Abstract
An investigation of the reaction of Pd(II) complexes with proflavine (3,6-diaminoacridine) resulted in the isolation of the compounds [Pd(terpy)(proflavine)](NO3)(HSO4)·3H2O, 1, (terpy = 2,2’:6’,2”-terpyridine), [Pd(en)(proflavineH))](NO3)(SO4), 2, (en = ethylenediamine), and [Pd(proflavineH)Cl2](SO4)0.5 ·H2O, 3. They have been isolated and characterized by NMR, IR, and electrospray ionization mass spectrometry techniques and by elemental analyses. The proflavine was bonded to the Pd(II) through the endocyclic nitrogen in 1, but through the proflavine NH2 in 2. Compound 3 appeared to be polymeric in the solid state with a 1:1 mole ratio of Pd(II):proflavine. Upon solution of 3 in DMSO, two unique species were formed. In one species the Pd(II) was bonded to Two proflavines through the endocyclic nitrogen (1:2 mole ratio) and in the other species, a Pd(II) was bonded to each NH2 group of a single proflavine (2:1 mole ratio). Molecular modeling of the equilibrium geometry by Spartan 8 produced structures which were consistent with the experimental data on the solutions of the three compounds. In vitro cytotoxicity testing against two breast cancer cell lines and one ovarian cancer cell line showed that compounds 1 and 3 had significant activity.
Keywords: proflavine; 3,6-diaminoacridine; palladium complex; NMR spectroscopy; cytotoxicity
1. Introduction
The success of cisplatin, oxaliplatin, and other Pt(II) complexes in the treatment of testicular, ovarian, head and neck, esophageal and non-small cell lung cancers [1-5], and the similarity of the properties of Pt(II) and its congener Pd(II), has led to a large effort in the search to find Pd(II) antitumor drugs that are effective against Pt(II)-resistant therapies and that have fewer side effects [6-9]. An important difference between the two metal ions is that the reaction kinetics of Pd(II) is 105 times faster than that of Pt(II), which could lead to the hydrolysis of Pd complexes before they reach their target DNA.
The antimicrobial properties of acridine and substituted acridines have been known for many years. 3,6-Diaminoacridine (proflavine) was used during World War I as a topical antiseptic; however, it was found to be too toxic to use as a systemic antibacterical [10]. Acridines have been used as antimalarial agents since the 1940’s. More recently they have been investigated as potential antitumor drugs because of their ability to bond to DNA, the mode of interaction being intercalation between the base pairs of the double helix [11-14]. 2,2’:6’,2”-Terpyridine is also a DNA intercalator and the antitumor activity of Pt-terpyridine complexes has been investigated [15].
It was hypothesized that a combination of a Pd(II) species and proflavine could lead to the formation of complexes with antitumor activity having both metal-DNA covalent bonding and intercalation of the proflavine [16, 17]. As a result, this might be expected to yield a more potent antineoplastic agent. Complexes of a number of Pt-acridines [16-22] have been synthesized and their therapeutic properties evaluated, but no report of a Pd(II) complex of proflavine has been found. In the complexes of Pt(II) with N,N’,N”,N’”-tetramethyl-3,6-diaminoacridine (agent orange), the Pt has been found to bond to the endocyclic nitrogen atom [20].
We have synthesized, characterized and performed cytotoxicity studies with several Pd(II) complexes of proflavine. Bonding of the Pd(II) moiety has been identified at the endocylic nitrogen in some of the compounds and at the amino group nitrogen in others. The preferred bonding site appears to be determined by the other ligands on the Pd(II) and the reaction conditions.
2. Experimental
2.1. Materials
Proflavine hemisulfate monohydrate (3,6-diaminoacridine), dichloro(ethylenediamine)palladium(II), potassium tetrachloropalladate(II), diamminedichloroplatinum(II), 2,2’:6’,2”-terpyridine (terpy), deuterated solvents and chemicals for cytotoxicity studies were obtained from Sigma Aldrich and used as received.
2.2. Synthetic Methods
[Pd(terpy)Cl]Cl was prepared according to the literature procedure [23]. [Pd(en)(H2O)2](NO3)2 and [Pd(terpy)(NO3)](NO3) were prepared from Pd(en)Cl2 or [Pd(terpy)Cl]Cl by precipitation of AgCl using AgNO3 according to literature methods [24].
2.2.1. [Pd(terpy)(proflavine)](NO3)(HSO4)·3H2O, (1)
[Pd(terpy)(NO3)](NO3) (50 mg, 1.0 × 10-4 mol) was combined with proflavine hemisulfate (25.8 mg, 1.00 × 10-4 mol) in 40 mL of ethanol. It was warmed using a water bath (40-50° C) for 4-5 hours with stirring, and left to stir at room temperature overnight. The solution was filtered to remove a small amount of impurities and was placed in a refrigerator at +2° C. In 3 days an orange-red precipitate was collected by vacuum filtration. Yield of [Pd(terpy)(proflavine)] (NO3)(HSO4)·3H2O: 52 mg (67 %). Anal. Calc. for C28H29N7O10PdS (Mr = 762.06): C, 44.13; H, 3.84; N, 12.87. Found: C, 43.74; H, 3.50; N, 12.99. IR (solid; cm-1; vs = very strong, s = strong, m = medium, w = weak, sh = shoulder) 3327m, 3180m, 3076m, 3043m, 1637s, 1599s, 1573s, 1480s, 1453w, 1379s, 1367s,sh, 1323vs, 1240w, 1173vs, 1142vs, 1026vs, 907m, 889m, 825w, 770m.
