Summary
Despite broad variability in study populations, methodologies for CMV detection, and analytic methods used, multiple studies have documented frequent CMV infection in non-immunocompromised adults with critical illness due to a variety of causes. Higher rates of CMV infection in studies of seropositive patients suggest that reactivation of latent infection rather than primary infection is the main mechanism in this setting. Risk factors for CMV reactivation (other than seropositivity) have not been clearly defined and there does not appear to be a consistent association with severity of illness. Furthermore, CMV reactivation in this setting has been associated with important adverse clinical outcomes, including increased duration of mechanical ventilation, longer length of stay, and all-cause mortality. There are several biologically plausible mechanisms that could link CMV reactivation with adverse outcomes, including: direct lung injury (CMV pneumonia), amplification of inflammation systemically and within the lung, or predisposition to other nosocomial infections, but clinical data in the ICU setting are limited. Further observational studies are unlikely to significantly advance our understanding of the role of CMV in critically-ill patients. Given the significant impact of critical illness, limited current therapeutic options, the availability of generally well-tolerated antiviral options for CMV, and the clinical data supporting a possible pathogenic role for CMV, there is a strong rationale for a randomized controlled trial of CMV prevention as a novel means of improving the outcomes of critically-ill patients.
Introduction
Seroprevalence for CMV in adults ranges from 50-90% and varies with the population studied. As with all herpesviruses, primary CMV infection is followed by lifelong latency and episodes of reactivation, occasionally progressing to symptomatic disease, especially during periods of immunosuppression. With the use of sensitive molecular tools, CMV infection has been detected in previously unexpected clinical settings, including in non-immunocompromised adults with critical illness. Although reactivated infection (in seropositive patients) and primary infection (blood products) are both possible mechanisms for acquisition of CMV infection, available evidence supports reactivation of latent infection as the main mechanism in the ICU setting. Many lines of evidence suggest that CMV reactivation in critically-ill, non-immunocompromised patients might have important clinical implications, but further research, including carefully-conducted clinical trials will be required to fully understand the role of CMV in this setting. In this review we will summarize available evidence on the incidence and clinical associations of CMV infection in non-immunocompromised, critically-ill patients, describe potential mechanisms through which these clinical adverse outcomes could be mediated, and outline an approach to test the hypothesis that prevention of CMV reactivation in critically-ill patients might improve clinical outcomes.
How common is CMV infection in non-immunocompromised adults with critical illness and what are the risk factors?
The incidence of CMV infection in critically-ill non-immunocompromised adults has been assessed in previous studies and reviewed in a recently published meta-analysis (Table 1). In all of these studies, overtly immunocompromised patients (transplant, HIV, etc.) have specifically been excluded, since CMV reactivation and association with adverse outcomes have been previously well-documented in these populations. The included patient populations, laboratory methods used to diagnose CMV infection, and sampling frequencies varied considerably among the included studies. Important factors that influenced the rate of CMV infection included: sensitivity of the diagnostic method used (Table 2) (PCR or antigenemia vs. culture), time of screening for CMV infection after admission to the intensive care unit (ICU) (>5 days vs. less than 5 days), and baseline CMV serostatus (Table 3) [1]. The significantly higher rates of CMV infection in studies that included only seropositive patients (rather than all patients without regard to serostatus) is consistent with reactivation of latent infection rather than acquisition of primary infection as the mechanism underlying CMV infection in this setting.
Table 1.
Rates of CMV infection in non-immunocompromised adults in the ICU. Adapted from Reference [1].
| Reference | Incidence | 95% CI |
|---|---|---|
| 11 | 1 / 120 | 0.00 – 0.06 |
| 9 | 1 / 96 | 0.00 – 0.07 |
| 6 | 1 / 24 | 0.00 – 0.26 |
| 8 | 12 / 142 | 0.05 – 0.14 |
| 5 | 8 / 86 | 0.05 – 0.18 |
| 12 | 10 / 104 | 0.05 – 0.17 |
| 13 | 40 / 237 | 0.13 – 0.22 |
| 4 | 29 / 115 | 0.18 – 0.34 |
| 14 | 8 / 25 | 0.17 – 0.52 |
| 7 | 11 / 34 | 0.19 – 0.50 |
| 3 | 39 / 120 | 0.25 – 0.41 |
| 15 | 35 / 99 | 0.27 – 0.45 |
| 10 | 20 / 56 | 0.24 – 0.49 |
| | ||
| Pooled | 215 / 1258 (17%) | 0.11 – 0.24 |
Table 2.
