Abstract
For many species, the presence of a significant social partner can lessen the behavioral and physiological responses to stressful stimuli. This study examined whether a single, individually specific, signature vocalization (phee call) could attenuate the physiological stress response that is induced in marmosets by housing them in short-term social isolation. Utilizing a repeated-measures design, adult marmosets (n = 10) were temporarily isolated from their long-term pair mate and exposed to three conditions: signature vocalizations from the pair mate, phee calls from an unfamiliar opposite sex individual, or no auditory stimuli. Levels of urinary cortisol were monitored as a physiological indicator of the stress response. Urinary cortisol levels were also monitored, while subjects remained undisturbed in their home cages to provide baseline levels. Temporarily isolated marmosets showed significantly higher levels of urinary cortisol than undisturbed marmosets. However, the nature of the acoustic stimulus experienced during isolation led to differences in the excretion of urinary cortisol. Isolated marmosets exposed to a familiar pair mate’s vocalization showed significantly lower levels of urinary cortisol than when exposed to unfamiliar marmoset vocalizations (P < 0.04) or to no auditory stimuli (P < 0.03). Neither the duration of pairing nor the quality of relationship in the pair (indexed by spatial proximity scores) predicted the magnitude of reduction in cortisol in the familiar vocalization condition. The results presented here provide the first evidence that a single, individually specific communication signal can decrease the magnitude of a physiological stress response in a manner analogous to the physical presence of a social partner, a process we term “vocal buffering.”
Keywords: Stress, Cortisol, Social buffering, Communication, Signature signals, Vocal buffering
Introduction
For members of a social species, one of the most profound moderators of function in the hypothalamic–pituitary–adrenal (HPA) axis during times of stress may be the presence of significant social partners (DeVries et al., 2003; Hennessy, 1997; Levine, 1993). This model of social support, sometimes known as buffering, postulates that during times of stress, the presence of significant social partners down-regulates activity in the HPA axis and hence serves to buffer the individual against the stressful stimulus (Cohen and Wills, 1985). Presumably, the beneficial effects associated with the physical presence of a significant social partner derive from the partner’s ability to either modify the perceived intensity of the stressor, and/or to down-regulate the magnitude and duration of the HPA response during exposure to the stressful stimulus. This phenomenon has been well studied in the context of mother–offspring interactions. In some species, offspring that are exposed to stressful situations in the presence of the mother display significantly reduced behavioral and glucocorticoid responses to those stressors, relative to offspring exposed to the same stressors in the absence of the mother (see review in Hennessy, 1997). However, this effect of social support is not just limited to mother–infant dyads. Social buffering of behavioral and physiological responses to stress has also been reported in studies involving separation in adult nonhuman primates (e.g., heterosexual pairs: Mendoza and Mason, 1986; Smith et al., 1998; same-sex pairs: Gust et al., 1994).
The physical presence of a social partner is defined by an amalgamation of several distinct stimulus attributes (e.g., visual, olfactory, and auditory signals) as well as by the nature and pattern of social interactions. Presumably, the interactions of these signals function to provide a representation of one individual (the sender) and perhaps the relationship it represents to another individual (the receiver). In the absence of one or more of these signals, as often occurs in visually obscured environments, isolated individuals may attempt to maintain the positive effects of social support by using a single individually specific, or signature, signal rather than having to rely on a combination of several signals. Signature signals have been identified across a wide variety of birds and mammals (e.g., Charrier et al., 2003; Randall, 1989; Sayigh et al., 1998; Searby et al., 2004). Individually distinct communication signals are also prevalent among many nonhuman primates (e.g., Cleveland and Snowdon, 1982; Hammerschmidt and Todt, 1995; Jones et al., 1993; Jorgensen and French, 1998; Snowdon and Cleveland, 1980; Snowdon et al., 1983; Symmes et al., 1979). Therefore, if the physical presence of a social partner can moderate responses (i.e., reduce HPA axis activity) to stressful stimuli, it may be that, in addition to identity, these signature signals also communicate the beneficial effects of social support by communicating representations of individuals and the relationship they represent, in turn moderating the consequences of exposure to stressful events.
