Abstract
Estrogen (E) and epidermal growth factors (EGF) receptors were assayed in the liver of nine patients with hepatocellular carcinoma (HCC). Total E and nuclear E receptors were decreased significantly in neoplastic tissue as compared to the levels found in surrounding nonneoplastic tissue. The EGF receptor was decreased also in neoplastic tissue. On the basis of binding data, a decrease in the number but not in affinity of both the E and EGF receptors was found.
Keywords: estrogen, epidermal growth factor receptors, hepatocellular carcinoma
Epidermal growth factor (EGF) is a polypeptide growth factor that was isolated originally from the submaxillary gland. It stimulates DNA synthesis in vitro in a variety of different cells (1–3). This effect is initiated by the interaction of EGF with a specific plasma membrane receptor (4). During the last five years, several lines of evidence have demonstrated that EGF receptor expression can be altered by hormones such as estrogen (E) (4), growth hormone (5), and thyroid stimulating hormone (TSH) (6) in target tissues for these hormones.
The relationship between E and EGF has been studied extensively in normal uterus and in breast cancer tissue (4–7). For example, it has been shown that E stimulates EGF receptor expression in uterine tissue obtained from immature rats (4). In contrast, an inverse relationship exists between the EGF and E receptors content in breast cancer tissues (8). Recently it has been shown that the liver is also a target tissue for sex hormones (9–11). E receptors (ER) appear to play an important role in hepatic regeneration where a strong temporal relationship between increased hepatic DNA synthesis and an increased activity and nuclear distribution of ER have been found. Because both ER and EGF are involved in hepatocyte proliferation, the pattern of E and EGF receptors in tissue samples obtained from patients with hepatocellular carcinoma (HCC) was studied.
MATERIALS AND METHODS
Liver Specimens
Samples of HCC-containing and normal adjacent liver were collected from patients undergoing resection in Milan, Bari, and Pittsburgh. Both tumor and adjacent normal liver tissue were collected and their identities confirmed by histology. The samples were wrapped in aluminum foil, snap-frozen, and stored at −70° C until assayed for their content of E and EGF receptors. Prior studies using fresh and frozen liver have showed that both the E and EGF receptors are stable during frozen storage.
Materials
Radioactive [2,3,6,7-3H]estradiol ([3H]E2), 90 Ci/mmol and [125I]EGF were purchased from New England Nuclear. The purity of the radiolabeled compounds was assessed periodically by thin-layer or column chromatography (12). Sources of other materials were as described previously (12–14).
Binding Studies
The preparation of cytosolic, nuclear, and plasma membrane fractions was as described previously (10, 15). The methods for the estrogen receptor assay (10) and the EGF binding assay have been described previously (15).
Other Methods
Protein concentrations were determined by the method of Lowry et al (16). DNA concentrations of homogenates and the nuclear preparations were determined by the method of Kissane and Robins (17). Statistical analyses were performed using the Hewlett-Packard 9815S. The radioactivity content of tritiated samples was determined using a Packard TriCarb 4530 with an automatic dpm option. ACS scintillant (Amersham) was used for single-phase counting of tritium-containing samples.
RESULTS
The clinical characteristics of the patients studied have been reported in an earlier paper (18). Figure 1 shows the E receptor activity which was quantitated in both the cytosolic and nuclear fraction as well as in whole tissue. The results are expressed as femtomoles E bound per gram wet liver. The binding capacity of the cytosolic ER was different between nonneoplastic and neoplastic tissue. A trend for an increase in cytosoiic E receptors content in cancerous tissue was evident. The nuclear ER content was significantly lower in neoplastic than in nonneoplastic tissue from the same patient (P < 0.05). Additionally total ER content was reduced in the neoplastic tissue as compared to the nonneoplastic tissue (P < 0.025).
Fig 1.
