Abstract
Melanoma is responsible for an estimated 62,000 new American cancer diagnoses and is projected to cause nearly 8,000 deaths in 2008 alone. Although the histogenesis of the tumor is not well understood, it is thought to originate from a rare melanocyte stem cell that resides in the skin. The transcription factor PAX3 has a well-established role in the development of melanocytes during embryogenesis, and has recently been characterized as a molecular switch in the mature melanocyte. In this capacity, PAX3 promotes a melanocytic phenotype but blocks terminal differentiation. This mechanism may also contribute to the uncontrolled cell growth and loss of terminal differentiation in melanomas. Here, we find PAX3 expression in 8/8 melanoma cell lines. We also see PAX3 commonly expressed in primary melanoma samples (21/58) but significantly less frequently in benign pigmented lesions (9/75). Further analysis of our melanoma set revealed that PAX3 expression is strongly correlated with younger patients with low or no evidence of sun damage. Our data suggest that PAX3-expressing melanomas may be less environmentally dependent and more genetically-linked.
Keywords: PAX3, melanoma, melanocyte, stem cell
INTRODUCTION
PAX3 (also known as Pax3 in the mouse) is an essential factor for embryonic melanoblast development and survival. Recently, Pax3 expression was described within the hair follicles of juvenile and adult mouse skin within the melanocyte stem niche and in the melanocyte stem cells themselves (1). The presence of Pax3 in melanocyte stem cells has been characterized by anatomic location of expression within the bulge region of the hair follicle, co-expression with other melanocyte stem cell markers (such as dopachrome tautomerase [Dct]), and co-expression with label-retaining cells identified by long-term retention of 5-bromodeoxyuridine (BrdU) DNA incorporation. Pax3 acts as a regulator of the stem cell phenotype by activating genes to initiate melanogenic lineage but also repressing downstream factors to prevent terminal differentiation (1). Pax3 has been suggested to play a role in lineage specificity in these melanocyte stem cells, but must be ultimately down-regulated for terminal differentiation to take place (1).
Expression of Pax3 has been described in a limited number of melanoma cell lines (2–4), but its precise role and mechanism of action in melanocytic oncogenic transformation is not understood. Melanoma is a deadly form of skin cancer that is occurring at an increasing incidence in the United States (5). Variations in clinical, histopathological, and molecular features suggest two major sub-groups of melanoma occurring in sun-exposed skin (6). One group demonstrates significant chronic sun damage as evidenced histologically by moderate or severe solar elastosis in the adjacent dermis. The second shows no or low levels of chronic sun damage (non-chronic sun damaged melanoma). This latter group tends to occur in younger patients and is linked with frequent mutations in the BRAF oncogene (7, 8).
Here, we find the expression of the melanocyte stem cell factor, PAX3, expressed in melanoma cell lines and primary melanomas but infrequently in melanocytic nevi. We find that the patients that have PAX3-expressing melanomas tend to be of the non- chronic sun damaged phenotype, having absent or low levels of solar elastosis and a significantly younger age at diagnosis. We propose that PAX3 is a marker for non- chronic sun damaged melanoma.
MATERIALS AND METHODS
Western blot analysis of melanoma cell lines
All cell lines were obtained through the University of Chicago Cancer Research Center. We studied the B16 murine melanoma cell line and eight human melanoma cell lines: 537, 624, 888, A375, HIM2, SK23, SKMEL5, and SKMEL28. The mouse fibroblast 3T3 and human pancreatic cancer cell line CFPAC1 are available from ATCC (Manassas, VA). For Western analysis detection of PAX6 and beta-tubulin, cell lysate (40 μg/well) was loaded on 4–12% gradient gels, transferred to PVDF membranes, and probed with PAX3 mouse monoclonal antibody (1:100 dilution, University of Iowa Hybridoma Bank, Iowa City, IA) and Western blotted (Western Breeze Chemiluminescent kit, Invitrogen, Carlsbad, CA). The membranes were stripped using Restore Western Blot Stripping Buffer (Pierce Biotechnology, Rockford, IL), checked for complete removal of antibodies and re-analyzed for beta-tubulin expression (1:400 mouse monoclonal antibody E7, University of Iowa Hybridoma Bank) as a loading control.
