Abstract
Objective
Anxiety is highly comorbid with depression, but little is known about the impact of anxiety disorders on the effectiveness of empirically supported psychotherapies for depression. We examined such outcomes for people with Multiple Sclerosis (MS) and depression, with versus without comorbid anxiety disorders.
Design
Participants with MS (N = 102) received 16 weeks of telephone-administered psychotherapy for depression and were followed for one year post-treatment.
Results
Participants with comorbid anxiety disorders improved to a similar degree during treatment as those without anxiety disorders. Outcomes during follow-up were mixed, and thus we divided the anxiety diagnoses into distress and fear disorders. The distress disorder (GAD) was associated with elevated anxiety symptoms during and after treatment. In contrast, fear disorders (i.e., panic disorder, agoraphobia, social phobia, specific phobia) were linked to depression, specifically during follow-up, across 3 different measures.
Conclusions
People with GAD receiving treatment for depression may benefit from additional services targeting anxiety more specifically, while those with comorbid fear disorders may benefit from services targeting maintenance of gains after treatment.
Keywords: Depression, Anxiety, Multiple Sclerosis, Tele-Mental Health, Comorbidity, Psychotherapy, Treatment Outcome
Anxiety disorders are highly comorbid with depressive disorders (Mineka et al., 1998), as over half of people with major depressive disorder (MDD) experience at least one comorbid anxiety disorder (Kessler et al., 2003). Thus, there is a need to understand the interplay between such disorders in psychological treatment. In pharmacological treatments of depression, comorbid anxiety symptoms often predict poorer prognosis (e.g., the STAR*D trial; Fava et al., 2008), although counterexamples are frequent (e.g., Lenze et al., 2003) and anxiety is inconsistently operationalized.
Few studies to our knowledge have examined the trajectory of individuals with comorbid anxiety pursuing empirically supported, psychological treatments for depression. In one example, participants referred from primary care who entered interpersonal therapy with comorbid lifetime generalized anxiety disorder (GAD) recovered halfway through treatment at equivalent rates to those without GAD or panic disorder. Participants with comorbid lifetime panic disorder, however, exhibited poorer recovery rates. Small sample sizes reduced power to substantiate this pattern of group differences at treatment cessation. Participants with current GAD and panic disorder were also undifferentiated from those with lifetime history but no current anxiety diagnoses (Brown, Schulberg, Madonia, Shear, & Houck, 1996). Limitations notwithstanding, this study highlights the need to understand the impact of differing anxiety disorders on treatment for depression. In another study, a small (N = 24) sample of participants completing cognitive therapy for MDD benefited similarly in terms of anxious and depressive symptoms, regardless of whether they experienced more comorbid anxiety symptoms at baseline (Gibbons & DeRubeis, 2008). However, neither of these studies included follow-up data.
There is also some suggestion that residual anxiety after the completion of treatment for depression can impact maintenance of gains. In one study, 37% of participants completing cognitive therapy for depression reported residual anxiety symptoms, and these anxiety symptoms predicted greater risk for relapse and recurrence during a 2 year follow-up period. This sample only included participants whose MDD diagnosis remitted after treatment (Taylor, Walters, Vittengl, Krebaum, & Jarrett, 2009), suggesting even people who respond well to treatment for depression often continue to experience anxiety and require additional services.
The prevalence of both anxiety and depressive disorders is often higher among people with chronic medical conditions (Scott et al., 2007). This is particularly true of people with MS (Beiske et al., 2008), the group examined in this study, in which lifetime prevalence of anxiety disorders is 36% (Korostil & Feinstein, 2007) and MDD is around 50% (Sadovnick et al., 1996). As in medically healthy people, the consequences of these disorders for people with MS include higher risk for suicide attempts (Feinstein, 2002) and decreased quality of life (D’Alisa et al., 2006). Depression may also exact a greater toll on the health of people with MS. Depression may affect MS indirectly by decreasing adherence to MS disease modifying medications (Mohr et al., 1997) and more directly by aggravating MS-related immune dysregulation (Foley et al., 1992; Mohr, Goodkin, Islar, Hauser, & Genain, 2001).
In summary, while anxiety disorders are common among individuals with MDD, there is a paucity of research on the impact of anxiety on outcomes during and after psychotherapy for depression. This gap in the literature obscures formulation of clear treatment guidelines for people with both disorders. We examined the effects of comorbid anxiety disorders on outcomes during and after telephone-administered, efficacious psychotherapy for depression among participants with MS and impairment in physical functioning. Anxiety disorders were assessed at baseline, and participants completed well-established measures of depression and anxiety symptoms during treatment and over a 1-year follow-up period. We hypothesized that participants entering treatment with comorbid anxiety disorders would improve on all measures due to the overlap between anxiety and depression. However, we also hypothesized that comorbid anxiety would predict reduced improvement and maintenance of treatment gains. Finally, we expanded on Brown et al.’s (1996) findings of differential effects of GAD and panic disorder on depression treatment by exploring differences between “Fear Disorders” and “Distress Disorders,” a grouping that reflects genetic and phenotypic clustering within the spectrum of anxiety and affective disorders (Watson, 2005; Watson, O’Hara, & Stuart, 2008).