Marmosets, small tropical primates from the New World family Callitrichidae, are characterized by strong socioemotional attachments (“pair bonds”) between adult males and females, as well as cooperative infant care and prolonged residence of offspring in extended family groups (Rylands, 1993). Thus, social interactions are an important attribute of callitrichid life, and the resulting social relationships have profound impacts on marmoset physiology and behavior including reproduction (Ginther et al., 2001; Smith et al., 1997; Ziegler and Sousa, 2002), endocrine regulation (Shepherd and French, 1999; Smith and French, 1997; Smith et al., 1998), and communication (Elowson and Snowdon, 1994; Rukstalis et al., 2003; Snowdon and Elowson, 1999; Snowdon and Hodun, 1981; Vitale et al., 2003). In addition to these important social characteristics, marmosets also possess rich vocal repertoires that are used in a wide variety of contexts, including intragroup cohesion and maintenance of territories (Cleveland and Snowdon, 1982; Epple, 1968; Heymann, 1987; Hubrecht, 1985; Stevenson and Poole, 1976). These vocalizations are also known to have “signature” or individually distinct properties which can be used to distinguish individuals among group members (Jones et al., 1993; Jorgensen and French, 1998; Snowdon and Cleveland, 1980). In addition to strong attachments and signature vocalizations, marmosets have also been shown to experience social buffering of the stress response. Smith et al. (1998) demonstrated that marmosets removed from their home cage and exposed to a novel environment had significantly lower levels of urinary cortisol when their heterosexual pair mate was present versus when they were not. Therefore, the existence of complex social interactions, highly conserved, individually identifiable vocalizations, and evidence of social buffering make callitrichid primates ideally suited for examining the effects of individually specific signals on physiological responses to stressful events.
The purpose of the present study was to evaluate whether exposure to a single, individually specific, signal (i.e., phee call) could reduce the consequences of social separation and exposure to environmental novelty. Specifically, we predicted that exposure to the phee call of a significant social partner (i.e., pair mate) would reduce urinary cortisol excretion, a common indicator of psychosocial stress, in marmosets isolated and exposed to a novel environment. Additionally, it may be that exposure to vocalizations from individuals other than the focal animal’s pair mate may also influence the physiological consequences of exposure to stressful events. However, given the individually specific nature of marmoset vocalizations and the long-term attachments formed between heterosexual pairs, we predicted that exposure to vocalizations from unfamiliar individuals would not reduce urinary cortisol production in socially isolated individuals exposed to novel environments. We also examined the effect of length of pairing and social proximity, if any, on urinary cortisol excretion upon exposure to a pair mate’s vocalization.
Materials and methods
Subjects and housing
Subjects for this study were 10 Wied’s black tufted-ear marmosets (Callithrix kuhlii) housed in five preexisting, long-term heterosexual pairs at the University of Nebraska at Omaha’s Callitrichid Research Center. Table 1 contains demographic information for all marmosets included in the study. The mean age and length of pairing prior to the beginning of the study were 6.8 and 4.4 years, respectively. Wire mesh enclosures for each pair measured approximately 1.2 × 0.9 × 2.4 m and were equipped with branches, nest boxes, and various enrichment devices. All colony rooms were maintained at a constant temperature of 20–22°C and were subject to a 12:12 light–dark cycle. Groups residing in the same colony room were denied visual contact but retained olfactory and auditory contact with neighboring enclosures. All animals were fed a mixture of various fresh fruits and vegetables, dairy products, Zupreem marmoset diet (Hills Brothers; Mission, KS, USA), and Mazuri primate fiber sticks (Mazuri; St. Louis, MO, USA). All protocols in this study were approved by the University of Nebraska at Omaha’s Institutional Animal Care and Use Committee (IACUC #03-096-011).
Table 1.
Demographic information for all marmosets included in the study
| Subject | Sex | Age (years) | Time paired (years) |
|---|---|---|---|
| Ye | M | 5 | 4 |
| Iz | F | 4 | |
| Ca | M | 10 | 4 |
| It | F | 6 | |
| Fl | M | 6 | 3 |
| Fo | F | 5 | |
| Ke | M | 12 | 8 |
| Ya | F | 10 | |
| Bu | M | 5 | 3 |
| Ne | F | 5 |
Auditory signals
Phee calls were collected with a Marantz PMD 201 portable analog cassette recorder (Marantz; Itasca, IL, USA) using a Sennheiser (Sennheiser USA; Old Lyme, CT, USA) ME 80 directional microphone (frequency range 50–15,000 Hz). Prior to each recording session, animals were allowed to habituate to the presence of an observer and recording equipment, located approximately 1.5 m from the enclosure. Individual callers were identified by speaking the name of the calling animal directly into the microphone following the end of the vocalization (Jorgensen and French, 1998). Unfamiliar vocalizations were collected from marmoset pairs residing in colony rooms other than the focal animal. Previous research in our laboratory (Rukstalis et al., 2003) and others (Elowson and Snowdon, 1994) has indicated that changes in the demographic makeup of colony rooms can significantly alter the morphology of marmoset vocalizations. In light of this information, all calls were obtained from animals residing in undisturbed colony rooms throughout the course of this study.
