Abstract
Objective
Elderly persons with mild cognitive impairment (MCI) are at increased risk of dementia and functional impairments. The present study investigated the contribution of three domains of executive cognition to everyday functioning among persons with MCI.
Methods
124 MCI patients and 68 cognitively normal elderly participants were administered a cognitive screening battery. These tests were used to divide patients into four subgroups (amnestic single domain, amnestic multiple domain, non-amnestic single domain, and non-amnestic multiple domain). Subjects were then administered 18 executive function tests that assess planning/problem-solving, working memory, and judgment. Performance of everyday activities and everyday cognition was rated with two informant-reported measures.
Results
All MCI subtypes had more difficulties in everyday activities than cognitively normal elderly participants. Multiple domain MCI patients had more functional impairments than single domain MCI patients. Contrary to our expectations, only one executive function component, working memory, contributed significantly to functional status after controlling for demographic, health-related and other cognitive factors.
Conclusions
Functional abilities are compromised in all MCI subtypes. Working memory may be associated with functional impairments, but general cognitive measures account for more unique variance.
Keywords: everyday functioning, executive cognition, working memory, mild cognitive impairment
Introduction
Mild cognitive impairment (MCI) is a clinical syndrome with multiple etiologies and outcomes that range from normal cognitive aging to dementia (Fischer et al., 2007). One of the defining features of MCI is ‘grossly’ preserved functional abilities and cognitive deficits that do not considerably disrupt daily activities (Winblad et al., 2004; Petersen, 2004b). However, there is accumulating evidence that approximately one third of MCI patients have difficulties in instrumental activities of daily living, especially in managing their finances, making medical decisions, and completing everyday tasks that rely heavily on memory and complex reasoning (Albert et al., 2002; Tuokko et al., 2005; Farias et al., 2006; Peres et al., 2006; Perneczky et al., 2006; Okonkwo et al., 2007; Allaire et al., 2008). Functional restrictions might comprise the independence, safety or quality of life of patients, and contribute to caregiver burden and community expenses (Gauthier et al., 2006). Identifying these restrictions and understanding their cognitive correlates could lead to an earlier and more effective intervention (Bell-McGinty et al., 2002).
Several clinical subtypes of MCI have been identified based on the nature of cognitive deficits (Winblad et al., 2004; Petersen, 2004a). Certain subtypes appear to be at higher risk of developing dementia than others (Alexopoulos et al., 2006). Identifying the degree of functional impairment in these subtypes could contribute to patient prognosis, since the presence of functional impairments is related with higher rates of conversion to dementia (Purser et al., 2005; Peres et al., 2006). However, few studies have compared the functional abilities of different MCI subtypes and they have produced contradictory findings. While two studies found that amnestic multiple domain (AM) MCI patients have greater functional decline than other MCI subtypes (Tam et al., 2007; Kim et al., 2009), Schmitter-Edgecombe et al. (2009) reported similar patterns of everyday functioning in amnestic and non-amnestic MCI groups.
Research on the cognitive correlates of functional decline in MCI patients has focused on verbal learning, memory, and executive cognition (Okonkwo et al., 2006; Pereira et al., 2008; Tomaszewski Farias et al., 2009). The contribution of distinct memory processes to everyday functioning has been recently examined in MCI patients (Schmitter-Edgecombe et al., 2009). However, to our knowledge, there are no studies investigating the contribution of specific domains of executive functions to the functional abilities in well-characterized MCI patient groups.
Studies of the effects of executive cognition on daily activities in non-demented geriatric individuals with various degrees of ‘subclinical’ impairment have yielded mixed results. Several authors have suggested that inhibitory control, mental flexibility, psychomotor speed, or sequencing ability contribute significantly to functional abilities (Bell-McGinty et al., 2002; Cahn-Weiner et al., 2002; Jefferson et al., 2006). Others have indicated that measures of planning and problem solving are stronger correlates (Lewis and Miller, 2007). Some of the differences in conclusions stem from the fact that different tasks or even different measures of the same cognitive task have been used and there has been no empirical validation of the executive domains underlying those tasks. Although abilities such as initiation, planning, judgment, self-monitoring, and mental flexibility are studied under the term executive cognition, there is no consensus on the definition of executive functions and their subcomponents (Burgess and Shallice, 1997; Miyake et al., 2000; Ardila, 2008). In addition, some studies have reported minimal variance explained by executive function measures (Richardson et al., 1995), while others have reported up to 54% explained variance (Bell-McGinty et al., 2002).
The first goal of this study was to investigate the functional abilities of four well-defined MCI patient groups. More specifically, we compared the performance of daily activities and everyday cognitive tasks of each MCI subgroup to a cognitively normal group. We then compared the functional status of amnestic (both single- and multiple-domain) to non-amnestic MCI (both single- and multiple-domain), and of those with single to multiple-domain impairments. Our second aim was to test whether specific, empirically defined domains of executive function components are uniquely associated with functional abilities in MCI patients. We sought to determine whether executive functions predict functional status above and beyond demographic and health-related factors and other cognitive variables.
Method
Participants
124 patients with MCI and 68 cognitively normal elderly individuals were studied. Most participants (81%) were recruited from the Johns Hopkins Alzheimer’s Disease Research Center (ADRC) and from other research studies. A small number of participants (19%) were referred from University clinics and physicians in the community. The majority of the participants were Caucasian (87.5%); 11.5% were African-American.
Inclusion criteria were normal overall cognitive status, defined as a score in the normal range (i.e., at or above the 20th percentile for age and education) on the Mini-Mental State Examination (MMSE) (Bravo and Hebert, 1997), and normal or ‘relatively preserved’ functional status defined by an overall score of 0 (for normal subjects) or 0.5 (for MCI subjects) on the Clinical Dementia Rating (CDR) (Hughes et al., 1982).
