical stimulation.
(B) Same as (A), but with 300 nM cocaine present throughout experiment.
(C) Same as (A), but with 300 nM cocaine and 200 nM sulpiride present throughout experiment.
(D) Summary of endogenous dopaminergic fiber stimulation experiments. Values normalized to baseline ΔG/R amplitudes. Dots are single cells. Grey bar represents 2x standard deviation of the cocaine+sulpiride condition, centered on its mean. Any decrements in Ca2+ transients below this bar were considered successes.
If this experiment was limited by our ability to place the stimulating electrode in the proper position to recruit TH+ fibers, then the success rate should improve by increasing the effective area of dopaminergic signaling. Therefore, we attenuated monamine reuptake with 300 nM cocaine. In cocaine, AIS Ca2+ transients were decreased in 4/7 cells. These effects were not observed in any of 8 cells in the presence of 200 nM sulpiride and 300 nM cocaine (Fig. 6B-D; p < 0.02, cocaine vs. cocaine+sulpiride), indicating that changes in AIS Ca2+ were indeed due to dopaminergic signaling.
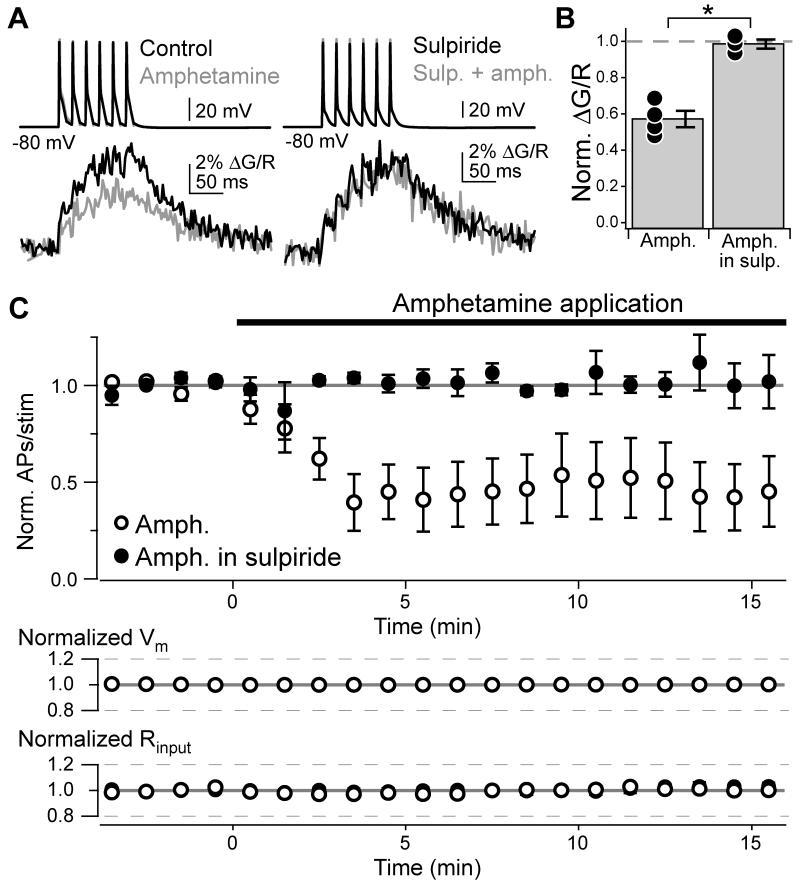
Because of the uncertainty of evoking release with local electrical stimulation, we did not test whether AP output was altered. Instead, we utilized a pharmacological approach and evoked dopamine release by reversing monoamine transporters with amphetamine (10 μM). In contrast to local electrical stimulation, amphetamine consistently decreased AIS Ca2+ transients (Fig. 7A, B; normalized ΔG/R: 0.57 ± 0.05, n = 4). Amphetamine effects were blocked by sulpiride (normalized ΔG/R: 0.99 ± 0.03, n = 3, p < 0.001, unpaired t-test). Similar to results with quinpirole and PMA, amphetamine reduced the number of APs evoked by 30-ms somatic current pulses (Fig. 7C; amphetamine: normalized APs/stim: 0.42 ± 0.15, n = 6; amphetamine in sulpiride: 1.01 ± 0.03, n = 5, p < 0.01) and raised AP threshold 1.7 ± 0.4 mV (n = 6, p < 0.02 vs. amphetamine in sulpiride; supplemental Fig. 1). Thus, these results establish that dopamine is released from TH+ fibers in the DCN and can act on cartwheel cell D3R to alter AIS Ca2+ and alter neuronal output.
Fig. 7. Amphetamine reduces AIS Ca2+ and spike output.
(A) AP train-evoked Ca2+ influx in the AIS, imaged with Fluo-5F. Colors correspond to drug conditions to right of AP trains.
(B) Summary of amphetamine effects on AIS Ca2+. Dots are single cells. Bars are SEM. Asterisk: p < 0.05.
(C) Time course of AP inhibition by amphetamine. When required, sulpiride was present throughout the recording. Data normalized to baseline number of APs evoked per stimulus. Bars are SEM.