Data collection
Pretrial urine samples were collected the morning of the experiment, between 0700 and 0800 h, and represented the first void of the day. At 1200 h, individual marmosets were removed from their home cages by trained technicians and placed in small transport cages (0.38 × 0.38 × 0.38 m). Isolated individuals were then moved to a quiet room located at least 20 m from the home colony room. Subjects were isolated from their long-term pair mates for 4 h (1200–1600 h) and exposed to one of three conditions: isolation with no auditory stimulation (no call), isolation and exposure to an unfamiliar opposite sex marmoset’s phee call (unfamiliar call), or isolation and exposure to the familiar pair mate’s phee call (familiar call). Familiar and unfamiliar vocalizations were played back using a Marantz model PMD 201 (Marantz) cassette recorder, located approximately 0.5 m from the enclosure. Playbacks consisted of a single vocal exemplar from either the focal animal’s familiar pair mate or an unfamiliar opposite sex animal presented at a rate of once every 2 min throughout the course of the separation. In order to control for possible order effects and habituation to the protocol, the sequence of conditions for each animal was assigned in a random manner. Additionally, for any individual, a period of at least 3 days elapsed between successive trials. Urine samples were collected every 30 min from clean aluminum pans placed under the transport cage. Posttrial urine samples were collected the following morning (0700–0800 h). Urine samples from these same subjects (control condition) residing undisturbed in their home cage were collected at 0700 h, during the trial periods (i.e., 1200–1600 h), and on the following morning to document circadian variation in urinary cortisol when marmosets did not undergo social isolation. All subjects had been previously trained to urinate in exchange for a preferred food item. Therefore, urine collection during control trials was collected in hand-held aluminum pans without the need for separation and/or isolation. Following collection, all samples were centrifuged to remove debris, transferred to clean vials, and stored at −20°C until assay.
During the control trial, a 15-min behavioral observation was conducted once an hour (1200–1600 h) by a trained technician located approximately 1.5 m from the enclosure using the Observer 4.1 software (Noldus USA; Leesburg, VA, USA). All animals were allowed 5 min to habituate to the presence of the observer. An all-occurrences method was used to record pair proximity and individual approaches and leaves. Approaches were scored when an animal approached its pair mate to within one marmoset body length and remained there for at least 5 s. Leaves were scored when an animal moved at least one marmoset body length away from its pair mate and remained there for at least 5 s.
Data analysis
Urinary cortisol concentrations were determined using an enzyme immunoassay (EIA) previously described and validated for use in C. kuhlii (Smith and French, 1997). Hormone concentrations were measured in 18 assays. Coefficients of variation (CVs) were measured from duplicate evaluations of pooled marmoset urine. Interassay CVs for high and low pools were 12.05% and 16.95%, respectively. Intraassay CVs for high and low pools were 4.4% and 3.84%, respectively. To account for differences in urine sample concentration, all hormone values were corrected for creatinine concentration. Creatinine values were determined using a modified Jaffé end-point assay, previously described and validated for use in this species (French et al., 1996).
Levels of urinary cortisol were examined in samples collected from individual marmosets exposed to the four conditions. For each subject, cortisol concentrations were determined for the first-void preisolation sample, the mean concentration excreted over the first 2 h of a trial, the mean cortisol concentration during the last 2 h of a trial, and the first-void sample collected on the morning following a trial. Previous studies in this laboratory have demonstrated that changes in urinary cortisol are readily detectable within 2 h of exposure to a mild stressor (Smith and French, 1997; Smith et al., 1998). A three-way mixed ANOVA [Sex(2) × Condition(4) × Time(4)] was used to assess the impact of these varying conditions on urinary concentrations of cortisol. Where appropriate, paired-sample, dependent t tests were used to compare possible differences at individual time points. Additionally, a two-way ANOVA [Order(3) × Time(4)] was used to assess the impact of presentation of the separation conditions on subsequent levels of urinary cortisol. An alpha level of 0.05 was adopted for all statistical tests.