Rates of CMV infection in non-immunocompromised adults in the ICU according to diagnostic method. Adapted from Reference [1].
| Viral Culture | Viral DNA or Antigen | ||||
|---|---|---|---|---|---|
| | |||||
| Reference | Incidence | 95% CI | Reference | Incidence | 98% CI |
| 8 | 12 / 142 | 0.05 – 0.14 | 11 | 1 / 20 | 0.00 – 0.06 |
| 5 | 8 / 86 | 0.05 – 0.18 | 9 | 1 / 96 | 0.00 – 0.07 |
| 12 | 10 / 104 | 0.05 – 0.17 | 6 | 1 / 24 | 0.00 – 0.26 |
| 4 | 29 / 115 | 0.18 – 0.34 | 13 | 40 / 237 | 0.13 – 0.22 |
| 14 | 8 / 25 | 0.17 – 0.52 | |||
| 7 | 11 / 34 | 0.19 – 0.50 | |||
| 3 | 39 / 120 | 0.25 – 0.41 | |||
| 15 | 35 / 99 | 0.27 – 0.45 | |||
| 10 | 20 / 56 | 0.24 – 0.49 | |||
| | |||||
| Pooled | 59 / 447 (13%) | 0.06 – 0.22 | Pooled | 156 / 811 (19%) | 0.13 – 0.31 |
Table 3.
Rates of CMV infection in non-immunocompromised adults in the ICU according to baseline CMV serology. Adapted from Reference [1].
| Active CMV Infection Rate by CMV Serology | |||||
|---|---|---|---|---|---|
| Not Reported | Reported | ||||
| | |||||
| Reference | Incidence | 95% CI | Reference | Incidence | 95% |
| 11 | 1 / 120 | 0.00-0.06 | 6 | 1 / 24 | 0.00-0.26 |
| 9 | 1 / 96 | 0.00-0.07 | 12 | 10 / 76 | 0.07-0.23 |
| 8 | 12 / 142 | 0.05-0.14 | 14 | 8 / 25 | 0.17-0.52 |
| 5 | 8 / 86 | 0.05-0.18 | 7 | 11 / 34 | 0.19-0.50 |
| 13 | 40 / 237 | 0.13-0.22 | 3 | 39 / 120 | 0.25-0.41 |
| 4 | 8 / 22 | 0.19-0.58 | |||
| 10 | 20 / 56 | 0.24-0.49 | |||
| 15 | 23 / 41 | 0.41-0.70 | |||
| | |||||
| Pooled | 62 / 681 (9%) | 0.03-0.14 | Pooled | 120 / 398 (30%) | 0.22-0.42 |
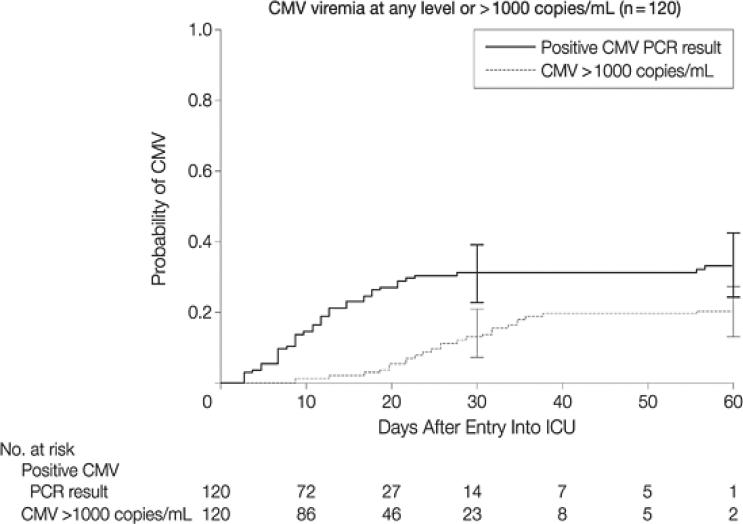
The detailed quantitative virologic aspects of blood CMV reactivation in the ICU setting are illustrated by a recently published prospective study of CMV seropositive non-immunocompromised adults with a broad range of critical illness (burn, trauma, myocardial infarction, sepsis) who were monitored thrice weekly with quantitative plasma CMV PCR from the time of ICU admission until death or hospital discharge, and are shown in Table 4 and Figure 1 [3]. Data regarding CMV reactivation at other sites, particularly the lung, are more limited, but suggest that CMV reactivation in lung might occur earlier and more frequently and at greater levels than in blood [2].
