Abstract
All female primates incur energetic costs associated with producing and caring for offspring, but females belonging to the New World primate family Callitrichidae, the marmosets and tamarins, appear to face even further demands. In fact, the energetic demands of rearing callitrichid infants are thought to have led to the evolution of cooperative infant care in these species. If this explanation is true, then one might expect that natural selection should also have shaped patterns of maternal behavior to be sensitive to the costs of reproduction and equipped females to reduce their investment in offspring under certain conditions. Therefore, we examined the maternal effort and postpartum endocrine profiles of individual female marmosets (Callithrix kuhlii) across conditions that represented two hallmarks of callitrichid reproduction—conception during the early postpartum period and alloparental assistance. When females conceived during the early postpartum period and faced the upcoming demands of caring for their newly conceived litters (Study 1), they significantly reduced their caregiving effort and had significantly higher postpartum levels of estradiol relative to breeding attempts in which conception occurred later in the postpartum period. Postpartum estradiol was negatively correlated with maternal carrying effort. When experienced alloparents were present (Study 2), females again reduced their caregiving effort relative to breeding attempts in which experienced alloparents were not present. Postpartum cortisol, however, did not vary as a function of experienced alloparental assistance. The results of these studies suggest that female marmosets have been subjected to similar selection pressures as females of other primate taxa—to maximize their reproductive success by reducing their investment in offspring under the worst and best of conditions—and suggest that hormones may mediate within-female variation in maternal care. These studies also provide support for the notion that mothers are “flexible opportunists” when it comes to providing care to their young.
Keywords: Wied’s black tufted-ear marmoset, Callitrichidae, Maternal care, Postpartum conception, Lactation, Alloparental care
Introduction
All female primates incur energetic costs associated with producing and caring for offspring (Altmann, 1983), but females belonging to the New World primate family Callitrichidae, the marmosets and tamarins, appear to face even further energetic demands. Callitrichid primates are the smallest (100–750 g; Fleagle, 1999) and most fecund (Tardif, 1996) of the anthropoid primates. Females typically produce dizygotictwin litters, which at birth can weigh as much as 15–25% of the female’s own body weight (Kleiman, 1977). Moreover, marmosets and tamarins do not exhibit lactational suppression of ovulation (e.g., Lunn and McNeilly, 1982; French, 1983; Sousa et al., 1999). Instead, a postpartum ovulation occurs two to four weeks following birth (e.g., Ziegler et al., 1990; French et al., 1996a), so that females can conceive while still nursing and carrying their current litter of large, twin infants.
These energetic demands have often been cited as factors necessitating some form of assistance for callitrichid females (e.g., Leutenegger, 1980; Garber, et al., 1984; Goldizen, 1987; Dunbar, 1988; Wright, 1990; Price 1992c), and, indeed, cooperative rearing of offspring is a hallmark of callitrichid reproduction (see review in Tardif, 1996). In every species studied to date, females share the responsibility of infant care with members of their family or social group (e.g., Cleveland and Snowdon, 1984; Goldizen, 1987). Breeding males and alloparents (i.e., older offspring and/or non-related individuals living in the family group) play an active role in every aspect of infant care, except for nursing (e.g., Cleveland and Snowdon, 1984; Goldizen, 1987), and the timing and/or extent of their involvement appears to be controlled by females (Schradin and Anzenberger, 2003).
If the energetic demands of rearing callitrichid infants did lead to the evolution of cooperative breeding in these species, then one might expect that natural selection should also have shaped patterns of maternal behavior to be sensitive to the costs of caring for offspring, and equipped females to reduce their investment in offspring under certain conditions. Decades of research on the reproductive ecology of maternal care have revealed that female mammals, including primates, are neither selfless nor indiscriminate about the care they provide their young (see reviews in Trivers, 1972, 1974; Clutton-Brock, 1991; Hrdy, 1999). Instead, they appear to have been shaped by natural selection to be “flexible opportunists” (Hrdy, 1999), whose motivation to produce and care for their offspring appears to be contingent on complex interactions, or trade-offs, between their ability to invest in current offspring and the probability of producing offspring in the future. When their physical condition is poor and when they have difficulty meeting their own subsistence needs, females may sacrifice their current offspring’s fitness, and possibly survival, in favor of maternal survival and the possibility of producing offspring in the future (Clutton-Brock, 1991; Hrdy, 1999). There is also good reason to believe that females in the best physical condition, and those with abundant resources, might also reduce their overall investment in offspring relative to females in lesser condition and/or with fewer resources available to them (Lee et al., 1991; Fairbanks and McGuire, 1995; Hrdy, 1999). Without jeopardizing offspring survival, these females can improve their own physical condition and prepare for future breeding attempts by providing offspring with the necessary resources in less time and with less effort than other mothers. Thus, one might expect that callitrichid primates have been subjected to similar selection pressures as other mammalian taxa, albeit within the context of their unique reproductive system, to maximize their reproductive success by reducing their investment in offspring under the worst and best of conditions. If this is true, it seems likely that natural selection should have favored callitrichid females equipped to relinquish infant care to other caregivers within their family group, and thereby reduce their behavioral investment in their current litters, under two conditions: 1) when conception occurred during lactation and infant carrying, and 2) when alloparental assistance for rearing offspring was available. To the best of our knowledge, however, within-female shifts in callitrichid caregiving effort in response to these conditions have not been previously explored.
What proximate mechanisms might have been shaped by natural selection to regulate within-female variation in callitrichid maternal behavior? One possibility is that experiential factors—which include the experiences of pregnancy, parturition, and rearing one’s own offspring, as well as the experience of providing care to siblings—might facilitate within-female changes in maternal motivation to care for offspring. Experiential factors are known to exert long-lasting, if not permanent, enhancement effects on female primates’ motivation to care for offspring (see review in Coe, 1990), and callitrichid females are no exception. Parturient and sibling-rearing experience have been shown to positively impact the expression of competent and nurturing maternal care and greatly improve infant survivorship in a variety of callitrichid species (e.g., black lion tamarins, Leontopithecus chrysopygus: French et al., 1996b; common marmosets, Callithrix jacchus: Tardif et al., 1984; cotton-top tamarins, Saguinus oedipus: Kirkwood et al., 1983; Tardif et al., 1984; Bardi et al., 2001; golden lion tamarins, L. rosalia: Hoage, 1978; French et al., 1996b; golden-headed lion tamarins, L. chrysomelas: French et al., 1996b; saddleback tamarins, S. fuscicollis: Epple, 1975). Once the prerequisite experience is gained, however, experiential factors appear to exert little impact on future variability in the expression of infant caregiving behavior. For example, among Wied’s black tufted-ear marmosets (C. kuhlii) with parturient and/or sibling-rearing experience, maternal carrying effort is not correlated with the number of previous litters females had produced (Fite and French, 2000). Washabaugh et al. (2002) examined the parental effort of cotton-top tamarins with sibling-rearing experience and found that maternal carrying effort did not vary with female parity. In fact, the carrying effort of females that had produced at least three sets of surviving litters did not differ significantly from less experienced females that had produced one or fewer surviving litters. Among golden lion tamarins with sibling-rearing experience, neither parity nor age accounted for a significant portion of variance in maternal carrying effort (Bales et al., 2002). During the first postpartum week, primiparous females exhibited a carrying rate (71.7 ± 17%) that was only slightly less than that of multiparous females (73.1 ± 6.5%). Experiential factors, therefore, appear to be unlikely candidates for mechanisms regulating within-female variation in callitrichid maternal care.
A second possibility is that physiological factors—which include the hormonal changes that accompany pregnancy, parturition, and lactation—might facilitate within-female variation in callitrichid maternal behavior and responsiveness. Primate maternal care was long thought to be relatively independent of endocrine processes (e.g., Coe, 1990; Pryce, 1992, 1996; Maestripieri, 1999). However, recent correlational and experimental studies have implicated hormonal factors in the regulation of maternal competency and caregiving motivation in a number of primate species (e.g., humans, Homo sapiens: Fleming et al., 1997a; Japanese macaques, Macaca fuscata: Bardi et al., 2003b; pigtail macaques, M. nemestrina: Maestripieri and Zehr, 1998; rhesus macaques, M. mulatta: Holman and Goy, 1995; savannah baboons, Papio hamadryas: Bardi et al., 2004; western lowland gorillas, Gorilla gorilla gorilla: Bahr, 1995), including marmosets and tamarins (e.g., common marmosets: Pryce, 1993; Pryce et al., 1993, 1995; red-bellied tamarins, S. labiatus: Pryce et al., 1988; Pryce, 1993; Wied’s black tufted-ear marmosets: Fite and French, 2000). There is also good reason to believe that within-female variation in callitrichid maternal caregiving effort corresponds to within-female variation in endocrine status. Utilizing a within-subjects design, Fite and French (2000) compared the endocrine profiles and caregiving effort of female Wied’s black tufted-ear marmosets when their infants did, and did not, survive a minimum of two weeks postpartum. Females exhibited significantly higher prepartum levels of the sex steroid hormone estradiol (E2), and significantly less infant-carrying effort, when their infants did not survive relative to when the same females’ infants did survive. Additionally, prepartum E2 levels were significantly, and negatively, correlated with postpartum carrying effort. To date, however, potential relationships between peripartum hormones and opportunistic, within-female shifts in maternal caregiving effort have not been assessed. For that reason, in this investigation we assessed within-female changes in the caregiving behavior and endocrinological profiles of female marmosets across conditions that represented conception during the early postpartum period (Study 1), and the opportunity to relinquish infant care to experienced alloparents (Study 2).
General methods
Subjects and housing
The subjects of these studies consisted of adult female C. kuhlii, and their families, housed at the University of Nebraska at Omaha’s Callitrichid Research Center. Marmosets were housed in wire mesh enclosures with wooden frames (1.6 × 0.9 × 2.4 m). Each enclosure contained a removable transport cage, natural branches, a feeding platform, a nest box, and a variety of enrichment devices. A 12 hr:12 hr light:dark cycle was controlled by automatic timers, with light onset occurring at 0800 hr. Enclosures containing neighboring groups were at least 1 m apart. Adjoining groups were denied visual, but not auditory or olfactory contact. Our routine husbandry practices were designed to minimize, as much as possible, any disturbance to the normal day-to-day activities of the animals. The marmosets were handled only when it was necessary to administer veterinary care. We also limited their exposure to unfamiliar humans. For further details of animal housing and husbandry, see Schaffner et al. (1995).
Behavioral measures
Observations of parental caregiving behavior were conducted from August 1996 through August 2001. Our observational protocol employed the “all-occurrences” recording technique (Martin and Bateson, 1993) using the Observer 3.0 (Noldus Information Technology, Leesburg, VA, USA) computerized behavioral recording program. Family groups were observed for 20 min five times per week for the first nine weeks of infant life (total observation time per litter, across nine weeks = 15 hr; total observation time for Studies 1 and 2 = 360 hr). Observations were conducted at randomly selected times between 0800 and 1600 hr, but never less than 1 hr preceding, or subsequent to, the morning feeding. All behavioral observations were conducted by one individual (J.E.F.), with whom the animals were familiar. The observer sat approximately 2 m from each cage, and began conducting a behavioral observation after a 10 min habituation period. Four forms of infant caregiving behavior were recorded: 1) Infant carrying: when one or more infants clung to the body or pelage of a parent; 2) Infant acquisition: when a parent actively transferred an infant from another carrier to itself with no resistance from the current carrier; 3) Infant rejection: when a parent removed an infant onto enclosure-wire or some other substrate (other than another animal) by biting, pulling, or rubbing; and 4) Infant grooming: when a parent licked an infant’s body, and when a parent manipulated an infant’s pelage by parting its hair with its hands and removed particles with the hands or mouth.
