Abstract
FHIT and WWOX are tumor suppressor genes that span the common fragile sites FRA3B and FRA16D, respectively. To analyze possible synergisms among these genes in cervical cancer progression, we considered 159 cervical intraepithelial neoplasias, and 58 invasive squamous cell carcinomas of the uterine cervix. FHIT and WWOX proteins were examined by immunohistochemistry and their expression was inversely correlated with precancerous versus invasive lesions. Statistics among biological markers indicated a strong association between FHIT and WWOX. Protein expression of these two genes was also absent or reduced in cancer cell lines. Thus, WWOX may be considered as a novel important genetic marker in cervical cancer and the association between the altered expression of FHIT and WWOX may be a critical event in the progression of this neoplasia.
Keywords: Cervical neoplasia, immunohistochemistry, tumor suppressors, fragile sites
Introduction
Since the introduction of cytological screening in the 1940s, the incidence of cervical carcinoma has declined in many developed countries [1]. Considerable efforts have been made to classify preneoplastic and neoplastic lesions [2], and an association between HPV infection, especially high risk HPV types 16 and 18, and the development of precancerous lesions has been proposed [3-6]. Nevertheless, prospective studies of cervical neoplasia suggest that HPV infection alone is not responsible for tumor development and additional genetic or epigenetic changes in tumor cells are required for tumorigenesis [7-9].
The fragile histidine triad (FHIT) gene is a candidate tumor suppressor gene located at chromosome 3p14.2 and encompassing the FRA3B common fragile site [10]. Frequent allelic losses and homozygous deletions, as well as loss of heterozygosity in microsatellites located in FHIT, have been observed in several types of tumor, particularly those resulting from exposure to environmental carcinogenesis such as lung, kidney, esophageal, head and neck, stomach and cervical cancer [11]. Aberrant protein expression and inactivation of the FHIT gene have been identified in a variety of tumors, including lung [12], breast [13,14], bladder [15], esophagus [16], and colon [17]. In cervical cancer, FHIT gene analysis showed a high frequency of inactivation of both alleles and aberrant mRNA transcripts [18, 19] while the FRA3B fragile site is also a candidate region for HPV 16 integration [20], suggesting that alterations and inactivation of the FHIT gene contribute and accelerate cervical carcinogenesis. Immunohistochemical studies showed down-regulation of FHIT in microinvasive and invasive cervical carcinomas and an aberrant expression has been reported as a poor prognosis factor independent of the human papilloma virus (HPV) [21-23].
Bednarek et al. described the gene WWOX when observing two WW domains at the NH2 terminus and a short-chain dehydrogenase/reductase (SDR) central domain (24). WWOX spans the second most active common fragile site in the human genome (FRA16D) at chromosome region 16q23.2 [25,26]. It has been reported that WWOX may have a role in regulating estradiol-ER interaction while the mouse homologue of the WWOX protein has been defined as an apoptogenic protein and a partner of p53 in cell death [24,27]. It was recently observed that WWOX altered expression is due not only to loss of heterozygosity and homozygous deletions but also to epigenetic modifications such as promoter hypermethylation [28]. The WWOX gene is altered at the genomic and expression level in several types of tumors, including breast [14, 29-30] ovarian [31], prostate [32], hepatocellular [33], pancreatic [34], esophageal [35], small cell lung [36] and gastric cancer [37]. Because no studies correlating FHIT and WWOX protein expression and cervical cancer progression have yet been reported, in the present study we investigated whether these genes might have a pathogenetic role in preinvasive and invasive primary cervical cancer and in cervical carcinoma cell lines.
Materials and Methods
Tissue specimens
From December 1998 to November 2007 we selected cervical tissues from archival paraffin blocks originating from 217 women, 159 of which were classified as precancerous lesions with 109 cases of cervical intraepithelial neoplasia (CIN) 1, 15 cases of CIN 2 and 35 cases of CIN 3. The 58 invasive squamous cell carcinoma, graded according to the WHO histopathological classification, were classified as follows: 20 cases were well-differentiated (G1), 29 cases were moderately differentiated (G2), and 9 cases were poorly differentiated. In order to conform, all cases of invasive carcinoma were chosen at stage Ib of the FIGO classification (International Federation of Gynaecology and Obstetrics). The mean age of patients was 40.58 (SD, ± 13.38 ; range, 22-81 years) from the date of biopsy. Informed consent was obtained from each subject after the purpose and nature of the study had been explained.