2.2.2. Pd(en)(proflavineH))](NO3)(SO4), (2)
[Pd(en)(H2O)2](NO3)2 (10 mL of 40 mM solution, 4.0 ×10-4 mol) in DI water was combined with 10 mL of 20 mM proflavine (2.0 ×10-4 mol) in DI water. The solution was warmed on a hot-plate (40-45°C), until half of the solvent was evaporated and then the solution was left to stand at room temperature until a dark red precipitate formed (3 days). The mixture was filtered, washed with cold water and ethanol, and air dried. Yield of [Pd(en)(proflavineH))](NO3)(SO4): 21 mg (24 %). Anal. Calc. for C15H20N6O7 PdS (Mr= 534.84): C, 33.68; H, 3.77; N, 15.71. Found C, 33.44; H, 4.00; N, 16.25. IR(solid; cm-1) 3349w, 3219w, ~3080vw, 1627s, 1592s, 1481m, 1461m,sh, 1386m 1337m, 1247s, 1157s, 1121s, 1022s, 937w, 853w, 754w, 632s, 572s.
2.2.3. [Pd(proflavineH)Cl2](SO4)0.5 ·H2O, (3)
An aqueous solution of K2PdCl4 (20 mL of 10 mM solution, 2.0 × 10-4 mol) was cooled in a beaker in an ice bath and added dropwise to 20 mL of 10 mM aqueous solution of proflavine (2.0 × 10-4 mol). Immediately upon addition of the first drop of the K2PdCl4 solution a fine, dark brown precipitate began to form. The K2PdCl4 solution was added slowly and the mixture was left to stand with stirring for two days to allow the fine precipitate to coagulate. The solution was suction filtered under N2; the dark maroon solid was washed with cold water and air dried. The only solvents in which it was soluble were DMSO and dimethylformamide. Yield of [Pd(proflavineH)Cl2](SO4)0.5 ·H2O: 12 mg (13 % ). Anal. Calc. for C13H14N3O3PdS0.5 (Mr = 453.63): C, 34.42; H, 3.11; N, 9.26. Found: C, 34.35; H, 3.15; N, 8.96. IR (solid; cm-1) 3330w, 3209w, 1637vs, 1592s, 1483s, 1393m 1309w, 1263w, 1236w, 1169s, 1119s, 939w, 850m, 810m, 754w, 648w, 590w.
2.3. Methods
1H NMR spectra were recorded at 499.68 MHz on a Varian INOVA 500 MHz spectrometer with variable temperature control. Spectra were referenced to the residual protons in the deuterated solvent, except for spectra at non-ambient temperatures, when the tetramethylammonium ion was used (3.185 ppm). FT-IR spectra of the solids were recorded at 4 cm-1 resolution on a Bruker VECTOR 22 spectrophotometer with an ATR attachment. There was considerable uncertainty in the IR bands in the 3000-3400 cm-1 region , especially for [Pd(en)(proflavineH)](NO3)(SO4) and [Pd(proflavineH)Cl2](SO4)0.5 ·H2O, because they were broad and weak. Electro-spray ionization mass spectra (ESI-MS) were recorded in the positive ion mode on a Finnigan LCQ Duo spectrometer in water or DMSO. MS conditions were: source voltage, 5.1 kV; capillary temperature, 230 °C; capillary voltage, 5-30 V depending on the sample; sheath flow rate, 40; auxil1ary flow rate, 20. Elemental analyses were performed by Midwest Microlab, LLC, Indianapolis, IN.
2.4. Spartan 8 Calculations
Equilibrium geometries were calculated using the Semi-Empirical Method with PM3 following an Energy minimization. In all cases, the geometry about the Pd(II) ion was constrained in the square planar configuration, the established geometry for the Pd(II) ion. If the constraints were not applied, an incorrect geometry resulted for the Pd(II) ion.