Given that infant carrying is likely the most costly form of investment that mothers can relinquish to others, we hypothesized that maternal carrying effort would be the form of maternal investment most sensitive to maternal energetics and resource availability (i.e., alloparents). No distinction was made as to whether females and males carried one versus two infants, because infants born into large social groups are not generally carried by the same individual (cotton-top tamarins: Price, 1992c), and because there is evidence that there are few additional energetic costs associated with carrying more than one infant at a time (e.g., Saddle-back tamarins, S.fuscicollis: Goldizen, 1987; cotton-top tamarins: Price, 1992c). We should note, however, that Schradin and Anzenberger (2001) reported a negative correlation between carrying weight and the distance marmosets could leap, such that an animal carrying two infants might have greater difficulty foraging and avoiding predators than an animal carrying one infant. We recorded rates of infant acquisition and infant rejection because we expected that these behaviors would be good indicators of maternal tolerance and motivation to spend time in contact with infants. We recorded rates of infant grooming because grooming is likely to be much less costly than infant carrying. We did not record nursing time, due to the difficulty in distinguishing between “time on the nipple” and suckling.
Urine collection
We collected urine samples two to five times per week from all of the breeding female and male marmosets in this study. A noninvasive, stress-free collection procedure previously described (French et al., 1996a) was utilized. Urine samples were collected between 0600 and 0800 hr, centrifuged at 7000 rpm for 2 min to remove detritus, and the supernatant was then transferred to a clean minivial for storage. All of the samples collected were catalogued and stored at −20°C until assayed.
Study 1: Postpartum conception and maternal care
In Study 1, we evaluated the sensitivity of patterns of maternal care to impending costs associated with the rearing of offspring, and tested the prediction that females would opportunistically reduce their investment in offspring when energetic constraints required them to do so. If females reduce their investment in current offspring in favor of future reproductive attempts when resources are low and/or body condition is challenged (Clutton-Brock, 1991; Lee et al., 1991), then we predicted that marmoset females would invest less in their current litters when they conceived during the period of critical infant dependence (DPCID) than when they conceived after the period of critical infant dependence (APCID). More specifically, we expected that conception DPCID, the time of maximal maternal involvement in infant care, represents a cue for upcoming energetic demands, as well as an opportunity for females to make a behavioral trade-off between investment in current versus future offspring. By investing less time and/or energy in their current litters, females might be better prepared to nurse and carry their newly conceived litters. Furthermore, it seems likely that the cooperative breeding system that is a trademark of callitrichid reproduction and sociality (see review in Tardif, 1996) should allow females to make such trade-offs without any loss of fitness to the current litters, because breeding males and alloparents are clearly equipped to assist them in every form of infant care, except nursing (e.g., Cleveland and Snowdon, 1984; Goldizen, 1987; Tardif, 1996).
To assess the possibility that the endocrine changes accompanying pregnancy might serve as cues for the upcoming costs of rearing newly conceived litters, we evaluated the postpartum excretion of urinary E2. Given previously described increases in levels of urinary E2 that are expected to follow conception (Wied’s black tufted-ear marmosets: Fite and French, 2000), we predicted that females would exhibit higher levels of postpartum urinary E2 when they conceived DPCID relative to breeding attempts in which they conceived APCID. Moreover, we predicted that elevated levels of E2 would be associated with decreased maternal effort.
Methods
Subjects
The selection criterion for individuals to include in this study was the timing of females’ postpartum conception. We identified adult females (n = 6) who gave birth to surviving litters followed by conception DPCID and APCID. All APCID conceptions were preceded by normal ovulatory functioning, as assessed by urinary PdG levels (see “Identification of Conception” below). All DPCID and APCID conceptions resulted in surviving, full-term litters. When more than one DPCID or APCID conception occurred for a female, we randomly selected one conception per female.
To distinguish between DPCID and APCID conditions, we relied on Tardif et al.’s (1998) identification of three distinct phases of early infant development, which emphasizes infants’ reliance on caregivers for nutrition and transport. For this study, conception DPCID was operationally defined as conception during the first three weeks postpartum because infants exhibit very little, if any, independent feeding or locomotion during this time (common marmosets, C. jacchus: Tardif et al., 1998). During this phase of infant development, marmoset females nurse their infants every few hours (common marmosets: Missler et al., 1992), and infants are carried more than 90% of the time by the adult female and other caregivers (common marmosets; cotton-top tamarins; golden lion tamarins, Leontopithecus rosalia; saddle-back tamarins; silvery marmosets, C. argentata; see review in Tardif et al., 1993). This care is energetically costly for females. Lactation is the most energetically expensive component of reproduction for female mammals (see review in Gittleman and Thompson, 1988), and callitrichid females have been observed to lose weight (cotton-top tamarins: Sánchez et al., 1999) and significantly increase their energetic intake (cotton-top tamarins: Kirkwood and Underwood, 1984; saddle-back tamarins: Goldizen, 1987) during lactation. Carrying callitrichid infants comes at a 21% increase in the caloric cost (per minute) of traveling (Tardif, 1996). Infant transport is also associated with other costs, such as decreased foraging and feeding time (e.g., saddle-back tamarins: Goldizen, 1987; cotton-top tamarins: Price, 1992c; also see review in Tardif, 1994) and increased risk of predation (cotton-top tamarins: Price, 1992c; see review in Tardif, 1994). Conception APCID was operationally defined as conception four weeks following birth, and later, as maternal caregiving effort is not expected to be as intensive during this time period. Weeks 4–6 postpartum are a transitional period for infants in which locomotion and feeding becomes increasingly independent (common marmosets: Tardif et al., 1998). By weeks 7–10 postpartum, marmoset infants exhibit locomotion that is completely independent, as well as independent feeding, although some nursing can still occur. Therefore, we presumed females to be maximally involved in the rearing of their current litters when conception occurred DPCID, and less involved when conception occurred APCID.
Demographic data for the females in Study 1 are presented in Table 1. We used paired t-tests and a chi-square test to compare these variables across conditions. Individual females did not differ significantly in age between DPCID and APCID conditions (t(5) = 0.57, NS), nor did their litter sizes (X2(1) = 0.00, NS). Neither the number of potential alloparents nor the number of experienced alloparents present to assist each female in the rearing of offspring differed significantly between conditions (number of potential alloparents: t(5) = −0.42, NS; number of experienced alloparents: t(5) = 0.76, NS). Further, the age of females’ male partners did not vary significantly between DPCID and APCID conditions (t(5) = 0.56, NS).
Table 1.
Demographic data for mothers when they conceived DPCID and APCID
| Female ID | Timing of conception (postpartum week)a |
Female age (yr) | Male age (yr) | Litter size | Number of alloparents/number of experienced alloparents |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Concept. DPCID |
Concept. APCID |
Concept. DPCID |
Concept. APCID |
Concept. DPCID |
Concept. APCID |
Concept. DPCID |
Concept. APCID |
Concept. DPCID |
Concept. APCID |
|
| Bas | 2 | 9 | 3.00 | 3.42 | 2.39 | 2.82 | 2 | 2 | 2/0 | 3/1 |
| Bon | 2 | 4 | 7.33 | 6.91 | 12.97 | 12.55 | 2 | 1 | 2/1 | 1/1 |
| Jin | 2 | 7 | 5.05 | 4.63 | 8.43 | 8.01 | 1 | 2 | 1/2 | 1/0 |
| Luc | 2 | 6 | 4.48 | 3.56 | 4.58 | 3.66 | 1 | 2 | 1/4 | 2/0 |
| Pix | 2 | 7 | 5.78 | 5.36 | 6.42 | 6.01 | 2 | 2 | 2/1 | 1/0 |
| Xux | 2 | 6 | 2.11 | 2.96 | 5.74 | 6.58 | 2 | 1 | 2/0 | 3/2 |
| Mean ± SE | 2.00 ± 0.00 | 6.50 ± 0.67 | 4.63 ± 0.77 | 4.47 ± 0.61 | 6.76 ± 1.49 | 6.61 ± 1.42 | 1.67 ± 0.21 | 1.67 ± 0.21 | 1.67 ± 0.21/1.33 ± 0.61 | 1.83 ± 0.40/0.67 ± 0.33 |
Postpartum week during which conception occurred (e.g., conception during week two postpartum occurred 8−14 days postpartum).
Each of the parents examined had experience caring for infants prior to the commencement of this study. All six females and their male partners had extensive sibling-rearing experience as alloparents. Three females (Bas, Jin, Pix) and their male partners had experience rearing their own offspring.
Hormone assays
Concentrations of E2 and PdG in postpartum urine samples were determined using enzyme immunoassays described and validated for use with C. kuhlii in prior work (E2: Fite and French, 2000; PdG: French et al., 1996a). E2 was extracted from samples with diethyl ether before performing assays (Fite and French, 2000). We measured hormone concentrations in 12 E2 assays and 12 PdG assays. Intra-assay coefficients of variation, determined from duplicate evaluations of pooled marmoset urine run between assays, were 2.03% and 6.91% for high concentration pools and 7.34% and 11.15% for low concentration pools in E2 and PdG assays, respectively. Interassay coefficients of variation, also determined from evaluations of pooled marmoset urine run within assays, were 5.50% and 6.48% for high concentration pools and 9.64% and 17.87% for low concentration pools in E2 and PdG assays, respectively. All hormone concentrations were corrected for the creatinine concentration of each sample. Creatinine concentrations were measured by a modified Jaffé end-point assay (Tietz, 1976), previously described and validated for C. kuhlii (French et al., 1996a).
Identification of conception
We monitored mothers’ urinary PdG concentrations across the first nine weeks of infant life. French et al. (1996a) previously described the pattern of urinary PdG excretion throughout the reproductive cycle in C. kuhlii, and these parameters were used to determine the postpartum week during which conception occurred. Although there were inter-individual differences in urinary PdG concentrations, qualitative changes in PdG profiles across the postpartum period were similar among females in each condition. Elevated urinary PdG excretion, above early postpartum levels, was indicative of postpartum ovulation. For nonconceptive cycles, PdG levels returned to preovulatory follicular levels within 25 days (mean duration in days from successive luteinizing hormone (LH) peaks: 24.9 ± 0.60 days; French et al., 1996a). For conceptive cycles, there was a rapid elevation in urinary PdG excretion during the first 30 days of pregnancy, and PdG levels remained high for the first two trimesters of pregnancy (mean first trimester urinary PdG levels: 33.7 ± 8.4 µg/mg Cr; mean second trimester urinary PdG levels: 39.0 ± 10.9 µg/mg Cr; mean gestation length: 143.1 ± 1.6 days; French et al., 1996a). The timing of each female’s conceptions is presented in Table 1.
Statistics
To compare levels of investment in offspring when females conceived DPCID versus APCID, 3-way mixed ANOVAs (sex × condition × weeks) were conducted on infant carrying effort, infant acquisition, infant rejection, and infant grooming. As breeding males are not faced with the same energetic demands as mothers, males were included in these analyses as a control for factors other than female reproductive energetics that may be influenced by the timing of the postpartum conception. To compare concentrations of urinary E2 when females conceived DPCID and APCID, a 2-way repeated measures ANOVA (condition × week postpartum) was conducted. We used Pearson correlation coefficients to examine the relationship between postpartum hormone levels and maternal behavior. Post hoc analyses were conducted using the Tukey test (Keppel, 1991), and an α level of 0.05 was adopted for all statistical tests. All data are presented as X̅ ± SE.