Exclusion criteria were any history of major mental illness, CNS disorder or active systemic illness (e.g., cancer). Volunteers with past or present depression were not excluded since depression is very common in MCI and may be related to outcome (Jorm, 2001; Lyketsos et al., 2002).
The following screening tests were administered to determine subjects’ group assignment: Logical Memory (story A) of the Wechsler Memory Scale-Revised (WMS-R) (Wechsler, 1987), the 30-item version of the Boston Naming Test (Goodglass and Kaplan, 1983; Brandt and Mellits, 1989), word list generation (for the letters FAS and the semantic categories animals and vegetables) (Salmon et al., 1999; Rascovsky et al., 2007) and clock drawing to request (Rouleau et al., 1992). Finally, each participant was required to have an ‘informant’, who could provide information on his/her everyday functioning. The majority of the informants were spouses of the participants (57.6%), 20.9% were adult children, 5.2% were siblings, 2.6% were other relatives, and 11% were friends and the average length of the association with the participant was 46.14 years (SD = 15.19). Depressive symptoms were evaluated with a short form of the Geriatric Depression Scale (GDS) (Sheik and Yesavage, 1986).
MCI group
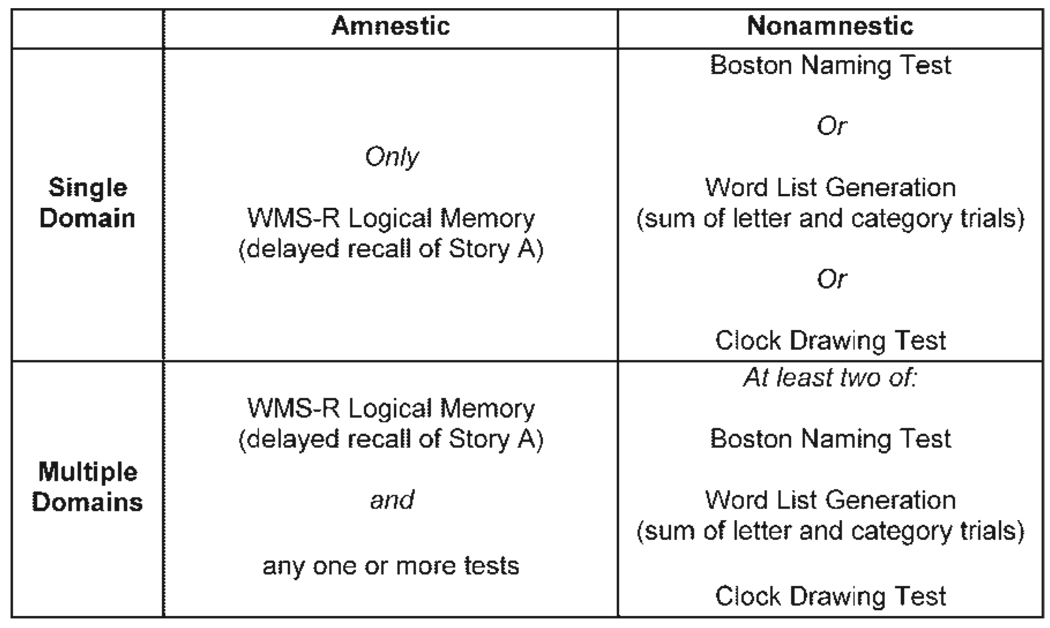
Participants were diagnosed with MCI according to Mayo Clinic criteria (Petersen, 2004b). An informant- or self-reported history of cognitive difficulties was required. Further inclusion criteria were an overall CDR score of 0.5, and performance at or below 1.5 standard deviations below the mean for age and education according to published norms on one or more of the screening tests. The MCI patients were then categorized into four subgroups based on the performances on the screening tests (see Figure 1): amnestic single domain (AS) (N = 36); amnestic multiple domain (AM) (N = 45); non-amnestic single domain (NAS) (N = 26), and non-amnestic multiple domain (NAM) (N = 17).
Figure 1.
Operational criteria for four groups of participants with mild cognitive impairment. Subjects in each group performed at or below 1.5 SD below age and education norms on the test(s) indicated (from Brandt et al. (2009)).
Normal control group
A self- and informant-reported history of unimpaired cognitive functioning was required for each cognitively normal control subject. Further inclusion criteria were an overall CDR score of 0 and scores in the normal range on all screening battery tests.
Procedures
Executive function measures
Eighteen clinical and experimental tests of executive functions were administered. The tests were initially chosen to represent the six following executive domains (three tests from each domain): spontaneous flexibility/generativity, inhibition of prepotent responses, planning/sequencing, concept-rule learning/set shifting, decision-making/ judgment, and working memory/resource sharing. Principal components analysis was performed to extract empirical factors and resulted in a three component solution: planning/problem-solving, working memory, and judgment. The tests that loaded higher on each component are summarized in Table 1. The details of the statistical procedures have been previously described (Brandt et al., 2009).
Table 1.
Summary of tests of executive function that load on the three components
| Components | Tests |
|---|---|
| Planning/problem solving |
Alternate Uses Test (Guilford et al 1978 |
| Random number generation (Brugger et al., 1996; Jahanshahi et al., 2000) | |
| Tinker Toy Test (Lezak, 1982; Koss et al., 1998) | |
| Porteus Maze Test (Porteus, 1965) | |
| D-KEFS Tower Test (Delis et al., 2001) | |
| D-KEFS Sorting Test (Delis et al., 2001) | |
| Stanford Binet Absurdities Test (Thorndike et al. 1986) | |
| Working memory | D-KEFS Stroop Test (Delis et al., 2001) |
| Completions and Corrections Test (Manning and Brandt, 2006) | |
| Brixton Test (Burgess and Shallice, 1997) | |
| Trail Making Test (Reitan, 1958) | |
| Brief Test of Attention (Schretlen et al 1996) | |
| TEA Telephone Search While Counting (Robertson et al., 1994) | |
| Judgment | Iowa Gambling Test (Bechara et al., 1998) |
| Experimental Judgment Test (Brandt et al., 2009) |
Measures of functional status
Two informant-reported ratings of daily functioning were used. We selected informant- over self-reported measures because the former have been shown to have greater validity in cognitively impaired persons (Albert et al., 2002; Mitchell and Miller, 2008).
