Abstract
Current classification of pulmonary adenocarcinoma includes non-invasive bronchioloalveolar carcinoma, mixed subtype adenocarcinoma and several patterns of invasive carcinoma. The extent of invasion in mixed subtype adenocarcinoma is variable, and prior studies suggest that estimates of extent of desmoplasia or invasion and gross tumor size are predictors of survival. Pathologic review of 178 consecutive primary lung adenocarcinoma resections from 1997-2000 was performed blinded to outcome. Lymph node metastases were not present in adenocarcinomas with less then 0.6 cm of invasion. In multivariate analysis and in strata adjusted for stage, measurement of linear extent of invasion was significantly associated with survival while gross size measurement alone was not. Significant differences in median survival were observed when patients were divided into non-invasive, micro-invasive (<0.6 cm invasion) and invasive subcategories. In conclusion, among lung adenocarcinomas, histologic assessment of invasive growth may provide valuable prognostic information, and tumors with invasion under 0.6 cm have a more indolent clinical course after resection.
Keywords: adenocarcinoma, bronchioloalveolar carcinoma, lung, invasion, microinvasion
Introduction
Non-small cell lung carcinoma (NSCLC) histology is heterogeneous, yet staging and conventional therapy for these tumors is homogeneous. Adenocarcinoma (AdCa) is the most common histological subtype of NSCLC. This group is categorized into distinct patterns and subtypes by the World Health Organization27. As defined in 1999, bronchioloalveolar carcinomas (BAC) are non-invasive AdCas that spread along alveolar walls by lepidic/replacement growth. The category of mixed subtype AdCa was added in 20042 to include tumors that had a component of invasion in a background of a lepidic/replacement growth pattern.
In lung AdCa, the extent of the non-invasive component varies considerably and is associated with clinical outcomes. This parallels malignancies in other organs, such as breast3,8,17 and cervix7,10, that are defined as non-invasive (in-situ carcinoma), microinvasive (microscopic invasion) or as invasive carcinomas. Notably, in the lung this scheme is most relevant in AdCa compared with other non-small cell carcinomas because the extent of non-invasive component is generally minor in squamous cell carcinoma and often absent in large cell carcinoma.
The clinical importance of lung adenocarcinoma invasion is supported by several recent studies12,20,21,25,29. These studies indicate that the risk of death in non-invasive BAC tumors and in tumors with less than 0.6 cm of fibrosis or linear invasion is significantly lower than that of AdCa with greater extent of invasion. These results suggest that prognosis of BAC is favorable and that prognosis is similarly favorable in a subset of mixed subtype tumors with limited invasion (<0.6cm). However, important issues such as interobserver variability in measurement of invasion, definitions of invasiveness in lung adenocarcinoma, and importance of gross tumor size remain. In this study, we address these issues in a large case series of primary lung adenocarcinomas consecutively resected at Columbia University Medical Center between 1997 and 2000.
Materials and Methods
Lung cancer specimens from 178 consecutive primary lung AdCa resections spanning 1997-2000 were reviewed by two pathologists (ACB, FQ), blinded to lymph node and survival status. Gross size was recorded as reported in the original pathology report. Slides were evaluated for measurements of invasion, nuclear grade, mitotic activity, histologic subtype, visceral pleural invasion and predominant histologic pattern. Criteria for nuclear grade were: Grade 1- small uniform nuclei with absent or indistinct nucleoli and low amount of pleomorphism, Grade 2 – intermediate size nuclei with irregular nuclear contours, identifiable nucleoli, and moderate pleomorphism, and Grade 3 – marked nuclear pleomorphism ranging to large nuclei with irregular nuclear contours and prominent nucleoli. Differentiation was assessed using degree of gland or papillary formation as low (well formed glands or papilla), intermediate (irregular or fused glands), or poor (solid growth or micropapillary). Mitotic rate was counted over 30 high power fields and averaged to yield a mitotic score per 10 HPF. For small tumors, a maximum number of fields were counted (up to 30) and the value adjusted to 10 HPF for analysis.
Invasion criteria were applied as follows: 1. invasion of pleura, vessels, and airways; 2. loss of alveolar architecture with desmoplastic stroma; 3. gland shapes not conforming to pre-existing architecture; and 4. solid or papillary growth or other WHO pattern of invasive adenocarcinoma. If invasive carcinoma was present on either side of a scar, the scar was included in the measurement, but scarring or alveolar collapse distant from carcinoma was excluded. Invasive size was measured linearly on histological sections (Figure 1F). The process included both low and higher power assessment, dotting the boundaries of the invasive areas, drawing a boundary line and taking the longest linear measurement. For tumors greater than 2.5 cm (approximate maximum size of a histologic section), that were predominantly invasive (>50% invasion) it was assumed that invasive size was equal to gross size.