Methods
Participants
This is a secondary analysis of a trial first reported by Mohr et al. (2005). Participants were recruited through Kaiser Permanente Medical Care Group of Northern California (KP) and the National Multiple Sclerosis Society. The consent process was approved by the University of California, San Francisco, and KP Human Subjects Review Committees. Participants provided initial verbal consent over the telephone, followed by written consent via mail. Inclusion criteria for this study were neurologist confirmation of MS diagnosis, functional impairment in physical activity indicated by a score of 3 or more on at least one functional domain assessed by the Guy’s Neurological Disability Scale (GNDS; Sharrack & Hughes, 1999), scores of at least 16 on the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996) and 14 on the Hamilton Depression Rating Scale (HDRS; Hamilton, 1960), and ability to read, write, and speak in English. Participants were excluded if they were under 18 years of age, were presently in psychotherapy, exhibited dementia or severe psychiatric disturbance (e.g., current psychosis), were currently experiencing MS exacerbation, or used medications that can affect mood (e.g. corticosteroids) with the exception of antidepressants.
Measures
Interview based measures were administered via telephone by evaluators who were unaware of participants’ treatment assignment, and self-reports were mailed with stamped return envelopes and instructions to complete them the same day as the telephone assessments. Assessments were administered at baseline, week 16 (treatment cessation), week 40 (6 month follow-up) and week 64 (12 month follow-up). Anxiety and depressive symptoms were also measured at 8 weeks (mid-treatment). Only depressive symptoms were assessed at 3 month (28 week) and 9 month (52 week) follow-ups.
Depression was assessed via 3 different instruments. The HDRS is a widely used, semi-structured interview comprised of depressive symptoms rated by the interviewer for severity (Hamilton, 1960). We used a version adapted for administration over the telephone (Potts, Daniels, Burnam, & Wells, 1990), and monthly reliability checks suggested good inter-rater reliability between our trained assessors (Mean ICC = .89; range = 75 - .97). We also used the BDI-II, a commonly used self-report measure of somatic, cognitive, and emotional symptoms of depression (Beck et al., 1996). Finally, the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 1995) was administered to establish diagnoses of the anxiety disorders and MDD. Telephone administration of the SCID yields high agreement with face-to-face administration (Ruskin et al., 1998; Simon, Revicki, & VonKorff, 1993).
Anxiety disorders were assessed via the SCID at baseline only. Classification of fear and distress disorders was based on Watson’s (2005) hierarchical classification system for the mood and anxiety disorders (see also Watson et al., 2008). Accordingly, participants were classified as having a “Fear Disorder” if they were diagnosed with current Panic Disorder, Agoraphobia, Social Phobia, or Specific Phobia. The fear and distress disorder groups were not mutually exclusive. The distress disorder group was comprised of participants with GAD. Posttraumatic Stress Disorder (PTSD) and Obsessive Compulsive Disorder (OCD), on the other hand, are not easily classified. PTSD and OCD involve diverse symptom clusters, which vary as to their associations with other fear or distress disorders (Watson, 2005). Therefore, PTSD and OCD were not included in either grouping. Participants with PTSD or OCD were removed from analyses involving fear or distress disorders, unless they also had a fear or distress disorder on which to base classification.
In order to control for concurrent anxiety symptoms as well as examine anxiety as an outcome, we used the Anxiety scale of the Hospital Anxiety and Depression Scale (HADS-A; Zigmond & Snaith, 1983). The HADS-A is a self-report measure in which participants rate frequency of various anxiety symptoms.
We also controlled for MS disease-related factors. The GNDS is a structured interview, in which a single score is produced from assessment of 11 basic areas of function (Sharrack & Hughes, 1999). In calculating this total score, an item assessing mood was dropped due to potential confounding with the depression outcome measures. We also assessed MS exacerbation using self-report (Verdier-Taillefer, Roullet, Cesaro, & Alperovitch, 1994).
Treatments
Participants were randomized to receive one of two empirically validated, manualized, 16 week psychotherapies. Randomization was conducted by a biostatistician after determination of eligibility, based upon randomly generated numbers. Randomization was also stratified for current use of antidepressants and MDD diagnostic status, as not all participants reported depressive symptoms meeting full criteria for MDD. As participants had MS-related impairments that could interfere with the ability to appear for regularly scheduled, face-to-face appointments, both treatments were administered via telephone (Hatzakis, Haselkorn, Williams, Turner, & Nichol, 2003). A growing body of literature supports the efficacy of telephone-administered psychological interventions (Mohr, Vella, Hart, Heckman, & Simon, 2008). Telephone-delivered therapy is an efficacious mode of intervention in people with MS (Mohr et al., 2005), and its accessibility is underscored by low attrition rates across a variety of medical populations (Mohr et al., 2008). Telephone-administrated cognitive behavioral therapy (T-CBT) is a structured, skill-focused treatment, guided by a therapist manual (Mohr, 2010a) and supported by a patient workbook (Mohr, 2010b). Telephone-administered supportive emotion-focused therapy (T-SEFT) is supported by a validated, manualized process-experiential treatment (Greenberg, Rice, & Elliot, 1993). Doctoral level clinicians provided the treatments in weekly sessions lasting 50 minutes, and they all had demonstrated allegiance to the treatments they provided. All therapists received two hours of supervision each week from senior clinicians who were experts in the therapy orientation. Therapists’ fidelity to treatment was coded by research assistants unaware of treatment assignment, and these ratings are detailed in the report on the parent trial (Mohr et al., 2005).