The Activities of Daily Living-Prevention Instrument (ADL-PI) (Galasko et al., 2006) consists of 15 items assessing performance on complex activities of daily living rated on a 3-point scale (0 = ‘no difficulty’ to 2=‘a lot of difficulty’) and five physical function questions requiring as a ‘yes’ or ‘no’ response. It is a sensitive measure for the detection of minor changes in functional status in the transition from cognitively normal to MCI. The sum of ratings on the 15 functional items constitutes the score. For the purposes of our study, we calculated an additional dichotomous variable (no difficulty/at least some difficulty) for each ADL-PI item. Our intention was to explore and document the presence of a restriction on specific ADL-PI activities rather than the severity/degree of the restriction. The number of reported physical restrictions (e.g., impaired visual or auditory acuity or mobility) is summed to create a measure of physical health obstacles to independent functioning.
The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) (Jorm and Jacomb, 1989) measures changes in an elderly subject’s everyday cognitive abilities that are manifested in daily life. It is considered ‘a measure of the disablement caused by cognitive decline’ (Jorm et al., 1996, p. 137). Twenty six cognitive activities of daily life are described and rated on a 5-point scale compared to 10 years previously (1=‘much better’, 5=‘much worse’). The mean of the 26 ratings was used in our analyses.
Procedure
The Johns Hopkins University Institutional Review Board fully reviewed and approved the study protocol. Written informed consent was obtained from all participants and their study partners.
Data analysis
Statistical analyses were performed using SPSS 15. One-way ANOVAs were used to compare the five groups on demographic and clinical characteristics. Group differences on the two measures of everyday functioning were investigated with non-parametric tests (Mann-Whitney U, Kruskal-Wallis H, or χ2) due to the fact that scores were not normally distributed. χ2 was used to test the association of participants’ group membership with the frequency of reported difficulties on specific ADL-PI items.
The contribution of executive cognition to everyday functioning among the MCI patients was examined with a series of stepwise multiple regression analyses. Because each of the MCI subgroups was quite small, we did not perform the regression analyses on the subgroups separately. In the first set of analyses, the relative contribution of the three executive function components to ADL-PI and IQCODE scores was investigated in stepwise models, since our goal was to investigate which executive domain (if any) has the greatest unique contribution to everyday functioning. In the second set of analyses, hierarchical regression models were used to address our hypothesis that executive functions would add significantly to the prediction of functional status above and beyond the potential contribution made by demographic (age, education), health-related (depression, physical limitations), and other cognitive variables (screening battery tests), all factors that have been shown to be associated with functional status and decline (Stuck et al., 1999).
Results
The demographic and clinical characteristics of the participants are summarized in Table 2. The groups differed in age, with normal elderly subjects being younger than the AM participants (p < 0.001). The groups had equivalent levels of education, but differed in sex distribution (p = 0.002); men predominated in the AS group and women in the NAM and NC group. Normal control participants had higher MMSE scores than the MCI subjects (p < 0.001) and lower GDS scores than the AM (p = 0.001) and the NAM group (p = 0.014).
Table 2.
Demographic and clinical characteristics of study participants
| Normal control | MCI patients | Amnestic MCI |
Non-amnestic MCI |
P | ||||
|---|---|---|---|---|---|---|---|---|
| Single domain | Multiple domain | Single domain | Multiple domain | |||||
| N | 68 | 124 | 36 | 45 | 26 | 17 | ||
| Age (years) | Mean (SD) | 72.41(7.25) | 76.28 (7.52) | 75.08 (5.77) | 78.36 (7.69) | 74.81 (8.62) | 75.59 (7.97) | <0.001 |
| Range | 59–85 | 55–96 | 61–84 | 60–96 | 55–87 | 57–87 | ||
| Education (years) | Mean (SD) | 15.93(2.49) | 15.72 (2.40) | 15.92 (1.17) | 15.93 (2.50) | 15.42 (2.73) | 15.18(2.16) | >0.05 |
| Range | 11–20 | 10–20 | 10–20 | 12–20 | 10–20 | 12–20 | ||
| Sex ratio (M:F) | 27:41 | 72:52 | 26:10 | 28:17 | 14:12 | 4:13 | 0.002 | |
| Ethnicity (% Caucasian) | 88.2% | 87.1% | 94.4% | 86.7% | 84.6% | 76.5% | >0.05 | |
| Mini-Mental State | Mean (SD) | 29.26 (0.87) | 28.20(1.22) | 28.33(1.21) | 28.11 (1.19) | 28.54(1.36) | 27.65(1.12) | <0.001 |
| Exam score | Range | 27–30 | 25–30 | 26–30 | 25–30 | 25–30 | 26–30 | |
| Geriatric Depression | Mean (SD) | 1.24 (2.05) | 2.41 (2.30) | 2.12 (2.28) | 2.76 (2.61) | 1.96(1.78) | 2.74 (2.14) | 0.007 |
| Scale score | Range | 0–11 | 0–10 | 0–8 | 0–10 | 0–7 | 0–7 | |
Everyday functioning in MCI subtypes
The correlation between ADL-PI and IQCODE scores in our MCI sample was r = 0.68, (p < 0.001). This indicates that although the two scales measure overlapping aspects of adaptive functioning, there is considerable unshared variance between them (53%). The IQCODE measures more cognitively demanding tasks that rely considerably on episodic memory, whereas the ADL-PI is a measure of general functioning that taps into a broader range of abilities (Galasko et al., 2006; Jorm et al., 1996).