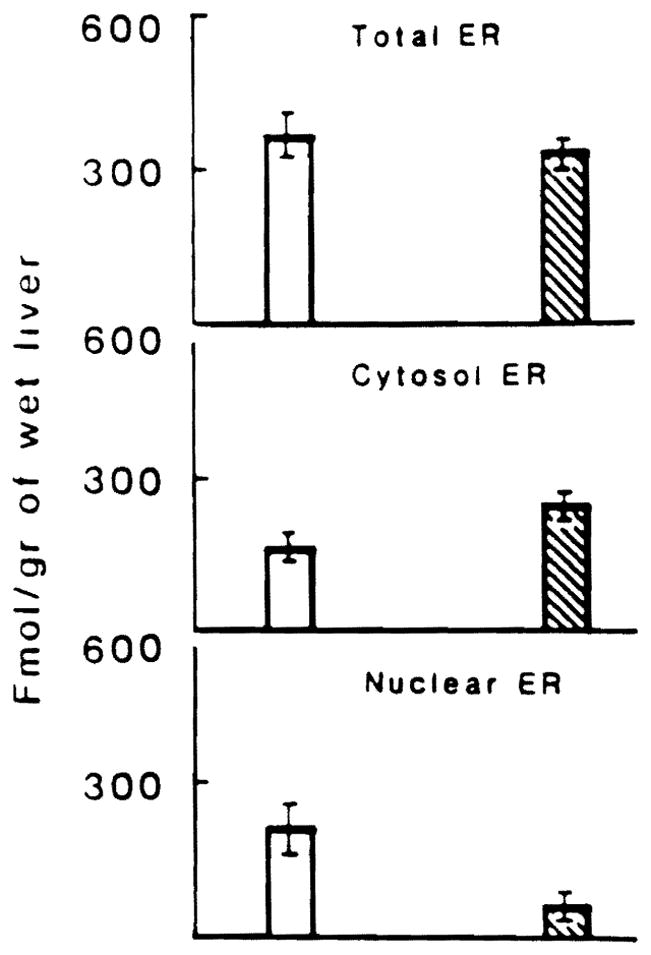
Estrogen receptor activity in HCC ( ) and surrounding normal tissues (
) and surrounding normal tissues ( ). ER activity was measured in cytosoiic (middle panel) and nuclear (lower panel) fractions of HCC and normal liver tissue. Total receptor (top panel) content was calculated by adding the values for nuclear and cytosolic receptor.
). ER activity was measured in cytosoiic (middle panel) and nuclear (lower panel) fractions of HCC and normal liver tissue. Total receptor (top panel) content was calculated by adding the values for nuclear and cytosolic receptor.
Figure 2 shows the EGF binding of plasma membranes isolated from cancerous and surrounding normal hepatic tissue. The values are expressed as femtomoles EGF bound per milligram of membrane protein. It can be seen that hepatic membranes isolated from neoplastic tissue have a lower content of EGF receptor than do membranes isolated from nonneoplastic tissue. The binding affinity data demonstrate that the KD values were similar for normal and neoplastic tissue, demonstrating that the observed reduction in binding is a result of a reduction in number, not affinity, of the receptor.
Fig 2.