RT-PCR for Pax7 expression in melanoma cell lines
RNA was isolated from freshly growing cells using Trizol (Invitrogen). RT-PCR was performed using SuperScript III First-Strand Synthesis System for RT-PCR following the manufacturer’s protocols (Invitrogen) using random hexamer primers to generate cDNA strands. As a negative control against genomic DNA contamination, each RNA sample was tested by PCR following an RT step in the absence of reverse transcriptase. As a positive control, each RNA sample was tested for the expression of the housekeeping gene G3PDH. 2 μl of cDNA was used for the PCR reaction. G3PDH (human) Primers: Sense 5′ TGA AGG TCG AGT CAA CGG ATT TGG T 3′ and antisense 5′ CAT GTG GGC ATG AGG TCC ACC AC 3′ with an expected PCR product of 983 basepairs and PAX7 primers: forward 5′ GCT CCG GGG CAG AAC TAC C 3′ and reverse 5′GCA CGC GGC TAA TCG AAC TC 3′ with expected PCR product of 436 basepairs. PCR conditions were 94°C for 2 min, followed by 20 cycles of 94°C (30 s), 57°C (30 s), and 72°C (30 s). 293T cells transfected with Pax7 plasmid served as a positive control. The plasmid pcDNA3-Pax7 was transfected into 293T cells using Qiagen Effectene reagent (Qiagen Inc, Valencia, CA). Transfection efficiency was determined by pCMV-betagal transfection and staining of cells for beta-galactosidase activity.
Primary melanoma and nevus samples
All archival blocks of skin samples were obtained through the Section of Dermatology’s clinical tissue bank at University of Chicago Medical Center. All tissue samples were obtained and analyzed through collaboration with the tissue bank coordinator and in accordance with the University of Chicago Institutional Review Board and Clinical Trials Committee. All tissue samples were selected based on diagnosis (nevus or melanoma) and assigned a number by the tissue bank coordinator, then processed and scored under blind study conditions. Typical and atypical (also known as Clark or dysplastic) nevi were diagnosed using previously published criteria (9). Both architectural features (circumscription, symmetry, cohesiveness of nests, suprabasal melanocytosis, confluence, and single-cell proliferation) and cytologic features (round/euchromatic nuclei, nuclear enlargement, cell enlargement, and prominent nucleoli) were considerations for this determination. By these criteria, none of the nevi attained a degree of atypia sufficient to warrant the diagnosis of malignant melanoma. After scoring and analysis of PAX3 expression and for solar elastosis grade, diagnosis and patient age (at time of biopsy) were obtained. All patient identification information was removed from the sample and the clinical report in accordance with HIPAA guidelines.
Immunofluorescent staining of PAX3 in tissue samples
Fluorescence, rather than other chromagens, was utilized to avoid potentially confusing results due to the presence of melanin in the skin samples. The 5-micrometer tissue sections on slides were blocked using ImmunoPure Normal Goat Serum (Pierce Biotechnology), and were washed and incubated with Pax3 primary antibody (University of Iowa Hybridoma bank) at a 1:100 dilution and incubated overnight at 4°C. The secondary antibody was Goat Anti-Mouse IgG Dylight 547 (Pierce Biotechnology) and was used at a 1:200 dilution for 30 min. at room temperature. For scoring and analysis of PAX3 expression, slides were simply scored as “positive for expression” (equal or greater intensity of staining of positive controls) or “negative for expression” (equal or lesser intensity of staining of normal adjacent tissue and negative controls.) Since the pattern of Pax3 expression is well described during murine development (10), sagittal sections of embryonic day 12.5 mouse embryos were used for both positive controls (neural crest, neural tube) and negative controls (non-Pax3 expressing tissues of the body).