Immunohistochemistry
Sections 5 μm thick were cut onto silanized glass slides and air-dried overnight at room temperature. Sections were dewaxed in xylene and rehydrated through graded alcohol. Incubating the slides for 10 min in 3% hydrogen peroxide quenched endogenous peroxidase activity. Sections for microwave antigen retrieval pre-treatment were immersed in citrate buffer (Zymed, San Francisco, CA, USA). They were irradiated twice in a microwave oven (800W) at full power for 4 min and then left to cool for 15 min in the hot buffer at room temperature. FHIT primary antibody (Zymed, San Francisco, CA, USA) was diluted 1:200 in PBS and incubated overnight at 4°C. WWOX expression was performed using a polyclonal rabbit anti-glutathione-Stransferase (anti-GST)-WWOX antibody with dilution 1: 4000 [14].
Sections were reacted with biotinylated anti-rabbit antibody and streptavidin-biotin-peroxidase (Histostain-SP Kit, Zymed Laboratories, San Francisco, CA). Diaminobenzidine was used as chromogene substrate. Finally, sections were washed in distilled water and weakly counterstained with Harry's modified hematoxylin. The primary antibodies were omitted and replaced with preimmune serum in the negative control. Keratinocyte ME 180 cell lines were used for FHIT positive control; breast cancer was used as positive control to perform WWOX protein expression.
Interpretation of immunoreactivity
The degree of all antibodies expression was evaluated semiquantitatively by measuring both intensity and extent of staining: −, 0; +, weak with <10% positive cells; ++, >10% but <50% strongly positive cells; and +++, >50% strongly positive cells. Negative immunoreactivity was scored − or +, and positive immunostaining as ++ or +++. For FHIT and WWOX a distinct brown cytoplasmic staining was scored as positive. Slides were scored independently by three pathologists (E.G., A.V., R.M.); the discordant cases were reviewed and reassigned scores based on consensus of opinion.
Cell lines
All carcinoma cell lines HT-3, SiHa, CaSki, C33a, HeLa, and HCT 116 were obtained from the American Type Culture Collection and maintained in the recommended media.
Protein isolation and Western blotting
Cells were collected and resuspended in lysis buffer (50 mM TRIS pH 7.5, 150 mM NaCl, 10%, 0.4% Nonidet P40, glycerol, protease inhibitors), and incubated in ice for 20 min. Samples were then centrifuged for 20 min at 13000 rpm. The supernatants were discharged, pellets dried and resuspended in 1X Sample buffer (2% SDS, 10% glycerol, 60 mM Tris pH 6.8).
For Western blotting, 50 μg of proteins were fractionated on SDS-polyacrylamide gel and transferred by electrophoresis on nitrocellulose membrane (Bio-Rad Laboratories, Melville, NY). Membranes were blotted overnight with rabbit polyclonal anti Wwox antibody (a gift from Kay Huebner, Ohio State University), rabbit polyclonal anti Fhit antibody (Zymed Laboratories, Carlsbad, CA, U.S.A.), and rabbit polyclonal anti actin antibody (Sigma-Aldrich U.S.A.). Antibodies bound to membrane-immobilized proteins were visualized by enhanced chemiluminescence using the ECLTM Western blotting detection reagents (GE Healthcare, Piscataway, NJ, USA).
Statistics
Statistical analyses were performed using the SPSS® computer program package (SPSS for Windows, version 11.5). Frequency tables were analysed using the Chi-square test and Spearman's rank (r) correlation coefficient. The statistical significance was set at P < 0.05.
Results
Immunohistochemical analysis of protein expression
The intensity of FHIT and WWOX expression was judged on the basis of immunoreactivity of precancerous and invasive lesions. Results are shown in Table I.
Table I.
FHIT and WWOX protein expression in histopathological lesions of uterine cervix
| FHIT |
WWOX |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative −/+ |
Positive ++/+++ |
Negative −/+ |
Positive ++/+++ |
Tot | |||||
| CIN 1 | 46 | 42.2% | 63 | 57.8% | 47 | 43.1% | 62 | 56.9% | 109 |
| CIN 2/3 | 37 | 74.0% | 13 | 26.0% | 25 | 50.0% | 25 | 50.0% | 50 |
| ISCCs | 35 | 60.3% | 23 | 39.7% | 40 | 69.0% | 18 | 31.0% | 58 |
|
|
|
||||||||
| Tot | 118 | 99 | 112 | 105 | 217 | ||||
Histopathological lesion vs FHIT: χ2=15.106, p=0.001; vs WWOX: χ2=10.194, p=0.006. CIN1 and CIN2/3 vs FHIT χ2=13.89, p=0.00019. CIN2/3 and ISCCs vs FHIT χ2=2.25 p=0.133. CIN1 and CIN2/3 vs WWOX χ2=0.65, p=0.418. CIN2/3 and ISCCs vs WWOX χ2=4.03, p=0.044.