2.5. Cytotoxicity Testing in vitro
Four complexes: [Pd(proflavineH)Cl2](SO4)0.5 ·H2O, [Pd(terpy)(NO3)](NO3), [Pd(terpy)(proflavine)](NO3)(HSO4)·3H2O, [Pd(en)(H2O)2](NO3)2, plus proflavine and cisplatin were tested against three cancer cell lines: breast (SK-BR-3; MCF-Her-2-6) and ovarian (SK-OV-3). Solutions (10 mM) of the palladium complexes, proflavine, and cisplatin were prepared in sterile deionized H2O and five dilutions (100 μM, 10 μM, 1 μM, 100 nM, 10 nM) were done with sterile water. Solid [Pd(proflavineH)Cl2](SO4)0.5 ·H2O was dissolved in DMSO and diluted in the same fashion as the other compounds. Each solution was filtered through a Fisher sterile nylon 0.22 μm syringe filter unit except Pd(proflavineH)Cl2](SO4)0.5 ·H2O, which was filtered through a 0.8 μm unit. The cells were plated as 104 cells per 200 μL well in a 96 well plate in DMEM medium supplemented with 10 % fetal bovine serum, glutamine, penicillin and streptomycin and incubated overnight at 5 % CO2 and 37°C. The solutions of the prepared concentrations were added to the wells and incubated again for 36 hours. The solutions were removed from the wells and the cells were washed with Hank’s balanced salt solution (HBSS). HBSS (100 μL) and 10 μL of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution was added and the plates were again incubated for one hour after which time the absorbance at 570 nm was measured [25]. The percent survival rate of the cells was measured as the ratio of intensities of the treated cells (with drug) divided by the control (no drug). Two independent experiments were done and each concentration was run in triplicate. [Pd(en)(H2O)2](NO3)2 was tested only in the first of the two experiments because it was found to be relatively ineffective and cisplatin was only tested in the second experiment.
3. Results and Discussion
The proflavine molecule has two potential types of bonding sites to a Pd(II) ion: the endocyclic N10 and the two exocyclic amino groups. The more basic of these two sites is N10, so much so that it remains protonated as N-H+ under neutral and somewhat basic conditions in water (pKa= 9.53 ± 0.05) [26]. However, we have observed metal bonding to the N10 site in some cases and to the amino groups in other cases. The 1H NMR spectrum of pure proflavine in D2O contains only four resonances as a result of the symmetrical nature of the molecule (Fig. 1A, Table 1). The spectrum is pH dependent as expected and all resonaces shift downfield with decreasing pH. The H4,5 protons are quite acidic and at pH ≤ 4, these resonance quickly decrease in intensity and vanish due to their exchange when the solvent is D2O.
Fig. 1.

1H NMR spectra in D2O: (A) proflavine hemisulfate, pD 4.5; (B) [Pd(terpy)(proflavine)](NO3)(HSO4), pD 5.0; (C) [Pd(en)(proflavineH)](NO3)(SO4), pD 5.0.
Table 1.
| H9 | H1,8 | H2,7 | H4,5 | Other | |
|---|---|---|---|---|---|
| Proflavine, pD 4.5, D2O | 7.57s | 7.00d J=8.8 | 6.46dd J=8.7, 2.0 | 5.75d J=1.6 | |
| Pd(terpy)(NO3)2 pD 4.2 D2O | 8.41m, 7.85m | ||||
| Pd(terpy)(Pro), pD 5.0, D2O, 1 | 7.75s | 6.93d J=8.3 | 6.17d J=8.8 | 5.59s | ~ 8.10, 7.41 |
| Pd(en)(Pro), pD 4.5, D2O, 2 | 8.34s | 7.85d (H8); 6.82d (H1) J=9.0; J=8.8 | 7.30 dd (H7); 5.97dd (H2) J=9.3, 2.0; J=8.7, 1.8 | 9.54s (H4); 8.90s (H5) | en: 3.07m |
| Proflavine , DMSO-d6 | 8.52s | 7.71d J=8.8 | 6.90dd J=9.3, ~1.6 | 6.71d J=~1.6 | NH2 6.54s |
| Pd((Pro)Cl (I), DMSO-d6, 3 | 8.74d J=1.9 | 7.81 d J=9.0 | 6,95 dd J=9.2,1.8 | 6.67d J=2.1 | NH2 7.26s N(10)H+ 13.4 |
| Pd(Pro)Cl (II), DMSO-d6, 3 | 8.25s | 6.91dd J=8.8,1.6 | d7.63d; 7.635d J=8.7; J=8.6 | 8.43d; 8.44 d J=1.7; J=5.2 | NH2 6.63s, 6.82s |
chemical shifts in ppm; coupling constants in Hz
s, singlet; d, doublet; dd, doublet of doublets; m, multiplet
Pro = proflavine
H2 and H7 are separate resonances which overlap each other. Chemical shift order is not known.
3.1. [Pd(terpy)(proflavine)](NO3)(HSO4)·3H2O, (1)
Of the Pd(II)-proflavine complexes reported herein, this complex is the most straight forward. [Pd(terpy)(NO3)](NO3) reacted readily with proflavine in water to form a 1:1 complex in which the proflavine was bonded to the Pd(II) via the N10 atom (Scheme 1A). This type of bonding was deduced from the presence of only four proflavine resonances in the 1H NMR spectrum in D2O at pD 5.0 (Fig. 1B, Table 1), indicative of a symmetrically bonded proflavine. The H9 proton shifted downfield by 0.18 ppm, reflecting the influence of the metal on the center ring, while the H2,7 protons shifted upfield by 0.29 ppm. All of the terpy resonances were shifted upfield in the complex, most likely due to aromatic stacking.