Results
Postpartum conception and maternal caregiving behavior
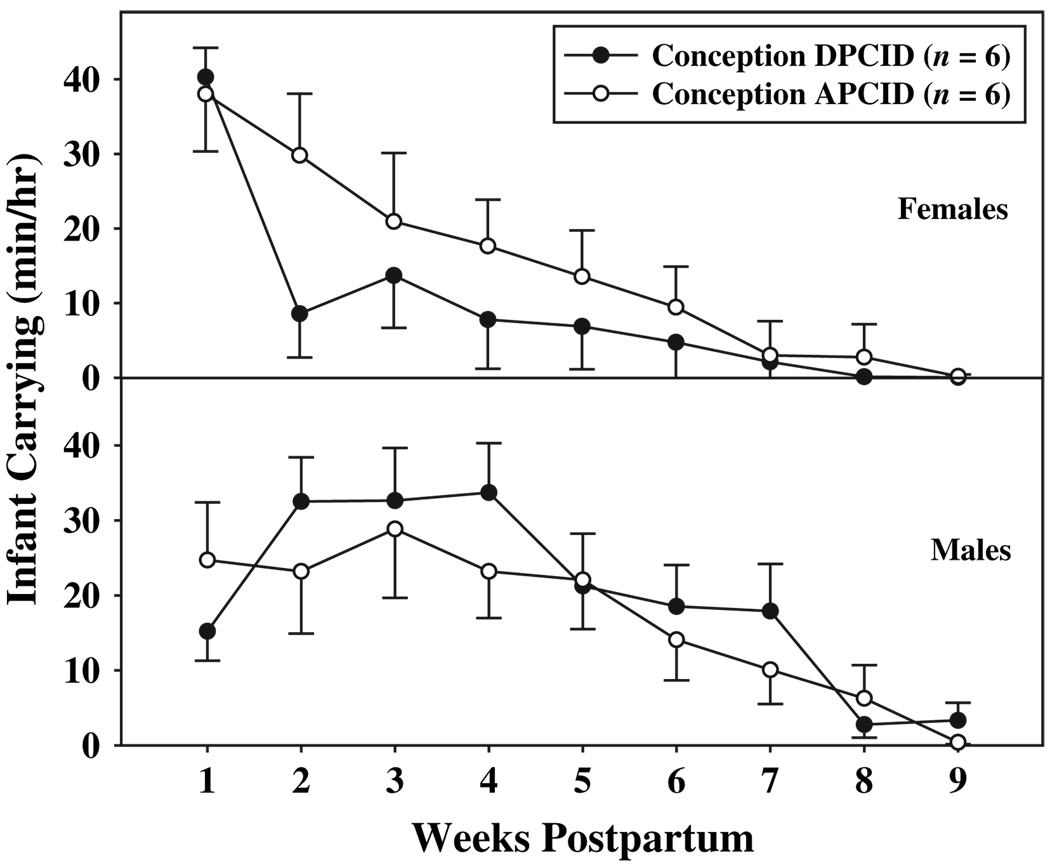
Maternal carrying effort was influenced by the timing of the postpartum conception. In fact, a significant interaction between sex, conception, and weeks (F(8, 80) = 2.16, p < 0.05) indicated that the carrying effort of females and males was differentially influenced by conception DPCID versus APCID. When females conceived DPCID, their carrying effort at week two post-partum was significantly less than their carrying effort when they conceived APCID (Fig. 1; p < 0.05). Further, when females conceived DPCID, their carrying effort at every time-point was significantly less than their effort at week one postpartum (p < 0.05). In contrast, female carrying effort slowly declined across the postpartum period when females conceived APCID, with the only statistical difference occurring between weeks six and seven postpartum (p < 0.05). Male carrying effort did not vary as a function of the timing of conception (Fig. 1). Because the significant interaction between sex, conception, and weeks indicated that these variables did not act alone to influence carrying effort, significant main effects will not be addressed.
Fig. 1.
Mean (±SE) infant carrying effort for females and males across the first nine weeks of infant life, when females conceived DPCID and APCID.
Parental motivation to be in contact with infants was most pronounced during the first few weeks of infant life, when infants were most dependent on their caregivers for nutrition and transport. Rates of infant acquisition differed significantly among weeks (F(8, 80) = 9.47, p < 0.001), such that parents were significantly more likely to acquire infants from other family members during the first four weeks postpartum. Rates of acquisition during weeks 1–4 postpartum (week 1: 0.87 ± 0.18 /hr; week 2: 0.71 ± 0.20 /hr; week 3: 0.88 ± 0.12 /hr; week 4: 0.96 ± 0.17 /hr) were significantly higher than during weeks 7–9 postpartum (week 7: 0.11 ± 0.06 /hr; week 8: 0.00 ± 0.00 /hr; week 9: 0.00 ± 0.00 /hr; p < 0.05). Rates of acquisition did not, however, vary as a function of the timing of the postpartum conception (F(1, 80) = 0.53, NS).
Parents rejected infants with the greatest frequency during the period of infant transition to independent feeding and locomotion. Rates of infant rejection differed significantly between weeks (F(8, 80) = 9.47, p < 0.001), such that rates of rejection increased across weeks 1–5 postpartum and decreased across weeks 6–9 postpartum. The rate of infant rejection at week five postpartum (1.42 ± 0.27 /hr) was significantly higher than at weeks 1–4 (week 1: 0.04 ± 0.04 /hr; week 2: 0.00 ± 0.00 /hr; week 3: 0.43 ± 0.21 /hr; week 4: 0.43 ± 0.21 /hr) and 7–9 postpartum (week 7: 0.86 ± 0.26 /hr; week 8: 0.34 ± 0.13 /hr; week 9: 0.17 ± 0.08 /hr; p < 0.05). Rates of rejection did not vary as a function of the timing of the postpartum conception (F(1, 80) = 0.43, NS).
Variation in infant grooming was independent of weeks and female reproductive status. Grooming effort did not differ significantly among weeks (F(8, 80) = 1.80, NS). Furthermore, it did not differ as a function of the timing of the postpartum conception (F(1, 80) = 1.37, NS).
Postpartum conception and urinary E2 excretion
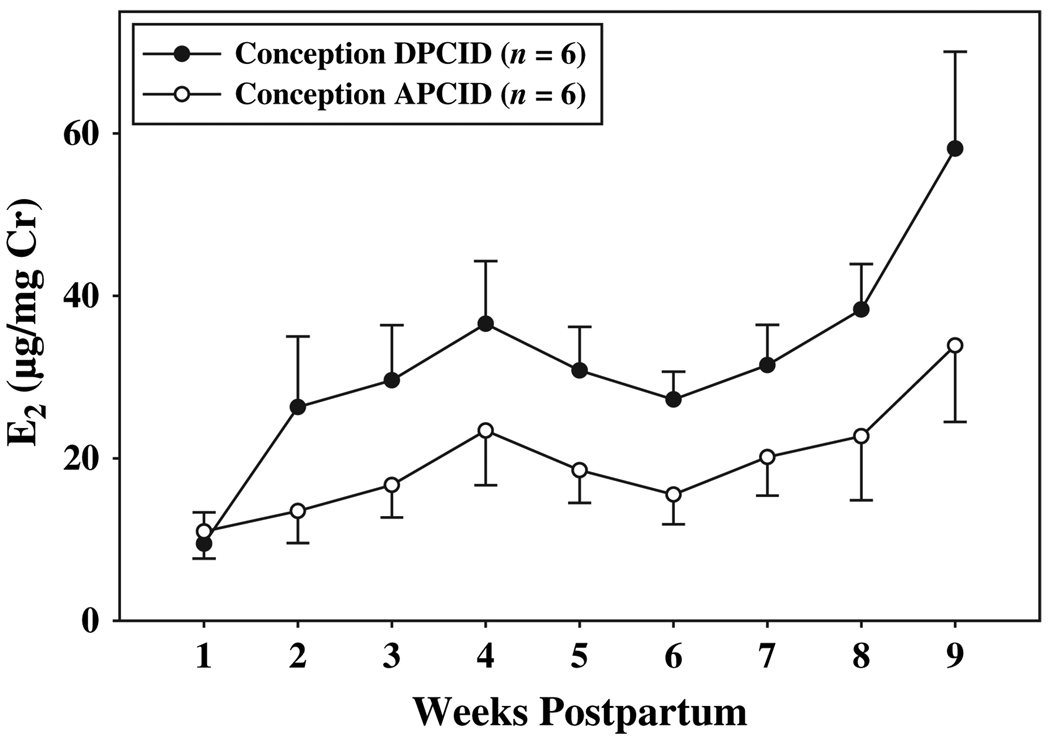
Urinary E2 excretion, which varied across the postpartum period, was related to the timing of conception and the expression of maternal caregiving behavior. Urinary E2 concentrations varied significantly among weeks (F(8, 40) = 6.65, p < 0.001). At week one postpartum, urinary E2 was significantly lower than at any other postpartum time point (10.24 ± 1.527 µg/mg Cr; p < 0.05); E2 steadily increased across the postpartum period, reaching its highest level at week nine postpartum (46.01 ± 5.56 µg/mg Cr). When females conceived DPCID, their overall urinary E2 concentrations were significantly higher (32.00 ± 3.03 µg/mg Cr) than at breeding attempts in which these same females conceived APCID (19.50 ± 3.23 µg/mg Cr; F(1, 5) = 9.07, p = 0.03). Although the main effect of the timing of conception was significant and the interaction between weeks and conception was not, different E2 levels for females in each condition can be accounted for by postpartum changes in E2 excretion that occurred after week one postpartum (Fig. 2).
Fig. 2.
Mean (±SE) weekly concentrations of E2 in the urine of females across the first nine weeks of infant life, when they conceived DPCID and APCID.
Urinary E2 excretion during the postpartum period was related to concurrent maternal behavior when conception occurred DPCID. Overall, females’ weekly postpartum urinary E2 concentrations were negatively and significantly correlated with their concurrent carrying effort (r(108) = −0.30, p < 0.003). However, examination of individual Pearson correlation coefficients (Table 2), by female and condition, reveals that the relationship between E2 and maternal effort varied by condition. When conception occurred DPCID, the maternal caregiving effort of all six females decreased as urinary E2 levels increased. For four females (Bon, Jin, Luc, Xux), the relationship between urinary E2 levels and carrying effort was significant (p < 0.05). For one female (Pix), this relationship approached statistical significance (p = 0.07). When conception occurred APCID, maternal caregiving effort was not found to be significantly related to urinary E2 levels for any female.
Table 2.
Correlations between weekly postpartum urinary E2 excretion and concurrent maternal carrying effort for females, when conception occurred DPCID and APCID
| Female ID | Conception DPCID |
Conception APCID |
||||
|---|---|---|---|---|---|---|
| n | r | p | n | r | p | |
| Bas | 9 | −0.34 | 0.38 | 9 | −0.27 | 0.49 |
| Bon | 9 | −0.87 | 0.003 | 9 | −0.50 | 0.17 |
| Jin | 9 | −0.75 | 0.02 | 9 | −0.50 | 0.18 |
| Luc | 9 | −0.72 | 0.03 | 9 | 0.20 | 0.61 |
| Pix | 9 | −0.63 | 0.07 | 9 | −0.51 | 0.16 |
| Xux | 9 | −0.77 | 0.02 | 9 | 0.53 | 0.14 |
| Mean r | −0.68 | −0.17 | ||||
Study 2: Alloparental assistance and maternal care
In Study 2, we evaluated the sensitivity of patterns of maternal care to the opportunity to relinquish infant care to other family members. We also tested the prediction that females would opportunistically reduce their investment in their current litters, perhaps in favor of future offspring, when they can. Previous investigations of the impact of alloparental assistance on callitrichid maternal care have produced mixed results (see review in Bales et al., 2000). Although studies have indicated that females reduce their caregiving effort when alloparental assistance is available (e.g., cotton-top tamarins: Cleveland and Snowdon, 1984; Ziegler et al., 1990; golden lion tamarins: Bales et al., 2002), there are also reports that females are unaffected by the presence of alloparents (e.g., black lion tamarins, L. chrysopygus: Santos et al., 1997; cotton-top tamarins: Tardif et al., 1990; golden lion tamarins and golden-headed lion tamarins, L. chrysomelas: Santos et al., 1997). Moreover, contradictory to what one might expect, some females might even spend more time carrying infants as group size increases and more alloparents are present (e.g., Wied’s black tufted-ear marmosets and Geoffroy’s marmosets, C. geoffroyi: Santos et al., 1997). We predicted that, if females do, in fact, reduce investment in current offspring in favor of future reproductive attempts when resources allow them to do so (Lee et al., 1991), then marmoset females would invest less in their current litters when experienced alloparents were present.