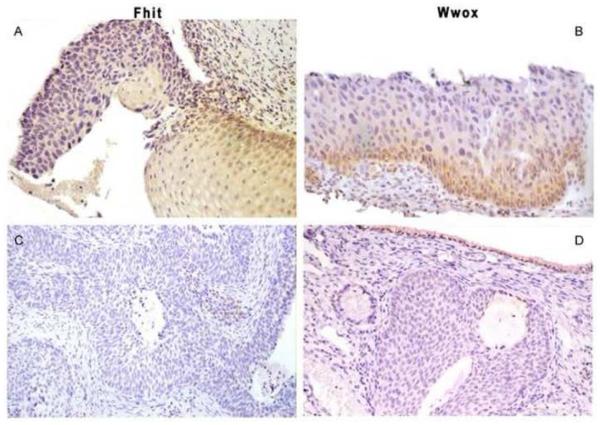
Strong and moderate FHIT immunostaining was detected in CIN1 and, when available on the same biopsy section, in normal epithelium (Figure 1 case 1). Low or undetectable immunoreactivity was found in 46 out of 109 cases (42.2%) with CIN 1 and 37 out of 50 (74%) cases with CIN 2-3 (Figure 1 case 2, 3). Thirty-five out of 58 (60.3%) ISCC cases proved negative (Figure 1, cases 4). Observing normal ectocervical squamous epithelium, we detected WWOX immunoreactivity in squamous epithelium and basal layer (Figure 1 case 1). Low or undetectable expression was found in 47 out of 109 (43.1%) with CIN 1 and 25 out of 50 (50%) with CIN 2-3 (Figure 1 case 2, 3). Forty out of 58 (69%) ISCCs were found to be negative (Figure 1 case 4).
Figure 1.
Illustration of representative immunohistochemistry in normal epithelium, cervical preneoplastic and invasive lesions.
Case 1 (magnification, ×10). Fhit and Wwox positive immunoreactivity in normal ectocervical squamous epithelium.
Case 2 (magnification, ×20). Low or undetectable immunoreactivity of Fhit and Wwox in high grade cervical intraepithelial neoplasia (CIN 3) lesion.
Case 3 (magnification, ×10). A case with Low or undetectable immunoreactivity of Fhit and Wwox protein expression observed in high grade cervical intraepithelial neoplasia (CIN 3) lesion. Note Wwox positive expression in normal columnar epithelial cells.
Case 4 (magnification, ×10). In invasive squamous cell carcinoma, Fhit and Wwox protein expression show negative immunoractivity.
Expression of specific proteins and histological grading of ISCCs
FHIT and WWOX protein expression correlated inversely with precancerous versus invasive lesions (P=0.001; P=0.006, respectively) (Table I). Moreover, a significant relationship was observed between the histopathological grading of ISCCs and low or undetectable immunoreactivity FHIT protein expression (p=0.016) (Table II).
Table II.
FHIT and WWOX protein expression and ISCC grading
| FHIT |
WWOX |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade ISCCs |
Negative −/+ |
Positive ++/+++ |
Negative −/+ |
Positive ++/+++ |
Tot | ||||
| G1 | 7 | 35.0% | 13 | 65.0% | 13 | 65.0% | 7 | 35.0% | 20 |
| G2 | 21 | 72.4% | 8 | 27.6% | 20 | 69.0% | 9 | 31.0% | 29 |
| G3 | 7 | 77.8% | 2 | 22.2% | 7 | 77.8% | 2 | 22.2% | 9 |
|
|
|
||||||||
| Tot | 35 | 23 | 40 | 18 | 58 | ||||
Grade vs FHIT: χ2=8.277, p=0.016; vs WWOX: χ2=0.473, p=0.789. G1 and G2/3 vs FHIT: χ2=8.19, p=0.004. G1 and G2/3 vs WWOX: χ2=0.22 , p=0.635.
Statistical analysis of biological markers showed a strong correlation between FHIT and WWOX [r=0.298; (P<0.0001)].