Table 4.
Rates of CMV infection in non –immunocompromised adults in the ICU as assessed by PCR [3].
| CMV Variable | Overall (n = 120) | Burn ICU (n = 20) | Cardiac ICU (n = 20) | Medical ICU (n = 40) | Trauma ICU (n = 40) |
|---|---|---|---|---|---|
| CMV viremia at any level, No. (%) | 39 (33) | 11 (55) | 3 (15) | 10 (25) | 15 (38) |
| CMV viremia at >1000 copies/mL, No. (%) | 24 (20) | 9 (45) | 1 (5) | 6 (15) | 8 (20) |
| CMV viremia at >10000 copies/mL, No. (%) | 11 (9) | 4 (20) | 0 | 4 (10) | 3 (8) |
| Maximum CMV load, median (range), log10 PCR copies | 3.3 (1.8-5.5) | 3.9 (2.5-5.5) | 2.4 (1.8-3.7) | 3.4 (2.3-4.8) | 3.1 (2.1-4.5) |
| Days to first detectable CMV viremia, median (range) | 12 (3-57) | 19 (7-57) | 15 (9-21) | 8 (3-13) | 11 (3-21) |
| Duration of viremia, median (range), d | 17 (2-45) | 20 (4-45) | 4 (2-17) | 18 (4-38) | 14 (2-32) |
Figure 1.
Cumulative incidence of CMV viremia in CMV seropositive, non-immunocompromised adults in the ICU [3].
Consistent risk factors for CMV reactivation have not been identified but the rates of CMV reactivation appeared to be higher in studies that included patients with higher disease severity [1]. However, important confounding factors known to influence CMV reactivation rates (as noted above) preclude definitive conclusions. Furthermore, in a large prospective study that utilized blinded endpoints and various statistical approaches to control for confounders, the severity of illness (as assessed by APACHE II [acute physiology and chronic health evaluation] score at admission) was not associated with an increased risk for CMV reactivation [3]. Thus, although existing data are limited, CMV reactivation does not appear to be simply a marker for severity of illness in this setting.
What adverse clinical outcomes have been associated with CMV reactivation in the ICU setting?
In previously published studies, the association of CMV with various adverse clinical outcomes was assessed using a range of statistical approaches [4-14,3,15,16]. Six of eight studies that assessed all-cause mortality found an association with CMV infection, with a pooled odds ratio of 1.93 (95% confidence interval 1.29 to 2.88), p=0.001 (Table 5). Other adverse clinical outcomes associated with CMV in this setting have included: increased hospital and/or ICU length of stay, longer duration of mechanical ventilation, and increased rates of nosocomial infections as summarized in Table 6.
Table 5.
Association of CMV infection with mortality in non-immunocompromised adults in the ICU. Adapted from Reference [1].
| Deaths / Total | |||
|---|---|---|---|
| | |||
| References | Active CMV Infection | No Active CMV Infection | P-Value |
| 15 | 10 / 35 | 7 / 64 | 0.031 |
| 13 | 20 / 40 | 11 / 40 | 0.041 |
| 4 | 16 / 29 | 31 / 86 | 0.073 |
| 12 | 5 / 10 | 25 / 94 | 0.132 |
| 3 | 1 / 39 | 9 / 81 | 0.146 |
| 10 | 11 / 20 | 13 / 36 | 0.174 |
| 14 | 5 / 8 | 6 / 17 | 0.209 |
| 7 | 7 / 11 | 17 / 23 | 0.540 |
| | |||
| Pooled | 75 / 192 (39%) | 119 / 441 (27%) | 0.001 |
Table 6.
Adverse clinical outcomes associated with CMV infection in non-immunocompromised adults in the ICU.
All of these studies had one or more important limitations, including: relatively small sample size, inclusion of only selected ICU populations, lack of quantitative CMV methods, non-blinded assessment of clinical endpoints, or analytic issues (time-varying endpoints, limited control for confounding factors). However, the general consistency among these studies with regard to CMV reactivation rates and association with adverse clinical outcomes, along with biologically-plausible mechanisms to explain the associations (discussed below), all are consistent with a pathogenic role for CMV in this setting. We emphasize that observational studies cannot conclusively establish causality and that the data are also consistent with the possibility that CMV reactivation could be a marker (rather than a determinant) of adverse clinical outcomes. The only definitive means of differentiating between a role of CMV as a cause vs. marker for adverse clinical outcomes is by means of a randomized controlled trial of CMV prevention or treatment in this setting.