Discussion
Our results describe a novel mechanism for inhibition of AP initiation by selective dopaminergic modulation of Ca2+ channels in the AIS. Dopamine decreased Ca2+ influx through AIS T-type channels without altering intrinsic membrane properties, allowing us to isolate the effects of AIS Ca2+ modulation on AP output. We found that lowered AIS Ca2+ current reduced the rate of local membrane depolarization, which in turn reduced activation rates of local Na+ channels (Bender and Trussell, 2009), raising AP threshold and thereby reducing AP output.
Several lines of evidence suggest that dopamine-mediated reductions in AIS Ca2+ were the result of decreased Ca2+ influx through T-type Ca2+ channels. AIS Ca2+ transients were reduced by D3R-PKC pathway activation in current clamp (Fig. 1), but also in voltage clamp when T-type currents were isolated (Fig. 2). This argues against the possibility that dopamine altered membrane potential in the AIS, which then indirectly altered local Ca2+ influx. Another possibility is that, since activation of AIS Ca2+ and Na+ channels mutually support regenerative responses (Bender and Trussell, 2009), reductions in local Na+ influx could in turn reduce Ca2+ influx; however, neither AP-evoked Na+ transients imaged in the AIS or persistent Na+ currents were affected by quinpirole (Fig. 4). Thus, dopamine receptor activation directly modulates AIS T-type channels.
While at this time AIS Ca2+ channels have been most thoroughly described in cartwheel cells, Ni2+-sensitive AIS Ca2+ transients have been observed in a variety of cell types (Bender and Trussell, 2009), raising the possibility that AIS Ca2+ channel modulation is a common mechanism for control of neuronal excitability. Indeed, dopamine reduces neuronal excitability, assessed via whole-cell somatic current injection, in a variety of cell types, including prefrontal cortex and hippocampal pyramidal cells (Gulledge and Jaffe, 1998; Stanzione et al., 1984; Tseng and O’Donnell, 2004), avian basal ganglia spiny neurons (Ding and Perkel, 2002), and striatal interneurons and medium spiny neurons (Hernandez-Lopez et al., 2000; Maurice et al., 2004). Direct tests will be needed to determine whether alterations to AIS Ca2+ influx contribute to reduced excitability in these cells types.
Axon initial segment specificity
Some reports show that T-type Ca2+ channels can be downregulated by both D2R family agonists and PKC, either by reducing open probability or by hyperpolarizing inactivation curves (Lledo et al., 1992; Marchetti et al., 1986), but others show that T-currents are not regulated (Chemin et al., 2006; Perez-Reyes, 2003). This disparity suggests that channel regulation depends on a specific set of conditions, fulfilled only when all components are present. We observed a similar mix of results in cartwheel cells: D3R activation reduced Ca2+ influx through axonal, but not dendritic, T-type channels. This specificity suggests that either key components of the D3R-PKC pathway are localized to the AIS or that T-type channels localized to the AIS are uniquely sensitive to neuromodulation. Of the three T-channel subtypes, the ascorbate and Ni2+-sensitive CaV3.2 subtype is the most common neuromodulatory target, due to the presence of unique phosphorylation sites found in the intracellular loop between transmembrane domains II and III (Chemin et al., 2006; Lambert et al., 2006). Therefore, we tested if CaV3.2 channels were exclusively localized to the AIS, but we found that both AIS and dendritic IT-evoked Ca2+ transients were blocked by CaV3.2-selective antagonists (Fig. 2). Thus, selective initial segment modulation is likely not due to the differential localization of T-type isoforms, though we cannot rule out the possibility that the AIS contains a unique splice variant of the CaV3.2 subtype (Chemin et al., 2006). Most likely, specificity is conferred by upstream components of the D3R-PKC pathway, or through co-localization of D3R, PKC, and T-type channels mediated by the cytoskeleton protein ankyrin G, which is restricted to the AIS (Kordeli et al., 1995; Rasband, 2010).
PKC-dependent reductions in AP output occurred within minutes of agonist application (Fig. 3, 7). While protein kinases can translocate upon activation (Zhao et al., 2006), this time scale limits the distance PKC could move before acting on AIS T-type channels. PKA has been shown to translocate from dendritic shaft to spine within seconds of PKA activation (Zhong et al., 2009), but longer distances (e.g., from nucleus to plasma membrane in HEK 293 cells, ~5 μm) require > 30 min (O’Flaherty et al., 2001). Therefore, we hypothesize that PKC is activated by D3Rs and acts on Ca2+ channels within a small domain, with both D3Rs and PKC localized to the AIS.
PKC comprises a family of protein kinases, some of which cluster with remarkable subcellular precision. In the AIS, PMA-induced reductions in Ca2+ influx were blocked by GF 109203X (Fig. 1), a high-affinity inhibitor of classical, Ca2+-dependent PKC isoforms, including PKC-α, PKC-β, PKC-γ, and PKC-ε (Toullec et al., 1991). Of these, D3R agonists have been shown to activate PKC-γ, inducing a translocation from the cytosol to the cell membrane (Glaser et al., 2003). Consistent with these results, PKC-γ is localized to the AIS in cerebellar Purkinje neurons (Cardell et al., 1998), which share considerable genetic, electrophysiological, and morphological homology with cartwheel cells (Berrebi et al., 1990), and PKC-γ mRNA is highly expressed in both Purkinje cells and cartwheel cells (Lein et al., 2007). Thus, PKC-γ expressed in the AIS may mediate dopaminergic regulation of AIS Ca2+.