Results of the four 15-min behavioral observations were pooled. The number of individual leaves and approaches to and from the pair mate was counted. These numbers were used to calculate an index of the maintenance of social proximity as an indicator of relationship quality (Hinde and Atkinson, 1970). A Pearson correlation was used to assess the relationship between these scores and the reduction in urinary cortisol excretion between the familiar call and no call conditions. We also used a Pearson correlation to assess the relationship between length of pairing and reduction in urinary cortisol between these same two conditions.
Results
Social isolation and exposure to environmental novelty induced a significant physiological stress response in marmosets. The results of our repeated-measures ANOVA revealed a significant interaction between time of sample collection and call condition on levels of urinary cortisol excretion [F(9) = 6.39, P < 0.0001]. After two h, marmosets separated from their pair mates had significantly higher levels of urinary cortisol when exposed to any of the three treatment conditions [unfamiliar calls: t(9) = 2.76, P = 0.022; familiar calls: t(9) = 2.553, P = 0.011; or no calls: t(9) = 4.11, P =0.003] than when they were left undisturbed (control) in their home cages. Although, after 2 h, there was no significant difference in levels of urinary cortisol when marmosets were exposed to familiar versus unfamiliar calls or unfamiliar versus no calls, exposure to familiar vocalizations resulted in significantly lower levels of urinary cortisol compared to when the marmosets were exposed to no vocalizations [t(9) = 2.65, P = 0.026; Fig. 1].
Fig. 1.
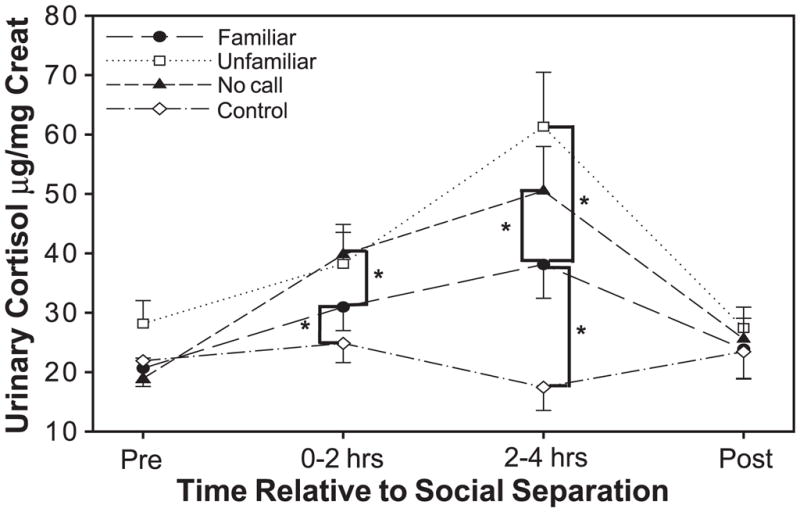
Mean (± SEM) urinary cortisol excretion (μg/mg creatinine) for all marmosets (N = 10), in the familiar condition, versus all other experimental conditions, across the 4-h separation period. *P < 0.05. See text for other significant effects.
Differences in the excretion of urinary cortisol between conditions continued to be evident after 4 h of separation. Again, isolation and exposure to any of the three treatment conditions resulted in significantly higher levels of urinary cortisol [familiar call: t(9) = 3.219, P = 0.011; unfamiliar call: t(9) = 5.113, P = 0.001; no call: t(9) =4.744, P = 0.001] than when marmosets were left undisturbed in their home cages. At 4 h, there was clear evidence that exposure to familiar calls reduced the stress response to isolation. Marmosets who had been exposed to familiar calls displayed significantly lower levels of urinary cortisol 4 h postisolation than marmosets exposed to unfamiliar calls [t(9) =2.397, P = 0.04] or no calls [t(9) =2.554, P = 0.031].
The results of the two-way ANOVA indicated that the order of presentation of the separation conditions had no effect on subsequent levels of urinary cortisol (mean urinary cortisol: Presentation 1, 30.52 ± 3.20 μg/ml; Presentation 2, 36.30 ± 4.37 μg/ml; Presentation 3, 34.06 ± 4.65 μg/ml). Additionally, neither the duration of pairing nor proximity maintenance scores appeared to influence the degree of vocal buffering.