Protein expression in cervical cancer cell lines
In order to see whether the clinical outcome had support in human cancer cells in vitro, five cervical cancer cell lines, HeLa, CaSki, HT-3, SiHa, and C33a, were tested for Fhit and Wwox expression by Western blot in comparison with the control cell line HTC 116 that is positive for the two proteins (Figure 2). As a result, both proteins were absent or under-expressed in all five cervical cell lines when compared with HTC 116. Only in CaSki cell line Wwox is present in a certain amount that is however 2 or 3-fold less than control. Thus, Western analysis for these two tumor suppressor proteins in cervical cancer cells in vitro confirms the immunoreactivity data described above.
Figure 2.
Western blot showing the reduced protein expression in vitro of Fhit and Wwox in five cervical carcinoma cell lines, in comparison with a carcinoma cell line positive for both proteins. Actin is shown to demonstrate the same amount of proteins loaded on the gel.
Discussion
Aberrant FHIT transcript was detected in cervical carcinoma cell lines and primary carcinomas [38], and correlated with significant loss of FHIT protein by immunohistochemistry [39]. Literature reported a loss of FHIT expression in 33-76% of cervical cancers, whereas high levels of FHIT expression were found in normal cervical epithelium but less frequently in squamous intraepithelial lesions [39-41] and a correlation between tumor progression, reduced FHIT expression and poor prognosis had been previously detected [22, 23]. In a previous study, we found an absence of FHIT protein in 65.2% of ISCCs and 57.1% of non invasive squamous cell carcinomas (NISCCs), observing that the FHIT protein was strongly expressed in dysplastic koilocytosis tissue but was reduced or actually disappeared in areas of invasive carcinoma [42]. Confirming previous data, in the present study we observed a significant association between FHIT low expression and precancerous lesions. Indeed, when observing cases from CIN1 to CIN2/3, we detected an increase of 31.8% with loss of or undetectable protein expression, confirming that alteration of FHIT could occur at the stage of CIN1, thus becoming an important molecular event in invasive cervical cancer progression. Finally, we observed a strong correlation between loss or undetectable FHIT protein expression and poor histologic differentiation, suggesting that FHIT absence or down-regulation correlates with an aggressive biological behavior of cervical cancer.
In vitro and in vivo strategy demonstrated that WWOX is a candidate tumor suppressor gene [43]. Among its functions, WWOX suppresses the transactivation of p73, AP2 γ and ErbB-4 via its WW domain containing Yes-Associated Protein (YAP) and, more recently, an important role for the WWOX/c-jun functional interaction was observed [27, 44-46]. The authors observed some similarity between WWOX and FHIT gene functions. For example, WWOX and FHIT are downregulated after exposure to environmental carcinogens involved in cellular stress and cooordinately inactivated in human cancer [47, 48, 14]. Correlated expression and association with failure of apoptosis in lymphocytes was observed in thyroid cancer [49] and, on examining the relationship between WWOX and FHIT methylation status in breast, lung and bladder cancer, it was observed that expression of both genes is reduced or lost coordinately with promoter region methylation [50]. Immunohistochemistry studies reported frequent loss of WWOX protein expression with a range of 63-84%. In non-small cell lung cancer, Donati et al. observed negative or weak staining intensity in 84% of squamous cell carcinomas showing a relatively high extent of staining but a very low intensity [36]. We found a reduction or absence of WWOX protein expression of 64.3% and 53%, respectively, in preinvasive and invasive cervical cancer. Interestingly, we also found abnormal WWOX protein immunoreaction in the early stage of cervical cancer, sometimes only in HPV infection and condylomatous lesions, suggesting a series of hypotheses. WWOX and FHIT genes are the most common fragile sites in human genome. The location of WWOX in fragile site and its inactivation pattern are similar to that of the FHIT gene, and so perhaps their involvement in cervical carcinogenesis is not surprising. Indeed, coordinated loss of WWOX and FHIT immunoreaction, confirmed also in vitro by western blot analysis in five cervical carcinoma cell lines, was detected in 30.8% of precancerous lesions and 48.3% of ISCCs, suggesting an involvement of both genes in the same molecular pathway in a subgroup of lesions. A possible interaction between HPV and FHIT was previously reported [51], although not, so far, between WWOX and HPV infection in cervical cancer.
In breast cancer, an association between WWOX immunoreactivity and a steroid hormone was reported when observing a reduction of protein expression prevalently in premenopausal women, while severely reduced WWOX staining was found in normal tissue only in postmenopausal women [14]. No relationship was found between WWOX protein expression and patient age: nevertheless, the cervical epithelium is subjected to cyclic hormonal influence at a fertile age and the immunoreactivity was performed only on biopsies with no normal counterpart.