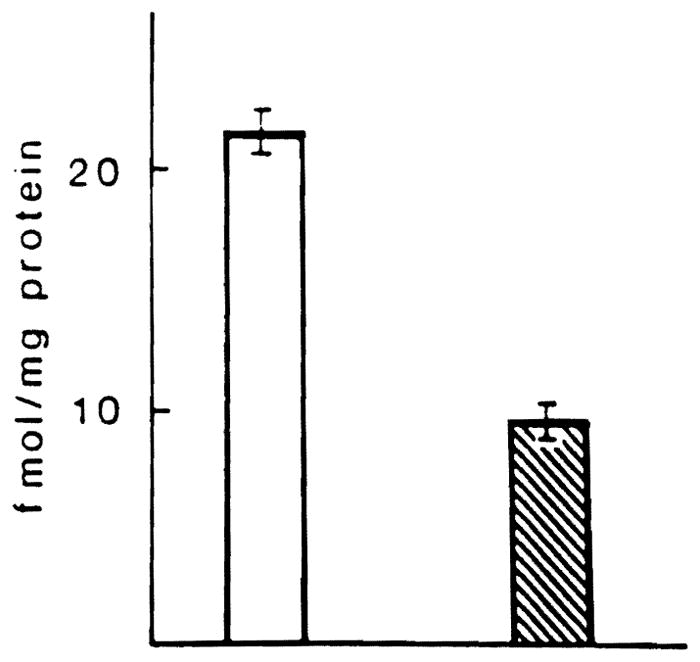
EGF receptor activity in plasma membranes derived from HCC ( ) and normal liver specimens (
) and normal liver specimens ( ).
).
DISCUSSION
Several lines of evidence have demonstrated a direct relationship between E and EGF receptors. First, the administration of estrogen increases the EGF receptor content in the uterus of immature female rats (4). Second, a similar effect has been reported also in normal breast tissue (7). Recently it has been demonstrated that E may play a role in the regulation of hepatic regeneration in that there is a strong temporal relationship between hepatic DNA synthesis and the nuclear ER content of regenerating liver. In this same model, a reduction in the EGF receptor binding capacity of the liver has been shown to correlate with the increase in EGF receptors. These results have been extended recently in an in vitro model where hepatocytes treated with estrogen have been shown to lose their capacity for EGF-induced stimulation of proliferation (19). These data suggest the existence of a relationship between E receptors, EGF receptors, and tissue growth. Several additional lines of evidence indicate that EGF is involved in proliferation of both normal and neoplastic hepatic tissue. Recently it has become clear that EGF is synthesized at several sites in the body (20–22) and that EGF functions more in a paracrine rather than endocrine manner. Considering these facts, it is possible that the regulation of EGF expression in a particular tissue such as the liver might be under endocrine control and help to explain the relationships between TSH and the EGF receptor production in the thyroid, estrogen and the EGF receptor in the uterus, and growth hormone as well as estrogens and the EGF receptor in the liver.
The usual relationship in normal tissue between E and EGF may be altered in neoplastic tissue. In fact, Sainsbury et al (8) recently have reported a significant inverse relationship exists between the activity of the EGF receptor and the E receptor in primary breast cancer tissue. Moreover, EGF receptor was found in tissues with a greater frequency of metastases, presumably having a greater proliferative activity but were E receptor-negative. The results reported here demonstrate that the usual relationship between E receptors and EGF receptors existing in normal tissue is altered in neoplastic hepatic tissue, particularly when there is an overall reduction in the number of EGF receptors (Figure 2).
It is significant that this reduction in EGF receptor content in HCC is proportional to the reduction in ER found in the same tissue and correlates best with a reduction in nuclear E receptor content. It is well known that the nuclear E receptor number is the most biologically important fraction of the total E receptor content of hepatic tissue. The number receptor fraction controls the synthetic activity specific for E (23, 24). In relation to these data it is interesting to note that a reduction in EGF receptor content occurs also in regenerating rat liver but is coupled to an increase in the number of nuclear E receptors. These data demonstrate that in HCC tissue, a reduction in EGF receptor content that is not coupled with an increase in E receptor content occurs. The reasons for and importance of this unusual relationship between E and EGF receptor content in HCC is not known. It may be that, while regenerating rat liver is characterized by rapid proliferation, the growth is tightly regulated and is transient; no permanent growth-controlling alterations in the tissue have occurred and the tissue becomes normal once appropriate growth has concluded. In the case of HCC, the proliferative activity is not regulated by the normal regulatory signals. It is also possible that in neoplastic tissue (25, 26), the EGF receptors are saturated by other growth factors that bind to the receptor such as aipha-TGF, a newly characterized growth substance, which also stimulates cell proliferation via an interaction with the EGF receptor.