To assess the possibility that endocrine changes associated with the presence of experienced helpers might be associated with predicted decreases in maternal behavior, we also examined patterns of postpartum urinary cortisol (CORT) excretion in individual females when they did and did not have experienced alloparental assistance. Previous reports have indicated that marmoset family members can be a source of comfort or stress, which is reliably reflected in urinary CORT excretion (Smith and French, 1997a,b; Smith et al., 1998). Therefore, we expected that females’ CORT levels would be affected by the presence of experienced alloparents, although we made no specific prediction regarding the direction of change. Such socially mediated changes in CORT excretion might impact the expression of maternal caregiving behavior. In western lowland gorillas (Gorilla gorilla gorilla), postpartum CORT has been associated with reduced maternal responsiveness (Bahr et al., 1998), and in Japanese macaques (Macaca fuscata) postpartum CORT is associated with more rejecting mothering styles (Bardi et al., 2003a). Among human females, however, postpartum CORT has been positively associated with affectionate contact with infants (Fleming et al., 1997b), as well as maternal responsiveness to infant cues (Stallings et al., 2001).
Methods
Subjects
The selection criterion for individuals to include in this study was the presence or absence of experienced alloparents within females’ family groups. We identified adult female C. kuhlii, housed with their male partners, to which surviving, full-weight twin-litters were born. We then identified females (n = 6) who gave birth to one litter when no experienced alloparents were present in the family group, and one litter when experienced alloparents were present. When more than one “with experienced alloparents” or “without experienced alloparents” reproductive attempt occurred for a female, we randomly selected one reproductive event per female. Five reproductive attempts from four females in Study 1 were included in this study.
In light of numerous reports that competent caregiving behavior increases with age (e.g., common marmosets: Ingram, 1977; Tardif et al., 1986; cotton-top tamarins: Tardif et al., 1986, 1992; Price, 1991, 1992b; but also see Achenbach and Snowdon, 1998; saddle-back tamarins: Epple, 1975) and experience (e.g., common marmosets: Tardif et al., 1984; cotton-top tamarins: Tardif et al., 1984; Johnson et al., 1991; Washabaugh et al., 2002; but also see Achenbach and Snowdon, 1998; golden lion tamarins: Hoage, 1978; saddleback tamarins: Epple, 1975), alloparents must have been present for the birth of at least one litter of younger siblings, and at least one year of age, to be considered experienced. The mean number of previous litters to which alloparents had been exposed was 2.50 ± 0.56 litters. The mean age of experienced helpers was 1.26 ± 0.07 yr.
Table 3 presents demographic data for the females in Study 2. As in Study 1, we used paired t-tests and a chi-square test to compare these variables across conditions. The age of individual females differed significantly between our “with” and “without” experienced alloparents conditions (t(5) = 4.06, p < 0.01). Females were older when they had experienced alloparents living in their family groups than when they did not have experienced alloparents living in their family groups (mean age with experienced alloparents: 4.55 ± 0.39; mean age without experienced alloparents: 3.63 ± 0.48). The age of females’ male partners also varied significantly between conditions (t(5) = 4.06, p < 0.01). Males were older when they had experienced alloparents living in their family groups than when they did not have experienced alloparents living in their family groups (mean age with experienced alloparents: 5.75 ± 0.81; mean age without experienced alloparents: 4.84 ± 0.86). These differences in age were not entirely unexpected, because, in our colony, alloparents were always older offspring residing within their natal groups. As breeding males and females aged, and their families naturally grew in size, the number of alloparents present increased. Neither litter sizes nor the timing of females’ postpartum conceptions varied significantly between conditions (litter size: X2(1) = 2.40, NS; timing of conception: t(5) = 0.07, NS).
Table 3.
Demographic data for mothers with and without experienced alloparental assistance
| Female ID | Female age (yr) | Male age (yr) | Litter size | Number of experienced alloparents |
Timing of Conception (postpartum week)a |
||||
|---|---|---|---|---|---|---|---|---|---|
| Without exp. alloparents |
With exp. alloparents |
Without exp. alloparents |
With exp. alloparents |
Without exp. alloparents |
With exp. alloparents |
Without exp. alloparents |
With exp. alloparents |
||
| Bas | 3.00 | 3.42 | 2.39 | 2.82 | 2 | 2 | 1 | 8 | 2 |
| Ind | 3.55 | 5.14 | 3.62 | 5.21 | 2 | 2 | 4 | 8 | 2 |
| Jin | 4.63 | 5.05 | 8.01 | 8.43 | 2 | 1 | 2 | 2 | 7 |
| Luc | 3.15 | 4.48 | 3.25 | 4.58 | 2 | 1 | 4 | 2 | 7 |
| Pix | 5.36 | 5.78 | 6.01 | 6.42 | 2 | 2 | 1 | 2 | 7 |
| Xux | 2.11 | 3.44 | 5.74 | 7.07 | 2 | 2 | 3 | 6 | 2 |
| Mean ± SE | 3.63 ± 0.48 | 4.55 ± 0.39 | 4.84 ± 0.86 | 5.75 ± 0.81 | 2.00 ± 0.00 | 1.67 ± 0.21 | 2.50 ± 0.56 | 4.67 ± 1.23 | 4.50 ± 1.12 |
Postpartum week during which conception occurred (e.g., conception during week two postpartum occurred 8–14 days postpartum).
Each of the parents in this study had experience caring for infants, even in the “without experienced alloparents” condition. All six females, and their male partners, had extensive sibling-rearing experience as alloparents. Three females (Bas, Jin, Pix) and their male partners had experience rearing their own offspring.
Hormone assays
Concentrations of CORT in postpartum urine samples were determined using enzyme immunoassays described and validated for use with C. kuhlii in prior work (Smith and French, 1997a). We measured hormone concentrations in 12 assays. Intra-assay coefficients of variation, determined from duplicate evaluations of pooled marmoset urine run within assays, were 3.59% for high concentration pools and 6.99% for low concentration pools. Interassay coefficients of variation, also determined from evaluations of pooled marmoset urine run between assays, were 3.46% for high concentration pools and 9.69% for low concentration pools. All hormone concentrations were corrected for the creatinine concentration of each sample. Creatinine concentrations were measured by a modified Jaffé end-point assay (Tietz, 1976), which was previously described and validated for C. kuhlii (French et al., 1996a).
Statistics
To compare levels of investment in offspring when experienced alloparental assistance was and was not available to females, 3-way mixed ANOVAs (sex × condition × weeks) were conducted on infant carrying effort, infant acquisition, infant rejection, and infant grooming. The care-giving behavior of breeding males was again included in these analyses as a control for factors other than female reproductive energetics that may be influenced by the presence of alloparents. To compare concentrations of urinary CORT when females did and did not have experienced alloparental assistance, a 2-way repeated measures ANOVA (condition × week postpartum) was conducted. When significant endocrine differences were indicated, we used Pearson correlation coefficients to examine the relationship between postpartum hormone levels and maternal behavior. Post hoc analyses were conducted using the Tukey test (Keppel, 1991), and an α level of 0.05 was adopted for all statistical tests. All data are presented as X̅ ±SE.
Results
Alloparental assistance and maternal caregiving behavior
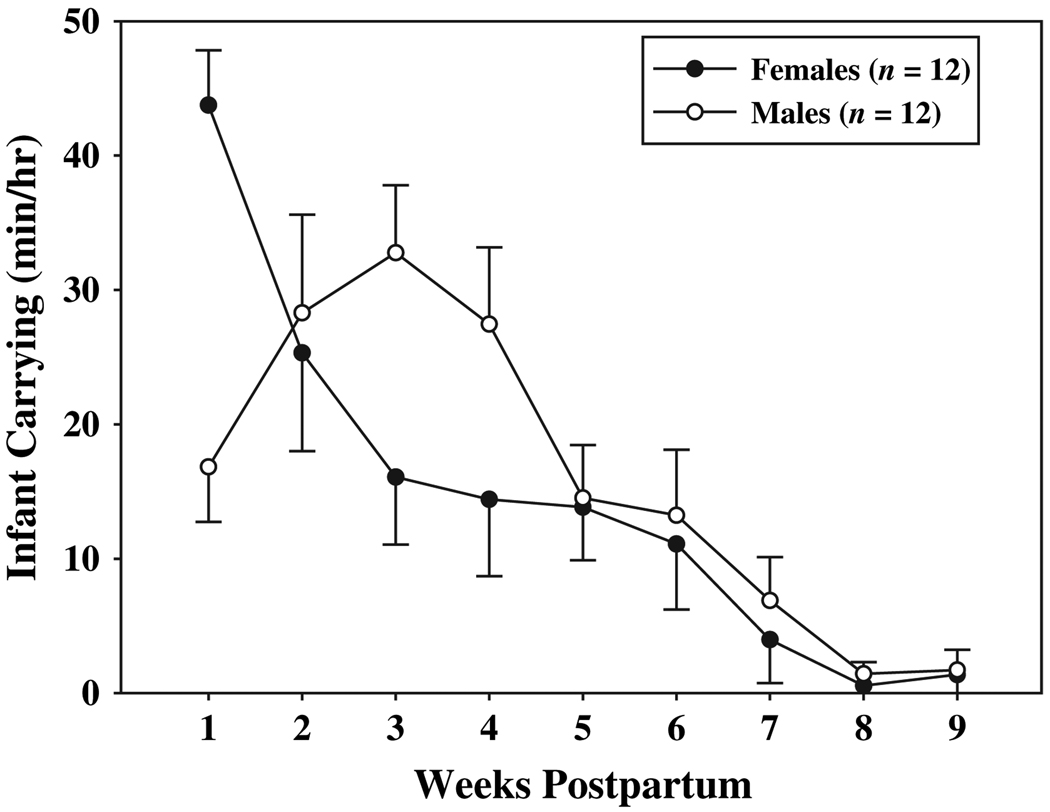
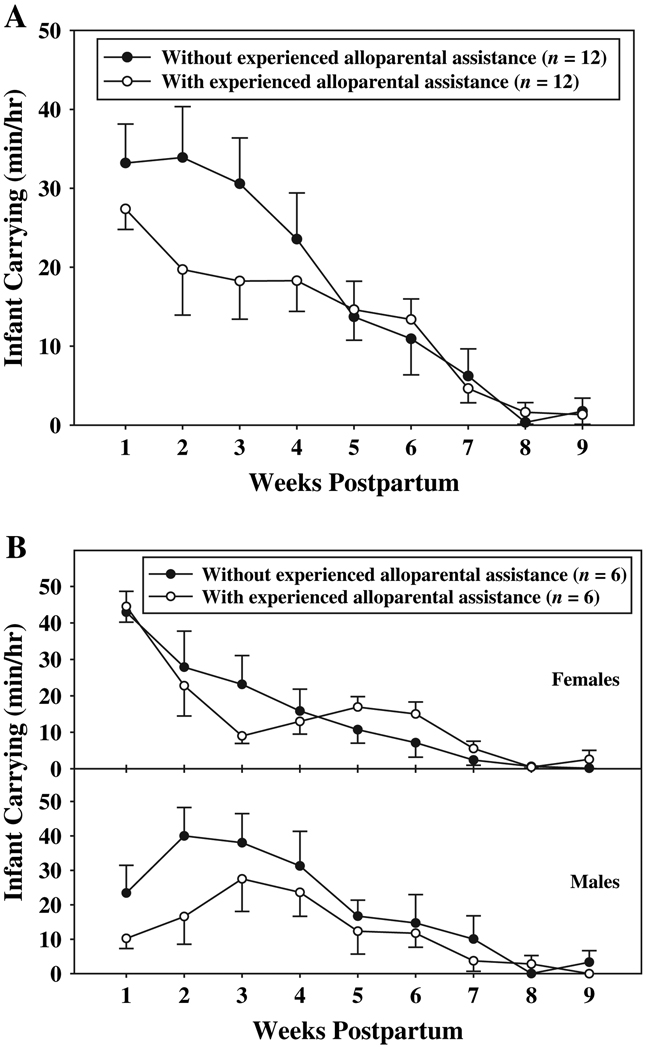
Females and males exhibited reciprocal decreases and increases, respectively, in carrying effort across the postpartum period. A significant interaction between sex and weeks (F(8, 80) = 5.57, p < 0.001) indicated that female and male carrying effort varied among postpartum weeks, such that females were the primary carriers of infants during the first week of infant life (Fig. 3; p < 0.05). By weeks three and four postpartum, however, females reduced their carrying effort to such a degree that they carried their infants at significantly lower rates than fathers (p < 0.05). Except for the first week of infant life, females carried their infants less than males did at every time-point. The carrying effort of both females and males, however, was influenced by the presence of experienced alloparents. A significant interaction between alloparental assistance and weeks [Fig. 4A; F(8, 80) = 2.34, p < 0.03] indicated that the effect of alloparental assistance on parental carrying effort varied across the postpartum period. When experienced alloparents were present to share in the carrying effort, both females and males carried infants significantly less at week two postpartum than when experienced alloparents were not present (p < 0.05). Although the interaction between alloparental assistance and weeks was significant, and the interaction between alloparental assistance and sex was not, in Fig. 4B we illustrate the impact of experienced alloparental assistance on carrying effort of females and males separately to show how female carrying effort was influenced by experienced helpers. At weeks two, three, and four postpartum, females spent less time carrying infants when experienced alloparents were present relative to when experienced alloparents were not present. At weeks 1–7 postpartum, and at week nine postpartum, males spent less time carrying infants when experienced alloparents were present relative to when experienced alloparents were not present. Because the significant interactions between sex and weeks and between alloparental assistance and weeks indicated that these variables did not act alone to influence carrying effort, significant main effects will not be addressed.