Data Analysis
Continuous outcome measures (i.e., the BDI-II, HDRS, and HADS-A) were analyzed using a random intercept and slope linear mixed model to allow for individual variation in participants’ trajectories over time. These analyses were run via SAS 9.2 (SAS Institute Inc., 2002-2008) using restricted maximum likelihood methods. For each dependent variable, time was modeled using a piecewise linear regression with an inflection point at the end of treatment (16 weeks) in order to capture the nonlinear effect of time across treatment and follow-up. Specifically, we used 2 time variables, one representing time during treatment (“Time1”) and the other representing time during follow-up (“Time2”). For all continuous outcomes, modeling both the intercept and the slope of Time1 as random effects significantly improved log likelihood values, over a random intercept alone. Treating the slope of Time2 as a random effect did not significantly improve fit, with the exception of the model for the HDRS involving comorbid anxiety disorders in general (as opposed to fear and distress disorders). Thus, this particular model also treated the slope for Time2 as a random effect, while the other models used only a random intercept and Time1 slope.
Our models included the main effect of comorbid anxiety disorders, as well as the interactions between comorbidity and time. The anxiety disorder variables were dummy-coded such that ‘0’ indicated no comorbid baseline anxiety disorders, while ‘1’ denoted the presence of at least one anxiety disorder. Thus, for the non-comorbid group, the main effect of comorbidity and the interaction terms dropped out of the models. Consequently, the main effects of the time variables reflected the symptom trajectory of the non-comorbid group, and the interaction effects reflected the difference in trajectory of the comorbid group relative to the non-comorbid group. As higher values of all outcome variables represented increased difficulties, a negative interaction effect denoted a more favorable trajectory for the comorbid group, while a positive interaction effect suggested a poorer trajectory for participants with comorbid anxiety. The main effect of comorbidity described the difference in symptoms experienced by the comorbid group relative to the non-comorbid group, independent of time.
We used generalized estimating equations (GEE) logistic regression to examine the binary repeated outcome measure of presence or absence of MDD diagnosis. The predictors included in this model were identical to those in the aforementioned linear mixed models. Using compound symmetry covariance structures produced the lowest Quasi-Likelihood Information Criterion (QIC) values relative to unstructured and autoregressive covariance structures. Thus, for models of MDD diagnosis, compound symmetry structures were used. In all models, we added years since MS diagnosis, concurrent GNDS score, and presence and severity of concurrent MS exacerbation as covariates. In the models for the MDD diagnosis, BDI-II, and HDRS, we also added anxiety symptoms per the HADS-A as a covariate, to control for concurrent anxiety and isolate any latent risk posed by baseline anxiety disorders. We standardized all variables in the models, with the exception of the binary variables representing presence or absence of diagnoses (Cohen, Cohen, West, & Aiken, 2003). All variables were entered simultaneously into the models, and only linear effects were modeled. All variables were also time-variant, with the exception that anxiety disorder diagnosis and number of years since being first diagnosed with MS were assessed only at baseline.
Results
Sample
Of 127 participants in the parent trial (Mohr et al., 2005), 25 could not be classified according to anxiety comorbidity due to potential for false negatives. This was because the full set of primary SCID anxiety modules was not added to the protocol until partway into the trial. Of the resulting sample (N = 102; 45 with comorbid anxiety disorders, 57 without), 9.8% were diagnosed with Panic Disorder, 2.9% with Agoraphobia, 3.9% with Social Phobia, 8.8% with Specific Phobia, 2.9% with OCD, 25.5% with GAD, and 3.9% with PTSD. The sample was 3.9% African American, 2.0% Hispanic, 1.0% Native American, 91.2% Caucasian, and 2.0% of other racial identification. Full-time employees comprised 24.5% of the sample, while 11.8% were unemployed and 54.9% were receiving disability benefits.
Age (M = 47.5, SD = 9.6), percentage of females (80.4%), education level (M = 15.4, SD = 2.7), monthly household income (M = $3803, SD = $2586), number of years since first being diagnosed with MS (M = 11.2, SD = 9.4), and baseline GNDS scores (M = 21.0, SD = 6.0) did not significantly differ between the groups with and without a baseline anxiety disorder (p’s > .15). The comorbid and non-comorbid groups also did not differ in terms of treatment assignment, χ2 (1) = .14, p = .71. The mean number of sessions completed was 14.7 (SD = 2.5) out of 16, and session participation did not appear to vary based on the presence of a comorbid baseline anxiety disorder, t (99) = 1.36, p = .18. Attrition was low and there appeared to be no systematic differences between the comorbid and non-comorbid groups. One participant from the non-anxiety disorder group and 2 participants with baseline anxiety disorders dropped out of the assessment process during treatment. Three participants (1 without baseline anxiety, 2 with baseline anxiety) failed to complete any follow-up assessments after treatment.
Outcomes and Comorbid Anxiety Disorders
Table 1 presents results from our piecewise linear mixed-effects models for changes on each of the study outcomes during treatment and follow-up phases1. As indicated by the main effect of Time1, there were significant improvements on MDD diagnostic frequency and the BDI-II, HDRS, and HADS-A during treatment for participants without comorbid anxiety disorders (p’s < .01). We did not find a significant relationship between comorbid baseline anxiety disorders and rate of improvement during treatment on any outcome measure (p’s > .06). All depression measures were associated with concurrent anxiety symptoms per the HADS-A (p’s < .01). Effects of time during follow-up and comorbidity, as well as the interaction between time during follow-up and comorbidity, varied per outcome measure and are described as follows.
Table 1.