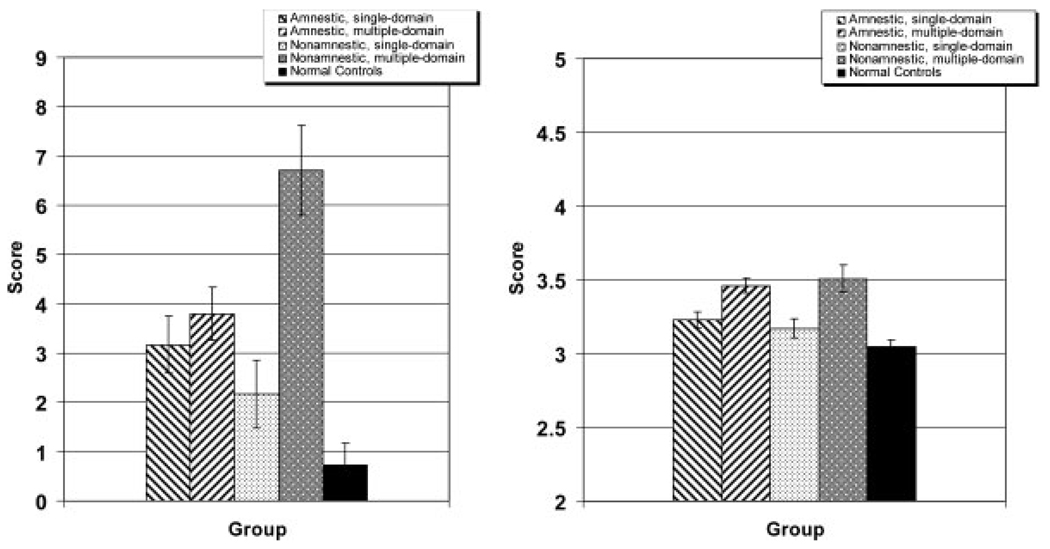
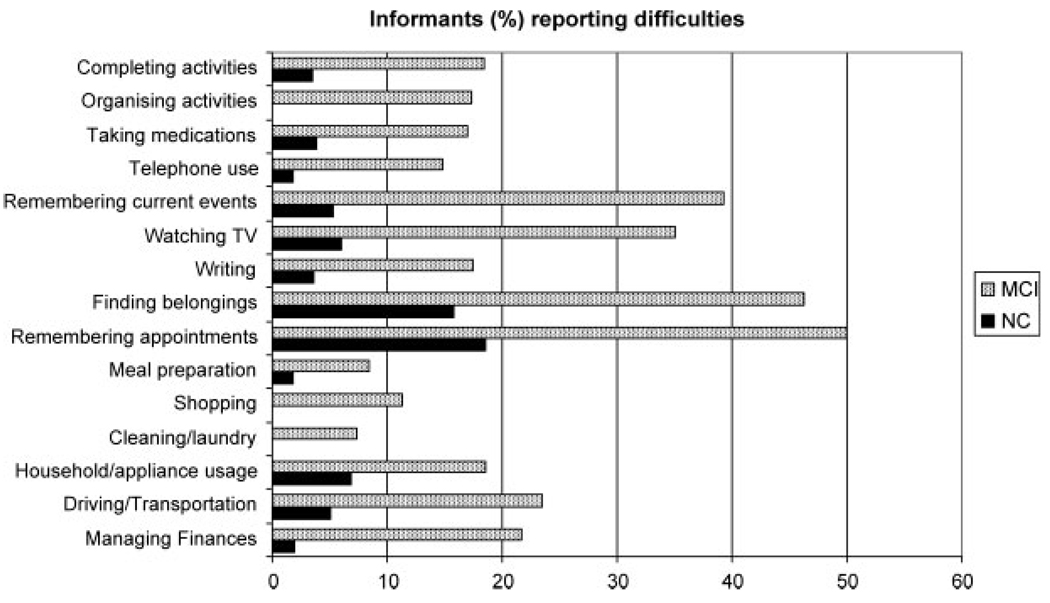
Figure 2 displays mean scores of the four MCI subgroups and the normal elderly participants on the two functional measures. MCI patients, as a group, had higher ratings on ADL-PI, indicating worse functional status (U = 1706, p < 0.001). A series of χ2 analyses on the individual items of the ADL-PI revealed that significantly more MCI subjects than NC subjects were reported to have at least some difficulty on 12 of 15 ADL-PI items. The exceptions were appliance usage (χ2 = 3.21, df = 1, p > 0.05), cleaning/laundry (χ2 = 3.607, df = 1, p > 0.05), and meal preparation (χ2 = 2.802, df = 1, p > 0.05). The greatest difficulties MCI patients faced were in keeping appointments (χ2 = 15.78, df = 1, p < 0.001), finding things at home (χ2 = 15.12, df = 1, p < 0.001), remembering current events (χ2 =21.78, df = 1, p < 0.001), and using the telephone (χ2 = 6.89, df = 1, p = 0.007) (see Figure 3).
Figure 2.
Mean ratings (±SE) of proxy on functional status measures: ADL-PI (left panel) and IQCODE (right panel).
Figure 3.
Frequency of reported difficulty by MCI patients and NC subjects’ informants on specific ADL activities.