Fig. 3.
Mean (±SE) parental carrying effort across the first nine weeks of infant life.
Fig. 4.
A) Mean (±SE) parental carrying effort, across the first nine weeks of infant life, when experienced alloparents were and were not present; B) mean (±SE) infant carrying effort for females and males across the first nine weeks of infant life, when experienced alloparents were and were not present.
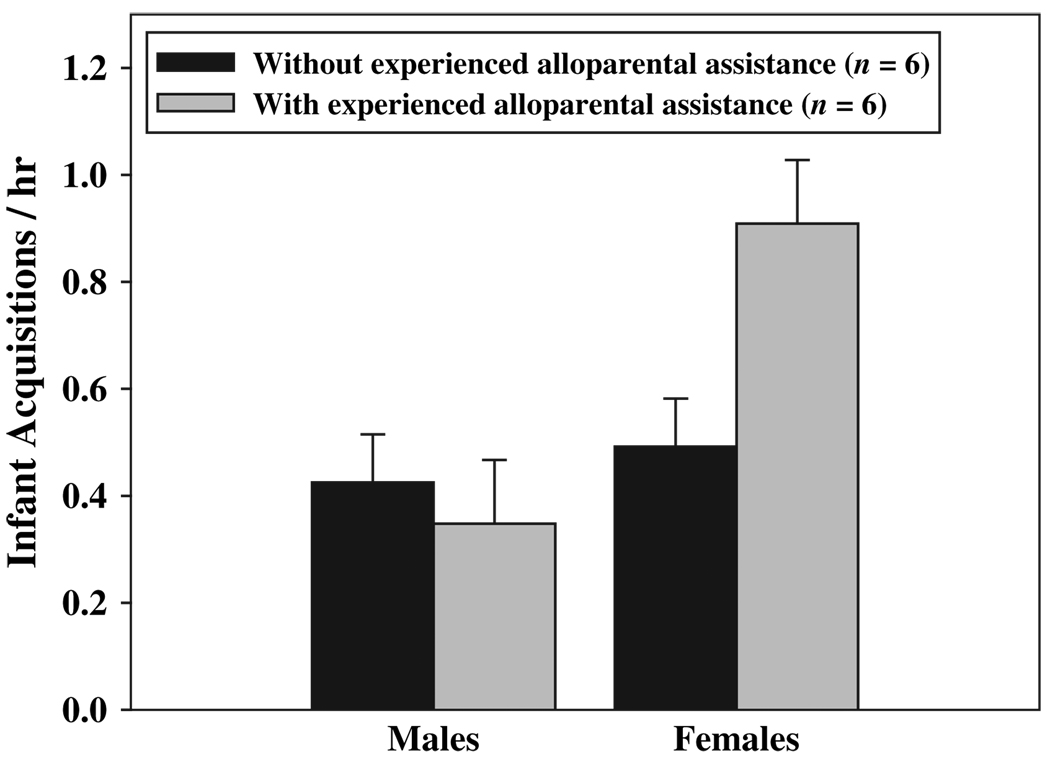
Parental motivation to be in contact with infants was most evident during the first few weeks of infant life. Rates of infant acquisition varied significantly among postpartum weeks (F(8, 80) = 11.19, p < 0.001), such that rates of acquisition at weeks 1–4 postpartum (week 1: 0.76 ± 0.14 /hr; week 2: 0.74 ± 0.18 /hr; week 3: 1.13 ± 0.22 /hr; week 4: 1.01 ± 0.19 /hr) were all significantly higher than at weeks 6–9 postpartum (week 6: 0.30 ± 0.09 /hr; week 7: 0.05 ± 0.03 /hr; week 8: 0.02 ± 0.03 /hr; week 9: 0.00 ± 0.00 /hr; p < 0.05). While both parents exhibited distinct changes in their motivation to be in contact with infants across the postpartum period, rates of infant acquisition for females and males were differentially impacted by the presence of experienced alloparental assistance (Fig. 5). A significant interaction between sex and alloparental assistance indicated that rates of infant acquisition by females and males were dependent on whether or not experienced alloparents were present (F(8, 80) = 5.11, p < 0.05). Females exhibited significantly higher rates of acquisition when experienced alloparents were present relative to breeding attempts in which experienced alloparents were not present (p < 0.05). Male rates of acquisition did not vary as a function of the presence of experienced alloparental assistance.
Fig. 5.
Mean (±SE) rates of infant acquisition for females and males, with and without experienced alloparental assistance, during the first nine weeks of infant life.
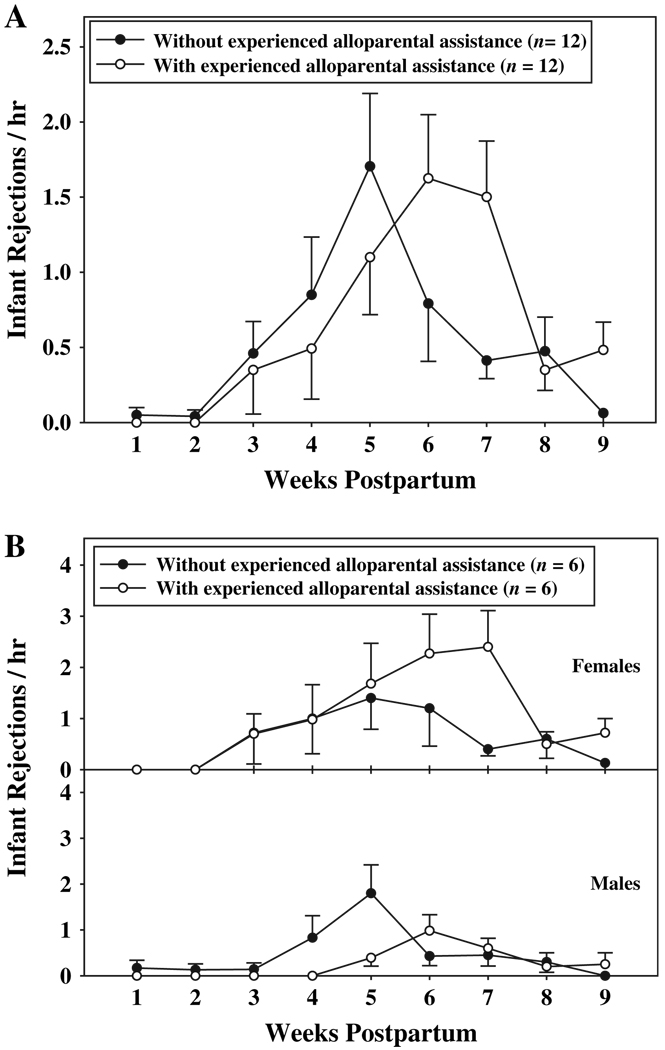
Both females and males exhibited a pattern of increasing intolerance for offspring as infants gained their independence; the timing of this change in parental responsiveness was related to the presence of experienced alloparents. A significant interaction between alloparental assistance and weeks (F(8, 80) = 2.56, p < 0.03) indicated that the impact of alloparental assistance on infant rejection varied among weeks (Fig. 6A). At weeks six, seven, and nine postpartum, parents exhibited higher rates of rejection when experienced alloparents were present (week 6: 1.63 ± 0.42 /hr; week 7: 1.50 ± 0.37 /hr; week 9: 0.48 ± 0.19 /hr) than when experienced alloparents were not present (week 6: 0.79 ± 0.39 /hr; week 7: 0.41 ± 0.12 /hr; week 9: 0.06 ± 0.06 /hr; p < 0.05). Although the interaction between alloparental assistance and weeks was significant, and the interaction between alloparental assistance and sex was not, in Fig. 6B we illustrate the impact of experienced alloparental assistance on the rejection rates of females and males separately to show more clearly how female intolerance of infants was influenced by experienced helpers. At weeks five, six, seven, and nine postpartum, females rejected infants at higher rates when experienced alloparents were present relative to when experienced alloparents were not present. At week six post-partum, males rejected infants at higher rates when experienced alloparents were present relative to when experienced alloparents were not present.
Fig. 6.
A) Mean (±SE) rates of infant rejection by parents, across the first nine weeks of infant life, when experienced alloparents were and were not present; B) mean (±SE) rates of infant rejection for females and males, across the first nine weeks of infant life, when experienced alloparents were and were not present.
Parental grooming of infants was most likely to occur during early infant life. Rates of infant grooming differed significantly among weeks (F(8, 80) = 2.59, p < 0.02). Rates of infant grooming at weeks 1–5 postpartum (week 1: 0.97 ± 0.21 /hr; week 2: 1.07 ± 0.56 /hr; week 3: 1.44 ± 0.51 /hr; week 4: 0.95 ± 0.31 /hr; week 5: 1.20 ± 0.39 /hr) were all significantly higher than at weeks 6–9 postpartum (week 6: 0.65 ± 0.15 /hr; week 7: 0.34 ± 0.18 /hr; week 8: 0.31 ± 0.12 /hr; week 9: 0.21 ± 0.11 /hr; p < 0.05). Rates of infant grooming did not differ significantly as a function of alloparental assistance (F(1, 80) = 4.79, NS).
Alloparental assistance and urinary CORT excretion
Patterns of postpartum urinary CORT excretion did not differ as a function of experienced alloparental assistance (F(1, 5) = 0.15, NS).
Discussion
When female marmosets conceived in the early postpartum period and were faced with the forthcoming energetic demands of lactation and infant carrying, they exhibited swift and dramatic reductions in levels of investment in their current litters relative to breeding attempts in which conception occurred later in the postpartum period. These behavioral changes might have endocrinological underpinnings. The hormonal profiles of females when they conceived during the period of critical infant dependence were characterized by elevated postpartum E2 levels, and postpartum E2 was significantly and negatively correlated with females’ carrying effort during the period of critical infant dependence. When experienced alloparents were present to assist in the rearing of offspring, thereby providing females with the opportunity to reduce the energetic costs associated with infant care and transport, females reduced their carrying effort and rejected their infants more frequently relative to breeding attempts in which there were no other experienced caregivers, other than the breeding male. However, postpartum urinary CORT excretion, when females had experienced alloparental assistance, did not differ from breeding attempts in which these same females did not have experienced helpers. Thus, even in a captive setting, in which marmosets are unlikely to incur the types or magnitude of energetic burdens faced in the wild (i.e., foraging for food and traveling 0.74–1.50 km per day; Tardif, 1994), individual patterns of maternal investment varied from litter to litter, apparently in response to the competing demands of caring for current and future litters.
Why might female marmosets spend less time and energy caring for infants when facing upcoming energetic demands and when they have an opportunity to reduce their investment in offspring? These seemingly conflicting findings are well explained by Lee et al.’s (1991) comparative model of maternal investment. Generated with life-history data from 32 primate species representing both catarrhine and platyrrhine taxa (but no species from the family Callitrichidae), this model states that weaning age, or the time and energy females devote to the care of offspring, is an inverted U-shaped function of maternal condition and resource availability.