Discussion
Individual marmosets, isolated from their long-term pair mate and exposed to a novel environment, showed significant increases in the production of glucocorticoids across the 4-h trial period, while urinary cortisol in marmosets that remained in their undisturbed home cages was quite stable. Thus, social isolation and exposure to a novel environment appear to have constituted a significant source of stress. However, the amount of urinary cortisol measured in each condition depended on the presence and identity of vocalizations presented to the isolated individuals. After 2 h of isolation, individuals exposed to phee calls from their long-term pair mates had significantly lower levels of urinary cortisol than when no calls were presented. After 4 h of separation, marmosets isolated and exposed to their pair mate’s phee call had significantly lower levels of urinary cortisol than when they were exposed to either no vocalizations or unfamiliar vocalizations. Therefore, exposure to a single, individually specific signal modality (i.e., conspecific vocalization) reduced the physiological consequences (i.e., glucocorticoid production) of social isolation and exposure to environmental novelty, a process we term vocal buffering.
The mechanisms or processes involved in this vocal buffering of the HPA axis are still unknown. However, two distinct possibilities exist as potential explanations for the ability of individually specific vocalizations to reduce a physiological response to stress. In the current study, we isolated individuals from their home cages and long-term pair mates prior to exposing them to various vocal conditions. As has been previously noted, environmental novelty is a potent stimulator of the HPA axis (Hennessy et al., 1997). It may be the case then that exposing individuals isolated in novel environments to familiar vocalizations simply reduced the overall environmental novelty and thereby diminished the perceived intensity of the stressor. In a similar study of marmosets, Smith et al. (1998) reported that removal of an individual’s pair mate from the home cage did not cause a subsequent rise in glucocorticoid production, suggesting that environmental novelty rather than social separation induced the stress response. Although the focal animals’ pair mates were removed from the home cage, other individuals (i.e., offspring) were not (Smith et al., 1998). Therefore, it may have been that social interactions with the remaining offspring were able to buffer the individual against the stress of pair mate removal. Alternatively, if social separation rather than exposure to environmental novelty is the true stressor, then the ability of familiar vocalizations to moderate an individual’s HPA axis may be due to the individually specific nature of the communication signals. In a study of the individually specific signaling system of the golden hamster (Mesocricetus auratus), Johnston and Bullock (2001) and Johnston and Jernigan (1994) provided evidence that identifying scent signals are an integral part of a lager multifactor representation of individuals. The results of these studies suggest that individually specific signals may go beyond mere markers of discrimination and may serve to elicit complex representations of individuality and quite possibly the relationships that identity entails. Callitrichid primates possess highly conserved, individually identifiable vocal signals, characterized by low within-individual and high between-individual variability (Jones et al., 1993; Jorgensen and French, 1998; Snowdon and Cleveland, 1980). In addition to identity, the signature system contained within the vocalizations of these nonhuman primates may also communicate more complex representations of individuality, thereby reducing the physiological consequences of exposure to stressful stimuli in a manner analogous to the physical presence of the social partner.
The results of this study also revealed that marmosets exhibited larger concentrations of urinary cortisol, although not significant, when individuals were exposed to vocalizations from unfamiliar conspecifics versus familiar conspecifics. Again, depending on the source of the stress, this trend might also be explained by either social separation or novelty exposure. Callitrichid primates are generally characterized as highly territorial, group dwelling animals (Heymann, 1987; Hubrecht, 1985; Stevenson and Poole, 1976). Thus, exposure to the calls of an unfamiliar individual when isolated from a social group may be perceived as a threat and therefore could intensify stress rather than alleviating it or having no effect. On the other hand, the vocalizations of callers unfamiliar to the focal animal might be perceived as an additional novel component of the separation environment. Therefore, any rise in physiological indices of stress may simply reflect a reaction to increased novelty rather than a perceived threat from a nongroup member. It should be noted, however, that the effects of novelty exposure and social separation may not be mutually exclusive, and the results presented here may reflect a combination of these two effects.