Parameter estimates for the outcome measures of depression and anxiety, by time and baseline anxiety disorder diagnosis (N = 102)
| Outcome Measures | ||||||||
|---|---|---|---|---|---|---|---|---|
| MDD 1 | BDI-II2 | HDRS3 | HADS-A4 | |||||
| β | p | β | p | β | p | β | p | |
| Intercept | −1.29 | <.01** | .05 | .51 | −.02 | .70 | −.24 | .02* |
| MS Exacerbation | .13 | .32 | .04 | .18 | .08 | <.01** | .03 | .40 |
| Years with MS | −.34 | .07 | −.11 | .06 | −.10 | .02* | −.15 | .04* |
| GNDS5 | .24 | .14 | .18 | <.01** | .19 | <.01** | .17 | <.01** |
| Concurrent HADS-A4 | .83 | <.01 ** | .38 | <.01 ** | .36 | <.01 ** | N/A | N/A |
|
| ||||||||
| Time1 | −.52 | <.01 ** | −.17 | < .01 ** | −.28 | <.01 ** | −.21 | <.01 ** |
| Time2 | −.86 | <.01 ** | −.05 | .15 | −.10 | .02 * | −.01 | .89 |
| Anx D/O6 | .43 | .27 | .02 | .87 | .08 | .42 | .45 | <.01 ** |
| Anx D/O * Time1 | −.42 | .10 | −.11 | .06 | −.06 | .29 | −.02 | .77 |
| Anx D/O * Time2 | .79 | .02 * | .13 | <.01 ** | .10 | .12 | .00 | .97 |
MDD = Major Depressive Disorder diagnosis.
BDI-II=Beck Depression Inventory-II (Beck, Steer, & Brown, 1996).
HDRS= Hamilton Depression Rating Scale (HDRS; Hamilton, 1960).
HADS-A= Hospital Anxiety and Depression Scale, Anxiety Scale (Zigmond & Snaith, 1983).
GNDS=Guy’s Neurological Disability Scale (Sharrack & Hughes, 1999).
Anx D/O = Presence or absence of any comorbid anxiety disorder at baseline.
p < .05
p < .01
MDD diagnosis
During follow-up the non-comorbid group decreased in terms of MDD frequency (β = −.86, p < .01). However, the interaction effect between time during follow-up and comorbid baseline anxiety disorder was significant and positive (β = .79, p = .02), indicating the comorbid group did not experience this degree of improvement.
BDI-II
During follow-up, the non-comorbid group did not change in depressive symptoms self-reported on the BDI-II (p = .15). However, there was a modest Time2 by comorbidity interaction effect on the BDI-II (β = .13, p < .01), suggesting participants with baseline anxiety disorders reported more depressive symptoms over time during follow-up.
HDRS
The non-anxious group decreased in depressive symptoms on the HDRS during follow-up (β = −.10, p = .02). However, the interaction effects of comorbid anxiety disorders and time during follow-up on depression were not replicated on the HDRS (p = .12).
HADS-A
The main effect of comorbid baseline anxiety disorders (β = .45, p < .01) suggested those with the comorbidity consistently reported more anxiety, independent of time.
Outcomes and Comorbid Fear or Distress Disorders
We analyzed the differential effects of distress and fear disorders in participants who could be classified by presence or absence of a fear and/or distress disorder (n = 97; 9 with both fear and distress disorders, 14 with only fear disorders, 17 with only the distress disorder, 57 with neither comorbidity). Five participants were not included from the full sample of 102 because they were diagnosed with OCD or PTSD in the absence of any fear or distress disorder on which to base classification. Again, two-tailed t-tests and chi-square tests revealed no significant differences on demographic variables between the groups with and without baseline fear disorders (p’s > .14), and those with and without fear disorders also did not differ in terms of treatment assignment, χ2 (1) = .83, p = .36, or number of sessions completed, t (94) = .97, p = .34. Similarly, those with and without the distress disorder did not differ in demographic variables (p’s > .14), treatment assignment, χ2 (1) = .59, p = .44, or number of sessions attended, t (94) = .67, p = .51.
We utilized identical data analytic procedures, this time replacing the main effect and time interactions involving any anxiety disorder with these more specific variables: 1) presence or absence of GAD at baseline, 2-3) the interactions between GAD at baseline and the two time variables, 4) presence or absence of any fear disorder at baseline, and 5-6) the interactions between fear disorder at baseline and the two time variables2.
Table 2 presents results from these models. Again, all depression measures were associated with concurrent anxiety symptoms on the HADS-A (p’s < .01). These effects were medium to large (Cohen, 1992). The participants with no anxiety disorders still showed significant improvements on all outcomes during treatment (p’s < .01). The non-significant Time1 by comorbidity interaction terms (p’s > .08) were not indicative of differential rates of improvement during treatment for participants with versus without comorbid fear disorders or GAD, excepting that participants with fear disorders improved more rapidly on the BDI-II during treatment than those without (β = −.17, p = .02).
Table 2.