Impact of AIS Ca2+ channel modulation on AP initiation
Na+ channel subtypes are differentially compartmentalized in the AIS, with NaV1.1/1.2 channels localized proximal and NaV1.6 channels localized distal to the soma (Hu et al., 2009; Lorincz and Nusser, 2008; Osorio et al., 2005). Because NaV1.6 channels have a low activation threshold, APs initiate in the distal AIS (Hu et al., 2009; Kole and Stuart, 2008; Palmer and Stuart, 2006; Royeck et al., 2008). Interestingly, NaV1.6 channels lack a key serine residue, S573, required for phosphorylation-mediated modulation (Cantrell and Catterall, 2001; Maurice et al., 2001), suggesting that the Na+ channels responsible for AP initiation may be insensitive to protein kinase-based modulation. Na+ imaging supports this idea (Fig. 4), indicating that quinpirole did not alter Na+ influx in the cartwheel cell AIS.
We found that the D3R-PKC pathway acted not on AIS Na+ channels but on AIS T-type Ca2+ channels. This may be an advantageous mechanism for regulating neuronal output. For example, if NaV1.6 channels were downregulated, not only would AP initiation probability be reduced, but the rising phase of the AP would also be altered significantly. This would likely alter neurotransmitter release at downstream axonal boutons, which is dependent on the waveform of the incoming AP (Kole et al., 2007). Since AIS Ca2+ channels are not directly responsible for the rising or falling phases of an AP, modification of AIS Ca2+ channels could determine whether or not a given stimulus results in AP initiation while leaving the AP waveform largely unaffected. Further, T-type Ca2+ channels are strongly linked to bursting activity in many neurons, especially when bursts are evoked from hyperpolarized potentials (Lisman, 1997). Indeed, quinpirole has been shown to reduce bursting behavior in hippocampal pyramidal cells (Stanzione et al., 1984), though it remains unclear whether these changes were mediated by AIS Ca2+ channel modulation. In cartwheel cells, bursts at the onset of a stimulus are preferentially evoked from more hyperpolarized potentials (Kim and Trussell, 2007), and as such, may serve to encode the recent membrane potential history of a cartwheel cell to postsynaptic targets (Uebachs et al., 2006). Alterations in AIS Ca2+ therefore may act as a switch, controlling how synaptic inputs are encoded as APs.
Ca2+ activates many neuronal signaling pathways, from transmitter release to gene regulation. Therefore, it is likely that AIS Ca2+ channels contribute not just to local membrane depolarization, but also to Ca2+-dependent signaling in the AIS. The overall length and location of the AIS relative to the soma dramatically influences neuronal excitability, and this location is fine tuned to the computational tasks of a given neuron (Kress et al., 2010; Kuba et al., 2006). Recent results in both chick auditory brainstem and cultured hippocampal neurons suggest that the AIS position and size are dynamically regulated by recent experience (Grubb and Burrone, 2010; Kuba et al., 2010). This regulation appears to be homeostatic: increased activity promotes a distal movement of the AIS from the soma, consequently lowering neuronal excitability (Grubb and Burrone, 2010), whereas sensory deprivation promotes an increase in the length of the AIS, increasing overall Na+ receptor number and thus enhancing neuronal excitability (Kuba et al., 2010). While it has yet to be tested in vivo, these plastic changes in AIS position were mediated by low-threshold Ca2+ channel activity in cultured neurons (Grubb and Burrone, 2010). These results, combined with those implicating Ca2+-dependent processes in the dismantling of the AIS after ischemic insult (Schafer et al., 2009), strongly suggest that AIS Ca2+ channels play an important role in the maintenance and plasticity of the initial segment.
Implications for auditory processing
Neuromodulators play a key role in sensory processing, altering neural circuit dynamics to aid in feature extraction (Hurley et al., 2004). While the cellular mechanisms of neuromodulation have been described primarily in higher brain regions, it has become increasingly clear that neuromodulation occurs at even the earliest stages of sensory processing (Hurley et al., 2004; Kothmann et al., 2009; Petzold et al., 2009). Dopaminergic projections from the brainstem to the cochlea have been proposed to have a protective effect on acoustic function (Darrow et al., 2006). Our data suggest that dopaminergic signaling plays a role in processing of auditory signals in the DCN. In this nucleus, direct auditory nerve input is compared with non-auditory modalities that may encode for head orientation and self-generated noise, aiding in sound localization (Oertel and Young, 2004; Shore and Zhou, 2006; Young and Davis, 2001). Synapses conveying non-auditory information exhibit robust synaptic plasticity (Fujino and Oertel, 2003; Tzounopoulos et al., 2004), suggesting that circuits in the DCN can be altered by experience. Catecholaminergic signaling potentially adds another level of experience-dependent modulation to DCN processing. Our results suggest that dopaminergic activity would dramatically alter the output of cartwheel cells, either by reducing overall activity or by selectively lowering burst output. Within a cartwheel cell, AIS dopaminergic signaling could affect dendritic processing, since bursts evoke large dendritic Ca2+ transients (Molitor and Manis, 2003; Roberts et al., 2008). Postsynaptic to cartwheel cells, this pathway could alter inhibitory filtering of non-auditory information, which is conveyed by parallel fibers that synapse on both cartwheel and fusiform cells (Fig. 5A). Dopamine could serve to alter the balance of excitation that fusiform cells receive from auditory and non-auditory streams, thereby altering DCN output. It will therefore be of great interest to determine the conditions under which dopaminergic signaling is recruited in the cochlear nucleus and the overall effect dopamine signaling has on sound processing.