To investigate whether only specific MCI subgroups had compromised functioning, we then compared the functional status of each of the four MCI subgroups to the NC group. On the ADL-PI, both the AM and NAM patients had worse functional status than the NC participants (U = 536.50, p< 0.001; and U = 182.50, p< 0.001, respectively). Even the single domain patients (AS and NAS) differed significantly from the NC subjects (U = 504.50, p< 0.001; and U = 482.50, p = 0.011). Finally, multiple domain MCI patients (AM and NAM) did not differ from single domain MCI patients (AS and NAS), and amnestic patients (AS and AM) did not differ from non-amnestic patients (NAS and NAM).
On the IQCODE as well, MCI patients, as a group, had higher ratings (worse functional status) than cognitively normal participants (U = 1382, p < 0.001). Comparisons of the NC group to each of the four MCI subgroups revealed that all four groups differed significantly from the NC group (AM: U = 216.50, p < 0.001; NAM: U = 140.00, p < 0.001; AS: U = 607.50, p = 0.001; NAS: U = 418.00, p = 0.003). Multiple domain MCI patients differed from single domain MCI patients on this functional measure (p <0.001), whereas amnestic patients did not differ from non-amnestic patients (p > 0.05).
Predictors of functional status
The potential contribution of the three executive domains to functional outcome was first evaluated with stepwise linear regression analyses. Only working memory contributed significantly to ADL-PI score, accounting for just 3.8% of the variance [F(1,112) = 5.467, β = −.217, p = 0.021] and to the IQCODE score, accounting for only 7.1% of the variance [F(1,110) = 9.350, β = −.281, p = 0.003]. Lower scores on working memory were associated with higher ratings on the ADL-PI and IQCODE, thus indicating poorer functional outcome. Neither planning/problem-solving nor judgment contributed significantly to ADL-PI or IQCODE score.
A series of hierarchical regression models were then developed to determine whether executive cognition predicts functional outcome in MCI patients above and beyond demographic, clinical, and other cognitive factors. In the first block, demographic and clinical variables (age, education, GDS score, and physical limitations) were allowed to enter; in the second block, the five tests of the screening battery (MMSE, Logical Memory subtest [delayed recall of story A], Boston Naming Test, word list generation, and clock drawing to request) could enter; finally, in the third block, only the working memory component score was allowed to enter, since this component was the only executive component associated with everyday functioning. The independent variables were entered in a stepwise fashion within each block.
For ADL-PI scores, only education (in step 1) and MMSE (in step 2) entered the model [F(2,108) = 8.836, p < 0.001] (see Table 3). Education accounted for 4.5% of the variance in the ADL-PI and MMSE contributed another 8.2%. Working memory did not contribute significant unique variance to ADL-PI ratings.
Table 3.
Summary of hierarchical regression analyses: contribution of demographic and clinical factors, executive function components and other cognitive factors on the ADL-PI and the IQCODE ratings
| Variable | Factor | B | SE B | Standardized β | R2 | |
|---|---|---|---|---|---|---|
| ADL-PI | ||||||
| Step 1 | Education | 0.408 | 0.165 | 0.232 | 0.045 | |
| Step 2 | Education | 0.456 | 0.159 | 0.260 | 0.143 | |
| MMSE | −1.052 | 0.317 | −0.299 | |||
| IQCODE | ||||||
| Step 2 | Clock drawing | −0.059 | 0.022 | −0.221 | 0.040 | |
| Step 3 | Clock drawing | −0.039 | 0.026 | −0.144 | 0.082 | |
| Working memory | −0.098 | 0.040 | −0.237 | |||
None of the demographic or clinical variables contributed significantly to the prediction of IQCODE score. Clock drawing entered on step 1 (4% variance) and working memory entered on step 2 (an additional 4.2% of variance) [F(2,106) = 5.730, p < 0.004].
Discussion
The present study replicated previous findings that daily functioning is notably comprised in a large, well-defined group of MCI patients. Regardless of cognitive subtype, MCI patients had more difficulties in daily functioning, as rated by knowledgeable informants, than cognitively normal elderly. More than one third of MCI patients have difficulty keeping appointments, finding their belongings, remembering current events, and following TV programs. About 20% reported difficulties driving and using transportation, managing their finances, organizing and completing activities, and even taking medications. Amnestic and non-amnestic MCI patients had similar levels of functional impairment. However, patients with cognitive impairments in more than one domain had more difficulties in daily activities than those with impairment in a single domain, possibly due to more widespread brain pathology.
These findings have important implications. The number of MCI informants reporting difficulties in multiple domains of daily functioning highlights the importance of a detailed assessment of functional abilities among these patients. Although the functional impairments in MCI are not as severe as in dementia, MCI patients still require assistance with more cognitively demanding daily activities. Second, since functional decline might be a harbinger of a dementing condition, we might predict that multiple-domain MCI patients are at higher risk of developing dementia than single-domain patients. This conclusion is consistent with several recent studies (Alexopoulos et al., 2006; Tabert et al., 2006).
Our second finding is that among three empirically validated executive function components (planning/problem solving, working memory, and judgment), only working memory was associated with ratings of daily functioning. Even then, the association was quite modest. When we controlled for other demographic, health-related and cognitive factors, working memory contributed unique variance on only one of the two measures, the IQCODE. We found that measures of global functioning and constructional praxis, the MMSE and the clock drawing test, were better predictors of ADL-PI, adding more unique variance than any other cognitive measure.
Most prior studies investigating the relationship of executive functions and performance on daily activities have not controlled for overall cognitive abilities (Cahn-Weiner et al., 2000; Bell-McGinty et al., 2002; Lewis and Miller, 2007; Mitchell and Miller, 2008). It might be argued that global cognitive measures, such as the MMSE, rely on several cognitive abilities and can therefore obscure the true influence of a specific cognitive domain (Peters and Pinto, 2008). Similarly, the clock drawing test, although primarily a measure of constructional praxis and visuospatial skills, relies on a broader range of cognitive abilities, including semantic memory and executive functions (Lowery et al., 2003). Thus, one reason why clock drawing contributes to everyday functioning may be that it requires planning, seriation, and other ostensibly executive skills (Lewis and Miller, 2007).