Parameter estimates for the outcome measures of depression and anxiety, by time and baseline GAD or fear disorder diagnosis (n = 97)
| Outcome Measures | ||||||||
|---|---|---|---|---|---|---|---|---|
| MDD 1 | BDI-II2 | HDRS3 | HADS-A4 | |||||
| β | p | β | p | β | p | β | p | |
| Intercept | −1.19 | <.01** | .07 | .38 | −.02 | .73 | −.19 | .06 |
| MS Exacerbation | .09 | .48 | .05 | .06 | .09 | <.01** | .02 | .55 |
| Years with MS | −.35 | .08 | −.12 | .047* | −.09 | .04* | −.17 | .02* |
| GNDS5 | .34 | .04* | .17 | <.01** | .20 | <.01** | .20 | <.01** |
| Concurrent HADS-A4 | .74 | <.01 ** | .38 | <.01 ** | .36 | <.01 ** | N/A | N/A |
|
| ||||||||
| Time1 | −.52 | <.01 ** | −.16 | < .01 ** | −.26 | <.01 ** | −.18 | <.01 ** |
| Time2 | −.72 | <.01 ** | −.04 | .12 | −.09 | .02 * | −.01 | .74 |
| GAD6 | −.48 | .32 | −.15 | .28 | −.12 | .26 | .36 | .048 * |
| GAD * Time1 | −.50 | .09 | .00 | .96 | −.07 | .32 | −.08 | .28 |
| GAD * Time2 | .21 | .48 | .01 | .80 | −.01 | .84 | −.01 | .83 |
| Fear D/O7 | .81 | .08 | .13 | .36 | .24 | .03 * | .21 | .28 |
| Fear D/O * Time1 | −.48 | .08 | −.17 | .02 * | −.07 | .34 | −.03 | .72 |
| Fear D/O * Time2 | .69 | .02 * | .18 | <.01 ** | .13 | .08 | .05 | .47 |
MDD = Major Depressive Disorder diagnosis.
BDI-II=Beck Depression Inventory-II (Beck, Steer, & Brown, 1996).
HDRS= Hamilton Depression Rating Scale (HDRS; Hamilton, 1960).
HADS-A= Hospital Anxiety and Depression Scale, Anxiety Scale (Zigmond & Snaith, 1983).
GNDS=Guy’s Neurological Disability Scale (Sharrack & Hughes, 1999).
GAD=Presence or absence of comorbid Generalized Anxiety Disorder at baseline.
Fear D/O = Presence or absence of at least one comorbid fear disorder at baseline.
p < .05
p < .01
GAD was not associated with differential changes on any outcomes during follow-up (p’s > .47). Main effects of time during follow-up and both comorbidities, as well as the interaction between time during follow-up and fear disorder comorbidity, varied per outcome measure as described below.
MDD diagnosis
During follow-up, participants with neither comorbidity decreased in rates of MDD (β = −.72, p < .01). However, the interaction effect between time during follow-up and comorbid baseline fear disorder was significant and positive (β = .69, p = .02), indicating the group with fear disorders did not experience this degree of improvement. These findings are consistent with graphical inspection (see Figure 1) suggesting participants without comorbid fear disorders at baseline continued to improve in MDD diagnostic status after treatment, whereas those entering treatment with comorbid fear disorders did not. Effects of fear disorders on MDD appear more characteristic of the follow-up phase, as there was only a trend toward a main effect of fear disorders (p = .08). GAD did not exert a significant main effect on MDD (p = .32).
Figure 1.
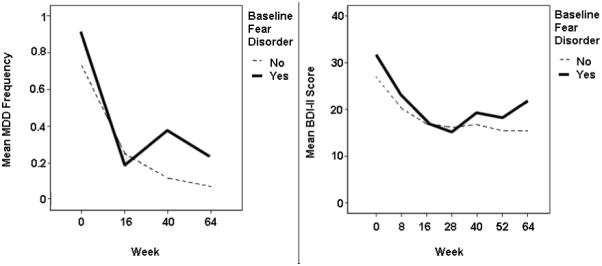
Major Depressive Disorder (MDD) diagnostic frequency and raw Beck Depression Inventory-II (BDI-II) scores over time, split by baseline fear disorder comorbidity.
BDI-II
During follow-up, the non-comorbid group did not change in BDI-II scores (p = .12). However, there was a small to medium(Cohen, 1992) interaction effect, suggesting participants with baseline fear disorders reported more depressive symptoms on the BDI-II over time post-treatment (β = .18, p < .01; see Figure 1). There was not a significant main effect of fear disorders (p = .36) or GAD (p = .28) on BDI-II scores.
HDRS
As in the other 2 depression measures, GAD did not exert a main effect (p = .26) on the HDRS. The non-anxious group evidenced a modest decrease in depressive symptoms on the HDRS during follow-up (β = −.09, p = .02). However, the interaction effect of comorbid fear disorders and time during follow-up was not replicated on the HDRS (p = .08). Rather, there was a medium-sized (Cohen, 1992) main effect of fear disorder such that HDRS scores were higher in the group with fear disorders independent of time (β = .24, p = .03; see Figure 2).
Figure 2.
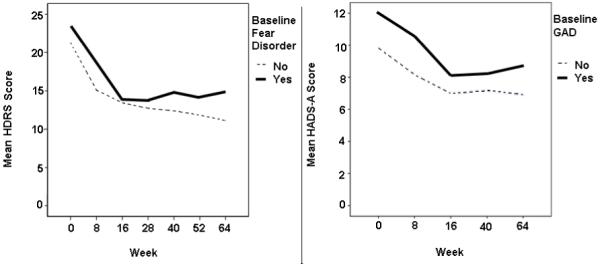
Raw Hamilton Depression Rating Scale (HDRS) and Hospital Anxiety and Depression Scale, Anxiety Scale (HADS-A) scores over time, split respectively by baseline fear and distress disorder (GAD) comorbidity.
HADS-A
In contrast to results on the depression measures, fear disorders did not exert a main effect (p = .28) or interaction effect with time during follow-up (p = .47) on anxiety symptoms per the HADS-A. There was, however, a medium to large (Cohen, 1992) main effect of comorbid GAD (β = .36, p < .05), suggesting those with this comorbid distress disorder consistently reported more anxiety symptoms independent of time (see Figure 2).