Experimental procedures
Electrophysiology
All procedures were in accordance with OHSU IACUC guidelines. Following anesthesia, coronal brainstem slices (210 μm) were made from P16-24 CBA, ICR, or C57 mice. Transgenic animals (BAC TH-GFP and D3−/−/+/−) were genotyped by PCR. No differences were observed across mouse strains, and results were pooled. Cutting solution contained (in mM): 87 mM NaCl, 25 mM NaHCO3, 25 mM glucose, 75 mM sucrose, 2.5 mM KCl, 1.25 mM NaH2PO4, 0.5 mM CaCl2 and 7 mM MgCl2; bubbled with 5%CO2/95%O2; 4°C. Following cutting, slices were incubated in the same solution for 30 min at 33°C, then at room temperature until recording. Recording solution contained (in mM): 130 NaCl, 3 KCl, 2.4 CaCl2, 1.3 MgSO4, 1.2 KH2PO4, 20 NaHCO3, 3 Na-HEPES, 10 glucose; bubbled with 5%CO2/95%O2; 32-34°C. To avoid dye saturation, CaCl2 was reduced to 1 mM and MgSO4 was raised to 2.7 mM for all experiments in which AIS Ca2+ transients were evoked by AP trains in current clamp and imaged with Fluo-5F. For all other experiments, including AP-evoked AIS Ca2+ imaging with Fluo-4FF, 2.4 mM CaCl2 was used. In all recordings, 10 μM NBQX, 0.5 μM strychnine, and 20 μM SR95531 were added to the recording solution to block synaptic activity. When endogenous dopamine sources were stimulated electrically, 50 μM D-AP5, 2 μM CGP-55845, 1 mM MCPG, and 100 μM LY 341495 were also added to the recording solution to block NMDA, GABAB, and mGluR transmission.
Cartwheel cells were visualized with Dodt contrast optics and identified based on their laminar position, dendritic morphology, and ability to fire complex spikes in response to somatic depolarization (Wouterlood and Mugnaini, 1984). For current clamp recordings, patch electrodes (Schott 8250 glass, 3-4 MΩ tip resistance, <10 MΩ series resistance) were filled with a solution containing (in mM): 113 K-Gluconate, 9 HEPES, 4.5 MgCl2, 0.1 EGTA, 14 Tris2-phosphocreatine, 4 Na2-ATP, 0.3 tris-GTP; ~290 mOsm, pH: 7.2-7.25. For Na+ imaging, 1 mM SBFI was added to the pipette solution. For Ca2+ imaging, EGTA was omitted while 250 μM Fluo-5F and 20 μM Alexa 594 were added to the pipette solution. Electrophysiological data were recorded at 20-50 kHz and filtered at 10 kHz using a Multiclamp 700B amplifier (Molecular Devices), and acquired with an ITC-18 interface (Instrutech) and Igor Pro (Wavemetrics). Vm was held < −75 mV with constant current injection. For imaging experiments, AP trains were evoked via somatic depolarization (1-2 nA, 2 ms), followed by hyperpolarizing steps to prevent AP burst generation (2-400 pA, 10 ms). For experiments assaying AP output following D3R-PKC pathway activation, AP bursts were evoked with 30 ms step depolarizations. Baseline and post-drug measurements of Vm and Rin are the average of −5 to 0 and 5 to 15 min following drug application, respectively. Vm was held to within 2% of baseline values, and cells were excluded if Rin changed by > ±7.5%. Data were corrected for a measured junction potential of 12 mV.
Two-photon imaging
A two-photon imaging system (Prairie Technologies) was used as described previously (Bender and Trussell, 2009). The laser was tuned to 810 and 790 nm for Ca2+ and Na+ imaging, respectively. Epi- and transfluorescence signals were captured through a 60x, 1.0 NA objective and a 1.4 NA oil immersion condenser (Olympus). Fluorescence was split into red and green channels using dichroic mirrors and band-pass filters (epi: 575 DCXR, HQ525/70, HQ607/45; trans: T560LPXR, ET510/80, ET620/60; Chroma). Green fluorescence (Fluo-5F, SBFI) was captured with R9110 or H8224 photomultiplier tubes (PMTs, Hamamatsu). H8224 PMTs have a higher signal-to-noise ratio than R9110 PMTs. This difference is evident in individual examples in Fig. 1, which were imaged with either R9110 (e.g., 1E, F) or H8224 PMTs (e.g., 1B-D). All SBFI and Fluo-4FF imaging experiments were performed with H8224 PMTs. Red fluorescence (Alexa 594) was captured with R9110 PMTs. Data were collected in linescan mode (2-2.4 ms/line, including mirror flyback). For Ca2+ imaging, data were presented as averages of 20-40 events per site, and expressed as Δ(G/R)/(G/R)max*100, where (G/R)max was the maximal fluorescence in saturating Ca2+ (2 mM; Yasuda et al., 2004). For Na+ imaging, data were presented as averages of 20 events per site, and expressed as ΔF/F0, where F0 is the baseline fluorescence 0-200 ms before stimulus onset. Ca2+ and Na+ transient peaks were calculated from the peak of exponential fits to the fluorescence decay following stimulus offset.