Scheme 1.
The IR spectrum provided solid state evidence that the Pd(II) was bonded to the N10 atom of proflavine (Fig. 2). There was a small shift in the νasym (N-H) and essentially no shift in the νsym (N-H). A larger shift was observed for the C=C and C=N stretching vibrations with the solid complex having absorptions at 1637 and 1573 cm-1, compared to 1630 and 1565 cm-1 for proflavine [27]. The shift to higher wavenumbers was indicative of electronic effects in the aromatic system due to bonding at the N10 site. The presence of the HSO4- anion was confirmed by strong, broad absorptions in the 1140-1175 cm-1 region [28], and the ionic nitrate was identified by νasym at 1367 cm-1 and a single weak combination band at 1710 cm-1 [29].
Fig. 2.

IR spectra as solids.(A) proflavine hemisulfate; (B) [Pd(terpy)(profavine)](NO3)(HSO4) •3H2O.
The ESI-MS spectrum of [Pd(terpy)(proflavine)](NO3)(HSO4)·3H2O dissolved in H2O contained several monomeric Pd(II) species and the interpretation was straight forward (Fig. 3), in contrast to the spectra of some other Pd(II) compounds in aqueous solution which exhibited multinuclear Pd(II) species believed to have been formed during the ESI vaporization process [30,31]. The Pd(proflavine)-containing species identified and matched by simulation were [Pd(terpy)(proflavine)](NO3)+, m/z 609.60, and [Pd(proflavine)(NO3)]+, m/z 376.20. Other Pd(terpy)-containing species were [Pd(terpy)(NO3)]+, m/z 400.10, [Pd(terpy)OH]+, m/z 356.13, and [Pd(terpyH)]+, m/z 338.40. Proflavine (m/z 210.60) and pyridine (m/z 77.87) from the decomposition of terpy were also observed. No sulfate-containing complexes of Pd were found.
Fig. 3.

ESI-MS of [Pd(terpy)(proflavine)](NO3)(HSO4) in H2O: A. full spectrum; B. expansion of 609 m/z; C. simulation for [Pd(terpy)(proflavine)(NO3)[+.
The energy minimized equilibrium geometry obtained from a Spartan calculation of the [Pd(terpy(proflavine))2+ cation is shown in Fig. 4. The N(terpy 1)-Pd-N(terpy 3) angle is 167.5° and the N(terpy 2)-Pd-N10 is 174.1°. The proflavine is nearly perpendicular with respect to the terpyridine ring with an average C-N10-Pd-N(terpy 2) dihedral angle of 88°.
Fig. 4.

Structure of [Pd(terpy)(proflavine)]2+ ion as modeled with Spartan. Color code: C gray; H white; N blue; Pd purple
3.2. [Pd(en)(proflavineH))(NO3)](SO4), (2)
This system is much more complex than that of Pd(terpy)-proflavine. Equilibrium was attained slowly upon mixing aqueous solutions of [Pd(en)(H2O)2](NO3)2 and proflavine hemisulfate. After 72 hours the solution contained predominantly one complex with small amounts of unreacted proflavine and other species. However, a pure 1:1 complex, [Pd(en)(proflavineH))](NO3)(SO4), could be isolated from a 2:1 mole ratio of [Pd(en)(H2O)2]2+ to proflavine in which the large excess of Pd(II) pushed the equilibrium in favor of the less soluble 1:1 species. A proposed structure based on the 1H NMR, IR, and ESI-MS data is shown in Scheme 1B. The 1H NMR spectrum (Fig. 1C, Table 1) of an aqueous solution prepared from the solid showed the presence of only one complex in which the proflavine was bonded in an unsymmetrical fashion to the Pd(en)2+ moiety, as confirmed by individual resonances for each of the seven proflavine non-exchangeable protons. The spectrum is consistent with bonding of the Pd(en)2+ to one NH2 group (N3) of the proflavine and assignments were made on the basis of a 1H COSY spectrum (Fig. S1). A downfield shift of the H4 and H5 protons upon bonding of the Pd(II) group to the amino group was expected, and the magnitude of the shift of 3.64 (H4) and 3.08 ppm (H5) was consistent with that observed for the aromatic protons in other acridine derivatives in which Pt(II) is bonded to an exocyclic amino group [22]. This indicates that the amino group, although exocyclic, had considerable electronic coupling with the aromatic ring system, which was affected strongly by the bonding of a metal ion to the NH2.
The en region (Fig.1C inset)) contained what appeared to be an AA‘BB’ multiplet and an AA’XX’ multiplet, the centers of which have nearly the same chemical shifts. These multiplets are consistent with conformational rigidity of the ethylene groups. Our interpretation of this is that when the solid complex (X ligand NO3-) was dissolved in D2O, some of the nitrato ligands were replaced by D2O, resulting in a mixture of environments for the en ligand.