Discussion
Compared to those without anxiety disorders, there was no evidence that participants with comorbid anxiety disorders at baseline responded differently to treatment on the depressive and anxiety outcome measures. Favorable response during treatment in participants with comorbid anxiety disorders is in contrast with some studies on pharmacotherapy suggesting people with comorbid anxiety have slower treatment response and are less likely to remit (e.g., Fava, 2008; Brown et al., 1996). The data during the follow-up period were mixed, suggesting increased depression in those entering treatment with anxiety disorders on 2 out of 3 depression measures. Participants with comorbid anxiety disorders also experienced more initial and residual anxiety symptoms, which is concerning given our findings that concurrent anxiety symptoms were moderately to strongly associated with all the depression outcomes.
We also examined whether the observed relationships between baseline comorbid anxiety disorders and treatment outcomes were due to fear or distress disorders, and whether these subsets of anxiety disorders could clarify the inconsistencies regarding comorbid anxiety disorders and post-treatment depression outcomes. Again, there was no suggestion that participants with comorbid fear disorders or GAD at baseline improved less during treatment than participants without these comorbidities. The data during the follow-up period, however, consistently suggested participants with baseline fear disorders experienced more depression. Participants without comorbid fear disorders continued to remit in terms of MDD diagnosis after treatment cessation, while those entering treatment with fear disorders did not. Participants with comorbid fear disorders also worsened on the BDI-II over time during follow-up. On the HDRS interview measure of depressive symptoms, fear disorders were associated with depressive symptoms severity independent of time. However, graphical inspection (see Figure 2) suggested that, like the other depression measures, participants with fear disorders ended treatment with similar symptom severity as those without comorbid fear disorders, and these two groups diverged again during follow-up. Thus, findings converged across measures to indicate fear disorders may not affect response to depression treatment, but do constitute a risk for poorer maintenance of gains following treatment cessation.
In contrast to results on the fear disorders, participants with a comorbid distress disorder (i.e., GAD in this study) did not evidence increased depressive symptoms during treatment or follow-up. Overlap between GAD and MDD is striking enough that it has been proposed they both be included together as “Distress Disorders” (Watson, 2005; Watson et al., 2008), while fear disorders introduce more distinct concerns that may have reduced depression-focused work during therapy sessions and resulted in more transient improvements in depression. However, GAD was associated with increased anxiety symptoms on the HADS-A during both treatment and follow-up. Thus, additional services may be indicated for people in depression treatment with comorbid fear disorders, as well as those with comorbid GAD. These supplemental interventions, however, may differ based on the type of comorbid anxiety disorder.
Targeting symptoms of anxiety more specifically may attain greater levels of symptom remission for people with GAD. In contrast, people with comorbid fear disorders may potentially benefit from booster sessions, longer protocols to assist in maintaining their gains, or treatments that more directly address the fears that appear to impact maintenance of gains. It remains a potentially fruitful topic of study to determine which of these treatment modifications, alone or in combination, might improve outcomes for those with fear disorders and depression. An alternative strategy would be to apply transdiagnostic psychotherapy (Allen, McHugh, & Barlow, 2008; Fairburn, Cooper, & Shafran, 2003), a promising but largely untested (Clark, 2009) approach that targets common factors underlying comorbid disorders (Barlow, Allen, & Choate, 2004). This strategy may help individuals with both types of comorbid anxiety disorders to apply knowledge gained in treatment more readily to their anxiety symptoms or other difficulties. Indeed, the latent risk for depression posed by baseline fear disorder, even controlling for concurrent anxiety symptoms, does suggest a broader, transdiagnostic mechanism meriting attention.
Session completion rates did not appear to differ based on anxiety comorbidity, and this may be related to the telephone-based treatment delivery format which is associated with low attrition for individuals with a variety of medical conditions (Mohr et al., 2008). The current findings provide preliminary evidence suggesting telephone-administered depression treatment may help to overcome not only illness-related barriers to face-to-face treatment, but perhaps also complications arising from the interplay of anxiety, depression, and MS. Although this study did not involve direct comparison of telephone-based and face-to-face treatment delivery, the high participation rates suggest providers serving individuals with MS should consider adding the option of telephone-based delivery to their depression treatment programs. Further, service providers should monitor individuals with MS and fear disorders after acute treatment of depression. The depressive symptoms encountered by participants with fear disorders during the follow-up period may reflect chronic uncertainty due to the unpredictability of MS course (Kroencke, Denny, & Lynch, 2001). These same uncertainties may also have contributed to the partial response, in terms of anxiety symptoms, of the participants with GAD. Mental health services for individuals with MS should address potential interactions between vulnerabilities common to anxiety and depression (e.g., intolerance of uncertainty; Miranda, Fontes, & Marroquín, 2008) and uncertainty regarding future changes in MS-related impairment.
Limitations of the current study include its post-hoc nature, as well as unknown generalizability to depressed populations who do not have MS or another chronic, disabling illness. While we are unaware of any unique effects that telephone-administered psychotherapy might have compared to face-to-face treatment, we cannot rule out the possibility that the distance intervention may have unique effects or that it may have resulted in a selection bias that limits generalizability. Results should also be replicated with larger samples of participants with both types of comorbid anxiety disorders, as well as OCD and PTSD. This study was not powered to detect differences between T-CBT and T-SEFT in their efficacy for people with both depression and anxiety disorders, and larger samples would also help to address this issue. Finally, although temporal order was clearly established, there was no evidence regarding a causal role of distress or fear disorders on depression and anxiety outcomes.