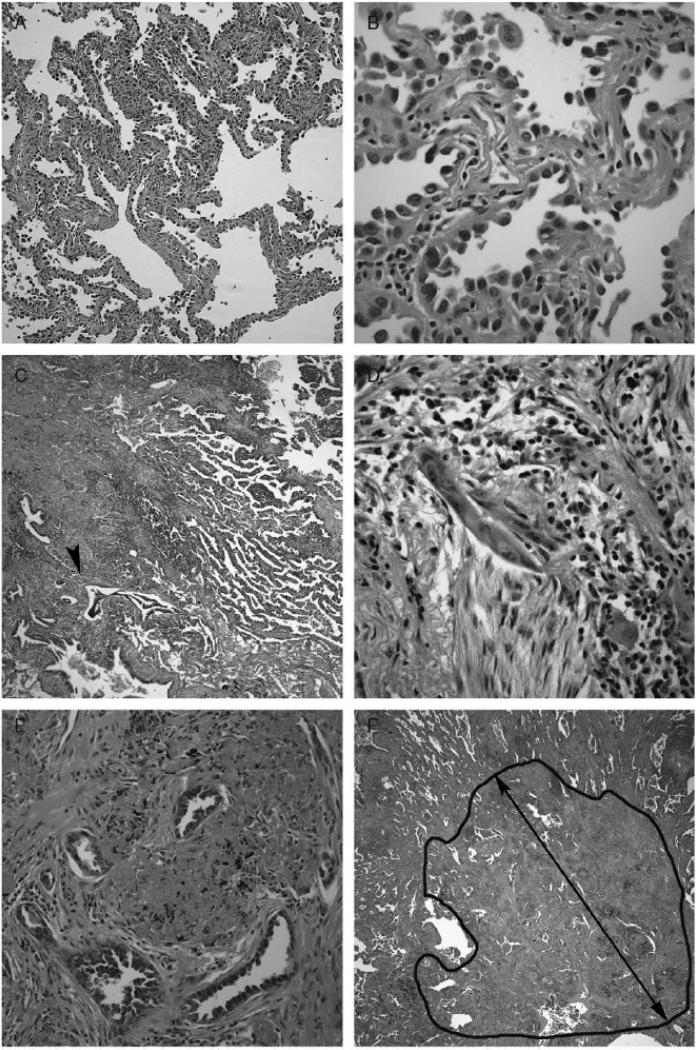
Figure 1. Histology of pre-invasive bronchioloalveolar carcinoma and invasion in AdCa.
(A) BAC shows irregularly collapsed slightly thickened alveolar walls.
(B) Alveolar walls are lined by uniform low columnar neoplastic cells.
(C) Microinvasive AdCa with a predominant BAC/lepidic pattern and a solid area (arrowhead).
(D) The solid area in (C) shows irregular glands amidst a fibroblastic stroma.
(E) Irregular angulated glands in a fibrous stroma within an invasive focus.
(F) The extent of the invasive focus (black circle) seen in (E). The black line represents the linear measurement of invasive size.
(A-F, Hematoxylin and eosin stain - Original magnification - Panel A × 10, Panel C, F × 5, Panel B, D and E × 150).
All tumors were staged pathologically using both the AJCC Cancer staging manual 6th edition and using the revised TNM staging system that will be deployed in 20094. Survival data were calculated as of September 30, 2007. Patient deaths were confirmed both by contact with the patient's physician and by query of the Social Security Death Index. Survival was confirmed by evidence of recent contact documented in the medical record or by report of the patient's physician. Six patients were lost to follow-up. Sensitivity analyses indicated that these missing data had no effect on the statistical analyses. The study was approved by the Columbia Institutional Review Board (IRB).
Statistical analysis was performed using SPSS v. 14.0 (Chicago, IL). One-way ANOVA was performed to test the association of variables with node status. For survival analysis, Cox regression analysis was performed to create univariate and multivariate models for survival analysis. Multivariate models were constructed using parameters identified as significant by univariate analysis (p<.05). Analyses were performed in the following subgroups – all patients, Stage 1 and 2 only, and Stage 1 only.