Lee et al.’s (1991) model revealed three distinct patterns of weaning, which appear to exist within primate species under different conditions and between species in different habitats. One pattern of early weaning, or reduced maternal investment in offspring, was associated with maternal inability to maintain offspring growth or lactation due to low resource availability or poor physical condition. Indeed, early weaning (e.g., vervet monkeys, Cercopithecus aethiops: Lee, 1984, 1987; Hauser and Fairbanks, 1988), unresponsive and neglectful parenting (e.g., vervet monkeys, Hauser, 1993; human: Belsky et al., 1991; also see review in Maestripieri and Carroll, 2000), infant abuse and abandonment (e.g., humans: Daly and Wilson, 1984; Hrdy, 1999), and infant mortality (e.g., barbary macaques, Macaca sylvanus: Ménard and Vallet, 1993; humans: Daly and Wilson, 1984; Hrdy, 1999) have all been associated with poor maternal condition and/or poor resource availability. In such cases, females may sacrifice their current offspring’s fitness in favor of maternal survival and the possibility of producing offspring in the future. A second pattern of early weaning was associated with high resource availability and good maternal condition. Comparisons of provisioned and non-provisioned populations have revealed that provisioned females exhibit shorter interbirth intervals and lactational periods, live longer, and exhibit higher infant survival rates than non-provisioned conspecifics (see reviews in Paul and Kuester, 1988; Asquith, 1989; Hendrickx and Dukelow, 1995). Additionally, there are reports that females with access to higher quality (but non-provisioned) diets (e.g., vervet monkeys: Hauser and Fairbanks, 1988) and those with high dominance rank (e.g., yellow baboons, Papio hamadryas cynocephalus: Altmann and Samuels, 1992) invest less time and energy in offspring than females in relatively moderate condition and/or access to resources of moderate quality. Thus, by rearing offspring more efficiently and investing less time and energy in the care of offspring, these females can improve their own physical condition and prepare for future reproductive attempts. Finally, a pattern of late weaning was predicted to be associated with moderate food or resource limitations, relatively slow infant growth, and the least rejecting mothering styles. Fairbanks and McGuire (1995) compared the caregiving behavior of vervet monkeys, whose reproductive condition was defined as marginal, prime, or average, and found that females in average reproductive condition were less rejecting and spent more time in ventral-ventral contact with their infants than females in prime or marginal condition. It appears, then, that natural selection has shaped patterns of primate maternal care to be sensitive to the costs of reproduction and equipped females with the ability to reduce their investment in offspring when attempting to reproduce under the worst and best of conditions.
The results of our studies suggest that patterns of marmoset maternal behavior are also sensitive to the costs of caring for offspring, and that female marmosets vary their caregiving effort in response to these costs. In Study 1, female marmosets faced with the impending demands of nursing and carrying newly conceived litters during early infant care significantly reduced their investment in their current litters—perhaps shifting investment away from their current litters to ensure sufficient resources for upcoming litters. Although studies have indicated that callitrichid caregivers sometimes reduce the energetic burden of infant care by spending less time foraging and traveling while transporting infants (e.g., saddle-back tamarins: Goldizen, 1987; cotton-top tamarins: Price, 1992c; see review in Tardif, 1994), and that some may be physically limited in their ability to travel while carrying infants (common marmosets: Schradin and Anzenberger, 2001), our results are consistent with recent reports that energetically challenged callitrichid females may actually provide less care to their offspring than females that are in better condition and/or have access to higher quality resources. Tardif et al. (2001) reported that, among common marmosets, smaller females engaged in fewer nursing bouts than larger females, and Bales et al. (2002) found that females with the lowest body weights also exhibited the lowest levels of carrying effort. Our results are also consistent with evidence that physiological components of callitrichid maternal investment vary with maternal condition. In common marmosets, for example, there was more within- than between-female variation in the number of ova females ovulated per cycle, and individual females ovulated significantly fewer ova at lower body weights than they did at higher body weights (Tardif and Jaquish, 1997). Similarly, Bales et al. (2001) reported that female golden lion tamarins at lower body weights gave birth to smaller litters than females at higher body weights. In a study of the relationship between nursing effort and ovulatory functioning in cotton-top tamarins, Ziegler et al. (1990) found that females nursing twin infants exhibited their first postpartum ovulation later in the postpartum period than females nursing singletons and those not nursing infants. Lactational performance also appears to be sensitive to maternal condition and resource availability (Tardif et al., 2001). Small common marmoset females rearing twins had lower milk fat percentages, lower gross milk energy output, and lower nursing bout frequencies than larger mothers nursing twins. The results of our studies, therefore, complement a growing body of research indicating that a broad array of callitrichid reproductive processes, both behavioral and physiological, are sensitive to the costs of reproduction. Consequently, our findings also suggest that, even in the context of their unique reproductive system, female marmosets have been subjected to similar selection pressures as females of other primate taxa (Lee et al., 1991) to partition their reproductive effort between current and future offspring when energetically challenged, because the investment of time, energy, or resources to the production of current offspring may diminish their ability to invest in future offspring (Trivers, 1972, 1974).
Female marmosets with an opportunity to reduce the costs of rearing their current litters—those with experienced alloparental assistance—also reduced their investment in offspring, possibly in favor of future reproductive opportunities. We found this result to be particularly interesting in light of reports that female common marmosets compensate for the care normally provided by their mates when males were experimentally removed from their family groups (Arruda et al., 1986; Yamamoto et al., 1987) and when normal patterns of paternal care were disrupted by housing changes (Rothe et al., 1993). Even though the females in Study 2 decreased their carrying effort when experienced alloparents were present, they also exhibited increased motivation to acquire infants from other carriers in this condition. It seems likely that the increase in rates of infant acquisition observed among females, but not males, when experienced alloparents were present might reflect the fact that only females are obligated to obtain infants from other caregivers for the purpose of nursing. In fact, there is evidence that alloparental assistance may benefit breeding males more than females. Among golden lion tamarins, for example, male caregiving effort decreased with increasing group size, and male reproductive tenure, but not female reproductive tenure, increased with group size (Bales et al., 2000). Additionally, Achenbach and Snowdon (2002) reported that, among cotton-top tamarins, male weight loss associated with infant care was negatively correlated with the number of alloparents present to assist males.
The results of Study 2 were not surprising given reports indicating that female carrying effort is negatively correlated with group size (common marmosets: Box, 1977; cotton-top tamarins: Cleveland and Snowdon, 1984; Ziegler et al., 1990; golden lion tamarins: Bales et al., 2002). However, an investigation into caregiving behavior in two taxa of callitrichid primates (Callithrix and Leontopithecus) demonstrated that Callithrix females living in large groups (i.e., with more alloparental assistance) spent more time carrying their infants during the first month of infant life than females living in smaller groups (Santos et al., 1997). Further, other studies have found females to be unaffected by the presence of alloparents (e.g., black lion tamarins: Santos et al., 1997: cotton-top tamarins: Tardif et al., 1990; Geoffroy’s marmosets: Santos et al., 1997; golden-headed lion tamarins: Santos et al., 1997; golden lion tamarins: Santos et al., 1997; Bales et al., 2000; Wied’s black tufted-ear marmosets: Santos et al., 1997). One likely explanation for these different findings regarding the impact of alloparents on maternal effort may be the difficulty of disentangling the effects of alloparental assistance from the effects of alloparental age and experience. Several studies have indicated that young alloparents (e.g., common marmosets: Ingram, 1977; Tardif et al., 1986; cotton-top tamarins: Tardif et al., 1986, 1992; Price, 1991, 1992c; saddle-back tamarins: Epple, 1975), and those without prior experience with infant siblings (e.g., common marmosets: Tardif et al., 1984; cotton-top tamarins: Tardif et al., 1984; Washabaugh et al., 2002; golden lion tamarins: Hoage, 1978; Johnson et al., 1991; saddle-back tamarins: Epple, 1975), are less involved in infant care or provide less competent care to infants. In our study, alloparents must have been present for the birth of at least one litter of younger siblings, and at least one year of age, to have been considered experienced, presumably resulting in their being old enough, large enough, and with enough infant-rearing experience to competently assist their mothers.
It was also the case that in Study 2 there were two singleton litters born to females in our “with experienced alloparents” condition, while only twin litters were born to females in our “without experienced alloparents” group. Nonetheless, we do not expect that litter size influenced the changes in maternal behavior that we observed between conditions. Price (1991, 1992a), for example, reported that, in cotton-top tamarins, singletons were carried more than twins and, although individuals are known to compete to care for infants (Pryce, 1988; Price, 1991), adult males and alloparents were unlikely to resist the attempts of adult females to take infants from another carrier for the purposes of transport or nursing (Price, 1991). In our study, females and males spent less time carrying infants when experienced alloparents were present relative to when experienced alloparents were not present. These results are not consistent with what one would expect if litter size had influenced our results.
The studies presented in this paper suggest that hormonal factors may play a role in the regulation of some litter-to-litter changes in marmoset maternal responsiveness and behavior. In Study 1, female marmosets had significantly higher postpartum levels of E2 when they conceived DPCID than when they conceived APCID, and these differences did not occur until the second postpartum week, when females had conceived in the DPCID condition. Furthermore, postpartum E2 was significantly and negatively correlated with maternal carrying effort when conception occurred DPCID. Clearly, experimental studies will be required to determine whether postpartum hormones are directly responsible for litter-to-litter variation in marmoset maternal behavior.
Unfortunately, the design of our study did not allow us to identify the source(s) of the E2 excreted by females when conception occurred DPCID versus APCID. It may be, however, that the earliest differences (i.e., weeks two and three postpartum) in urinary E2 excretion were indicative of implantation having occurred when females conceived DPCID; Heger and Neubert (1983) described a characteristic increase in urinary E2 excretion in common marmosets that occurs with conceptive, but not nonconceptive, cycles and coincides with the timing of implantation. Future investigations into the sources of E2 in early pregnancy and the timing of implantation in Wied’s black tufted-ear marmosets may help to shed light on this issue. To date, there are no published reports on the timing of implantation in this species.
Ours is not the first study to indicate a link between E2 and the expression of primate maternal behavior. Among red bellied tamarins (S. labiatus), for example, prepartum urinary E2 levels were found to be significantly higher in “good”-inexperienced mothers than in “poor”-inexperienced mothers who lost their infants within the first week of infant life due to neglect (Pryce et al., 1988). In an operant setting, nulliparous common marmosets treated with exogenous E2 and progesterone in concentrations designed to mimic those of late-pregnancy exhibited greater motivation to bar-press for infant stimuli than they did when they received a control vehicle (Pryce et al., 1993). Similarly, group-living ovariectomized rhesus macaques treated with early- to mid-pregnancy levels of E2 handled infants at higher rates than untreated ovariectomized females and untreated intact (nonpregnant) females (Maestripieri and Zehr, 1998). There is also reason to believe that prepartum E2 might interfere with the expression of competent, responsive maternal caregiving behavior and the development of socio-emotional attachments to offspring. Among Wied’s black tufted-ear marmosets, for instance, females had higher prepartum urinary E2 concentrations when their infants did not survive a minimum of two weeks postpartum relative to when their infants did survive, and females with higher urinary E2 levels spent less time carrying their infants than females with lower E2 levels (Fite and French, 2000). Additionally, Fleming et al. (1997a) reported that human mothers with low feelings of attachment toward their infants during the early postpartum period had higher plasma E2 levels and higher plasma E2:PdG ratios during early pregnancy than mothers with high attachment. While the majority of these studies have specifically focused on the involvement of prepartum E2 in the priming of females for the postpartum care of offspring, the results of Study 1 suggest an important role for postpartum E2 in the maintenance of maternal motivation following birth.