Conclusions
In conclusion, results did not suggest that comorbid anxiety disorders upon entry into treatment for depression affected the degree of improvement during treatment. Findings on anxiety outcomes and maintenance of gains after treatment, however, were more clearly understood by dividing the anxiety disorders into fear and distress subgroups. This showed the comorbid distress disorder (GAD) increased risk of anxiety symptoms independent of time. Comorbid fear disorders were, in contrast, associated with depression specifically during the follow-up period, and these results emerged in terms of unremitting MDD, increased interviewer rated depressive symptom severity, and increased self-reported depressive symptoms. Future studies on comorbid depressive and anxiety disorders should clarify mechanisms by which differing types of anxiety disorders can undermine maintenance of gains. Substantial follow-up time periods should also be used to examine whether residual anxiety symptoms and difficulty maintaining mood-related gains can be ameliorated through use of transdiagnostic approaches or additional disorder-specific treatment.
Acknowledgements
This study was supported by grant MH59708 R01 from the National Institute of Mental Health to David C. Mohr, Ph.D.
Footnotes
Although randomization resulted in equal proportions of participants with anxiety disorders between treatments, we tested the model for each outcome with treatment added as a covariate. The pattern of all results involving time and comorbidity remained identical. We did not present these data because the type of treatment received was not relevant to the hypotheses.
We again tested the model for each outcome with treatment added as a covariate. The same pattern of main effects and interactions involving time, fear, and distress disorders emerged with two exceptions. The main effect of distress disorder no longer reached statistical significance on HADS-A anxiety symptoms. However, the beta weight remained almost identical (β = .35, p = .055). The only other exception was that the main effect of fear on MDD frequency became significant (β = .89, p = .047).
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/REP
References
- Allen L, McHugh R, Barlow D. Emotional disorders: A unified protocol. In: Barlow DH, editor. Clinical handbook of psychological disorders: A step-by-step treatment manual. Guilford Press; New York: 2008. pp. 216–249. [Google Scholar]
- Barlow D, Allen L, Choate M. Toward a unified treatment for emotional disorders. Behavior Therapy. 2004;35:205–230. doi: 10.1016/j.beth.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. Beck Depression Inventory: Second Edition: Manual. Psychological Corp.; San Antonio, TX: 1996. [Google Scholar]
- Beiske A, Svensson E, Sandanger I, Czujko B, Pedersen E, Aarseth J, Myhr K. Depression and anxiety amongst multiple sclerosis patients. European Journal of Neurology. 2008;15:239–245. doi: 10.1111/j.1468-1331.2007.02041.x. [DOI] [PubMed] [Google Scholar]
- Brown C, Schulberg H, Madonia M, Shear M, Houck P. Treatment outcomes for primary care patients with major depression and lifetime anxiety disorders. American Journal of Psychiatry. 1996;153:1293–1300. doi: 10.1176/ajp.153.10.1293. [DOI] [PubMed] [Google Scholar]
- Clark D. Cognitive behavioral therapy for anxiety and depression: Possibilities and limitations of a transdiagnostic perspective. Cognitive Behaviour Therapy. 2009;38:29–34. [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West S, Aiken L. Applied Multiple Regression / Correlation Analysis for the Behavioral Sciences. 3rd ed. Lawrence Erlbaum Associates, Inc.; Mahwah, NJ: 2003. [Google Scholar]
- D’Alisa S, Miscio G, Baudo S, Simone A, Tesio L, Mauro A. Depression is the main determinant of quality of life in multiple sclerosis: A classification-regression (CART) study. Disability & Rehabilitation. 2006;28:307–314. doi: 10.1080/09638280500191753. [DOI] [PubMed] [Google Scholar]
- Fairburn C, Cooper Z, Shafran R. Cognitive behaviour therapy for eating disorders: A “transdiagnostic” theory and treatment. Behaviour Research & Therapy. 2003;41:509–528. doi: 10.1016/s0005-7967(02)00088-8. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, Trivedi MH. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: A STAR*D report. American Journal of Psychiatry. 2008;165:342–351. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- Feinstein A. An examination of suicide intent in patients with multiple sclerosis. Neurology. 2002;59:674–678. doi: 10.1212/wnl.59.5.674. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I disorders – patient edition (SCID-I/P, Version 2.0) New York State Psychiatry Institute; New York: 1995. [Google Scholar]
- Foley FW, Traugott U, LaRocca NG, Smith CR, Karen RP, Caruso LS, Scheinberg LC. A prospective study of depression and immune dysregulation in multiple sclerosis. Archives of Neurology. 1992;49:238–244. doi: 10.1001/archneur.1992.00530270052018. [DOI] [PubMed] [Google Scholar]