Results
Patient characteristics of the four primary lung AdCa groups using previously described strata29 of BAC, Microinvasive (<0.6 cm invasion), Mixed Subtype (AdCa with invasive and BAC pattern), and Invasive (No BAC pattern) indicated that age and gender were similar across the groups (Table 1). No lymph node metastases were seen in the BAC and microinvasive groups. The most common invasive histologic pattern was acinar; no microinvasive tumors had a micropapillary pattern.
Table 1.
Patient characteristics and histopathology
| ALL | BAC (b) | Microinvasive (mi) | Mixed (m) | Invasive (i) | |
|---|---|---|---|---|---|
| Number of cases | 178 | 8/178 (5%) | 24/178 (13%) | 87/178 (49%) | 59/178 (33%) |
| Age | 66 +/- 9.2 | 65 +/- 7 | 69 +/- 8 | 68 +/- 9 | 64 +/- 9 |
| Gross size | 2.78 +/- 1.5 | 1.4 +/-.7 | 2.7 +/- 2.2 | 2.8 +/- 1.3 | 2.9 +/- 1.5 |
| Invasive size | 2.05 +/- 1.67 | 0 | .35 +/- .13 | 2.19 +/- 1.5 | 2.8 +/- 1.5 |
| Node positive | 45 (25%) | 0 | 0 | 20 (23%) | 25 (41%) |
| Gender | 69M; 109F | 3M; 5F | 5M;19F | 37M;50F | 24M;35F |
| Predominant invasive pattern | |||||
| Acinar | 97 (57%) | N/A | 19 | 51 | 28 |
| Papillary | 35 (21%) | N/A | 4 | 21 | 10 |
| Micropapillary | 10 (6%) | N/A | 0 | 4 | 6 |
| Solid | 22 (13%) | N/A | 0 | 10 | 12 |
| Mucinous | 5 (3%) | N/A | 1 | 1 | 3 |
The interobserver reliability of invasion measurements was examined in sixty consecutive cases reviewed by three pathologists (ACB, AA, JE). For each case, the linear extent of invasion (cm) and invasion category [BAC, microinvasion (< 0.6 cm invasion) and invasive (>= 0.6 cm invasion)] were recorded. The intraclass correlation coefficient (ICC) for invasion measurement was .854. For determination of invasion subclasses of BAC, microinvasive adenocarcinoma and invasive adenocarcinoma, the kappa statistic between observers were .49, .64, and .57, indicating a moderate level of agreement. These results are similar to those reported by Noguchi and colleagues15 in their study of 32 cases evaluated by expert and non-expert lung pathologists. Furthermore, our kappa values are comparable with those reported for pathologic measurements of melanoma thickness22 and for subclassification of breast and prostate carcinoma5,11.
We examined pathologic parameters associated with risk of lymph node metastasis. Nuclear grade, visceral pleural invasion, differentiation, and invasive size, but not gross tumor size, mitotic activity or necrosis was associated with lymph node metastasis (Table 2).
Table 2.
Univariate analysis, all patients, of pathologic parameters and lymph node status.
| Parameter | Statistic* | p-value |
|---|---|---|
| Gross size | .319 | .573 |
| Invasive size | 12.8 | .001 |
| Nuclear grade | 4.1 | .046 |
| Mitotic activity | .227 | .634 |
| Differentiation | 8.2 | .001 |
| Visceral pleural invasion | 5.9 | .018 |
| Necrosis | .396 | .532 |
One way ANOVA
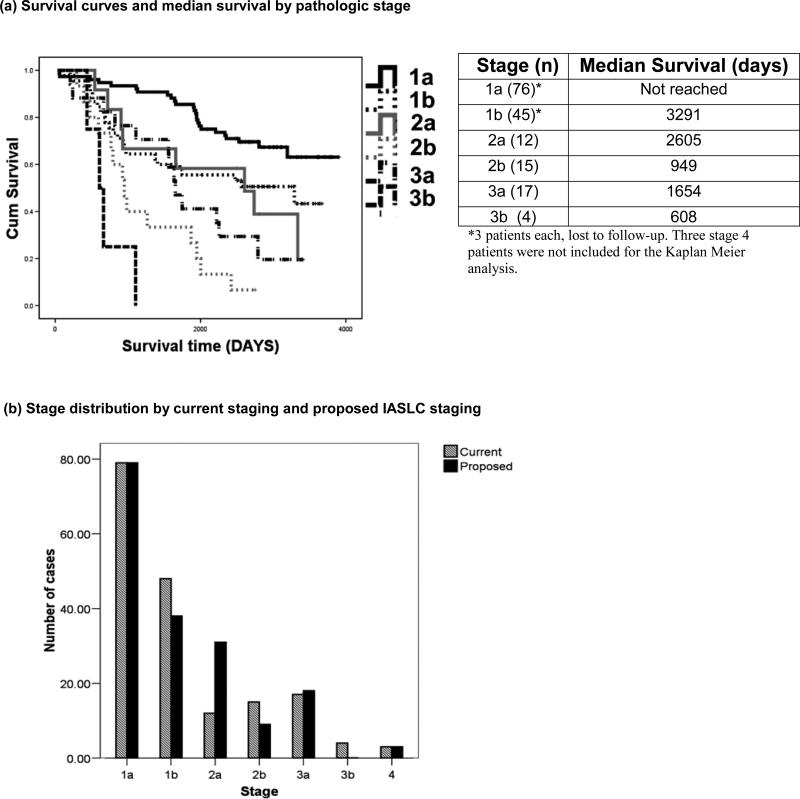
The majority of patients had stage 1 disease (71%), with Stage 1a patients not reaching median survival time (Figure 2a). In anticipation of implementation of the new IASLC TNM Lung Cancer Staging system, all patients were re-staged using the new scheme (Figure 2b). The main impact of this re-staging was an increase in Stage 2a patients and decrease in Stage 1b and 2b patients. Results from all subsequent analyses were similar for both staging algorithms.