Our results are not directly comparable with previous studies, since we used empirically derived executive function component scores rather than individual tests or composite scores with only face validity. We believe this approach to be a significant improvement over prior studies as it emphasizes the role of underlying neurocognitive deficits rather than test scores. Nevertheless, we suggest that the present findings are in general agreement with studies showing that Trail Making Test (part B) and D-KEFS Color-Word Interference are significant predictors of IADLs (Bell-McGinty et al., 2002; Cahn-Weiner et al., 2002; Schmitter-Edgecombe et al., 2009). Both tasks load on our working memory component (Brandt et al., 2009), which was found to predict functional outcome in this study. This component is, of course, not an unambiguous measure of working memory, but it captures the contributions of tests requiring multiple tracking, divided attention, and inhibitory control (see Table 1 and also Brandt et al., 2009).
The specific mechanisms whereby working memory affects everyday functioning remain incompletely specified. Multi-step tasks in everyday life clearly depend on temporary active maintenance of specific elements in working memory while other elements are being performed (Humphrey et al., 2001). These elements include representation of goals, stimuli in the environment, response states, and response contingencies and production rules (e.g., if condition X, then perform action Y) (Baddeley, 1986; Kimberg and Farah, 1993). Kimberg and Farah (1993) suggested that selective damage or ‘weakening of associations’ (p. 114) between these elements in working memory can disrupt the successful completion of a task. Moreover, three other functions necessary for everyday functioning depend on working memory: (a) the ability to ‘maintain the finer temporal details of the structure of a script’ (Sirigu et al., 1996, p. 297), (b) the ability to monitor conflicts between the actual and the required sequence of actions (Shallice and Burgess, 1996), and (c) the inhibition of environmental stimuli distraction and the ‘rejection of action alternatives presented by the stimulus situation’ (Zanini et al., 2002, p. 88). Further studies on the pattern of difficulties and errors in everyday tasks are necessary to clarify how working memory affects everyday functioning in MCI patients.
Regarding the magnitude of the contribution of executive cognition to functional status, our results stand in contrast to those of Bell-McGinty et al. (2002) who found up to 54% of variance in functioning explained by executive cognition. However, a recent meta-analysis of 68 studies in demented and non-demented individuals supports our findings; Royall et al. (2007) found that the unique variance in functional outcome explained by executive function and other neurocognitive measures is small to modest (no more than 12%).
Our study has several limitations. First, our MCI sample is a non-random sample of convenience, with a higher proportion of men. Second, our selection of criteria for defining MCI may be questioned, since there is no universally accepted prescription for how the Petersen/Mayo criteria should be operationalized. However, we applied both clinical criteria (interview with an informant, yielding a CDR = 0.5), as well as psychometric criteria based on well-recognized neuropsychological procedures. Third, one might argue that proxy reports used to assess functional status are less valid than performance-based tests. The latter are, of course, much more labor-intensive, and performance of an activity while being examined is likely to be different than performance in everyday life. Finally, the differential contribution of the executive function domains to everyday functioning was examined to the MCI patients as a group due to small subgroup sample sizes. This warrants further investigation.
Key Points.
Functional abilities are impaired in all MCI subtypes. Working memory impairment contributes significantly to IQCODE. General cognitive impairment accounts for more unique variance than executive impairment.
Acknowledgements
The authors thank Laura Wulff, Ph.D., Chiadi Onyike, M.D., Marilyn Albert, Ph.D., and the staff and participants of the Johns Hopkins Alzheimer’s Disease Research Center. Eleanor Neijstrom, M.S., Jaclyn Samek, B.S., and Kevin Manning, M.S. collected the data. This study was supported by grant AG-005146 from the National Institute on Aging.
References
- Albert SM, Tabert MH, Dienstag A, Pelton G, Devanand D. The impact of mild cognitive impairment on functional abilities in the elderly. Curr Psychiatry Rep. 2002;4(1):64–68. doi: 10.1007/s11920-002-0015-8. [DOI] [PubMed] [Google Scholar]
- Alexopoulos P, Grimmer T, Perneczky R, Domes G, Kurz A. Progression to dementia in clinical subtypes of mild cognitive impairment. Dement Geriatr Cogn Disord. 2006;22(1):27–34. doi: 10.1159/000093101. [DOI] [PubMed] [Google Scholar]
- Allaire JC, Gamaldo A, Ayotte BJ, Sim R, Whitfield K. Mild cognitive impairment and objective instrumental everyday functioning: the everyday cognition battery memory test. J Am Geriatr Soc. 2009;57(1):120–125. doi: 10.1111/j.1532-5415.2008.02054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A. On the evolutionary origins of executive functions. Brain Cogn. 2008;68(1):92–99. doi: 10.1016/j.bandc.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working Memory. Oxford, UK: Oxford University Press; 1986. [Google Scholar]
- Bell-McGinty S, Podell K, Franzen M, Baird AD, Williams MJ. Standard measures of executive function in predicting instrumental activities of daily living in older adults. Int J Geriatr Psychiatry. 2002;17(9):828–834. doi: 10.1002/gps.646. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation of working memory from decision making within the human prefrontal cortex. J Neurosci. 1998;18(1):428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Mellits ED, Rovner B, et al. Relation of age at onset and duration of illness to cognitive functioning in Alzheimer’s disease. Neuropsychiatry Neuropsychol Behav Neurol. 1989;2(2):93–101. [Google Scholar]