The N-H stretching region in the IR spectrum (Fig. S2) of the solid complex was difficult to interpret due to the presence of amino groups on both the proflavine and en ligands. However, two broad bands (3349 and 3219 cm-1) were observed at wavenumbers different from those of either of the reactants. The aromatic C=C and C=N region (1627 and 1592 cm-1) showed little shift between pure proflavine and the Pd(en)-proflavine complex, as expected if the Pd(II) was bonded to the proflavine NH2. The fourth position about the square planar Pd(II) appeared to be occupied by a coordinated monodentate nitrato ligand as shown by a strong absorption at 1247 cm-1. Additional strong bands at 1121 and 1157 cm-1 indicated the presence of a sulfate or hydrogen sulfate anion in the solid [29].
Most of the major peaks in the ESI-MS were assignable with good matches to the simulated spectrum (Fig. 5). They were consistent with the NMR and IR data and included [Pd(en)(proflavine)-H)]+, m/z 374.33, [Pd(en)(proflavine)(NO3]+, m/z 437.33, and a dinuclear species {2[Pd(en)(proflavine)(NO3)2] - 3NO3 − 2H}+, m/z 811.93, which was consistent with a nitrato- bridged dipalladium complex. The origin of a peak at m/z 1002.20 which arose from a 2+ ion, did not match the pattern for a complex containing 1, 2, 3 Pd centers, is unknown.
Fig. 5.
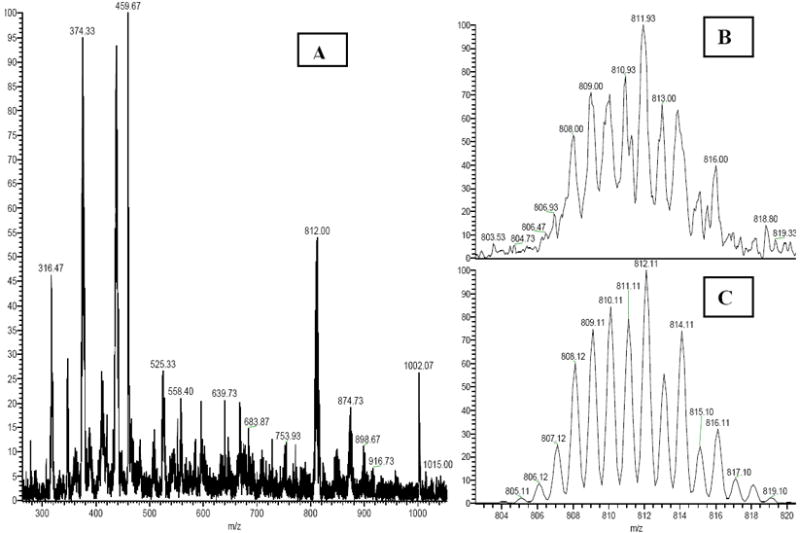
ESI-MS of [Pd(en)(proflavineH)](NO3)(SO4) in H2O (A) full spectrum; (B) expansion of 811.9 m/z; (C) simulation for the dimer [Pd(en)(proflavine)]2(NO3)-2H+.
The energy minimized equilibrium geometry obtained from a Spartan calculation of the [Pd(en)(proflavineH)(NO3)]+ cation is shown in Fig. 6. The Pd-N3 (amino)-C3 angle is 110.5°. The nitrate N-O (Pd) bond length is considerably longer than that of the terminal N-O length, 1.315 Å compared to 1.194 Å, respectively.
Fig. 6.
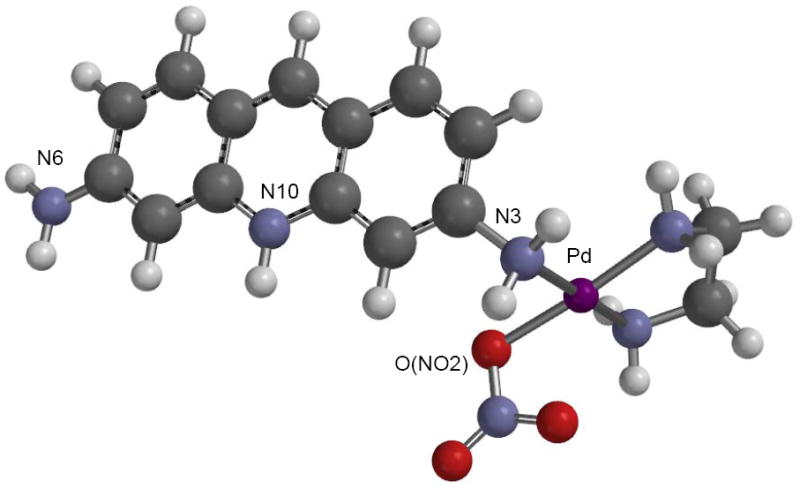
Structure of [Pd(en)(proflavineH)(NO3)]2+ ion as modeled with Spartan. Color code: C gray; H white; N blue; Pd purple; O red.