Figure 2. Distribution and survival times of patients by pathologic staging.
(a) Kaplan Meier curves of patient survival by pathologic stage and median survival
(b) Comparison of stage distribution of patients using existing pathologic staging with the proposed IASLC staging projected for year 2009 implementation.
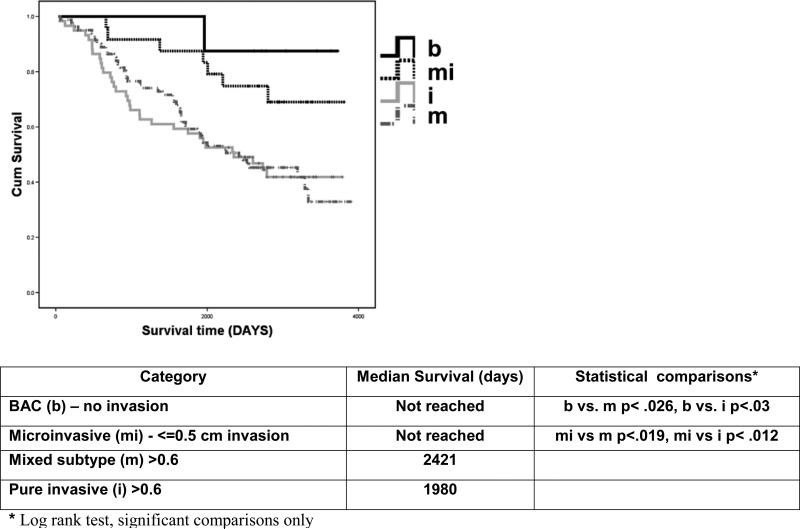
Survival was associated with AdCa invasive groups (Figure 3). The median survival for the mixed subtype and pure invasive groups were 2421 days and 1980 days respectively, while the median survival of the BAC (b) group and of the microinvasive group (mi) was not reached. Survival of mixed and invasive tumors was significantly shorter than that of BAC or microinvasive tumors (P < .05 in each instance).
Figure 3. Survival curves by invasive size parameter, all patients.
Kaplan Meier curves showing survival of patients divided into 4 groups based in invasiveness with BAC (solid black), Microinvasive (dotted black), Mixed subtype (dash dot) and invasive (solid gray). The median survival is recorded by group. Log rank testing was performed between all categories and only significant statistical comparisons are reported.
Univariate and multivariate analysis was performed for all patients (n=172), all Stage 1 and 2 (n=146) and all Stage 1 (n=121) patients. In all patients using parameters significant by univariate analysis in a multivariate model, risk of death was associated with age, invasive size, lymph node metastasis, and visceral pleural invasion (Table 3), but not differentiation, gender or gross size that were significant by univariate analysis. For Stage 1 and 2 patients, age, invasive size, visceral pleural invasion and nodal metastases were independently associated with risk of death, but gross tumor size and invasive differentiation were not (Table 4). Among Stage I patients, only age and invasive size were associated with risk for death indicating that invasive size was an independent histopathological predictor of prognosis within Stage 1 lung AdCa patients (Table 5).
Table 3.