The ability of specific social partners to buffer an individual against stressful stimuli or to stimulate a stress response upon separation can vary greatly depending on the nature and quality of the relationship they share. Filial attachment, the bond that forms from an offspring to its caregiver, can be observed in many species (Mason and Mendoza, 1998). For example, when placed in a novel environment, guinea pig pups show greater elevations of plasma cortisol and ACTH when they are tested alone versus with they are tested with their mothers (Hennessy and Moorman, 1989; Hennessy and Tamborski, 1989; Hennessy et al., 2002). Similarly, squirrel monkey mother–infant dyads experience significant increases in plasma cortisol when separated from each other (Coe et al., 1978; Levine et al., 1993; Mendoza et al., 1978; Wiener et al., 1990). Documentation of attachment between adult heterosexual pairs is less common. However, a notable exception has been reported in a previous study of a socially monogamous primate, the titi monkey. Mendoza and Mason (1986) demonstrated that titi monkey heterosexual pair mates showed a significant increase in plasma cortisol when separated from each other, but not from their offspring. Like titi monkeys, the socially monogamous marmosets are also known for their strong heterosexual pair-bonds. These long-term relationships can take several weeks to form and represent an important social bond for an adult marmoset (Schaffner et al., 1995). In the present study, we used both length of pairing and Hinde and Atkinson index scores (proximity maintenance) to quantify relationship quality. However, in this case, neither was an indicator of the amount of reduction in urinary cortisol (buffering) an individual experienced. However, given the small sample size and restricted range of data (i.e., time paired), a correlation may be difficult to find. Future studies assessing social buffering in callitrichid primates would be benefited by examining the relationship between quantitative measures of relationship quality and amount of social buffering experienced by individuals.
The results of the present study may provide a possible framework for disentangling the potential of various relationships and the attachments they represent to influence behavioral and physiological responses to stress. The data presented here showed a significant “vocal buffering” effect of individually specific vocalizations on urinary cortisol excretion in isolated pair mates. Future studies would do well to examine the effect of individually specific stimuli from various social companions (i.e., offspring, same-sex conspecific, siblings, etc.), mating strategies, and social systems in order to shed additional light on the strengths of attachments and existence of vocal buffering in complex social systems.
Acknowledgments
We wish to thank Heather Jensen and the volunteers and staff of the Callitrichid Research Center at UNOmaha for their excellent care of the marmoset colony. We would also like to thank Jeff Fite for comments on this manuscript. The work was supported, in part, by funds from the National Science Foundation (IBN 00-91030) and the National Institutes of Health (HD- HD 42882).
References
- Charrier I, Mathevon N, Jouventin P. Vocal signature recognition of mothers by fur seal pups. Anim Behav. 2003;65:543–550. [Google Scholar]
- Cleveland J, Snowdon CT. The complex vocal repertoire of the adult cotton-top tamarin (Saguinus oedipus oedipus) Z Tierpsychol. 1982;58:231–270. [Google Scholar]
- Coe CL, Mendoza SP, Smotherman WP, Levine S. Mother–infant attachment in the squirrel monkey: adrenal responses to separation. Behav Biol. 1978;22:253–256. doi: 10.1016/s0091-6773(78)92305-2. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- DeVries AC, Glasper ER, Detillion CE. Social modulation of stress responses. Physiol Behav. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- Elowson MA, Snowdon CT. Pygmy marmosets, Cebuella pygmea, modify vocal structure in response to changed social environment. Anim Behav. 1994;47:1267–1277. [Google Scholar]
- Epple G. Comparative studies on vocalization in marmoset monkeys (Hapalidae) Folia Primatol. 1968;8:1–40. doi: 10.1159/000155129. (Basel) [DOI] [PubMed] [Google Scholar]