What biologically-plausible mechanism(s) could link CMV with observed adverse clinical outcomes?
CMV could potentially cause adverse clinical outcomes in ICU patients through several biologically-plausible mechanisms. Although CMV organ involvement typically has been documented in overtly immunocompromised persons, recent studies have documented CMV pneumonia in critically-ill patients without known immunocompromise [17]. Whether functional immunocompromise from critical illness alone or the inflammatory milieu within the lung of such patients could facilitate CMV pneumonia in this setting is unknown. In three studies that specifically assessed for CMV pneumonia in biopsy or autopsy lung samples from ICU patients with acute lung injury/acute respiratory distress syndrome (ARDS) and respiratory failure [18,19,5], CMV was detected by histopathology in approximately 30% of cases overall (range, 29-50%) and the sensitivity of detection was reportedly higher for histopathology than culture (10/100 [10%]) [17]. The indications for lung biopsy varied and the specific impact of CMV pneumonia was not systematically assessed in these studies, making it difficult to estimate the true incidence and clinical impact of CMV pneumonia in ICU patients with respiratory failure and/or acute lung injury. Thus, based on these limited data, direct CMV-mediated lung injury (i.e., CMV pneumonia) is an important potential mechanism through which CMV might cause adverse clinical outcomes such as longer length of stay, longer duration of mechanical ventilation, or mortality in ICU patients.
Critical illness secondary to trauma or sepsis frequently leads to the complication of acute lung injury (ALI) or its more severe form, ARDS. Acute lung injury/ARDS are associated with high rates of morbidity and mortality despite current standards of ICU care. Dysregulated inflammation is thought to play a major role in the pathogenesis of ALI/ARDS [20]. Two specific inflammatory mediators (IL-6, IL-8) have been shown to be elevated in blood and/or bronchoalveolar lavage fluid (BAL) of patients with ALI/ARDS, and higher levels either at onset or during the course of illness are associated with worse outcomes [21-24]. Furthermore, the intervention of lung protective ventilation in ALI/ARDS, which has been shown to improve mortality in randomized controlled trials, is associated with reductions in levels of IL-6 and IL-8, suggesting that these cytokines either are integrally involved or are potentially useful biomarkers in this clinical setting [23]. Importantly, CMV has been linked to both of these cytokines both in vitro and in vivo [25-33]. Local reactivation of CMV within the lung appears to be common in ICU patients [2], and could therefore provide a mechanistic link between CMV and dysregulated inflammation within the lungs of patients with ALI/ARDS. Based on the relatively later onset of CMV reactivation compared to the onset of acute lung injury/ARDS, it is more plausible that CMV might exacerbate or prolong rather than initiate the process of ALI/ARDS. Thus, CMV could potentially worsen clinical outcomes (lung injury, duration of mechanical ventilation) through upregulation of these key cytokines (or other inflammatory mediators) in the lungs of patients with critical illness and ALI/ARDS.
Another potential mechanism through which CMV could contribute to worse clinical outcomes in ICU patients is through its immunomodulatory properties [34]. A number of studies in immunocompromised populations (solid organ and hematopoietic cell transplant recipients) have linked CMV with an increased risk for subsequent bacterial, fungal or other opportunistic infections, and nosocomial infections have been associated with longer lengths of stay and increased mortality [35-41]. Consistent with this hypothesis are the findings of prior studies that reported an increased rate of nosocomial infections (the majority of which were ventilator-associated pneumonias) in ICU patients with CMV reactivation [12,13,16].
Although there are multiple plausible mechanisms for a pathogenic role of CMV in ICU patients, much of the data are extrapolated from other clinical settings, and thus their applicability in non-immunocompromised patients remains unproven. Thorough investigation into the specific mechanisms by which CMV mediates adverse clinical outcomes should be an important component of future clinical trials of CMV prevention in the ICU setting.
What should be the next steps to further define the relationship between CMV and adverse outcomes in patients with critical illness?
As discussed earlier, given the inherent difficulty in ascertaining causality from prior observational studies, additional data from observational studies is unlikely to significantly advance our understanding of the role of CMV in ICU patients. Rather, given the existing large body of evidence demonstrating frequent CMV reactivation and association with adverse clinical outcomes in critically-ill patients, biologically-plausible mechanisms through which CMV could mediate these outcomes, and the urgent clinical need for new treatment options, a controlled trial of CMV prevention in ICU patients is warranted and should be the next appropriate step. Since the association of CMV with adverse outcomes is far from proven, and because robust data on the potential effect size and appropriate endpoints are not yet available, an initial phase II pilot study using a randomized controlled trial design would be more appropriate than proceeding directly to a large-scale phase III efficacy trial.