Effect of clinical and pathologic parameters on survival time by univariate and multivariate analysis, all patients*
| Parameters | Univariate | Multivariate |
|---|---|---|
| Age | 1.033 (1.01-1.057), p=.005 | 1.039 (1.01 - 1.062), p=.001 |
| Female Gender | 0.63 (.414 -.958), p=.031 | NS, .72 (.469 - 1.103), p=.131 |
| Gross size | 1.142 (1.01 - 1.027), p=.034 | NS, 1.12 (.966 - 1.287), P=.137 |
| Invasive size | 1.194 (1.074 - 1.31), p=.001 | 1.16 (1.03 - 1.31), p=.016 |
| Nodes | 2.6 (1.65 - 3.91), p=.0001 | 2.02 (1.39 - 3.2) p=.002 |
| Visceral pleural invasion | 2.12 (1.36 - 3.30), p=.001 | 1.61 (1.006 - 2.6), p=.047 |
| Predominant histology | NS | NS |
| Mitotic rate | NS | NS |
| Necrosis | NS | NS |
| Nuclear Grade | NS | NS |
| Invasive differentiation | 1.46 (1.10 -1.95), p=.01 | NS |
| Invasive diff (poor vs well/mod) | 1.51 (.973 - 2.33), p=.066 | NS |
n=172, 6 patients lost to follow-up
Table 4.
Effect of clinical and pathologic parameters on survival time by univariate and multivariate analysis, Stage 1 & 2 only*
| Parameters | Univariate | Multivariate |
|---|---|---|
| Age | 1.03 (1.01 - 1.06), p= 0.028 | 1.03 (1.01 - 1.06) p=.03 |
| Gender | NS | NS |
| Gross size | NS, 1.14 (.992 - 1.32), p=.064 | NS, 1.15 (.979 - 1.346) p=.088 |
| Invasive size | 1.22 (1.09 - 1.36) p= .001 | 1.19 (1.04 - 1.34) p=.006 |
| Nodes | 2.65 (1.58 - 4.47) p=.0001 | 2.2 (1.29 - 3.74) p=.004 |
| Visceral pleural invasion | 2.21 (1.33 - 3.66) p=.002 | 1.70 (1.007 - 2.88) p= .047 |
| Predominant histology | NS | NS |
| Mitotic rate | NS | NS |
| Necrosis | NS | NS |
| Nuclear Grade | NS | NS |
| Invasive differentiation | 1.49 (1.085 - 2.04) p=.014 | NS |
| Invasive diff (poor vs well/mod) | 1.53 (.94 - 2.5) p=.09 | NS |
n=146, 6 patients lost to follow-up
Table 5.
Effect of clinical and pathologic parameters on survival time by univariate and multivariate analysis, Stage 1 only*
| Parameters | Univariate | Multivariate |
|---|---|---|
| Age | 1.04 (1.01 -1.08) p=.016 | 1.05 (1.01 - 1.08) p=.006 |
| Gender | NS | NS |
| Gross size | NS, 1.14 (.973-1.342) p=.104 | NS |
| Invasive size | 1.20 (1.06 - 1.37), p= .004 | 1.25 (1.09 - 1.42) p=.002 |
| Visceral pleural invasion | NS, p=.179 | NS |
| Predominant histology | NS | NS |
| Mitotic rate | NS | NS |
| Necrosis | NS | NS |
| Nuclear Grade | NS | NS |
| Invasive differentiation | NS | NS |
| Invasive diff (poor vs well/mod) | NS | NS |
n=121, 6 patients lost to follow-up
Discussion
Our results indicate that the extent of invasion is independently associated with lymph node metastasis and risk of death. In contrast, gross tumor size in AdCa was not independently associated with risk of death. Prognostic factors in stage 1 NSCLC include tumor size and this has been reported in tumor size strata of 3.0 cm16 and 2.0 cm19. However, not all studies have shown such a relationship18. A potential explanation for the discrepancy between the association of prognosis with gross tumor size is that this measurement assumes the majority of the tumor mass reflects invasive tumor. Although this assumption is accurate for most squamous carcinomas and large cell carcinomas, it does not apply to a substantial proportion of lung AdCas. Therefore, results of prior studies could be confounded by the proportion of mixed subtype AdCA included.
In 1999, the WHO classification defined bronchioloalveolar carcinoma as completely non-invasive, and in 2004 created the term mixed subtype adenocarcinoma that included tumors with invasion along with replacement or lepidic growth pattern. The mixed subtype adenocarcinoma category however can include tumors with small foci of invasion or with invasion that involves the majority of the tumor. This raises the question as to the threshold extent of invasion that increases the risk of metastatic disease and death in solitary mixed subtype tumors. Similar to parameters established for microinvasive cervical cancer or microinvasive breast carcinoma, our results and those of others indicate that for lung adenocarcinoma, 5 mm or less of invasion is biologically indolent.