In conclusion, in the present study, observing that the Wwox protein is reduced in high rates of cervical cancer, we speculate that alterations of Wwox gene may contribute to cervical tumorigenesis. Moreover, the risk of cervical cancer is associated with HPV infection, and, because an interaction is assumed between the oncovirus and FHIT/FRA3B, further investigation will be necessary into the possible relationship between WWOX/FRA16D and HPV infection in cervical tumorigenesis.
Acknowledgements
Work was supported by a Sidney Kimmel Cancer Research Foundation grant (to N.Z.) and National Cancer Institute grants (to C.M.C.). We thank Dr. Kay Huebner for supplying the Wwox antiserum and for her critical review of the manuscript and Drs Rami Aqeilan, Eugenio Gaudio, and Flavia Pichiorri for helpful advice and technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None declared.
References
- 1.Brinton LA. Epidemiology of cervical cancer. In: Munoz N, Bosch FX, Shah KV, Meheus A, editors. Epidemiology of Cervical Cancer and Human Papillomavirus. Lyon, France: 1992. pp. 2–23. (IARC Scientific Publication No.109). Overview, in. [Google Scholar]
- 2.The revised Bethesda System for reporting cervical/vaginal cytological diagnoses: report of the 1991 Bethesda workshop. Acta Cytol. 1992;36:273–276. [PubMed] [Google Scholar]
- 3.Parker MF, Arroyo GF, Gerardts J, Sabichi AL, Park RC, Taylor RR. Molecular characterization of adenocarcinoma of the cervix. Gynecol. Oncol. 1997;64:242–251. doi: 10.1006/gyno.1996.4580. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen HN, Nordquist SR. The Bethesda system and evaluation of abnormal pap smears. Semin. Surg. Oncol. 1999;16:217–221. doi: 10.1002/(sici)1098-2388(199904/05)16:3<217::aid-ssu4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.McLachin CM, Tate JE, Zitz JC, Sheets EE, Crum CP. Human papillomavirus type 18 and intraepithelial lesions of the cervix. Am. J. Pathol. 1994;144:141–147. [PMC free article] [PubMed] [Google Scholar]
- 6.Paquette RL, Lee YY, Wilczynsky SP, Karmakar A, Kizaki M, Miller CW. Mutations of p53 and human papillomavirus infection in cervical carcinoma. Cancer (Phila.) 1993;72:1272–1280. doi: 10.1002/1097-0142(19930815)72:4<1272::aid-cncr2820720420>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Spriggs AL, Boddington MM. Progression and regression of cervical lesions: review of smears from women followed without initial biopsy or treatment. J. Clin. Pathol. 1980;33:517–522. doi: 10.1136/jcp.33.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brescia RJ, Jenson AB, Lancaster WD, Kurman RJ. The role of human papillomaviruses in the pathogenesis and histologic classification of precancerous lesions of the cervix. Hum. Pathol. 1986;17:552–559. doi: 10.1016/s0046-8177(86)80126-5. [DOI] [PubMed] [Google Scholar]
- 9.Aaltonen LA, Peltomaki P, Leach FS. Clues to the pathogenesis of familial colorectal cancer. Science (Washington DC) 1993;260:812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 10.Ohta M, Inoue H, Cotticelli MG, Kastury K, Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, Croce CM, Huebner K. The FHIT gene, spanning the chromosome 3p14. 2 fragile site and renal carcinoma-associated t(3;8) breakpoint, is abnormal in digestive tract cancers. Cell. 1996;84:587–597. doi: 10.1016/s0092-8674(00)81034-x. [DOI] [PubMed] [Google Scholar]
- 11.Negrini M, Monaco C, Vorechovsky I, Ohta M, Druck T, Baffa R, Huebner K, Croce CM. The FHIT gene at 3p14. 2 is abnormal in breast carcinomas. Cancer Res. 1996;56:3173–3179. [PubMed] [Google Scholar]
- 12.Sozzi G, Pastorino U, Moiraghi L, Tagliabue E, Pezzella F, Ghirelli C, Tornielli S, Sard L, Huebner K, Pierotti MA, Croce CM, Pilotti S. Loss of FHIT function in lung cancer and preinvasive bronchial lesions. Cancer Res. 1998;15:5032–7. [PubMed] [Google Scholar]