- Gibbons C, DeRubeis R. Anxiety symptom focus in sessions of cognitive therapy for depression. Behavior Therapy. 2008;39:117–125. doi: 10.1016/j.beth.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Greenberg L, Rice L, Elliott R. Facilitating emotional change: The moment by moment process. Guilford Press; New York: 1993. [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzakis M, Jr., Haselkorn J, Williams R, Turner A, Nichol P. Telemedicine and the delivery of health services to veterans with multiple sclerosis. Journal of Rehabilitation Research & Development. 2003;40:265–282. [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Wang PS. The Epidemiology of Major Depressive Disorder: Results from the National Comorbidity Survey Replication (NCS-R) Journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Multiple Sclerosis. 2007;13:67–72. doi: 10.1177/1352458506071161. [DOI] [PubMed] [Google Scholar]
- Kroencke D, Denney D, Lynch S. Depression during exacerbations in multiple sclerosis: The importance of uncertainty. Multiple Sclerosis. 2001;7:237–242. doi: 10.1177/135245850100700405. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Mulsant BH, Dew MA, Shear MK, Houck P, Pollock BG, Reynolds CF., III Good treatment outcomes in late-life depression with comorbid anxiety. Journal of Affective Disorders. 2003;77:247–254. doi: 10.1016/s0165-0327(02)00177-5. [DOI] [PubMed] [Google Scholar]
- Mineka S, Watson D, Clark L. Comorbidity of anxiety and unipolar mood disorders. Annual Review of Psychology. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- Miranda R, Fontes M, Marroquín B. Cognitive content-specificity in future expectancies: Role of hopelessness and intolerance of uncertainty in depression and GAD symptoms. Behaviour Research & Therapy. 2008;46:1151–1159. doi: 10.1016/j.brat.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Mohr D. The stress and mood management program for individuals with multiple sclerosis: Therapist guide. Oxford Press; New York: 2010a. [Google Scholar]
- Mohr D. The stress and mood management program for individuals with multiple sclerosis: Workbook. Oxford Press; New York: 2010b. [Google Scholar]
- Mohr D, Goodkin D, Islar J, Hauser S, Genain C. Treatment of depression is associated with suppression of nonspecific and antigen-specific T(H)1 responses in multiple sclerosis. Archives of Neurology. 2001;58:1081–1086. doi: 10.1001/archneur.58.7.1081. [DOI] [PubMed] [Google Scholar]
- Mohr D, Goodkin D, Likosky W, Gatto N, Baumann K, Rudick R. Treatment of depression improves adherence to interferon beta-1b therapy for multiple sclerosis. Archives of Neurology. 1997;54:531–533. doi: 10.1001/archneur.1997.00550170015009. [DOI] [PubMed] [Google Scholar]
- Mohr D, Hart S, Julian L, Catledge C, Honos-Webb L, Vella L, Tasch ET. Telephone-Administered Psychotherapy for Depression. Archives of General Psychiatry. 2005;62:1007–1014. doi: 10.1001/archpsyc.62.9.1007. [DOI] [PubMed] [Google Scholar]
- Mohr D, Vella L, Hart S, Heckman T, Simon G. The effect of telephone-administered psychotherapy on symptoms of depression and attrition: A meta-analysis. Clinical Psychology: Science & Practice. 2008;15:243–253. doi: 10.1111/j.1468-2850.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts M, Daniels M, Burnam M, Wells K. A structured interview version of the Hamilton Depression Rating Scale: evidence of reliability and versatility of administration. Journal of Psychiatric Research. 1990;24:335–350. doi: 10.1016/0022-3956(90)90005-b. [DOI] [PubMed] [Google Scholar]
- Ruskin PE, Reed S, Kumar R, Kling MA, Siegel E, Rosen M, Hauser P. Reliability and acceptability of psychiatric diagnosis via telecommunication and audiovisual technology. Psychiatric Services. 1998;49:1086–1088. doi: 10.1176/ps.49.8.1086. [DOI] [PubMed] [Google Scholar]
- Sadovnick AD, Remick RA, Allen J, Swartz E, Yee IML, Eisen K, Paty DW. Depression and multiple sclerosis. Neurology. 1996;46:628–632. doi: 10.1212/wnl.46.3.628. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS Version 9.2. SAS Institute Inc; Cary, NC: 2002-2008. [Google Scholar]
- Scott KM, Bruffaerts R, Tsang A, Ormel J, Alonso J, Angermeyer MC, Von Korff M. Depression-anxiety relationships with chronic physical conditions: Results from the World Mental Health surveys. Journal of Affective Disorders. 2007;103:113–120. doi: 10.1016/j.jad.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Sharrack B, Hughes R. The Guy’s Neurological Disability Scale (GNDS): A new disability measure for multiple sclerosis. Multiple Sclerosis. 1999;5:223–233. doi: 10.1177/135245859900500406. [DOI] [PubMed] [Google Scholar]
- Simon G, Revicki D, VonKorff M. Telephone assessment of depression severity. Journal of Psychiatric Research. 1993;27:247–252. doi: 10.1016/0022-3956(93)90035-z. [DOI] [PubMed] [Google Scholar]
- Taylor D, Walters H, Vittengl J, Krebaum S, Jarrett R. Which depressive symptoms remain after response to cognitive therapy of depression and predict relapse and recurrence? Journal of Affective Disorders. 2009 doi: 10.1016/j.jad.2009.08.007. doi:10.1016/j.ad.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier-Taillefer M, Roullet E, Cesaro P, Alperovitch A. Validation of self-reported neurological disability in multiple sclerosis. International Journal of Epidemiology. 1994;23:148–54. doi: 10.1093/ije/23.1.148. [DOI] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM-V. Journal of Abnormal Psychology. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Watson D, O’Hara M, Stuart S. Hierarchical structures of affect and psychopathology and their implications for the classification of emotional disorders. Depression & Anxiety. 2008;25:282–288. doi: 10.1002/da.20496. [DOI] [PubMed] [Google Scholar]
- Zigmond A, Snaith R. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]