3.3. [Pd(proflavineH)Cl2](SO4)0.5 ·H2O, (3)
This compound was very insoluble in water and precipitated immediately upon mixing of K2PdCl4 and proflavine. Elemental analysis confirmed a mole ratio of Pd(II):proflavine of 1:1 and it was concluded that the compound was polymeric. It was soluble in DMSO with decomposition into two different Pd(II)-proflavine complexes with the proposed structures shown in Scheme 2. The 1H NMR spectrum is shown in Fig. 7. By obtaining the spectrum at three temperatures (15, 23, 40 °C) it was possible to discern the presence of the two species by the variation in their relative integrated intensities at the different temperatures. One of the species, I, had four resonances from non-exchangeable protons, plus one from the NH2 and a resonance at 13.4 ppm from N(10)H+, consistent with a completely symmetrical proflavine environment, and a second unsymmetrical species, II, which had six resonances plus two non-equivalent NH2 signals (Table 1). The two species were in equilibrium with each other and the symmetrical complex was favored at the lower temperatures. The ratio of II (unsymmetrical) to I (symmetrical) was approximately 0.80, 1.0, and 1.4 at 15, 23, and 40 °C, respectively.
Scheme 2.
Fig. 7.

1H NMR spectra in DMSO-d6. (a) proflavine hemisulfate; (B) [Pd(proflavineH)Cl2](SO4)0.5 ·H2O, * indicates the resonances of NH2 (II).
A NOESY spectrum obtained in DMSO-d6 showed dipolar interaction of the N10-H+ resonance at 13.4 ppm in species I with the H4 and H5 and was used to identify the resonances that belonged to this structure. The considerable downfield chemical shift of the NH2 group with respect to that in pure proflavine is consistent with bonding to the Pd(II) moiety and the presence of only one chemical shift suggests that there was a Pd(II) bonded to each of the NH2 groups (Scheme 2). An equilibrium geometry obtained from Spartan is shown in Fig. 8A, with the O-bonded DMSO model having a slightly lower energy than the S-bonded model. The calculated Pd-O(DMSO) bond distance was 2.007 Å and the average Pd-Cl bond distance was 2.323 Å. The Pd-N(H2)-C3 angle was 102.1°.
Figure 8.


Proposed structures for the DMSO solution species of [Pd(proflavineH)Cl2](SO4)0.5 ·H2O as modeled with Spartan. (A) Structure I (N10 protonated), O-bonded DMSO; (B) Structure II, O-bonded DMSO. Color code: C gray; H white; N blue; Pd purple; O red; Cl green; S yellow.
The 1H NMR spectrum of species II (Scheme 2) indicated it was essentially unsymmetrical with respect to the two proflavines, but still retained some symmetry. The H1 and H8 protons were still equivalent and the H2 and H7 appeared as two intermingled doublets whose chemical shifts were only separated by 3.5 Hz. Clearly some type of perturbation had affected the chemical shifts of the protons on the N10 side of the proflavine, and ESI-MS and equilibrium geometry modeling provided insight into this (vide infra). The assignments for the H4 and H5 protons are ambiguous because there were two resonances very close in chemical shift at 8.41-8.43 ppm. Both of these have been assigned to H4 and H5, but the resonance at 8.42 ppm was a doublet exhibiting long range coupling to the H9 (J ≈ 1.5 Hz) while the resonance at 8.43 ppm was a doublet with J = 5.2 Hz, There were no other resonance in the spectrum that had the latter coupling constant and the source of the coupling is unknown. However, the temperature dependence of the integrated intensities clearly indicated they both belonged to species II.
The ESI-MS of the species II in DMSO provided information on its composition with a peak present at 674.04 m/z+ as a +1 ion (Fig. 9). Simulation of the pattern gave an excellent fit for the experimental data for species II with a DMSO molecule bonded as a fifth ligand on Pd(II) (Scheme 2). As a consequence, the equilibrium geometry computations using Spartan were done with a coordinated DMSO. The minimum energy for species II with a weakly coordinated DMSO was lower by 9.1 kJ/mol for an O-bonded DMSO compared to an S-bonded DMSO (Fig. 8B). The calculated Pd-O (DMSO) bond length was 5.045 Å and the oxygen was additionally hydrogen bonded to an amino group on each of the two proflavine molecules. This, plus the cant of the two proflavines with respect to each other, might provide an explanation for the 1H NMR spectrum of this species. Species I was not observed in the ESI MS, possibly because it may be an anion, depending on the nature of the X ligand.
Figure 9.

ESI-MS of [Pd(proflavineH)Cl2](SO4)0.5 ·H2O in DMSO. (A) full spectrum; (B) expansion of 674 m/z; (C) simulation for [Pd(proflavine)2Cl2(DMSO)H]+.
The IR spectrum of the isolated solid provided rather contradictory results. There was a shift of 34 cm-1 to higher wavenumber for the νsym(NH2) vibration, but no shift in the νasym(NH2). There was also a 7 cm-1 shift to higher wavenumber for the C=C vibration (1638 cm-1) and a 5 cm-1 shift to lower wavenumber for the C=N vibration. Although the formula of the solid was well established by elemental analysis, the IR data might be interpreted as a mixture of two types of structures occurring fortuitously in a 1:1 ratio. The sulfate was observed as a strong, broad band centered at 1119 cm-1.