Sun damage analysis
Duplicate slides from each specimen were cut, with one used for PAX3 protein expression and the other stained with hematoxylin and eosin (H/E). The H/E slides were analyzed by a board-certified dermopathologist for sun damage using a solar elastosis index using a scale from 0 to 3 following previously described methods (8). Absent solar elastosis was classified as a 0 score, low level as 1, moderate as a 2, and severe as 3.
Statistical analysis
Differences in sample sets were determined by Student’s t-test using statistical analysis software (JMP IN software, SAS institute, Inc. Cary, NC). A p-value of less than 0.05 was considered statistically significant.
RESULTS
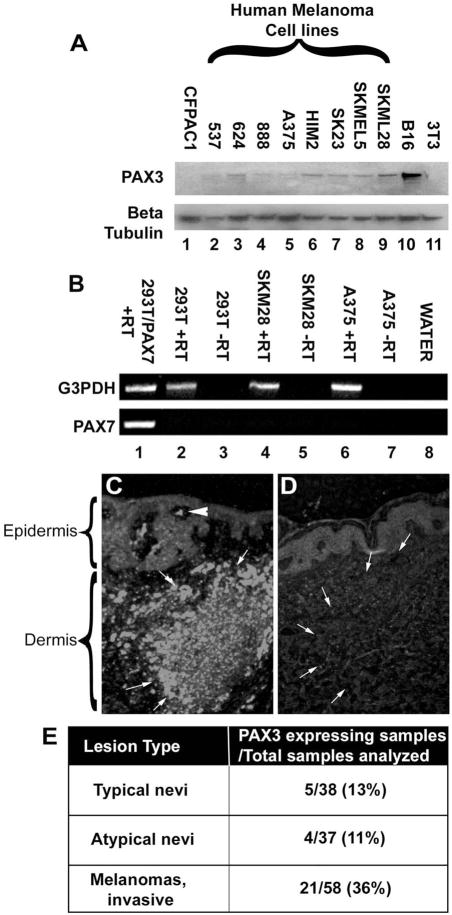
All the melanoma cell lines (one murine and eight human melanoma cell lines) examined by Western blot analysis were found to express varying levels of Pax3 (Figure 1A). As negative controls, non-melanoma cell lines CFPAC1 (a pancreatic carcinoma cell line, Figure 1A, lane 1) and 3T3 (mouse fibroblasts Figure 1A, lane 11) that do not express PAX3 are shown. The melanoma cell lines were also screened for the expression of PAX7, a closely related family member of PAX3 that is also expressed in melanocyte precursors. No PAX7 expression was detected in any of the cell lines examined (representative RT-PCR analysis, Figure 1B). Two major groups of primary pigmented lesions were also tested for in situ expression of PAX3. Out of 133 samples analyzed, 75 were benign pigmented lesions, with 38 characterized as typical and 37 as atypical (dysplastic) nevi. The vast majority of these samples (9/75) were negative for PAX3 expression (Figure 1D). In contrast, a significant number (21/58) (p<0.005) of metastatic melanoma samples demonstrated PAX3 immunoreactivity (Figure 1C). The tissue samples were scored either as “PAX3 protein expression positive” or “PAX3 protein expression negative.” These data are summarized in Figure 1E.
Figure 1. PAX3 is expressed in melanoma cell lines and primary tumor samples.