- 13.Campiglio M, Pekarsky Y, Menard S, Tagliabue E, Pilotti S, Croce CM. FHIT loss of function in human primary breast cancer correlates with advanced stage of the disease. Cancer Res. 1999;15:3866–9. [PubMed] [Google Scholar]
- 14.Guler G, Uner A, Guler N, Han SY, Iliopoulos D, Hauck WW, McCue P, Huebner K. The fragile genes FHIT and WWOX are inactivated coordinately in invasive breast carcinoma. Cancer. 2004;15:1605–14. doi: 10.1002/cncr.20137. [DOI] [PubMed] [Google Scholar]
- 15.Baffa R, Gomella LG, Vecchione A, Bassi P, Mimori K, Sedor J, Calviello CM, Gardiman M, Minimo C, Strup SE, McCue P, Kovatich AJ, et al. Loss of FHIT expression in transitional cell carcinoma of the urinary bladder. Am. J. Pathol. 2000;156:419–24. doi: 10.1016/S0002-9440(10)64745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori M, Mimori K, Shiraishi T, Alder H, Inoue H, Tanaka Y, Sugimachi K, Huebner K, Croce CM. Altered expression of Fhit in carcinoma and precarcinomatous lesions of the esophagus. Cancer Res. 2000;60:1177–82. [PubMed] [Google Scholar]
- 17.Hao XP, Willis JE, Pretlow TG, Rao JS, MacLennan GT, Talbot IC, Pretlow TP. Loss of fragile histidine triad expression in colorectal carcinomas and premalignant lesions. Cancer Res. 2000;60:18–21. [PubMed] [Google Scholar]
- 18.Yoshino K, Enomoto T, Nakamura T, Nakashima R, Wada H, Saitoh J, Noda K, Murata Y. Aberrant FHIT transcripts in squamous cell carcinoma of the uterine cervix. Int. J. Cancer. 1998;76:176–81. doi: 10.1002/(sici)1097-0215(19980413)76:2<176::aid-ijc2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 19.Yoshino K, Enomoto T, Nakamura T, Sun H, Ozaki K, Nakashima R, Wada H, Saitoh J, Watanabe Y, Noda K, Murata Y. FHIT alterations in cancerous and non-cancerous cervical epithelium. Int. J. Cancer. 2000;85:6–13. doi: 10.1002/(sici)1097-0215(20000101)85:1<6::aid-ijc2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Wilke CM, Hall BK, Hoge A, Paradee W, Smith DI, Glover TW. FRA3B extends over a broad region and contains a spontaneous HPV16 integration site: direct evidence for the coincidence of viral integration sites and fragile sites. Hum. Mol. Genet. 1996;5:187–95. doi: 10.1093/hmg/5.2.187. [DOI] [PubMed] [Google Scholar]
- 21.Birrer MJ, Hendricks D, Farley J, Sundborg MJ, Bonome T, Walts MJ, Geradts J. Abnormal Fhit expression in malignant and premalignant lesions of the cervix. Cancer Res. 1999;59:5270–4. [PubMed] [Google Scholar]
- 22.Takizawa S, Nakagawa S, Nakagawa K, Yasugi T, Fujii T, Kugu K, Yano T, Yoshikawa H, Taketani Y. Abnormal Fhit expression is an independent poor prognostic factor for cervical cancer. Br. J. Cancer. 2003;88:1213–6. doi: 10.1038/sj.bjc.6600892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang LW, Chao SL, Chen TJ. Reduced Fhit expression in cervical carcinoma: correlation with tumor progression and poor prognosis. Gynecol. Oncol. 2003;90:331–7. doi: 10.1016/s0090-8258(03)00318-4. [DOI] [PubMed] [Google Scholar]
- 24.Bednarek AK, Keck-Waggoner CL, Daniel RL, Laflin KJ, Bergsagel PL, Kiguchi K, Brenner AJ, Aldaz CM. WWOX, the FRA16D gene, behaves as a suppressor of tumor growth. Cancer Res, 2001;61:8068–73. [PubMed] [Google Scholar]
- 25.Paige AJ, Taylor C, Hillier SG, Farrington S, Scott D, Porteous DJ, Smyth JF, Gabra H, Watson JE. WWOX: a candidate tumor suppressor gene involved in multiple tumor types. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11417–22. doi: 10.1073/pnas.191175898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ried K, Finnis M, Hobson L, Mangelsdorf M, Dayan S, Nancarrow JK, Woollatt E, Kremmidiotis G, Gardner A, Venter D, Baker E, Richards RI. Common chromosomal fragile site FRA16D sequence: identification of the FOR gene spanning FRA16D and homozygous deletions and translocation breakpoints in cancer cells. Hum. Mol. Genet. 2000;9:1651–63. doi: 10.1093/hmg/9.11.1651. [DOI] [PubMed] [Google Scholar]