3.4. Cytotoxicity Studies
Complexes 1 and 3 were tested for in vitro cytotoxicity against two breast cancer (SK-BR-3; MCF-HER-2-6) and one ovarian (SK-OV-3) cancer cell lines, all of which were cisplatin resistant. The results are reported in Table 2. Complex 3 is the material isolated from the initial synthesis. The LC50 values of compounds 1 and 3 were significantly lower compared to cisplatin against the SK-BR-3 cell line (6.8 μM) [32]. The values were also significantly lower than those for [Pd(terpy)(NO3)]NO3 and somewhat lower than proflavine alone for all of the tested cell lines. The greater activity of 1 compared to [Pd(terpy)(NO3)]NO3 was apparently due to the presence of proflavine in 1. The LC50 values for proflavine alone were nearly as good as for 3. The general order of activity against the three cancer cell lines can be summarized as 1 > 3 > [Pd(terpy)(NO3)]NO3 > [Pd(en)(H2O)2](NO3)2. The results from the cisplatin experiments confirmed that these cell lines were indeed cisplatin resistant. It is concluded from this, and from the much higher LC50 values of [Pd(en)(H2O)2](NO3)2, that the Pd(II) did not make a large contribution to the cytotoxicity against these cell lines. The results of the cytotoxicity studies indicated that compounds 1 and 3 show some promise as antineoplastic agents for SK-BR-3 and MCF-HER-2-6 cell lines. Compound 2 was not tested due to the high LC50 values found for [Pd(en)(H2O)2](NO3)2.
Table 2.
Results of in vitro cytotoxicity testing, LC50 (μM)
| Compounds | SK-OV-3a | SK-BR-3b | MCF-HER-2-6c |
|---|---|---|---|
| [Pd(terpy)(proflavine)](NO3)(HSO4)·3H2O (1) | –d | 1.0 | 0.75 |
| [Pd(proflavineH)Cl2](SO4)0.5 ·H2O (3) | –d | 2.7 | 2.4 |
| [Pd(terpy)(NO3)]NO3 | 15.5 | 12.0 | 14.0 |
| [Pd(en)(H2O)2](NO3)2e | 68 | 26 | 23 |
| Proflavine | 5.8 | 3.5 | 3.0 |
| cis-platin | >1000 | inactive | >1000 |
Human ovarian
Human breast
Human breast with HER 2 overexpression (stable transfection)
Poor agreement between Data Set 1 and Data Set 2
Data from Set 1 only
4. Conclusions
As a result of the presence of two types of bonding sites in the proflavine molecule, the reaction of Pd(II) complexes with it produced a variety of types of compounds, depending on the nature of the ligands in the original Pd(II) complex and the solvent in the case of solutions. Since the N(10) position is protonated in water at neutral pH, it must be deprotonated for the Pd(II) complex to bond at this site, while bonding to the NH2 groups does not require deprotonation. It is known that Pd(II) is capable of lowering the pKa of a proton by at least several pK units [33]. The data on DMSO solutions of [Pd(proflavineH)Cl2](SO4)0.5 ·H2O showed that in this coordinating solvent the stability of the N(10)-bonded Pd structure, II, was slightly more stable than that of the NH2-bonded Pd structure. Under aqueous conditions the presence of an aromatic ligand on the starting Pd(II) complex favored the N(10)-bonded structure while en and chloro ligands favored the NH2-bonded structure.
The in vitro cytotoxicity data indicated that compounds 1 and 3 showed promise in the treatment of some human breast cancer cell lines. Although some of the solid compounds were microcrystalline, attempts to obtain crystals of suitable quality for an Xray structure determination have so far been unsuccessful.
Supplementary Material
Acknowledgments
The authors thank the NIH SCORE program (S06-08194) and the Welch Foundation for support of this research.