In a study of 100 adenocarcinomas from 1987-1992 that were 3 cm or less, Suzuki et al25 measured invasion as defined by the size of fibrosis on low-power view. Similar to the finding of Shimosato et al23 published 20 years earlier, stratification of gross tumor size with a 2.0 cm cut-off did not have survival impact. Using strata of less than or equal to 5 mm, 6 to 15 mm, and > 15mm of fibrosis size, they reported 100% 5-yr survival for <0.6 cm group, 72% 5 yr survival for 0.6-1.5 cm group and 57% 5-yr survival for >1.5 cm. Multivariate analysis indicated that fibrosis size but not pleural invasion were associated with lymph node metastasis and vascular invasion. No lymph node metastases were detected in tumors with less than 0.6 cm of fibrosis, which is consistent with our findings. More recently, Sakurai et al21 examined 380 peripheral AdCa less than or equal to 2 cm. Only 3.3% of the 91 patients with fibrosis less than 0.6 cm had recurrence, and importantly 100% were alive at seven years.
All of the above studies were conducted in Japan, raising concern that these findings may not generalize internationally. However, in a European study, Rena et al20 examined 28 BAC and 80 AdCas, and reported a lower rate of recurrence and a higher rate of disease-free survival in BAC patients. Most recently, a US based study 29 demonstrated increased survival in tumors with less than or equal to 5 mm of invasion in stage 1 and 2 lung AdCa. The data presented in our current study are consistent with these prior studies from Europe and North America and suggest that the cut-off of 0.5 cm of invasion for acquisition of nodal metastatic potential and the importance of invasive size in mixed subtype tumors established by studies from Japan generalize to lung AdCa worldwide.
In the course of these studies there has been a focus on non-mucinous bronchioloalveolar carcinoma. Pure non-mucinous bronchioloalveolar carcinomas, when solitary, are associated with excellent 5 and 10-year survival and are not associated with lymph node metastasis. When measured invasive size (linear extent of invasion) or size of fibrosis as a surrogate for invasion is greater than 5 mm, the rate of node metastasis increases and survival rates decrease.
Recent reports of computed tomography imaging of pulmonary nodules suggest correlations between nodule image features and invasion. The correlation between a bubble-like30 or air-density containing tumor (called a ground glass opacity or GGO) and BAC histology has been reported, and conversely, the detection of solid components within a nodule is frequently associated with invasion. In a series of 69 cases of CT scan detected GGO, Suzuki et al24 found that of 38 pure GGO nodules without solid areas, 32 were BAC and 6 were AdCa. Similarly, Nakata14 reported 62 of 70 pure GGO were BAC, and that of pure GGO and mixed GGO lesions less than 1.0 cm, 4 of 60 or 6.6% were AdCa. Takashima et al26 reported that patients with Noguchi A (replacement growth, no fibrosis) and Noguchi B (replacement growth, fibrosis) tumors had better survival than those with Noguchi C and D (both invasive) lesions, and that lesions with a GGO percentage of greater than 57% were associated with longer survival. Other studies have shown that high percentage GGO lesions are more likely BAC, although mixed adenocarcinomas are also seen as GGO lesions, and BAC lesions are identified among tumors with <50% and <10% CT scan GGO1. In another series, 94% of Noguchi A were pure GGO, and Noguchi A, B and C lesions had 92%, 52% and 20% GGO component, respectively28. Thus while the evaluation of GGO contribution in lung adenocarcinoma nodules is a potentially useful prognostic predictor1,13, it is most useful in tumors less than 1 cm.
Thus, although small pure and mixed GGO may correlate with AdCa pathology, it is also clear that some cases are not predicted by this approach and that interobserver reliability may impact this clinical decision26. It is possible that measurement strata (such as 0.5 cm) that create categories will be replaced by numerical measurements to be used in prognostic nomograms. This may reduce the impact of interobserver error as evidenced by only moderate kappa score for categories and higher intraclass correlation for the absolute measurement. In addition, PET scanning may provide useful correlative information, as a positive PET lesion may have a greater likelihood of invasion6,9. It is possible that the contribution of molecular data acquired from biopsy material (such as EGFR and Kras mutation status and selected gene amplification studies) may also help stratify patient prognostic groups and guide therapy.