A. Western analysis for PAX3 expression in cell lines. Lane 1, CFPAC-1 pancreatic carcinoma cells (negative control). Lanes 2–9, human melanoma cell lines. Lane 10, B16 mouse melanoma cells (positive control). Lane 11, 3T3 mouse fibroblast cells (negative control). 40 micrograms of total protein were loaded in each lane. The protein sizes are approximately 56 kD (PAX3) and 50 kD (beta-Tubulin). B. A representative RT-PCR analysis for PAX7 expression. No PAX7 expression was detected in melanoma cell lines, including SKM28 (lane 4) and A375 (lane 6). Template cDNA was tested for expression of the housekeeping gene G3PDH as a positive control (lanes 2,4,6). Expression of G3PDH and PAX7 was also tested in RT-PCR reactions performed in the absence of reverse transcriptase (lanes 3,5,7) or RNA template (lane 8) as a negative control. 293T cells either transfected with PAX7 (lane 1) or untransfected (lane 2) served as an additional control. C, D. Immunohistochemistry for PAX3 expression in a primary melanoma (C) and a nevus (D). Melanocytic lesions are indicated with arrows. PAX3 expressing cells are evident in the melanoma mass in the dermis as well as pagetoid cells in the epidermis (arrowhead) in C. E. Tabular summary of PAX3 immunoreactivity in pigmented lesions, total n=133, benign pigmented nevi n=75, primary melanomas, n=58. Differences in PAX3 expression between groups is statistically significant, p<0.005. Western Analysis shown is a representative of three independent experiments. Immunohistochemistry shown in C and D is an example for the majority of samples analyzed and summarized in E.
The melanoma tumor samples were then split into two major groups, melanomas that expressed PAX3 (PAX3(+) tumors) and those that did not express PAX3 (PAX3(-) tumors). The PAX(+) tumor patients were significantly younger at age of biopsy and diagnosis. The PAX(+) patients were on average 45.3 years +/− 3.3 years, in comparison to the PAX3(−) patients with a mean age of 63.0 years +/− 3.3 years (standard error of the mean) (Figure 2). This age difference is highly significant (p <0.005).
Figure 2. Age differences between melanoma patients with or without PAX3-expressing tumors.
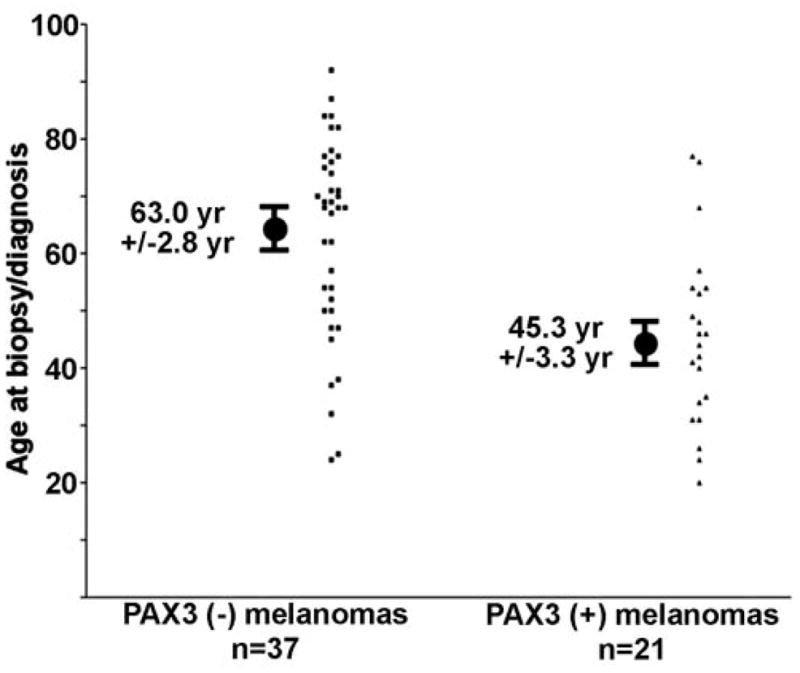
Patients lacking PAX3 protein expression in their melanoma tissues are older (63.0 years +/−2.8 years) than those who have PAX expressing tumors (45.3 years +/− 3.3 years). Ages are calculated at time of biopsy and diagnosis. Age ranged from 20 to 92 years old. Ages are calculated as mean +/− S.E.M. Median ages for each group is 68 years (PAX negative) and 44 years (PAX3 positive).