Abbreviations
- terpy
2,2’:6’,2”-terpyridine
- en
ethylenediamine
- MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- ESI-MS
electrospray ionization mass spectrometry
Appendix A. Supplementary Material
1H COSY spectra of [Pd(en)(proflavineH))(NO3)](SO4) (aq) and [Pd(proflavineH)Cl2](SO4)0.5 (DMSO) and IR spectra of these two compounds as solids can be found in the online version.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Todd RC, Lippard SJ. Metallomics. 2009;1:280–291. doi: 10.1039/b907567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eastman A. In: Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug. Lippert SJ, editor. Wiley-VCH; Weinheim, Germany: 1999. pp. 111–115. [Google Scholar]
- 3.Montana AM, Batalla C. Curr Med Chem. 2009;16:2235–2260. doi: 10.2174/092986709788453087. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Surrah AS, Kettuner M. Curr Med Chem. 2006;13:1337–1357. doi: 10.2174/092986706776872970. [DOI] [PubMed] [Google Scholar]
- 5.Łakomska I. Inorg Chim Acta. 2009;362:669–681. [Google Scholar]
- 6.Garoufis A, Hadjikakou SK, Hadjilia N. Coord Chem Revs. 2009;253:1384–1397. [Google Scholar]
- 7.Abu-Surrah AS, Al-Sa’doni H, Abdalla MY. Cancer Therapy. 2008;6:1–10. [Google Scholar]
- 8.Gao E, Liu C, Zhu M, Lin H, Wu Q, Liu L. Anti-Cancer Agents Med Chem. 2009;9:356–368. doi: 10.2174/1871520610909030356. [DOI] [PubMed] [Google Scholar]
- 9.Štarha P, Trávníček Z, Popa I. J Inorg Biochem. 2009;103:978–988. doi: 10.1016/j.jinorgbio.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Acheson RM, editor. Acridines. second. John Wiley and Sons; New York, NY: 1973. [Google Scholar]
- 11.Denny WA. Curr Med Chem. 2002;9:1655–1665. doi: 10.2174/0929867023369277. [DOI] [PubMed] [Google Scholar]
- 12.Benchabane Y, Di Giorgio C, Boyer G, Sabatier A-S, Allegro D, Peyrot V, De Méo M. J Med Chem. 2009;44:2459–2467. doi: 10.1016/j.ejmech.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Wheate NJ, Brodie CR, Collins JG, Kemp S, Aldrich-Wright JR. Mini-Rev Med Chem. 2007;7:627–648. doi: 10.2174/138955707780859413. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz R, Garcia B, Ruisi G, Silvestri A, Barone G. J Mol Struct: Theochem. 2009;915:86–92. [Google Scholar]
- 15.Lo Y-C, Ko T-P, Su W-C, Su T-L, Wang AH-J. J Inorg Biochem. 2009;103:1082–1092. doi: 10.1016/j.jinorgbio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Guddneppanavar R, Saluta G, Kucera GL, Bierbach U. J Med Chem. 2006;49:3204–3214. doi: 10.1021/jm060035v. [DOI] [PubMed] [Google Scholar]
- 17.Bowler BE, Ahmed KJ, Sundquist WI, Hollis LS, Whang EE, Lippard SJ. J Am Chem Soc. 1989;111:1299–1306. [Google Scholar]
- 18.Belmont P, Bosson J, Godet T, Tiano M. Anti-Cancer Agents Med Chem. 2007;7:139–169. doi: 10.2174/187152007780058669. [DOI] [PubMed] [Google Scholar]
- 19.Baruah H, Day CS, Wright MW, Bierbach U. J Am Chem Soc. 2004;126:4492–4493. doi: 10.1021/ja038592j. [DOI] [PubMed] [Google Scholar]
- 20.Crispini A, Pucci D, Sessa S, Cataldi A, Napoli A, Valentini A, Ghedini M. New J Chem. 2003;27:1497–1503. [Google Scholar]
- 21.Ciatto C, D’Amico ML, Natile G, Secco F, Venturini M. Biophys J. 1999;77:2717–2724. doi: 10.1016/S0006-3495(99)77105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundquist WI, Bancroft DP, Lippard SJ. J Am Chem Soc. 1990;112:1590–1596. [Google Scholar]
- 23.Karkali R, Bugari D. Monatsh Chem. 2000;131:819–824. [Google Scholar]
- 24.Tercero JM, Matilla A, Sanjuan Ma A, Moreno CF, Martin JD, Walmsley JA. Inorg Chim Acta. 2003;342:77–87. [Google Scholar]
- 25.Mosmann T. J Immun Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 26.Harris RG, Johnson BB, Wells JD. Clays Clay Miner. 2006;54:435–448. [Google Scholar]
- 27.Silverstein RM, Bassler GC, Morill TC. Spectrophotometric Identification of Organic Compounds. fifth. Wiley; New York: 1991. p. 123. [Google Scholar]
- 28.Nakamoto K. Infrared and Raman Spectra of Inorgaic and Coordination Compounds, Part B. fifth. Wiley Interscience; New York: 1997. pp. 79–82. [Google Scholar]
- 29.Nakamoto K. Infrared and Raman Spectra of Inorgaic and Coordination Compounds, Part B. fifth. Wiley Interscience; New York: 1997. pp. 87–89. [Google Scholar]
- 30.Bach SBH, Sepeda TG, Merrill G, Walmsley JA. J Am Soc Mass Spectrometry. 2005;16:1461–1469. doi: 10.1016/j.jasms.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Yoshikawa A, Bach SBH, Merrill GN. J Am Soc Mass Spectrometry. 2009;20:1015–1029. doi: 10.1016/j.jasms.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 32.Cheng K, Peng S, Xu C, Sun S. J Am Chem Soc. 2009;131:10637–10644. doi: 10.1021/ja903300f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walmsley JA, Zhu S, Matilla A, Donowick TG, Cramp JE, Tercero JM, Dalrymple T. Inorg Chem. 2007;46:9945–9953. doi: 10.1021/ic700830v. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