- 27.Chang NS, Pratt N, Heath J, Schultz L, Sleve D, Carey GB, Zevotek N. Hyaluronidase induction of a WW domain-containing oxidoreductase that enhances tumor necrosis factor cytotoxicity. J. Biol. Chem. 2001;276:3361–70. doi: 10.1074/jbc.M007140200. [DOI] [PubMed] [Google Scholar]
- 28.Iliopoulos D, Guler G, Han SY, Druck T, Ottey M, McCorkell KA, Huebner K. Roles of FHIT and WWOX fragile genes in cancer. Cancer Lett. 2006;232:27–36. doi: 10.1016/j.canlet.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 29.Nunez MI, Ludes-Meyers J, Abba MC, Kil H, Abbey NW, Page RE, Sahin A, Klein-Szanto AJ, Aldaz CM. Frequent loss of WWOX expression in breast cancer: correlation with estrogen receptor status. Breast Cancer Res. Treat. 2005;89:99–105. doi: 10.1007/s10549-004-1474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pluciennik E, Kusinska R, Potemski P, Kubiak R, Kordek R, Bednarek AK. WWOX--the FRA16D cancer gene: expression correlation with breast cancer progression and prognosis. Eur. J. Surg. Oncol. 2006;32:153–7. doi: 10.1016/j.ejso.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Gourley C, Paige AJ, Taylor KJ, Scott D, Francis NJ, Rush R, Aldaz CM, Smyth JF, Gabra H. WWOX mRNA expression profile in epithelial ovarian cancer supports the role of WWOX variant 1 as a tumour suppressor, although the role of variant 4 remains unclear. Int. J. Oncol. 2005;26:1681–9. doi: 10.3892/ijo.26.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson JE, Doggett NA, Albertson DG, Andaya A, Chinnaiyan A, van Dekken H, Ginzinger D, Haqq C, James K, Kamkar S, Kowbel D, Pinkel D, et al. Integration of high-resolution array comparative genomic hybridization analysis of chromosome 16q with expression array data refines common regions of loss at 16q23-qter and identifies underlying candidate tumor suppressor genes in prostate cancer. Oncogene. 2004;23:3487–94. doi: 10.1038/sj.onc.1207474. [DOI] [PubMed] [Google Scholar]
- 33.Park SW, Ludes-Meyers J, Zimonjic DB, Durkin ME, Popescu NC, Aldaz CM. Frequent downregulation and loss of WWOX gene expression in human hepatocellular carcinoma. Br. J. Cancer. 2004;91:753–9. doi: 10.1038/sj.bjc.6602023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuroki T, Yendamuri S, Trapasso F, Matsuyama A, Aqeilan RI, Alder H, Rattan S, Cesari R, Nolli ML, Williams NN, Mori M, Kanematsu T, et al. The tumor suppressor gene WWOX at FRA16D is involved in pancreatic carcinogenesis. Clin. Cancer Res. 2004;10:2459–65. doi: 10.1158/1078-0432.ccr-03-0096. [DOI] [PubMed] [Google Scholar]
- 35.Kuroki T, Trapasso F, Shiraishi T, Alder H, Mimori K, Mori M, Croce CM. Genetic alterations of the tumor suppressor gene WWOX in esophageal squamous cell carcinoma. Cancer Res. 2002;62:2258–60. [PubMed] [Google Scholar]
- 36.Donati V, Fontanini G, Dell'Omodarme, Prati MC, Nuti S, Lucchi M, Mussi A, Fabbri M, Basolo F, Croce CM, Aqeilan RI. WWOX expression in different histologic types and subtypes of non-small cell lung cancer. Clin. Cancer Res. 2007;13:884–91. doi: 10.1158/1078-0432.CCR-06-2016. [DOI] [PubMed] [Google Scholar]
- 37.Aqeilan RI, Kuroki T, Pekarsky Y, Albagha O, Trapasso F, Baffa R, Huebner K, Edmonds P, Croce CM. Loss of WWOX expression in gastric carcinoma. Clin. Cancer Res. 2004;10:3053–8. doi: 10.1158/1078-0432.ccr-03-0594. [DOI] [PubMed] [Google Scholar]