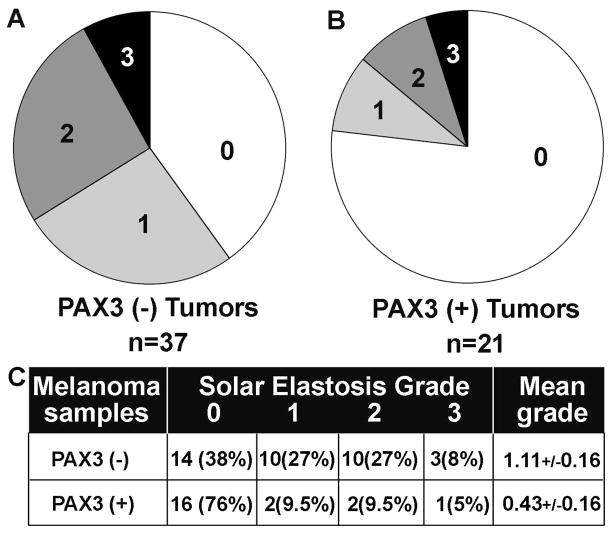
Melanoma samples were scored for solar elastosis grade, on a scale from 0 (no solar elastosis) to 3 (severe solar elastosis) (8). For the PAX3(-) tumors, 62% had some evidence of sun damage, with 35% demonstrating moderate to severe solar elastosis (Figure 3A). In contrast, PAX3(+) tumors overwhelmingly lacked signs of sun damage (76%) and only infrequently showed moderate or severe sun exposure (14.5%) (Figure 3C). The solar elastosis grades between groups, 1.11+/−0.16 for PAX3(−) tumors, 0.43+/−0.16 for PAX3(+) tumors was significantly different, with a p<0.02. These data are summarized in Figure 3C.
Figure 3. PAX3 expression in correlated with low or no evidence of sun damage in melanomas.
A, B. Percent of tumors (in pie chart format) for each grade of solar elastosis in the PAX3(−) expressing tumors (A) and in PAX3(+) expressing tumors (B). Hematoxylin and eosin stained sections were scored by previously defined methods (8), with a scale ranging from no evidence of sun damage = 0 to severe sun damage = 3. Levels of sun damage are defined by histological signs of solar elastosis in surrounding tissues. C. Tabular summary of solar elastosis/sun damage analysis of PAX3 negative or expressing tumors. Differences in solar elastosis grade score between groups are significantly different (p<0.02).
DISCUSSION
Somatic stem cells are characterized by a unique self-renewing capability within the adult organism. These cells play a key role in specialized tissue maintenance and repair by establishing a balance between unspecialized proliferation and differentiation. Essentially, these cells utilize the same molecular pathways that characterize early phases of organ development in the embryo (12). Cancer’s proliferative nature closely resembles the stem cell’s undifferentiated phenotype and self-renewing characteristics, suggesting a possible link in nature and/or mechanism.
PAX3 (also known as Pax3 in the mouse), a member of the PAX family of paired-domain proteins, plays an essential role in the development of neural crest cells. The neural crest is an embryonic tissue type that migrates to highly specific regions within the developing embryo. These embryonic cells possess a high degree of pluripotency and give rise to a multitude of different daughter cells including melanocytes (10, 11). The importance of PAX3 in the melanocyte population during development is demonstrated through the murine Splotch phenotype and in the clinical manifestations of Waardenburg syndrome, both of which result from mutations of the PAX3 (or murine pax3) gene. Loss of Pax3 expression in the mouse embryo leads to a greatly reduced number of melanoblasts. Although these mice retain a viable population of melanoblasts, the loss of Pax3 inhibits their precursors from expanding their numbers and therefore lessens the available pool of committed melanoblasts (13). In this cell population, Pax3 functions as a transcription factor by regulating the expression of lineage-specific genes (14).