Acknowledgments
Supported by the Veterans Administration and Project Grant DK 29961 from the National Institutes of Health, Bethesda, Maryland, and grant 87/01291-44 from Consiglio Nazionale delle Ricerche, Italy.
Footnotes
Presented at the Proceedings of the International Meeting on Normal and Neoplastic Growth in Hepatology, Bari, Italy, June 1989.
References
- 1.Carpenter G. Epidermal growth factor. Handb Exp Pharmacol. 1981;57:90–126. [Google Scholar]
- 2.Carpenter G, Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J Cell Physiol. 1976;88:227–237. doi: 10.1002/jcp.1040880212. [DOI] [PubMed] [Google Scholar]
- 3.King LE, Jr, Carpenter GF. Biochemistry and physiology of the skin. In: Goldsmith LA, editor. Epidermal Growth Factor. 1. New York: Oxford University Press; 1983. pp. 269–281. [Google Scholar]
- 4.Mukku VR, Stancel GM. Regulation of epidermal growth factor receptor by estrogen. J Biol Chem. 1985;260:9820–9824. [PubMed] [Google Scholar]
- 5.Ekberg S, Carlsson L, Carisson B, Billig H, Jansson JO. Plasma growth hormone pattern regulates epidermal growth factor (EGF) receptor messenger ribonucleic acid levels and EGF binding in rat liver. Endocrinology. 1989;125:2158–2167. doi: 10.1210/endo-125-4-2158. [DOI] [PubMed] [Google Scholar]
- 6.Westermark K, Westermark K, Karlsson FA, Ericson LE. Location of epidermal growth factor receptors on procine thyroid follicle cells and receptror regulation by thyrotropin. Endocrinology. 1986;118:1040. doi: 10.1210/endo-118-3-1040. [DOI] [PubMed] [Google Scholar]
- 7.Dickson RB, Lippman ME. Estrogenic regulation of growth and polypeptide growth factor secretion in human breast carcinoma. Endocrinol Rev. 1987;8:29–43. doi: 10.1210/edrv-8-1-29. [DOI] [PubMed] [Google Scholar]
- 8.Sainsbury JRC, Sherbet GV, Farndon JR, Harris AL. Epidermal-growth-factor receptors and oestrogen receptors in human breast cancer. Lancet. 1985;1:364–366. doi: 10.1016/s0140-6736(85)91385-6. [DOI] [PubMed] [Google Scholar]
- 9.Eagon PK, Porter LE, Francavilla A, DiLeo A, Van Thiel DH. Estrogen and androgen receptors in liver: their role in liver disease and regeneration. Semin Liver Dis. 1985;5:59–69. doi: 10.1055/s-2008-1041758. [DOI] [PubMed] [Google Scholar]
- 10.Francavilla A, DiLeo A, Eagon PK, Wu S-Q, Ove P, Van Thiel DH, Starzl TE. Regenerating rat liver: Correlations between estrogen receptor localization and DNA synthesis. Gastroenterology. 1984;86:1410–1416. [PMC free article] [PubMed] [Google Scholar]
- 11.Francavilla A, Eagon PK, DiLeo A, Polimeno L, Panella C, Aquilino AM, Ingrosso M, Van Thiel DH, Starzl TE. Sex hormone related functions in regenerating male rat liver. Gastroenterology. 1986;91:1263–1270. doi: 10.1016/s0016-5085(86)80026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eagon PK, Fisher SE, Imhoff AF, Porter LE, Stewart RR, Van Thiel DH, Lester R. Estrogen-binding proteins of male rat liver: influences of hormonal changes. Arch Biochem Biophys. 1980;201:486–499. doi: 10.1016/0003-9861(80)90537-8. [DOI] [PubMed] [Google Scholar]
- 13.Porter LE, Elm MS, Van Thiel DH, Dugas MC, Eagon PK. Characterization and quantitation of human hepatic estrogen receptor. Gastroenterology. 1983;84:704–712. [PubMed] [Google Scholar]