- Brandt J, Aretouli E, Neijstrom E, et al. Selectivity of executive function impairments in mild cognitive impairment. Neuropsychology. 2009 doi: 10.1037/a0015851. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo G, Hebert R. Age- and education-specific reference values for the Mini-Mental and modified Mini-Mental State Examinations derived from a non-demented elderly population. Int J Geriatr Psychiatry. 1997;12(10):1008–1018. doi: 10.1002/(sici)1099-1166(199710)12:10<1008::aid-gps676>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Brugger P, Monsch AU, Salmon DP, Butters N. Random number generation in dementia of the Alzheimer type: a test of frontal executive functions. Neuropsychologia. 1996;34(2):97–103. doi: 10.1016/0028-3932(95)00066-6. [DOI] [PubMed] [Google Scholar]
- Burgess P, Shallice T. The Hayling and Brixton Tests Manual. England: Bury St. Edmunds; 1997. [Google Scholar]
- Cahn-Weiner DA, Boyle PA, Malloy PF. Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Appl Neuropsychol. 2002;9(3):187–191. doi: 10.1207/S15324826AN0903_8. [DOI] [PubMed] [Google Scholar]
- Cahn-Weiner DA, Malloy PF, Boyle PA, Marran M, Salloway S. Prediction of functional status from neuropsychological tests in community-dwelling elderly individuals. Clin Neuropsychol. 2000;14(2):187–195. doi: 10.1076/1385-4046(200005)14:2;1-Z;FT187. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System examiner’s manual. San Antonio: Psychological Corporation; 2001. [Google Scholar]
- Farias ST, Mungas D, Reed BR, et al. MCI is associated with deficits in everyday functioning. Alzheimer Dis Assoc Disord. 2006;20(4):217–223. doi: 10.1097/01.wad.0000213849.51495.d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68(4):288–291. doi: 10.1212/01.wnl.0000252358.03285.9d. [DOI] [PubMed] [Google Scholar]
- Galasko D, Bennett DA, Sano M, et al. ADCS Prevention Instrument Project: assessment of instrumental activities of daily living for community-dwelling elderly individuals in dementia prevention clinical trials. Alzheimer Dis Assoc Disord. 2006;20(4 Suppl 3):S152–S169. doi: 10.1097/01.wad.0000213873.25053.2b. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Guilford JP, Christensen PR, Merrifield PR, Wilson RC. Alternate Uses: Manual of Instructions and Interpretation. Orange, CA: Sheridan Psychological Services Inc; 1978. [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Humphrey GW, Forde EM, Riddoch MJ. The planning and execution of everyday actions. In: Rapp B, editor. The Handbook of Cognitive Neuropsychology: What Deficits Reveal About the Human Mind. Philadelphia: Psychology Press; 2001. pp. 565–598. [Google Scholar]
- Jahanshahi M, Dirnberger G, Fuller R, Frith CD. The role of the dorsolateral prefrontal cortex in random number generation: a study with positron emission tomography. Neuroimage. 2000;12(6):713–725. doi: 10.1006/nimg.2000.0647. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Paul RH, Ozonoff A, Cohen RA. Evaluating elements of executive functioning as predictors of instrumental activities of daily living (IADLs) Arch Clin Neuropsychol. 2006;21(4):311–320. doi: 10.1016/j.acn.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry. 2001;35(6):776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- Jorm A, Broe A, Creasy H, et al. Further data on the validity of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) Int J of Geriatr Psychiatry. 1996;11:131–139. [Google Scholar]
- Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19(4):1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- Kim KR, Lee KS, Cheong HK, et al. Characteristic profiles of instrumental activities of daily living in different subtypes of mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;27(3):278–285. doi: 10.1159/000204765. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Farah MJ. A unified account of cognitive impairments following frontal lobe damage: the role of working memory in complex, organized behavior. J Exp Psychol Gen. 1993;122(4):411–428. doi: 10.1037//0096-3445.122.4.411. [DOI] [PubMed] [Google Scholar]
- Koss E, Patterson MB, Mack JL, Smyth KA, Whitehouse PJ. Reliability and Validity of the Tinkertoy Test in Evaluating Individuals with Alzheimer’s Disease. The Clinical Neuropsychologist. 1998;12(3):325–329. [Google Scholar]
- Lewis MS, Miller LS. Executive control functioning and functional ability in older adults. Clin Neuropsychol. 2007;21(2):274–285. doi: 10.1080/13854040500519752. [DOI] [PubMed] [Google Scholar]
- Lezak MD. The Problem of Assessing Executive Functions. International Journal of Psychology. 1982;17(1):281–297. [Google Scholar]
- Lowery N, Giovanni L, Mozley LH, et al. Relationship between clock-drawing and neuropsychological and functional status in elderly institutionalized patients with schizophrenia. Am J Geriatr Psychiatry. 2003;11(6):621–628. doi: 10.1176/appi.ajgp.11.6.621. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- Manning KJ, Brandt J. “Completions and Corrections” as a test of executive control; Presented at the 34th Annual Meeting of the International Neuropsychological Society; Massachusetts, Boston: 2006. [Google Scholar]
- Mitchell M, Miller LS. Executive functioning and observed versus self-reported measures of functional ability. Clin Neuropsychol. 2008;22(3):471–479. doi: 10.1080/13854040701336436. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, et al. The unity and diversity of executive functions and their contributions to complex ‘Frontal Lobe’ tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Okonkwo O, Griffith HR, Belue K, et al. Medical decision-making capacity in patients with mild cognitive impairment. Neurology. 2007;69(15):1528–1535. doi: 10.1212/01.wnl.0000277639.90611.d9. [DOI] [PubMed] [Google Scholar]