- French JA, Brewer KJ, Schaffner CM, Schalley J, Hightower-Merritt D, Smith TE, Bell SM. Urinary steroid and gonadotropin excretion across the reproductive cycle in female Wied’s black tufted-ear marmosets (Callithrix kuhlii) Am J Primatol. 1996;40 (3):231–245. doi: 10.1002/(SICI)1098-2345(1996)40:3<231::AID-AJP2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Ginther AJ, Ziegler TE, Snowdon CT. Reproductive biology of captive male cotton-top tamarin monkeys as a function of social environment. Anim Behav. 2001;61:65–78. doi: 10.1006/anbe.2000.1587. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Brodie AR, McClure HM. Effect of a preferred companion in modulating stress in adult female rhesus monkeys. Physiol Behav. 1994;53:681–684. doi: 10.1016/0031-9384(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt K, Todt D. Individual differences in vocalizations of young Barbary macaques (Macaca sylvanus): a multi-parametric analysis to identify critical cues in acoustic signalling. Behaviour. 1995;132:381–399. [Google Scholar]
- Hennessy MB. Hypothalamic–pituitary–adrenal responses to brief social separation. Neurosci Biobehav Rev. 1997;21 (1):11–29. doi: 10.1016/s0149-7634(96)00013-9. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Moorman L. Factors influencing cortisol and behavioral responses to maternal separation in guinea pigs. Behav Neurosci. 1989;103 (2):378–385. doi: 10.1037//0735-7044.103.2.378. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Tamborski PS. The influence of maternal separation on plasma concentrations of ACTH, epinephrine, and norepinephrine in guinea pig pups. Physiol Behav. 1989;45:11452–11472. doi: 10.1016/0031-9384(89)90101-7. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, McInturf SM, Mazzei SJ. Evidence that endogenous corticotropin-releasing factor suppresses behavioral responses of guinea pig pups to brief isolation in novel surroundings. Dev Psychobiol. 1997;31:39–47. doi: 10.1002/(sici)1098-2302(199707)31:1<39::aid-dev4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, O’Leary SK, Hawke JL, Wilson SE. Social influences on cortisol and behavioral responses of preweaning, periadolescent, and adult guinea pigs. Physiol Behav. 2002;76:305–314. doi: 10.1016/s0031-9384(02)00712-6. [DOI] [PubMed] [Google Scholar]
- Heymann E. Behaviour and communication of moustached tamarins, Saguinus mystax mystax (Primates: Callitrichidae), in an outdoor enclosure. Primate Rep. 1987;17:45–52. [Google Scholar]
- Hinde RA, Atkinson S. Assessing the roles of social partners in maintaining mutual proximity, as exemplified by mother–infant relations in rhesus monkeys. Anim Behav. 1970;18:169–176. [Google Scholar]
- Hubrecht RC. Home-range and use and territorial behavior in the common marmoset, Callithrix jacchus jacchus, at the Tapacura field station, Recife, Brazil. Int J Primatol. 1985;6 (5):533–550. [Google Scholar]
- Johnston RE, Bullock TA. Individual recognition by use of odours in golden hamsters: the nature of individual representations. Anim Behav. 2001;61:545–557. [Google Scholar]
- Johnston RE, Jernigan P. Golden hamsters recognize individuals, not just individual scents. Anim Behav. 1994;48:129–136. [Google Scholar]
- Jones BS, Harris DHR, Catchpole CK. The stability of the vocal signature in phee calls of the common marmoset, Callithrix jacchus. Am J Primatol. 1993;31:67–75. doi: 10.1002/ajp.1350310107. [DOI] [PubMed] [Google Scholar]
- Jorgensen D, French JA. Individuality but not stability in marmoset long calls. Ethology. 1998;104:729–742. [Google Scholar]
- Levine S. The influence of social factors on the response to stress. Psychother Psychosom. 1993;60:33–38. doi: 10.1159/000288677. [DOI] [PubMed] [Google Scholar]
- Levine S, Wiener SG, Coe CL. Temporal and social factors influencing behavioral and hormonal responses to separation inmother and infant squirrel monkeys. Psychoneuroendocrinology. 1993;19 (8):297–306. doi: 10.1016/0306-4530(93)90026-h. [DOI] [PubMed] [Google Scholar]