- 14.Eagon PK, Seguiti SM, Rogerson BJ, Mcguire TF, Porter LE, Seeley DH. Androgen receptor in rat liver: characterization and separation from a male-specific estrogen binding protein. Arch Biochem Biophys. 1989;268:161–175. doi: 10.1016/0003-9861(89)90577-8. [DOI] [PubMed] [Google Scholar]
- 15.Francavilla A, Ove P, Polimeno L, Sciascia C, Coetzee M, Pellici R, Todo S, Kam I, Starzl TE. Different response to epidermal growth factor of hepatocytes in cultures isolated from male or female rat liver: Inhibitor effect of estrogen on binding and mitogenic effect of epidermal growth factor. Gastroenterology. 1987;93:597–605. doi: 10.1016/0016-5085(87)90924-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 17.Kissane JM, Robins E. The fluorometric measurement of dexoyribonucleic acid in animal tissues with specific reference to the central nervous system. J Biol Chem. 1958;233:184–188. [PubMed] [Google Scholar]
- 18.Eagon PK, Francavilla A, DiLeo A, Elm MS, Gennan L, Mazzaferro V, Colella G, Van Thiel DH, Starzl TE. Quantitation of estrogen and androgen receptors in hepatocellular carcinoma and adjacent normal human liver. Dig Dis Sci. doi: 10.1007/BF01307527. (in press) [DOI] [PubMed] [Google Scholar]
- 19.Francavilla A, Polimeno A, DiLeo A, Barone M, Ove P, Coetzee M, Eagon P, Makowka L, Ambrosino G, Mazzaferro V, Starzl TE. The effect of estrogen and tamoxifen on hepatocyte proliferation in vivo and in vitro. Hepatology. 1989;4:614–620. doi: 10.1002/hep.1840090417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elder JB, Williams G, Lacey E, Gregory H. Cellular localization of human urogestrone/epidermal growth factor. Nature. 1978;271:464–467. doi: 10.1038/271466a0. [DOI] [PubMed] [Google Scholar]
- 21.Fallon JH, Loughlin SE, Morrison RS, Bradshaw RA. Epidermal growth factor immunoreactive material in the central nervous system: location and development. Science (Washington, DC) 1984;224:1107–1109. doi: 10.1126/science.6144184. [DOI] [PubMed] [Google Scholar]
- 22.Shikata H, Utsumi N, Hiramatsu M, Minami N, Nemoto N, Skidata T. Immunohistochemical localization of nerve growth factor and epidermal growth factor in guinea pig prostate gland. Histochemistry. 1984;80:411–413. doi: 10.1007/BF00495427. [DOI] [PubMed] [Google Scholar]
- 23.Gustafsson JA, Mode A, Norstedt G, et al. The hypothalam-opituitary-liver axis: A new hormonal system in control of hepatic steroid and drug metabolism. Biochem Action Horm. 1980;7:47–89. [Google Scholar]
- 24.Gustaffson JA, Mode A, Norstedt G, Skett P. Sex steroid induced changes in hepatic enzymes. Annu Rev Physiol. 1983;45:51–60. doi: 10.1146/annurev.ph.45.030183.000411. [DOI] [PubMed] [Google Scholar]
- 25.Twardtik DR, Sherwin SA, Ranchalis J, Todaro GJ. Transforming growth factors in the uterus of normal, pregnant and tumor-bearing humans. J Natl Cancer Inst. 1982;69:793–798. [PubMed] [Google Scholar]
- 26.Fausto N, Mead JE. Biology of disease. Regulation of liver growth: Protoncogenes and transforming growth factors. Lab Invest. 1989;60:4–13. [PubMed] [Google Scholar]