- Okonkwo OC, Wadley VG, Griffith HR, Ball K, Marson DC. Cognitive correlates of financial abilities in mild cognitive impairment. J Am Geriatr Soc. 2006;54(11):1745–1750. doi: 10.1111/j.1532-5415.2006.00916.x. [DOI] [PubMed] [Google Scholar]
- Pereira FS, Yassuda MS, Oliveira AM, Forlenza OV. Executive dysfunction correlates with impaired functional status in older adults with varying degrees of cognitive impairment. Int Psychogeriatr. 2008;20(6):1104–1115. doi: 10.1017/S1041610208007631. [DOI] [PubMed] [Google Scholar]
- Peres K, Chrysostome V, Fabrigoule C, et al. Restriction in complex activities of daily living in MCI: impact on outcome. Neurology. 2006;67(3):461–466. doi: 10.1212/01.wnl.0000228228.70065.f1. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Pohl C, Sorg C, et al. Impairment of activities of daily living requiring memory or complex reasoning as part of the MCI syndrome. Int J Geriatr Psychiatry. 2006;21(2):158–162. doi: 10.1002/gps.1444. [DOI] [PubMed] [Google Scholar]
- Peters R, Pinto EM. Predictive value of the Clock Drawing Test: a review of the literature. Dement Geriatr Cogn Disord. 2008;26(4):351–355. doi: 10.1159/000162261. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Challenges of epidemiological studies of mild cognitive impairment. Alzheimer Dis Assoc Disord. 2004a;18(1):1–2. doi: 10.1097/00002093-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004b;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Porteus SD. Porteus Maze Test. Fifty Year’s Application. New York: Psychological Corporation; 1965. [Google Scholar]
- Purser JL, Fillenbaum GG, Pieper CF, Walloce RB. Mild cognitive impairment and 10-year trajectories of disability in the Iowa established populations for epidemiologic studies of the elderly cohort. J Am Geriatr Soc. 2005;53(11):1966–1972. doi: 10.1111/j.1532-5415.2005.53566.x. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Hansen LA, Thal LJ, Galasko D. Disparate letter and semantic category fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer’s disease. Neuropsychology. 2007;21(1):20–30. doi: 10.1037/0894-4105.21.1.20. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- Richardson ED, Nadler JD, Malloy PF. Neuropsychologic prediction of performance measures of daily living skills in geriatric patients. Neuropsychology. 1995;9(4):565–572. [Google Scholar]
- Robertson IH, Ward T, Ridgeway V, Nimmo-Smith I. The Test of Everyday Attention (TEA) Manual. Bury St. Edmunds, England: Thames Valley Test Company; 1994. [Google Scholar]
- Rouleau I, Salmon DP, Butters N, Kennedy C, McGuirek Quantitative and qualitative analyses of clock drawings in Alzheimer’s and Huntington’s disease. Brain Cogn. 1992;18(1):70–87. doi: 10.1016/0278-2626(92)90112-y. [DOI] [PubMed] [Google Scholar]
- Royall DR, Lauterbach EC, Kaufer D, et al. The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2007;19(3):249–265. doi: 10.1176/jnp.2007.19.3.249. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Heindel WC, Lange KL. Differential decline in word generation from phonemic and semantic categories during the course of Alzheimer’s disease: implications for the integrity of semantic memory. J Int Neuropsychol Soc. 1999;5(7):692–703. doi: 10.1017/s1355617799577126. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Woo E, Greeley DR. Characterizing multiple memory deficits and their relation to everyday functioning in individuals with mild cognitive impairment. Neuropsychology. 2009;23(2):168–177. doi: 10.1037/a0014186. [DOI] [PubMed] [Google Scholar]
- Schretlen D, Bobholz JH, Brandt J. Development and psychometric properties of the brief test of attention. The Clinical Neuropsychologist. 1996;10(1):80–89. [Google Scholar]
- Shallice T, Burgess P. The domain of supervisory processes and temporal organization of behaviour. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1405–1411. doi: 10.1098/rstb.1996.0124. (Discussion 1411–1402) [DOI] [PubMed] [Google Scholar]
- Sheik J, Yesavage J. Geriatric depression scale (GDS): recent evidence and development of a shorter version. In: Brink D, editor. Clinical Gerontology: A Guide to Assessment and Intervention. New York: Hawthorn Press; 1986. pp. 165–173. [Google Scholar]
- Sirigu A, Zalla T, Pillon B, et al. Encoding of sequence and boundaries of scripts following prefrontal lesions. Cortex. 1996;32(2):297–310. doi: 10.1016/s0010-9452(96)80052-9. [DOI] [PubMed] [Google Scholar]
- Stuck AE, Walthert JM, Nikolaus T, et al. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48(4):445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- Tabert MH, Manly JJ, Liu X, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63(8):916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- Tam CW, Lam LC, Chiu HF, Lui VW. Characteristic profiles of instrumental activities of daily living in Chinese older persons with mild cognitive impairment. Am J Alzheimers Dis Other Demen. 2007;22(3):211–217. doi: 10.1177/1533317507301597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike RL, Hagen EP, Sattler JM. The Stanford-Binet Intelligence Scale: Fourth edition, Guide for Administration and Scoring. Chicago, IL: Riverside Publishing Company; 1986. [Google Scholar]
- Tomaszewski Farias S, Cahn-Weiner DA, Harvey DJ, et al. Longitudinal Changes in Memory and Executive Functioning are Associated with longitudinal change in instrumental activities of daily living in older Adults. Clin Neuropsychol. 2009;23(3):446–461. doi: 10.1080/13854040802360558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuokko H, Morris C, Ebert P. Mild cognitive impairment and everyday functioning in older adults. Neurocase. 2005;11(1):40–47. doi: 10.1080/13554790490896802. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale—Revised. San Antonio: The Psychological Corporation, TX; 1987. [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment- beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Zanini S, Rumiati RI, Shallice T. Action sequencing deficit following frontal lobe lesion. Neurocase. 2002;8(1–2):88–99. doi: 10.1093/neucas/8.1.88. [DOI] [PubMed] [Google Scholar]