The role for Pax proteins in mature tissues is not well defined. In a few contexts, such as that of Pax8 in the thyroid, these proteins have been characterized as essential for tissue maintenance (15). However, most cells tend to down-regulate the expression of Pax proteins following organogenesis. Persistent expression of these proteins may have a deleterious effect under many circumstances. In the kidney, Pax2 is expressed in cystic and hyperproliferative dysplastic diseases (16–18). Sustained expression of PAX genes may also contribute to oncogenesis (2, 19, 20). PAX 2, 5, and 8 expression has been identified in various cancers. Additionally, PAX3 and PAX7 are expressed in rhabdomyosarcomas (21, 22).
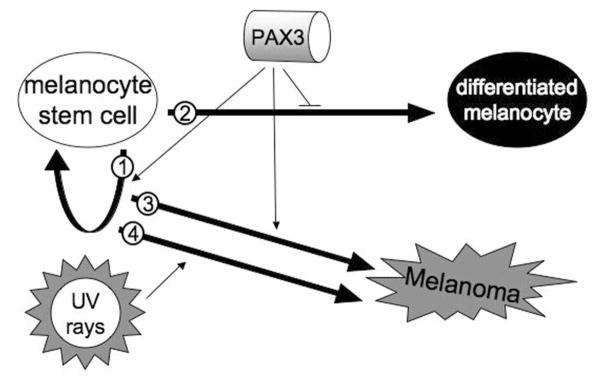
While Pax3 is essential for melanoblast development and survival in the embryo, Pax3 is also expressed in melanocyte stem cells and in pluripotent skin-derived precursor (SKP) cells in the post-embryonic skin (1, 23, 24). The function of PAX3 in the stem cell is not well understood, although evidence suggests that it plays a role in stem cell maintenance (Figure 4, arrow 1) and inhibition of terminal differentiation (Figure 4, arrow 2) (1). A possible duty for PAX3 in maintaining the stem cell is by inhibiting apoptosis, since inhibition of PAX genes by siRNA promotes apoptosis (2, 21, 25). In embryos lacking pax3, there is an increase in cell death, and this apoptosis can be partially reversed if it is coupled with the loss of function of the p53 gene (26). There is also evidence for a direct role in apoptosis since Pax3 directly regulates expression of the anti-apoptotic protein Bcl-XL (27). PAX3 is down-regulated as cells differentiate both in melanocytes (S. K. Powell, D. Lang unpublished observations) as well in myoblasts, and is uncommonly co-expressed with differentiation markers. Pax3 also represses the expression of genes involved with the differentiated phenotype such as Dct (also known as TRP2) (1).
Figure 4. A model for melanoma development.
Within the mature tissues, melanocyte stem cells reside and self-renew (arrow 1) or generate daughter cells to produce pigment for the skin (arrow 2). PAX3 protein helps maintain the stem cell phenotype while inhibiting terminal differentiation. Genetic susceptibility leaves the stem cell vulnerable to uncontrolled cell growth and loss of terminal differentiation in melanomas (arrow 3). Melanomas are also induced through environmental factors, such as by sun damage through ultraviolet irradiation (arrow 4). In our present study, we find a correlation between PAX3 expression in non-chronically sun-damaged melanomas but not in chronically sun-damaged melanomas.