The postpartum CORT profiles of marmoset females, when they did and did not have experienced alloparental assistance, did not differ significantly. Postpartum CORT has been associated with reduced maternal responsiveness in western lowland gorillas (Bahr et al., 1998). Females with elevated postpartum urinary CORT spent less time in ventro-ventral contact with their infants and spent less time carrying and supporting their infants in a ventral position. In a study of the hormonal correlates of maternal behavior in Japanese macaques, Bardi et al. (2003a) reported that females with high CORT levels were more likely to reject their infants than females with low CORT levels. Also, females with high CORT/E2 ratios were more rejecting and responded to their distress calls more slowly, and they were less responsive to infants, than females with low CORT/E2 ratios. Postpartum CORT has also been associated with elevated maternal responsiveness to newborn infant odors (Fleming et al., 1997b), as well as increased maternal sympathy toward infant cries (Stallings et al., 2001), among human females. Although we were unable to identify a hormonal correlate to the changes in maternal behavior that occurred in the presence of experienced helpers, the results of our study did indicate that the presence of experienced helpers was not reliably associated with an increase or decrease in HPA activity.
Conclusions
The energetic demands of producing and rearing callitrichid infants are often cited as factors necessitating some form of assistance for callitrichid females (e.g., Leutenegger, 1980; Garber, et al., 1984; Goldizen, 1987; Dunbar, 1988; Wright, 1990; Price 1992c), yet the response of individual females to two of the hallmarks of callitrichid reproduction—conceptionduring lactation, and alloparental assistance—have not been previously investigated. The results of the studies presented here suggest that female marmosets are equipped with the ability to relinquish infant care to other caregivers when conception occurs during the early postpartum period and when experienced alloparental assistance is available. Our results also provide further support for the notion that mothers are “flexible opportunists” (Hrdy, 1999) when it comes to providing care to their offspring. Indeed, the females in our studies reduced their investment in offspring under two distinct conditions—when they had to and when they could.
Acknowledgments
This study was a portion of the dissertation research of J.E. Fite in the Department of Psychology at the University of Nebraska at Omaha. The research presented here was described in Animal Research Protocol No. 95-103-07, and was approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center/University of Nebraska at Omaha. Financial support for this project was provided by grants from the National Science Foundation (IBN 97-23842 and 00-91030), the National Institute of Child Health and Development (HD 42882), and from the University of Nebraska at Omaha’s Animal Care Fund.
Contributor Information
Jeffrey E. Fite, Email: jfite@mail.unomaha.edu.
Kimberly J. Patera, Email: kpatera@vet.ksu.edu.
Jeffrey A. French, Email: jfrench@mail.unomaha.edu.
Michael Rukstalis, Email: mrukstalis@mail.unomaha.edu.
Elizabeth C. Hopkins, Email: ehopkins@unmc.edu.
Corinna N. Ross, Email: cross@bigred.unl.edu.
References
- Achenbach GG, Snowdon CT. Response to sibling birth in juvenile cotton-top tamarins (Saguinus oedipus) Behaviour. 1998;135:845–862. [Google Scholar]
- Achenbach GG, Snowdon CT. Costs of caregiving: weight loss in captive adult male cotton-top tamarins (Saguinus oedipus) following the birth of infants. Int. J. Primatol. 2002;23:179–189. doi: 10.1023/A:1013210226793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann J. Costs of reproduction in baboons. In: Apsey WP, Lustick SI, editors. Behavioral Energetics: The Cost of Survival in Vertebrates. Columbus, OH: Ohio State University Press; 1983. pp. 67–88. [Google Scholar]
- Altmann J, Samuels A. Costs of maternal care: infant-carrying in baboons. Behav. Ecol. Sociobiol. 1992;29:391–398. [Google Scholar]
- Arruda MF, Yamamoto ME, Bueno OFA. Interactions between parents and infants, and infant-father separation in the common marmoset (Callithrix jacchus) Primates. 1986;27:215–228. [Google Scholar]
- Asquith PJ. Provisioning and the study of free-ranging primates: history, effects, and prospects. Yearb. Phys. Anthropol. 1989;32:129–158. [Google Scholar]
- Bahr NI. Environmental factors and hormones: their significance for maternal behavior in captive gorillas. In: Pryce CR, Martin RD, Skuse D, editors. Motherhood in Human and Nonhuman Primates. Basel: Karger; 1995. pp. 94–105. [Google Scholar]
- Bahr NI, Pryce CR, Döbeli M, Martin RD. Evidence from urinary cortisol that maternal behavior is related to stress in gorillas. Physiol. Behav. 1998;64:429–437. doi: 10.1016/s0031-9384(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Bales K, Dietz J, Baker A, Miller K, Tardif SD. Effects of allo-caregivers on fitness of parents and infants in callitrichid primates. Folia Primatol. 2000;71:27–38. doi: 10.1159/000021728. [DOI] [PubMed] [Google Scholar]
- Bales K, French JA, Dietz JM. Explaining variation in maternal care in a cooperatively breeding mammal. Anim. Behav. 2002;63:453–461. [Google Scholar]
- Bales K, O’Herron M, Baker AJ, Dietz JM. Sources of variability in numbers of live births in wild golden lion tamarins (Leontopithecus rosalia) Am. J. Primatol. 2001;54:211–221. doi: 10.1002/ajp.1031. [DOI] [PubMed] [Google Scholar]
- Bardi M, French JA, Ramirez SM, Brent L. The role of the endocrine system in baboon maternal behavior. Biol. Psychiatry. 2004;55:724–732. doi: 10.1016/j.biopsych.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Bardi M, Petto AJ, Lee-Parritz DE. Parental failure in captive cotton-top tamarins (Saguinus oedipus) Am. J. Primatol. 2001;54:159–169. doi: 10.1002/ajp.1020. [DOI] [PubMed] [Google Scholar]
- Bardi M, Shimizu K, Barrett GM, Borgognini-Tarli SM, Huffman MA. Peripartum cortisol levels and mother-infant interactions in Japanese macaques. Am. J. Phys. Anthropol. 2003a;120:298–304. doi: 10.1002/ajpa.10150. [DOI] [PubMed] [Google Scholar]
- Bardi M, Shimizu K, Barrett GM, Borgognini-Tarli SM, Huffman MA. Peripartum sex steroid changes and maternal style in Rhesus and Japanese macaques. Gen. Comp. Endocrinol. 2003b;133:323–331. doi: 10.1016/s0016-6480(03)00193-x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization. Child Dev. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Box HO. Quantitative data on the carrying of young captive monkeys (Callithrix jacchus) by other members of their family groups. Primates. 1977;18:475–484. [Google Scholar]
- Cleveland J, Snowdon CT. Social development during the first twenty weeks in the cotton-top tamarin (Saguinus oedipus) Anim. Behav. 1984;32:432–444. [Google Scholar]
- Clutton-Brock TH. The Evolution of Parental Care. Princeton, NJ: Princeton University Press; 1991. [Google Scholar]
- Coe CL. Psychobiology of maternal behavior in nonhuman primates. In: Krasnegor NA, Bridges RS, editors. Mammalian Parenting, Biochemical, Neurobiological, and Behavioral Determinants. New York: Oxford University Press; 1990. pp. 157–183. [Google Scholar]
- Daly M, Wilson M. A sociobiological analysis of human infanticide. In: Hausfater G, Hrdy SB, editors. Infanticide: Comparative and Evolutionary Perspectives. New York: Aldine; 1984. pp. 487–502. [Google Scholar]
- Dunbar RIM. Primate Social Systems. Ithaca, NY: Chapman Hall; 1988. [Google Scholar]
- Epple G. Parental behavior in Saguinus fuscicollis ssp. (Callitrichidae) Folia Primatol. 1975;24:221–238. doi: 10.1159/000155691. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, McGuire MT. Maternal condition and the quality of maternal care in vervet monkeys. Behaviour. 1995;132:733–754. [Google Scholar]
- Fite JE, French JA. Pre- and postpartum sex steroids in female marmosets (Callithrix kuhlii): is there a link with infant survivorship and maternal behavior? Horm. Behav. 2000;38:1–12. doi: 10.1006/hbeh.2000.1607. [DOI] [PubMed] [Google Scholar]
- Fleagle JG. Primate Adaptation and Evolution. second ed. San Diego: Academic Press; 1999. [Google Scholar]
- Fleming AS, Ruble DN, Krieger H, Wong PY. Hormonal and experiential correlates of maternal responsiveness during pregnancy and the puerperium in human mothers. Horm. Behav. 1997a;31:145–158. doi: 10.1006/hbeh.1997.1376. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Steiner M, Corter C. Cortisol, hedonics, and maternal responsiveness in human mothers. Horm. Behav. 1997b;32:85–98. doi: 10.1006/hbeh.1997.1407. [DOI] [PubMed] [Google Scholar]
- French JA. Lactation and fertility: an examination of nursing and interbirth intervals in cotton-top tamarins (Saguinus o. oedipus) Folia Primatol. 1983;40:276–282. doi: 10.1159/000156110. [DOI] [PubMed] [Google Scholar]
- French JA, Brewer KJ, Schaffner CM, Schalley J, Hightower-Merritt D, Smith TE, Bell SM. Urinary steroid and gonadotropin excretion across the reproductive cycle in female Wied’s black tufted-ear marmosets (Callithrix kuhli) Am. J. Primatol. 1996a;40:231–246. doi: 10.1002/(SICI)1098-2345(1996)40:3<231::AID-AJP2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- French JA, Pissinatti A, Coimbra-Filho AF. Reproduction in captive lion tamarins (Leontopithecus): seasonality, infant survival, and sex ratios. Am. J. Primatol. 1996b;39:17–33. doi: 10.1002/(SICI)1098-2345(1996)39:1<17::AID-AJP2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Garber PA, Moya L, Malaga C. A preliminary field study of the moustached tamarin monkey (Saguinus mystax) in northeastern Peru: questions concerned with the evolution of a communal breeding system. Folia Primatol. 1984;42:17–32. [Google Scholar]
- Gittleman JL, Thompson SD. Energy allocation in mammalian reproduction. Am. Zool. 1988;28:863–875. [Google Scholar]
- Goldizen AW. Facultative polyandry and the role of infant-carrying in wild saddle-back tamarins (Saguinus fuscicollis) Behav. Ecol. Sociobiol. 1987;20:99–109. [Google Scholar]
- Hauser MD. Do vervet monkey infants cry wolf? Anim. Behav. 1993;45:1242–1244. [Google Scholar]
- Hauser MD, Fairbanks LA. Mother-offspring conflict in vervet monkeys: variation in response to ecological conditions. Anim. Behav. 1988;36:802–813. [Google Scholar]
- Heger HW, Neubert D. Timing of ovulation and implantation in the common marmoset, Callithrix jacchus, by monitoring of estrogens and 6β-hydroxypregnanolone in urine. Arch. Toxicol. 1983;54:41–52. doi: 10.1007/BF00277814. [DOI] [PubMed] [Google Scholar]
- Hendrickx AG, Dukelow WR. Reproductive biology. In: Bennett BT, Abee CR, Henrickson R, editors. Nonhuman Primates in Biomedical Research. New York, San Diego: Academic Press; 1995. pp. 147–191. [Google Scholar]
- Hoage RJ. Parental care in Leontopithecus rosalia rosalia: sex and age differences in carrying behavior and the role of prior experience. In: Kleiman DG, editor. The Biology and Conservation of the Callitrichidae. Washington, D.C.: Smithsonian Institute Press; 1978. pp. 293–305. [Google Scholar]
- Holman SD, Goy RW. Experiential and hormonal correlates of care-giving in rhesus macaques. In: Pryce CR, Martin RD, Skuse D, editors. Motherhood in Human and Nonhuman Primates. Basel: Karger; 1995. pp. 87–93. [Google Scholar]
- Hrdy SB. Mother Nature: A History of Mothers, Infants, and Natural Selection. New York: Pantheon Books; 1999. [DOI] [PubMed] [Google Scholar]