Frequently, a small solitary GGO nodule is managed by a period of observation, and resection follows if there is evidence of interval growth. As noted above, radiologicpathologic correlation studies suggest that pure GGO lesions are associated with excellent prognosis. Our pathologic data and those of others consistently indicate that a small focus of invasion, less than 0.6 cm in these lesions does not affect prognosis. Prospective studies to examine pathologic and radiologic correlation of nodule invasion with clinical risk for metastasis, recurrence and death will provide useful information that will guide clinical practice and future classification schemes for AdCa. It is clear that the extent of invasion is an independent prognostic factor for lung adenocarcinoma. Consideration of modifying staging criteria for AdCa to include a pre-invasive Tcis and microinvasive Tmic category is warranted and may have considerable clinical importance.
Contributor Information
Alain C. Borczuk, Dept of Pathology, Columbia University Medical Center..
Fang Qian, Dept of Pathology, Newark Beth Israel Medical Center, Newark NJ.
Angeliki Kazeros, Dept of Medicine, Columbia University Medical Center.
Jennifer Eleazar, Dept of Pathology, Columbia University Medical Center.
Adel Assaad, Dept of Pathology, Virginia Mason Seattle Main Clinic, Seattle, WA.
Joshua R Sonett, Dept of Surgery, Columbia University Medical Center.
Mark Ginsburg, Dept of Surgery, Columbia University Medical Center.
Lyall Gorenstein, Dept of Surgery, Columbia University Medical Center.
Charles A Powell, Dept of Medicine, Pulmonary, Allergy and Critical Care Medicine, Columbia University Medical Center.
References
- 1.Aoki T, Tomoda Y, Watanabe H, et al. Peripheral lung adenocarcinoma: correlation of thin-section CT findings with histologic prognostic factors and survival. Radiology. 2001;220:803–9. doi: 10.1148/radiol.2203001701. [DOI] [PubMed] [Google Scholar]
- 2.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40:90–7. doi: 10.1053/j.ro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 3.de Mascarel I, MacGrogan G, Mathoulin-Pelissier S, et al. Breast ductal carcinoma in situ with microinvasion: a definition supported by a long-term study of 1248 serially sectioned ductal carcinomas. Cancer. 2002;94:2134–42. doi: 10.1002/cncr.10451. [DOI] [PubMed] [Google Scholar]
- 4.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 5.Harnden P, Coleman D, Moss S, et al. Prostatic pathology reporting in the UK: development of a national external quality assurance scheme. Histopathology. 2008;52:147–57. doi: 10.1111/j.1365-2559.2007.02922.x. [DOI] [PubMed] [Google Scholar]
- 6.Higashi K, Ueda Y, Ayabe K, et al. FDG PET in the evaluation of the aggressiveness of pulmonary adenocarcinoma: correlation with histopathological features. Nucl Med Commun. 2000;21:707–14. doi: 10.1097/00006231-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins MP, Morley GW. Microinvasive squamous cell carcinoma of the cervix. J Reprod Med. 1994;39:671–3. [PubMed] [Google Scholar]
- 8.Jimenez RE, Visscher DW. Clinicopathologic analysis of microscopically invasive breast carcinoma. Hum Pathol. 1998;29:1412–9. doi: 10.1016/s0046-8177(98)90009-0. [DOI] [PubMed] [Google Scholar]
- 9.Lee KS, Jeong YJ, Han J, et al. T1 non-small cell lung cancer: imaging and histopathologic findings and their prognostic implications. Radiographics. 2004;24:1617–36. doi: 10.1148/rg.246045018. discussion 32-6. [DOI] [PubMed] [Google Scholar]
- 10.Leman MH, Jr., Benson WL, Kurman RJ, et al. Microinvasive carcinoma of the cervix. Obstet Gynecol. 1976;48:571–8. [PubMed] [Google Scholar]
- 11.Longacre TA, Ennis M, Quenneville LA, et al. Interobserver agreement and reproducibility in classification of invasive breast carcinoma: an NCI breast cancer family registry study. Mod Pathol. 2006;19:195–207. doi: 10.1038/modpathol.3800496. [DOI] [PubMed] [Google Scholar]