Voltage-clamp of Ca2+, Na+, and K+ currents
For Ca2+ and Na+ currents, internal solution contained (in mM): 110 CsMeSO3, 40 HEPES, 1 KCl, 4 NaCl, 4 Mg-ATP, 10 Na-phosphocreatine, 0.4 Na2-GTP, 0.5 Fluo-5F, 0.02 Alexa-594; ~290 mOsm, pH: 7.22, voltages adjusted for 10 mV junction potential. For K+ currents, internal solution was the same as for current clamp recordings.
T-type Ca2+ currents were activated with 100 ms voltage steps from −100 to −60 mV. Leak currents were subtracted using a P/4 protocol with −10 mV steps from −80 mV. Experiments were performed in the presence of 500 nM TTX, 20 μM SR95531, 500 nM strychnine, 10 μM NBQX, 1 mM Cs+, and 2.4 mM external Ca2+. Local Ni2+ iontophoretic block of AIS Ca2+ channels was performed as described previously (Bender and Trussell, 2009). Phosphates were omitted from the recording solution for these experiments.
Persistent Na+ currents were activated with 500 ms voltage steps from −90 to −60, with leak currents subtracted with −7.5 mV steps from −80 mV. Current amplitudes were calculated as the average of the last 100 ms of each step. Experiments were performed in 20 μM SR95531, 500 nM strychnine, 10 μM NBQX, 10 mM TEA, 2 mM 4-AP, 200 μM Cd2+, 3 μM mibefradil, and 1 mM Cs+.
K+ currents were activated with 500 ms voltage steps from −80 to 0 mV in 10 mV increments. Current amplitudes were calculated as the average of the last 10 ms of each step. Experiments were performed in 500 nM TTX, 20 μM SR95531, 500 nM strychnine, 10 μM NBQX, and 1 mM Cs+. Ca2+ channels were not blocked to allow for activation of Ca2+-dependent K+ channels.
Electrochemistry
Glass-encased carbon fiber electrodes (diameter 7 μm, length 30-50 μm) were placed ~100 μm below the slice surface, parallel to the ependyma, in the center of each DCN layer. Electrodes were fabricated as described previously (Ford et al., 2009; Stamford, 1990). Prior to use, the cut electrode tip was placed in isopropanol purified with activated carbon for 10 min. Monoamine release was evoked with a glass stimulating electrode (stimulation: 10x at 40 Hz, 33 μA, 0.5 ms each) placed in the same layer. This stimulus evoked near-maximal dopamine release within each train, and mimicked burst activity commonly observed in dopaminergic neurons. 10 Hz triangular waveforms (−0.4 to +1.3 V vs. Ag/AgCl, 400 V/s) were used for voltammetric recording of monoamines. Background-subtracted cyclic voltammogram currents were obtained by subtracting 10 cyclic voltammograms obtained before stimulation from voltammograms obtained after stimulation. After subtraction, two-dimensional voltammetric color plots were used to examine the data. To determine the voltammetrically-detected monoamine time course, the current at the peak oxidation was plotted against time. As observed in other brain regions, voltammetric responses showed run down (Rice et al., 1997). Therefore, for laminar profiles, we stimulated only once in each layer. To assess the effects of GBR 12909, we limited run down by stimulating every 5 minutes. GBR 12909 was added to the bath only after responses stabilized. Due to limitations in maintaining stable whole-cell recordings >1 hr, trains were evoked at higher frequencies (30x at 0.05 Hz) for experiments described in Fig. 6. Transmission may have run down during these protocols, but effects of dopaminergic signaling were still observed. Decay kinetics were determined by a single exponential fit from 90% of the peak (~1.5s post-stimulation) to 10s post stimulation. Currents were calibrated against dopamine standards ranging from 0.05 to 1 μM.
Chemicals
Fluo-5F pentapotassium salt, SBFI tetraammonium salt, and Alexa Fluor 594 hydrazide Na+ salt were from Invitrogen. SR95531, D-AP5, and NBQX were from Ascent. (−)-quinpirole hydrochloride, PMA, (S)-MCPG, CGP-55845, LY-341495, tetrodotoxin (TTX), and GF 109203X were from Tocris. PKC19-31 was from Calbiochem. All others were from Sigma.
Statistics
All data are shown as mean ± standard error (SEM). An ANOVA followed by Fisher’s PLSD post-hoc test was used unless otherwise noted (significance: p < 0.05).