In this study we see expression of PAX3 in a subgroup of melanomas (Figure 4, arrow 3). It is thought that there are two major origins of melanomas, “genetic dependent” and “environment dependent” (28, 29). Although these terms are over-generalized, they are representative of the basic model that some melanomas result from chronic sun damage caused by ultraviolet radiation exposure (Figure 4, arrow 4) while others, often occurring in areas of limited sun-exposure (non-chronic sun damaged) originate from pre-existing nevi or a genetic predisposition (Figure 4, arrow 3). Our data suggest that PAX3 may be important in non-chronic sun damaged tumors, due to the link between PAX3 immunoreactivity, younger age at biopsy, and lower solar elastosis grade (Figure 2, 3). Chronic sun damage may induce melanoma by an independent mechanism, or work on pathway components down-stream of PAX3, thereby making expression of PAX3 protein dispensable. Other factors correlated with non-chronic sun damaged tumors are mutations in the BRAF or N-RAS genes (7). Signals through receptor tyrosine kinases can promote proliferation through BRAF and the MAP kinase pathway as well as promote survival through the PI3 kinase cascade. Future studies will be designed to examine if PAX3 is involved in this pathway. Perhaps, in non-chronic sun damaged tumors, PAX3 actively promotes lesion survival while BRAF mutations support tumor proliferation.
Acknowledgments
We would like to thank Jacob Plummer for advice and support. This work has been supported by the American Cancer Society, the Cancer Research Foundation, the Louis Block Fund/University of Chicago, the Friends of Dermatology/University of Chicago, and the American Skin Association.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
Authors have no conflicts of interest to declare.
References
- 1.Lang D, Lu MM, Huang L, et al. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature. 2005;433:884–7. doi: 10.1038/nature03292. [DOI] [PubMed] [Google Scholar]
- 2.Muratovska A, Zhou C, He S, Goodyer P, Eccles MR. Paired-Box genes are frequently expressed in cancer and often required for cancer cell survival. Oncogene. 2003;22:7989–97. doi: 10.1038/sj.onc.1206766. [DOI] [PubMed] [Google Scholar]
- 3.Parker CJ, Shawcross SG, Li H, et al. Expression of PAX 3 alternatively spliced transcripts and identification of two new isoforms in human tumors of neural crest origin. Int J Cancer. 2004;108:314–20. doi: 10.1002/ijc.11527. [DOI] [PubMed] [Google Scholar]
- 4.Vachtenheim J, Novotna H. Expression of genes for microphthalmia isoforms, Pax3 and MSG1, in human melanomas. Cell Mol Biol. 1999;45:1075–82. [PubMed] [Google Scholar]
- 5.Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004;150:179–85. doi: 10.1111/j.1365-2133.2004.05708.x. [DOI] [PubMed] [Google Scholar]
- 6.Rivers JK. Is there more than one road to melanoma? Lancet. 2004;363:728–30. doi: 10.1016/S0140-6736(04)15649-3. [DOI] [PubMed] [Google Scholar]
- 7.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 8.Landi MT, Bauer J, Pfeiffer RM, et al. MC1R germline variants confer risk for BRAF-mutant melanoma. Science. 2006;313:521–2. doi: 10.1126/science.1127515. [DOI] [PubMed] [Google Scholar]
- 9.Shea CR, Vollmer RT, Prieto VG. Correlating architectural disorder and cytologic atypia in Clark (dysplastic) melanocytic nevi. Hum Pathol. 1999;30:500–5. doi: 10.1016/s0046-8177(99)90191-0. [DOI] [PubMed] [Google Scholar]
- 10.Le Douarin NM, Dupin E. Multipotentiality of the neural crest. Curr Opin Genet Dev. 2003;13:529–36. doi: 10.1016/j.gde.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Teillet MA, Le Douarin N. The migration of pigmentary cells studies by the method of heterospecific grafts of neural tube in bird embryo. C R Acad Sci Hebd Seances Acad Sci D. 1970;270:3095–8. [PubMed] [Google Scholar]