- 12.Maeshima AM, Niki T, Maeshima A, et al. Modified scar grade: a prognostic indicator in small peripheral lung adenocarcinoma. Cancer. 2002;95:2546–54. doi: 10.1002/cncr.11006. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura H, Saji H, Ogata A, et al. Lung cancer patients showing pure ground-glass opacity on computed tomography are good candidates for wedge resection. Lung Cancer. 2004;44:61–8. doi: 10.1016/j.lungcan.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Nakata M, Sawada S, Saeki H, et al. Prospective study of thoracoscopic limited resection for ground-glass opacity selected by computed tomography. Ann Thorac Surg. 2003;75:1601–5. doi: 10.1016/s0003-4975(02)04815-4. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 15.Noguchi M, Minami Y, Iijima T, et al. Reproducibility of the diagnosis of small adenocarcinoma of the lung and usefulness of an educational program for the diagnostic criteria. Pathol Int. 2005;55:8–13. doi: 10.1111/j.1440-1827.2005.01782.x. [DOI] [PubMed] [Google Scholar]
- 16.Ou SH, Zell JA, Ziogas A, et al. Prognostic factors for survival of stage I nonsmall cell lung cancer patients : a population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer. 2007;110:1532–41. doi: 10.1002/cncr.22938. [DOI] [PubMed] [Google Scholar]
- 17.Padmore RF, Fowble B, Hoffman J, et al. Microinvasive breast carcinoma: clinicopathologic analysis of a single institution experience. Cancer. 2000;88:1403–9. [PubMed] [Google Scholar]
- 18.Patz EF, Jr., Rossi S, Harpole DH, Jr., et al. Correlation of tumor size and survival in patients with stage IA non-small cell lung cancer. Chest. 2000;117:1568–71. doi: 10.1378/chest.117.6.1568. [DOI] [PubMed] [Google Scholar]
- 19.Port JL, Kent MS, Korst RJ, et al. Tumor size predicts survival within stage IA non-small cell lung cancer. Chest. 2003;124:1828–33. doi: 10.1378/chest.124.5.1828. [DOI] [PubMed] [Google Scholar]
- 20.Rena O, Papalia E, Ruffini E, et al. Stage I pure bronchioloalveolar carcinoma: recurrences, survival and comparison with adenocarcinoma of the lung. Eur J Cardiothorac Surg. 2003;23:409–14. doi: 10.1016/s1010-7940(02)00830-8. [DOI] [PubMed] [Google Scholar]
- 21.Sakurai H, Maeshima A, Watanabe S, et al. Grade of stromal invasion in small adenocarcinoma of the lung: histopathological minimal invasion and prognosis. Am J Surg Pathol. 2004;28:198–206. doi: 10.1097/00000478-200402000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Scolyer RA, Shaw HM, Thompson JF, et al. Interobserver reproducibility of histopathologic prognostic variables in primary cutaneous melanomas. Am J Surg Pathol. 2003;27:1571–6. doi: 10.1097/00000478-200312000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Shimosato Y, Suzuki A, Hashimoto T, et al. Prognostic implications of fibrotic focus (scar) in small peripheral lung cancers. Am J Surg Pathol. 1980;4:365–73. doi: 10.1097/00000478-198008000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki K, Asamura H, Kusumoto M, et al. “Early” peripheral lung cancer: prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg. 2002;74:1635–9. doi: 10.1016/s0003-4975(02)03895-x. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki K, Yokose T, Yoshida J, et al. Prognostic significance of the size of central fibrosis in peripheral adenocarcinoma of the lung. Ann Thorac Surg. 2000;69:893–7. doi: 10.1016/s0003-4975(99)01331-4. [DOI] [PubMed] [Google Scholar]
- 26.Takashima S, Maruyama Y, Hasegawa M, et al. Prognostic significance of high-resolution CT findings in small peripheral adenocarcinoma of the lung: a retrospective study on 64 patients. Lung Cancer. 2002;36:289–95. doi: 10.1016/s0169-5002(01)00489-5. [DOI] [PubMed] [Google Scholar]
- 27.Travis WDCT, Corrin B, Shimosato Y, Brambilla E, et al. World Health Organization, Histological Typing of lung and pleural tumours. Springer; Berlin: 1999. [Google Scholar]
- 28.Yang ZG, Sone S, Takashima S, et al. High-resolution CT analysis of small peripheral lung adenocarcinomas revealed on screening helical CT. AJR Am J Roentgenol. 2001;176:1399–407. doi: 10.2214/ajr.176.6.1761399. [DOI] [PubMed] [Google Scholar]
- 29.Yim J, Zhu LC, Chiriboga L, et al. Histologic features are important prognostic indicators in early stages lung adenocarcinomas. Mod Pathol. 2007;20:233–41. doi: 10.1038/modpathol.3800734. [DOI] [PubMed] [Google Scholar]
- 30.Zwirewich CV, Vedal S, Miller RR, et al. Solitary pulmonary nodule: high-resolution CT and radiologic-pathologic correlation. Radiology. 1991;179:469–76. doi: 10.1148/radiology.179.2.2014294. [DOI] [PubMed] [Google Scholar]