Supplementary Material
Acknowledgements
We are grateful to J. Trapani, J. Deignan, and members of the Trussell and Williams labs for comments, and to J. Williams for reading the manuscript. We thank D. Grandy and J. Williams for knockout and transgenic mice, and to A. Truitt, M. Grandy, K. Suchland, A. Matsui, and N. Quillinan for genotyping expertise. This research was supported by NIH grants K99DC011080 (KJB), K99DA026417 (CPF), and NS028901 (LOT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astman N, Gutnick MJ, Fleidervish IA. Persistent sodium current in layer 5 neocortical neurons is primarily generated in the proximal axon. J Neurosci. 2006;26:3465–3473. doi: 10.1523/JNEUROSCI.4907-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Dynamic spike threshold reveals a mechanism for synaptic coincidence detection in cortical neurons in vivo. Proc Natl Acad Sci U S A. 2000;97:8110–8115. doi: 10.1073/pnas.130200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Bender KJ, Trussell LO. Axon initial segment Ca2+ channels influence action potential generation and timing. Neuron. 2009;61:259–271. doi: 10.1016/j.neuron.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrebi AS, Morgan JI, Mugnaini E. The Purkinje cell class may extend beyond the cerebellum. J Neurocytol. 1990;19:643–654. doi: 10.1007/BF01188033. [DOI] [PubMed] [Google Scholar]
- Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Callewaert G, Eilers J, Konnerth A. Axonal calcium entry during fast ‘sodium’ action potentials in rat cerebellar Purkinje neurones. J Physiol. 1996;495(Pt 3):641–647. doi: 10.1113/jphysiol.1996.sp021622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- Cardell M, Landsend AS, Eidet J, Wieloch T, Blackstad TW, Ottersen OP. High resolution immunogold analysis reveals distinct subcellular compartmentation of protein kinase C gamma and delta in rat Purkinje cells. Neuroscience. 1998;82:709–725. doi: 10.1016/s0306-4522(97)00305-9. [DOI] [PubMed] [Google Scholar]
- Chemin J, Traboulsie A, Lory P. Molecular pathways underlying the modulation of T-type calcium channels by neurotransmitters and hormones. Cell Calcium. 2006;40:121–134. doi: 10.1016/j.ceca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum. J Neurosci. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JS, Curtis DR, Eccles JC. The interpretation of spike potentials of motoneurones. J Physiol. 1957;139:198–231. doi: 10.1113/jphysiol.1957.sp005887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Jordan LM, Fedirchuk B. Modulation of transient and persistent inward currents by activation of protein kinase C in spinal ventral neurons of the neonatal rat. J Neurophysiol. 2009;101:112–128. doi: 10.1152/jn.01373.2007. [DOI] [PubMed] [Google Scholar]
- Darrow KN, Simons EJ, Dodds L, Liberman MC. Dopaminergic innervation of the mouse inner ear: evidence for a separate cytochemical group of cochlear efferent fibers. J Comp Neurol. 2006;498:403–414. doi: 10.1002/cne.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Perkel DJ. Dopamine modulates excitability of spiny neurons in the avian basal ganglia. J Neurosci. 2002;22:5210–5218. doi: 10.1523/JNEUROSCI.22-12-05210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleidervish IA, Lasser-Ross N, Gutnick MJ, Ross WN. Na+ imaging reveals little difference in action potential-evoked Na+ influx between axon and soma. Nat Neurosci. 2010;13:852–860. doi: 10.1038/nn.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Phillips PE, Williams JT. The time course of dopamine transmission in the ventral tegmental area. J Neurosci. 2009;29:13344–13352. doi: 10.1523/JNEUROSCI.3546-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino K, Oertel D. Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus. Proc Natl Acad Sci U S A. 2003;100:265–270. doi: 10.1073/pnas.0135345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser S, Alvaro D, Roskams T, Phinizy JL, Stoica G, Francis H, Ueno Y, Barbaro B, Marzioni M, Mauldin J, et al. Dopaminergic inhibition of secretin-stimulated choleresis by increased PKC-gamma expression and decrease of PKA activity. Am J Physiol Gastrointest Liver Physiol. 2003;284:G683–694. doi: 10.1152/ajpgi.00302.2002. [DOI] [PubMed] [Google Scholar]
- Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron. 2008;58:387–400. doi: 10.1016/j.neuron.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010;465:1070–1074. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Jaffe DB. Dopamine decreases the excitability of layer V pyramidal cells in the rat prefrontal cortex. J Neurosci. 1998;18:9139–9151. doi: 10.1523/JNEUROSCI.18-21-09139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien ML, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76:5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- Heintz N. Gene expression nervous system atlas (GENSAT) Nat Neurosci. 2004;7:483. doi: 10.1038/nn0504-483. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J Neurosci. 2000;20:8987–8995. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Khaliq ZM, Raman IM. Relative contributions of axonal and somatic Na channels to action potential initiation in cerebellar Purkinje neurons. J Neurosci. 2006;26:1935–1944. doi: 10.1523/JNEUROSCI.4664-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Trussell LO. Ion channels generating complex spikes in cartwheel cells of the dorsal cochlear nucleus. J Neurophysiol. 2007;97:1705–1725. doi: 10.1152/jn.00536.2006. [DOI] [PubMed] [Google Scholar]