- 12.Lang D, Powell SK, Plummer RS, Young KP, Ruggeri BA. PAX genes: roles in development, pathophysiology, and cancer. Biochem Pharmacol. 2007;73:1–14. doi: 10.1016/j.bcp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Hornyak TJ, Hayes DJ, Chiu LY, Ziff EB. Transcription factors in melanocyte development: distinct roles for Pax-3 and Mitf. Mech Dev. 2001;101:47–59. doi: 10.1016/s0925-4773(00)00569-4. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe A, Takeda K, Ploplis B, Tachibana M. Epistatic relationship between Waardenburg syndrome genes MITF and PAX3. Nat Genet. 1998;18:283–6. doi: 10.1038/ng0398-283. [DOI] [PubMed] [Google Scholar]
- 15.Santisteban P, Bernal J. Thyroid development and effect on the nervous system. Rev Endocr Metab Disord. 2005;6:217–28. doi: 10.1007/s11154-005-3053-9. [DOI] [PubMed] [Google Scholar]
- 16.Dressler GR, Wilkinson JE, Rothenpieler UW, et al. Deregulation of Pax-2 expression in transgenic mice generates severe kidney abnormalities. Nature. 1993;362:65–7. doi: 10.1038/362065a0. [DOI] [PubMed] [Google Scholar]
- 17.Murer L, Caridi G, Della Vella M, et al. Expression of nuclear transcription factor PAX2 in renal biopsies of juvenile nephronophthisis. Nephron. 2002;91:588–93. doi: 10.1159/000065017. [DOI] [PubMed] [Google Scholar]
- 18.Winyard PJ, Risdon RA, Sams VR, Dressler GR, Woolf AS. The PAX2 tanscription factor is expressed in cystic and hyperproliferative dysplastic epithelia in human kidney malformations. J Clin Invest. 1996;98:451–9. doi: 10.1172/JCI118811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumann Kubetzko FB, Di Paolo C, Maag C, et al. The PAX5 oncogene is expressed in N-type neuroblastoma cells and increases tumorigenicity of a S-type cell line. Carcinogenesis. 2004;25:1839–46. doi: 10.1093/carcin/bgh190. [DOI] [PubMed] [Google Scholar]
- 20.Gnarra JR, Dressler GR. Expression of Pax-2 in human renal cell carcinoma and growth inhibition by antisense oligonucleotides. Cancer Res. 1995;55:4092–8. [PubMed] [Google Scholar]
- 21.Bernasconi M, Remppis A, Fredericks WJ, Rauscher FJ, 3rd, Schafer BW. Induction of apoptosis in rhabdomyosarcoma cells through down-regulation of PAX proteins. Proc Natl Acad Sci U S A. 1996;93:13164–9. doi: 10.1073/pnas.93.23.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiffin N, Williams RD, Shipley J, Pritchard-Jones K. PAX7 expression in embryonal rhabdomyosarcoma suggests an origin in muscle satellite cells. Br J Cancer. 2003;89:327–32. doi: 10.1038/sj.bjc.6601040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toma JG, Akhavan M, Fernandes KJ, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–84. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 24.Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–37. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 25.Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development. 1990;109:787–95. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- 26.Pani L, Horal M, Loeken MR. Rescue of neural tube defects in Pax-3-deficient embryos by p53 loss of function: implications for Pax-3- dependent development and tumorigenesis. Genes Dev. 2002;16:676–80. doi: 10.1101/gad.969302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Margue CM, Bernasconi M, Barr FG, Schafer BW. Transcriptional modulation of the anti-apoptotic protein BCL-XL by the paired box transcription factors PAX3 and PAX3/FKHR. Oncogene. 2000;19:2921–9. doi: 10.1038/sj.onc.1203607. [DOI] [PubMed] [Google Scholar]
- 28.Mishima Y. Melanocytic and nevocytic malignant melanomas. Cellular and subcellular differentiation. Cancer. 1967;20:632–49. doi: 10.1002/1097-0142(1967)20:5<632::aid-cncr2820200510>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Mishima Y, Matsunaka M. Pagetoid premalignant melanosis and melanoma: differentiation from Hutchinson’s melanotic freckle. J Invest Dermatol. 1975;65:434–40. doi: 10.1111/1523-1747.ep12608179. [DOI] [PubMed] [Google Scholar]