- 38.Muller CY, O' Boyle JD, Fong KM, Wistuba II, Biesterveld E, Ahmadian M, Miller DS, Gazdar AF, Minna JD. Abnormalities of fragile histidine triad genomic and complementary DNA in cervical cancer: association with human papilloma virus type. J. Natl. Cancer Inst. 1998;90:433–439. doi: 10.1093/jnci/90.6.433. [DOI] [PubMed] [Google Scholar]
- 39.Greenspan DL, Connolly DC, Wu R, Lei RY, Vogelstein JT, Kim YT, Mok JE, Munoz N, Bosch FX, Shah K, Cho KR. Loss of FHIT expression in cervical carcinoma cell lines and primary tumors. Cancer Res, 1997;57:4692–4698. [PubMed] [Google Scholar]
- 40.Croce CM, Sozzi G, Huebner K. Role of FHIT in human cancer. J. Clin. Oncol. 1999;17:1618–1624. doi: 10.1200/JCO.1999.17.5.1618. [DOI] [PubMed] [Google Scholar]
- 41.Wistuba II, Montellano FD, Milchgrub S, Virmani AK, Behrens C, Chen H, Ahmadian M, Nowak JA, Muller C, Minna JD, Gazdar AF. Deletions of chromosome 3p are frequent and early events in the pathogenesis of uterine cervical carcinoma. Cancer Res. 1997;57:3154–3158. [PubMed] [Google Scholar]
- 42.Giarnieri E, Mancini R, Pisani T, Alderisio M, Vecchione A. Msh2, Mlh1, Fhit, p53, Bcl-2, and Bax Expression in Invasive and in Situ Squamous Cell Carcinoma of the Uterine Cervix. Clin. Cancer Res. 2000;6:3600–3606. [PubMed] [Google Scholar]
- 43.Fabbri M, Iliopoulos D, Trapasso F, Aqeilan RI, Cimmino A, Zanesi N, Yendamuri S, Han SY, Amadori D, Huebner K, Croce CM. WWOX gene restoration prevents lung cancer growth in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 2005;102:15611–6. doi: 10.1073/pnas.0505485102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aqeilan RI, Donati V, Palamarchuk A, Trapasso F, Kaou M, Pekarsky Y, Sudol M, Croce CM. WW domain-containing proteins, WWOX and YAP, compete for interaction with ErbB-4 and modulate its transcriptional function. Cancer Res. 2005;65:6764–72. doi: 10.1158/0008-5472.CAN-05-1150. [DOI] [PubMed] [Google Scholar]
- 45.Gaudio E, Palamarchuk A, Palumbo T, Trapasso F, Pekarsky Y, Croce CM, Aqeilan RI. Physical association with WWOX suppresses c-Jun transcriptional activity. Cancer Res. 2006;66:11585–9. doi: 10.1158/0008-5472.CAN-06-3376. [DOI] [PubMed] [Google Scholar]
- 46.Aqeilan RI, Palamarchuk A, Weigel RJ, Herrero JJ, Pekarsky Y, Croce CM. Physical and functional interactions between the Wwox tumor suppressor protein and the AP-2gamma transcription factor. Cancer Res. 2004;64:8256–61. doi: 10.1158/0008-5472.CAN-04-2055. [DOI] [PubMed] [Google Scholar]
- 47.Smith DI, McAvoy S, Zhu Y, Perez DS. Large common fragile site genes and cancer. Semin. Cancer Biol. 2007;17:31–41. doi: 10.1016/j.semcancer.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Thavathiru E, Ludes-Meyers JH, MacLeod MC, Aldaz CM. Expression of common chromosomal fragile site genes, WWOX/FRA16D and FHIT/FRA3B is downregulated by exposure to environmental carcinogens, UV, and BPDE but not by IR. Mol. Carcin. 2005;44:174–82. doi: 10.1002/mc.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sbrana I, Veroni F, Nieri M, Puliti A, Barale R. Chromosomal fragile sites FRA3B and FRA16D show correlated expression and association with failure of apoptosis in lymphocytes from patients with thyroid cancer. Genes Chrom. Cancer, 2006;45:429–36. doi: 10.1002/gcc.20305. [DOI] [PubMed] [Google Scholar]
- 50.Iliopoulos D, Guler G, Han SY, Johnston D, Druck T, McCorkell KA, Palazzo J, McCue P, Baffa R, Huebner K. Fragile genes as biomarkers: epigenetic control of WWOX and FHIT in lung, breast and bladder cancer. Oncogene. 2005;24:1625–33. doi: 10.1038/sj.onc.1208398. [DOI] [PubMed] [Google Scholar]
- 51.Vecchione A, Zanesi N, Trombetta G, French D, Visca P, Pisani T, Botti C, Vecchione A, Croce CM, Mancini R. Cervical dysplasia, ploidy, and human papillomavirus status correlate with loss of Fhit expression. Clin. Cancer Res. 2001;7:1306–12. [PubMed] [Google Scholar]