- Klepper A, Herbert H. Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Res. 1991;557:190–201. doi: 10.1016/0006-8993(91)90134-h. [DOI] [PubMed] [Google Scholar]
- Kole MH, Letzkus JJ, Stuart GJ. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron. 2007;55:633–647. doi: 10.1016/j.neuron.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Kole MH, Stuart GJ. Is action potential threshold lowest in the axon? Nat Neurosci. 2008 doi: 10.1038/nn.2203. [DOI] [PubMed] [Google Scholar]
- Kordeli E, Lambert S, Bennett V. AnkyrinG. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem. 1995;270:2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- Kothmann WW, Massey SC, O’Brien J. Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. J Neurosci. 2009;29:14903–14911. doi: 10.1523/JNEUROSCI.3436-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress GJ, Dowling MJ, Eisenman LN, Mennerick S. Axonal sodium channel distribution shapes the depolarized action potential threshold of dentate granule neurons. Hippocampus. 2010;20:558–571. doi: 10.1002/hipo.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress GJ, Dowling MJ, Meeks JP, Mennerick S. High threshold, proximal initiation, and slow conduction velocity of action potentials in dentate granule neuron mossy fibers. J Neurophysiol. 2008;100:281–291. doi: 10.1152/jn.90295.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress GJ, Mennerick S. Action potential initiation and propagation: upstream influences on neurotransmission. Neuroscience. 2009;158:211–222. doi: 10.1016/j.neuroscience.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba H, Ishii TM, Ohmori H. Axonal site of spike initiation enhances auditory coincidence detection. Nature. 2006;444:1069–1072. doi: 10.1038/nature05347. [DOI] [PubMed] [Google Scholar]
- Kuba H, Oichi Y, Ohmori H. Presynaptic activity regulates Na(+) channel distribution at the axon initial segment. Nature. 2010;465:1075–1078. doi: 10.1038/nature09087. [DOI] [PubMed] [Google Scholar]
- Lambert RC, Bessaih T, Leresche N. Modulation of neuronal T-type calcium channels. CNS Neurol Disord Drug Targets. 2006;5:611–627. doi: 10.2174/187152706779025544. [DOI] [PubMed] [Google Scholar]
- Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block alpha1H. Biophys J. 1999;77:3034–3042. doi: 10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Homburger V, Bockaert J, Vincent JD. Differential G protein-mediated coupling of D2 dopamine receptors to K+ and Ca2+ currents in rat anterior pituitary cells. Neuron. 1992;8:455–463. doi: 10.1016/0896-6273(92)90273-g. [DOI] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci. 2008;28:14329–14340. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Lipp P, Luscher HR, Niggli E. Control of action potential propagation by intracellular Ca2+ in cultured rat dorsal root ganglion cells. J Physiol. 1996;490(Pt 2):319–324. doi: 10.1113/jphysiol.1996.sp021146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C, Carbone E, Lux HD. Effects of dopamine and noradrenaline on Ca channels of cultured sensory and sympathetic neurons of chick. Pflugers Arch. 1986;406:104–111. doi: 10.1007/BF00586670. [DOI] [PubMed] [Google Scholar]
- Martina M, Vida I, Jonas P. Distal initiation and active propagation of action potentials in interneuron dendrites. Science. 2000;287:295–300. doi: 10.1126/science.287.5451.295. [DOI] [PubMed] [Google Scholar]
- Maurice N, Mercer J, Chan CS, Hernandez-Lopez S, Held J, Tkatch T, Surmeier DJ. D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J Neurosci. 2004;24:10289–10301. doi: 10.1523/JNEUROSCI.2155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ. D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J Neurosci. 2001;21:2268–2277. doi: 10.1523/JNEUROSCI.21-07-02268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough SI, Bean BP. Mibefradil inhibition of T-type calcium channels in cerebellar purkinje neurons. Mol Pharmacol. 1998;54:1080–1087. doi: 10.1124/mol.54.6.1080. [DOI] [PubMed] [Google Scholar]
- Molitor SC, Manis PB. Dendritic Ca2+ transients evoked by action potentials in rat dorsal cochlear nucleus pyramidal and cartwheel neurons. J Neurophysiol. 2003;89:2225–2237. doi: 10.1152/jn.00709.2002. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Joksovic PM, Su P, Kang HW, Van Deusen A, Baumgart JP, David LS, Snutch TP, Barrett PQ, Lee JH, et al. Molecular mechanisms of subtype-specific inhibition of neuronal T-type calcium channels by ascorbate. J Neurosci. 2007;27:12577–12583. doi: 10.1523/JNEUROSCI.2206-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Flaherty JT, Chadwell BA, Kearns MW, Sergeant S, Daniel LW. Protein kinases C translocation responses to low concentrations of arachidonic acid. J Biol Chem. 2001;276:24743–24750. doi: 10.1074/jbc.M101093200. [DOI] [PubMed] [Google Scholar]
- Oertel D, Young ED. What’s a cerebellar circuit doing in the auditory system? Trends Neurosci. 2004;27:104–110. doi: 10.1016/j.tins.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Osorio N, Alcaraz G, Padilla F, Couraud F, Delmas P, Crest M. Differential targeting and functional specialization of sodium channels in cultured cerebellar granule cells. J Physiol. 2005;569:801–816. doi: 10.1113/jphysiol.2005.097022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LM, Stuart GJ. Site of action potential initiation in layer 5 pyramidal neurons. J Neurosci. 2006;26:1854–1863. doi: 10.1523/JNEUROSCI.4812-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Hagiwara A, Murthy VN. Serotonergic modulation of odor input to the mammalian olfactory bulb. Nat Neurosci. 2009;12:784–791. doi: 10.1038/nn.2335. [DOI] [PubMed] [Google Scholar]