- Ingram JC. Interactions between parents and infants, and the development of independence in the common marmoset (Callithrix jacchus) Anim. Behav. 1977;25:811–827. [Google Scholar]
- Johnson LD, Petto AJ, Sehgal PK. Factors in the rejection and survival of captive cotton top tamarins. Am. J. Primatol. 1991;25:91–102. doi: 10.1002/ajp.1350250203. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A Researcher’s Handbook. third ed. Englewood Cliffs, NJ: Prentice Hall; 1991. [Google Scholar]
- Kirkwood JK, Epstein MA, Terlecki AJ. Factors influencing population growth of a colony of cotton-top tamarins. Lab. Anim. 1983;17:35–41. doi: 10.1258/002367783781070867. [DOI] [PubMed] [Google Scholar]
- Kirkwood JK, Underwood SJ. Energy requirements of captive cotton-top tamarins (Saguinus oedipus oedipus) Folia Primatol. 1984;42:180–187. doi: 10.1159/000156160. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. Q. Rev. Biol. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Lee PC. Ecological constraints on the social development of vervet monkeys. Behaviour. 1984;91:245–262. [Google Scholar]
- Lee PC. Nutrition, fertility and maternal investment in primates. J. Zool. 1987;213:409–422. [Google Scholar]
- Lee PC, Majluf P, Gordon IJ. Growth, weaning and maternal investment from a comparative perspective. J. Zool. 1991;225:99–114. [Google Scholar]
- Leutenegger W. Monogamy in callitrichids: a consequence of phyletic dwarfism? Int. J. Primatol. 1980;1:95–98. [Google Scholar]
- Lunn SF, McNeilly AS. Failure of lactation to have a consistent effect on interbirth interval in the common marmoset, Callithrix jacchus jacchus. Folia Primatol. 1982;37:99–105. doi: 10.1159/000156023. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. The biology of human parenting: insights from nonhuman primates. Neurosci. Biobehav. Rev. 1999;23:411–422. doi: 10.1016/s0149-7634(98)00042-6. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Carroll KA. Causes and consequences of infant abuse and neglect in monkeys. Aggress. Violent Behav. 2000;5:245–254. [Google Scholar]
- Maestripieri D, Zehr JL. Maternal responsiveness increases during pregnancy and after estrogen treatment in macaques. Horm. Behav. 1998;34:223–230. doi: 10.1006/hbeh.1998.1470. [DOI] [PubMed] [Google Scholar]
- Martin P, Bateson P. Measuring Behaviour: An Introductory Guide. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Ménard N, Vallet D. Population dynamics of Macaca sylvanus in Algeria: an 8-year study. Am. J. Primatol. 1993;30:101–118. doi: 10.1002/ajp.1350300203. [DOI] [PubMed] [Google Scholar]
- Missler M, Wolff JR, Rothe H, Heger W, Merker H-J, Treiber A, Scheid R, Crook GA. Developmental biology of the common marmoset: proposal for a “postnatal staging”. J. Med. Primatol. 1992;21:285–298. [PubMed] [Google Scholar]
- Paul A, Kuester J. Life-history patterns of barbary macaques (Macaca sylvanus) at Affenberg Salem. In: Fa JE, Southwick CH, editors. Ecology and Behavior of Food-Enhanced Primate Groups. New York: Alan R. Liss, Inc; 1988. pp. 199–228. [Google Scholar]
- Price EC. Competition to carry infants in captive families of cotton-top tamarins (Saguinus oedipus) Behav. Brain Res. 1991;118:66–88. [Google Scholar]
- Price EC. The benefits of helpers: effects of group and litter size on infant care in tamarins (Saguinus oedipus) Am. J. Primatol. 1992a;26:179–190. doi: 10.1002/ajp.1350260304. [DOI] [PubMed] [Google Scholar]
- Price EC. Contributions to infant care in captive cotton-top tamarins (Saguinus oedipus): the influence of age, sex, and reproductive status. Int. J. Primatol. 1992b;13:125–141. [Google Scholar]
- Price EC. The costs of infant carrying in captive cotton-top tamarins. Am. J. Primatol. 1992c;26:23–33. doi: 10.1002/ajp.1350260106. [DOI] [PubMed] [Google Scholar]
- Pryce CR. Individual and group effects on early caregiver-infant relationships in red-bellied tamarin monkeys. Anim. Behav. 1988;36:1455–1464. [Google Scholar]
- Pryce CR. A comparative systems model of the regulation of maternal motivation in mammals. Anim. Behav. 1992;43:417–441. [Google Scholar]
- Pryce CR. The regulation of maternal behaviour in marmosets and tamarins. Behav. Processes. 1993;30:201–224. doi: 10.1016/0376-6357(93)90133-C. [DOI] [PubMed] [Google Scholar]
- Pryce CR. Socialization, hormones, and the regulation of maternal behavior in nonhuman simian primates. Adv. Study Behav. 1996;25:423–473. [Google Scholar]
- Pryce CR, Abbott DH, Hodges JK, Martin RD. Maternal behavior is related to prepartum urinary estradiol levels in red-bellied tamarin monkeys. Physiol. Behav. 1988;44:717–726. doi: 10.1016/0031-9384(88)90052-2. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Döbeli M, Martin RD. Effects of sex steroids on maternal motivation in the common marmoset (Callithrix jacchus): development and application of an operant system with maternal reinforcement. J. Comp. Psychol. 1993;107:99–115. doi: 10.1037/0735-7036.107.1.99. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Mutschler T, Döbeli M, Nievergelt C, Martin RD. Prepartum sex steroid hormones and infant-directed behaviour in primiparous marmoset mothers (Callithrix jacchus) In: Pryce CR, Martin RD, Skuse D, editors. Motherhood in Human and Nonhuman Primates. Basel: Karger; 1995. pp. 78–86. [Google Scholar]
- Rothe H, Darms K, Koenig A, Radespiel U, Juenemann B. Long-term study of infant-carrying behavior in captive common marmosets (Callithrix jacchus): effect of nonreproductive helpers on the parents’ carrying performance. Int. J. Primatol. 1993;14:79–93. [Google Scholar]
- Sánchez S, Peláez F, Gil-Bürmann C, Kaumanns W. Costs of infant-carrying in the cotton-top tamarin (Saguinus oedipus) Am. J. Primatol. 1999;48:99–111. doi: 10.1002/(SICI)1098-2345(1999)48:2<99::AID-AJP2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Santos CV, French JA, Otta E. Infant carrying behavior in callitrichid primates: Callithrix and Leontopithecus. Int. J. Primatol. 1997;18:889–908. [Google Scholar]
- Schaffner CM, Shepherd RE, Santos CV, French JA. Development of heterosexual relationships in Wied’s black tufted-ear marmosets (Callithrix kuhli) Am. J. Primatol. 1995;36:185–200. doi: 10.1002/ajp.1350360303. [DOI] [PubMed] [Google Scholar]
- Schradin C, Anzenberger G. Costs of infant carrying in common marmosets, Callithrix jacchus: an experimental analysis. Anim. Behav. 2001;62:289–295. [Google Scholar]
- Schradin C, Anzenberger G. Mothers, not fathers, determine the delayed onset of male carrying in Goeldi’s monkey (Callimico goeldii) J. Hum. Evol. 2003;45:389–399. doi: 10.1016/j.jhevol.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Smith TE, French JA. Psychosocial stress and urinary cortisol excretion in marmoset monkeys (Callithrix kuhli) Physiol. Behav. 1997a;62:225–232. doi: 10.1016/s0031-9384(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Smith TE, French JA. Social and reproductive conditions modulate urinary cortisol excretion in black tufted-ear marmosets (Callithrix kuhli) Am. J. Primatol. 1997b;42:253–267. doi: 10.1002/(SICI)1098-2345(1997)42:4<253::AID-AJP1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Smith TE, McGreer-Whitworth B, French JA. Close proximity of the heterosexual partner reduces the physiological and behavioral consequences of novel-cage housing in black tufted-ear marmosets (Callithrix kuhli) Horm. Behav. 1998;34:211–222. doi: 10.1006/hbeh.1998.1469. [DOI] [PubMed] [Google Scholar]
- Sousa MBC, Silva HPA, Vidal JF. Litter size does not interfere with fertility in common marmosets, Callithrix jacchus. Folia Primatol. 1999;70:41–46. doi: 10.1159/000021674. [DOI] [PubMed] [Google Scholar]
- Stallings J, Fleming AS, Corter C, Worthman C, Steiner M. The effects of infant cries and odors on sympathy, cortisol, and autonomic responses in new mothers and nonpostpartum women. Parent.: Sci. Pract. 2001;1:71–100. [Google Scholar]
- Tardif SD. Relative energetic cost of infant care in small-bodied neotropical primates and its relation to infant-care patterns. Am. J. Primatol. 1994;34:133–143. doi: 10.1002/ajp.1350340205. [DOI] [PubMed] [Google Scholar]
- Tardif SD. The bioenergetics of parental behavior and the evolution of alloparental care in marmosets and tamarins. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge: Cambridge University Press; 1996. pp. 11–33. [Google Scholar]
- Tardif SD, Carson RL, Gangaware BL. Comparison of infant care in family groups of the common marmoset (Callithrix jacchus) and the cotton-top tamarin (Saguinus oedipus) Am. J. Primatol. 1986;11:103–110. [PubMed] [Google Scholar]
- Tardif SD, Carson RL, Gangaware BL. Infant-care behavior of mothers and fathers in a communal-care primate, the cotton-top tamarin (Saguinus oedipus) Am. J. Primatol. 1990;22:73–85. doi: 10.1002/ajp.1350220202. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Carson RL, Gangaware BL. Infant-care behavior of non-reproductive helpers in a communal-care primate, the cotton-top tamarin (Saguinus oedipus) Ethology. 1992;92:155–167. doi: 10.1002/ajp.1350220202. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Harrison ML, Simek MA. Communal infant care in marmosets and tamarins: relation to energetics, ecology, and social organization. In: Rylands AB, editor. Marmosets and Tamarins: Systematics, Behaviour, and Ecology. New York: Oxford University Press; 1993. pp. 220–234. [Google Scholar]
- Tardif SD, Jaquish C, Layne D, Bales K, Power M, Power R, Oftedal O. Growth variation in common marmoset monkeys (Callithrix jacchus) fed a purified diet: relation to care-giving and weaning behaviors. Lab. Anim. Sci. 1998;48:264–269. [PubMed] [Google Scholar]
- Tardif SD, Jaquish CE. Number of ovulations in the marmoset monkey (Callithrix jacchus): relation to body weight, age and repeatability. Am. J. Primatol. 1997;42:323–329. doi: 10.1002/(SICI)1098-2345(1997)42:4<323::AID-AJP7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Power M, Oftedal OT, Power RA, Layne DG. Lactation, maternal behavior and infant growth in common marmoset monkeys (Callithrix jacchus): effects of maternal size and litter size. Behav. Ecol. Sociobiol. 2001;51:17–25. [Google Scholar]
- Tardif SD, Richter CB, Carson RL. Effects of sibling-rearing experience on future reproductive success in two species of Callitrichidae. Am. J. Primatol. 1984;6:377–380. doi: 10.1002/ajp.1350060408. [DOI] [PubMed] [Google Scholar]
- Tietz N. Fundamentals of Clinical Chemistry. Philadelphia: W. Saunders; 1976. [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual Selection and the Descent of Man. Chicago: Aldine-Atherton; 1972. [Google Scholar]
- Trivers RL. Parent-offspring conflict. Am. Zool. 1974;14:249–264. [Google Scholar]
- Washabaugh KF, Snowdon CT, Ziegler TE. Variations in care for cotton-top tamarin, Saguinus oedipus, infants as a function of parental experience and group size. Anim. Behav. 2002;63:1163–1174. [Google Scholar]
- Wright PC. Patterns of paternal care in primates. Int. J. Primatol. 1990;11:89–102. [Google Scholar]
- Yamamoto ME, Arruda MF, Bueno OFA. Compensation in abnormal conditions of infant care in the common marmoset (Callithrix jacchus) Int. J. Comp. Psychol. 1987;1:97–109. [Google Scholar]
- Ziegler TE, Widowski TM, Larson ML, Snowdon CT. Nursing does affect the duration of the post-partum to ovulation interval in cotton-top tamarins (Saguinus oedipus) J. Reprod. Fertil. 1990;90:563–570. doi: 10.1530/jrf.0.0900563. [DOI] [PubMed] [Google Scholar]