- Mason WA, Mendoza SP. Generic aspects of primate attachments: parents, offspring, and mates. Psychoneuroendocrinology. 1998;23 (8):765–778. doi: 10.1016/s0306-4530(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Mason WA. Parental division of labour and differentiation of attachments in a monogamous primate (Callicebus moloch) Anim Behav. 1986;34:1336–1347. [Google Scholar]
- Mendoza SP, Mason WA, Miner MT, Kaplan J, Levine S. Pituitary–adrenal response to separation in mother and infant squirrel monkeys. Dev Psychobiol. 1978;11:169–175. doi: 10.1002/dev.420110209. [DOI] [PubMed] [Google Scholar]
- Randall JA. Individual foot drumming signatures in banner-tailed kangaroo rats Dipodomys spectabilis. Anim Behav. 1989;38:620–630. [Google Scholar]
- Rukstalis M, Fite JE, French JA. Social change affects vocal structure in a callitrichid primate (Callithrix kuhlii) Ethology. 2003;109:327–340. [Google Scholar]
- Rylands AB. Marmosets and Tamarins: Systematics, Behavior and Ecology. Oxford Univ. Press; Oxford: 1993. [Google Scholar]
- Sayigh LS, Tyack PL, Wells RS, Solow AR, Scott MD, Irvine AB. Individual recognition in wild bottlenose dolphins: a field test using playback experiments. Anim Behav. 1998;57:41–50. doi: 10.1006/anbe.1998.0961. [DOI] [PubMed] [Google Scholar]
- Schaffner CM, Shepherd RE, Santons CV, French JA. Development of heterosexual relationships in Wied’s black tufted-ear marmosets (Callithrix kuhlii) Am J Primatol. 1995;36:185–200. doi: 10.1002/ajp.1350360303. [DOI] [PubMed] [Google Scholar]
- Searby A, Jouventin P, Aubin T. Acoustic recognition in macaroni penguins: an original signature system. Anim Behav. 2004;67:615–625. [Google Scholar]
- Shepherd RE, French JA. Comparative analysis of sociality in lion tamarins (Leontopithecus rosalia) and marmosets (Callithrix kuhlii): responses to separation from long-term pair mates. J Comp Psychol. 1999;113:24–32. [Google Scholar]
- Smith TE, French JA. Psychosocial stress and urinary cortisol excretion in marmoset monkeys (Callithrix kuhlii) Physiol Behav. 1997;62:225–232. doi: 10.1016/s0031-9384(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Smith TE, Schaffner CM, French JA. Social and developmental influences on reproductive function in female Wied’s black tufted-ear marmosets (Callithrix kuhlii) Horm Behav. 1997;31 (2):159–168. doi: 10.1006/hbeh.1997.1380. [DOI] [PubMed] [Google Scholar]
- Smith TE, McGreer-Whitworth B, French JA. Close proximity of the heterosexual partner reduces the physiological and behavioral consequences of novel-cage housing in black tufted-ear marmosets (Callithrix kuhlii) Horm Behav. 1998;34:211–222. doi: 10.1006/hbeh.1998.1469. [DOI] [PubMed] [Google Scholar]
- Snowdon CT, Cleveland J. Individual recognition of contact calls by pygmy marmosets. Anim Behav. 1980;28:717–727. [Google Scholar]
- Snowdon CT, Elowson MA. Pygmy marmosets modify call structure when paired. Ethology. 1999;105:893–908. [Google Scholar]
- Snowdon CT, Hodun A. Acoustic adaptations in pygmy marmoset contact calls: locational cues vary with distances between conspecifics. Behav Ecol Sociobiol. 1981;9:295–300. [Google Scholar]
- Snowdon CT, Cleveland J, French JA. Responses to context- and individual-specific cues in cotton-top tamarin long calls. Anim Behav. 1983;31:92–101. [Google Scholar]
- Stevenson MF, Poole TB. An ethogram of the common marmoset (Callithrix jacchus): general behavioral repertoire. Anim Behav. 1976;24:428–451. doi: 10.1016/s0003-3472(76)80053-x. [DOI] [PubMed] [Google Scholar]
- Symmes D, Newman JD, Talmage-Riggs G, Lieblich AK. Individuality and stability of isolation peeps in squirrel monkeys. Anim Behav. 1979;27:1142–1152. [Google Scholar]
- Vitale A, Zanzoni M, Queyras A, Chiarotti F. Degree of social contact affects the emission of food calls in the common marmoset (Callithrix jacchus) Am J Primatol. 2003;59:21–28. doi: 10.1002/ajp.10060. [DOI] [PubMed] [Google Scholar]
- Wiener SG, Bayart F, Faull KF, Levine S. Behavioral and physiological responses to maternal separation in squirrel monkeys (Saimiri sciureus) Behav Neurosci. 1990;104 (1):108–115. doi: 10.1037//0735-7044.104.1.108. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Sousa MB. Parent–daugther relationships and social controls on fertility in female common marmosets, Callithrix jacchus. Horm Behav. 2002;42:356–367. doi: 10.1006/hbeh.2002.1828. [DOI] [PubMed] [Google Scholar]