- Rasband MN. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci. 2010 doi: 10.1038/nrn2852. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ, Greenfield SA. Characteristics of electrically evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitro. J Neurophysiol. 1997;77:853–862. doi: 10.1152/jn.1997.77.2.853. [DOI] [PubMed] [Google Scholar]
- Roberts MT, Bender KJ, Trussell LO. Fidelity of complex spike-mediated synaptic transmission between inhibitory interneurons. J Neurosci. 2008;28:9440–9450. doi: 10.1523/JNEUROSCI.2226-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Jiang Z, Yao L, Vagts A, Diamond I, Gordon A. Coordinated movement of RACK1 with activated betaIIPKC. J Biol Chem. 1999;274:27039–27046. doi: 10.1074/jbc.274.38.27039. [DOI] [PubMed] [Google Scholar]
- Rosker C, Lohberger B, Hofer D, Steinecker B, Quasthoff S, Schreibmayer W. The TTX metabolite 4,9-anhydro-TTX is a highly specific blocker of the Na(v1.6) voltage-dependent sodium channel. Am J Physiol Cell Physiol. 2007;293:C783–789. doi: 10.1152/ajpcell.00070.2007. [DOI] [PubMed] [Google Scholar]
- Royeck M, Horstmann MT, Remy S, Reitze M, Yaari Y, Beck H. Role of axonal NaV1.6 sodium channels in action potential initiation of CA1 pyramidal neurons. J Neurophysiol. 2008;100:2361–2380. doi: 10.1152/jn.90332.2008. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Jha S, Liu F, Akella T, McCullough LD, Rasband MN. Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J Neurosci. 2009;29:13242–13254. doi: 10.1523/JNEUROSCI.3376-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J, Helmchen F, Sakmann B. Spatial profile of dendritic calcium transients evoked by action potentials in rat neocortical pyramidal neurones. J Physiol. 1995;487(Pt 3):583–600. doi: 10.1113/jphysiol.1995.sp020902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Action potential initiation and propagation in hippocampal mossy fibre axons. J Physiol. 2008;586:1849–1857. doi: 10.1113/jphysiol.2007.150151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JE, Fischbach PS, McCleskey EW. T-type calcium channels: heterogeneous expression in rat sensory neurons and selective modulation by phorbol esters. J Neurosci. 1990;10:947–951. doi: 10.1523/JNEUROSCI.10-03-00947.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE, Zhou J. Somatosensory influence on the cochlear nucleus and beyond. Hear Res. 2006;216-217:90–99. doi: 10.1016/j.heares.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Shu Y, Duque A, Yu Y, Haider B, McCormick DA. Properties of action-potential initiation in neocortical pyramidal cells: evidence from whole cell axon recordings. J Neurophysiol. 2007;97:746–760. doi: 10.1152/jn.00922.2006. [DOI] [PubMed] [Google Scholar]
- Stamford JA. Fast cyclic voltammetry: measuring transmitter release in ‘real time’. J Neurosci Methods. 1990;34:67–72. doi: 10.1016/0165-0270(90)90043-f. [DOI] [PubMed] [Google Scholar]
- Stanzione P, Calabresi P, Mercuri N, Bernardi G. Dopamine modulates CA1 hippocampal neurons by elevating the threshold for spike generation: an in vitro study. Neuroscience. 1984;13:1105–1116. doi: 10.1016/0306-4522(84)90291-4. [DOI] [PubMed] [Google Scholar]
- Stuart G, Schiller J, Sakmann B. Action potential initiation and propagation in rat neocortical pyramidal neurons. J Physiol. 1997;505(Pt 3):617–632. doi: 10.1111/j.1469-7793.1997.617ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddese A, Bean BP. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron. 2002;33:587–600. doi: 10.1016/s0896-6273(02)00574-3. [DOI] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7:719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Uebachs M, Schaub C, Perez-Reyes E, Beck H. T-type Ca2+ channels encode prior neuronal activity as modulated recovery rates. J Physiol. 2006;571:519–536. doi: 10.1113/jphysiol.2005.103614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouterlood FG, Mugnaini E. Cartwheel neurons of the dorsal cochlear nucleus: a Golgi-electron microscopic study in rat. J Comp Neurol. 1984;227:136–157. doi: 10.1002/cne.902270114. [DOI] [PubMed] [Google Scholar]
- Yasuda R, Nimchinsky EA, Scheuss V, Pologruto TA, Oertner TG, Sabatini BL, Svoboda K. Imaging calcium concentration dynamics in small neuronal compartments. Sci STKE. 2004;2004:pl5. doi: 10.1126/stke.2192004pl5. [DOI] [PubMed] [Google Scholar]
- Young ED, Davis KA. Circuitry and Function of the Dorsal Cochlear Nucleus. In: Oertel D, Popper AN, Fay RR, editors. Integrative Functions in the Mammalian Auditory Pathway. 2001. [Google Scholar]
- Zhao Y, Leal K, Abi-Farah C, Martin KC, Sossin WS, Klein M. Isoform Specificity of PKC Translocation in Living Aplysia Sensory Neurons and a Role for Ca2+-Dependent PKC APL I in the Induction of Intermediate-Term Facilitation. J. Neurosci. 2006;26:8847–8856. doi: 10.1523/JNEUROSCI.1919-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Sia GM, Sato TR, Gray NW, Mao T, Khuchua Z, Huganir RL, Svoboda K. Subcellular dynamics of type II PKA in neurons. Neuron. 2009;62:363–374. doi: 10.1016/j.neuron.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.