Based on currently available data, the most appropriate study populations for initial clinical trials should include adult CMV seropositive patients with either sepsis or pneumonia-associated ALI/ARDS. These populations are known to have high morbidity and mortality despite current state of the art ICU care and represent those in whom previous studies have documented high rates of CMV reactivation [1]. Furthermore, these are populations in whom specific biomarkers (IL-6, IL-8) have been shown to be associated with important clinical endpoints [21-24], thereby providing rational surrogate endpoints for initial “proof of concept” clinical trials.
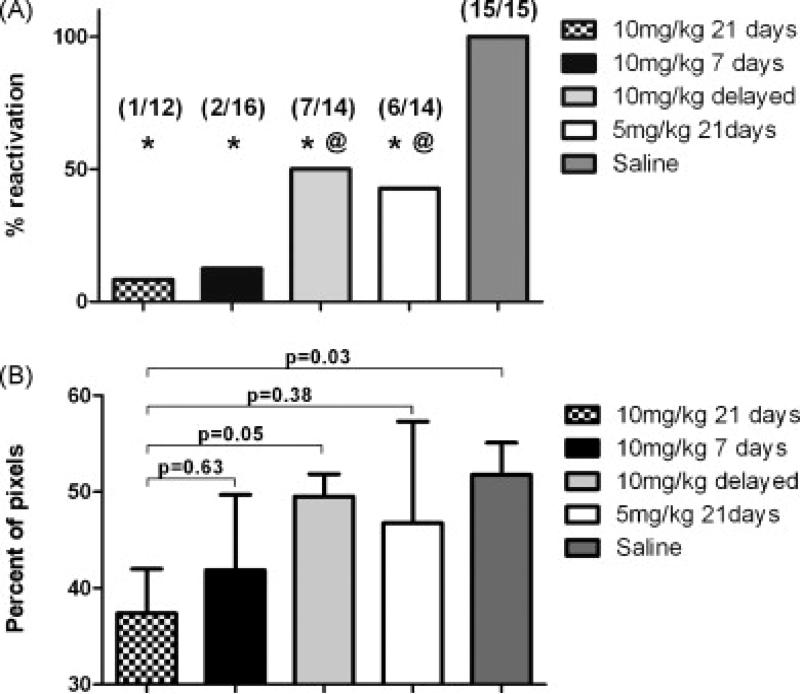
The choice of antiviral drug and appropriate strategy for employing antiviral drug requires careful consideration. Among currently available options, ganciclovir is favored over alternative agents based on its potent activity against CMV, availability of intravenous and oral formulations (via valganciclovir), and better efficacy vs. comparators in previous clinical trials [42-46]. The two major approaches for CMV prevention that have been used in immunocompromised patient populations are antiviral prophylaxis (antiviral drug given to all patients at risk for CMV infection/reactivation) and preemptive therapy (antiviral drug given only to patients with early laboratory evidence of CMV infection). At least for initial clinical trials, there is a stronger rationale for the approach of antiviral prophylaxis over preemptive therapy. Although preemptive therapy and prophylaxis appear to both be effective for prevention of symptomatic CMV infection (i.e., CMV disease), there are emerging data suggesting that antiviral prophylaxis might have a greater beneficial effect on other CMV-associated effects [47-50], such as those hypothesized to be responsible for the association with adverse clinical outcomes in ICU patients. Importantly, in a murine model of sepsis-induced CMV reactivation, early (prophylactic) administration of high dose ganciclovir was significantly more effective in preventing both CMV reactivation and subsequent pulmonary fibrosis than a delayed (i.e., treatment) approach [51] (Figure 2). Also, from a logistical standpoint, few clinical laboratories have the capability of providing real-time results for a large multicenter trial without significant delays in instituting antiviral drug. This might be particularly relevant in the ICU setting where a prior study has demonstrated an apparent dose effect of CMV with adverse outcomes [3]. Since in a preemptive approach antiviral drug would not be started (after some delay) until some degree of viral replication had already occurred, this could potentially minimize the effect size seen in the active drug arm if a preemptive strategy were used. These potential disadvantages of a preemptive therapy approach must be carefully weighed against the likely higher risk for toxicity, cost and potential for resistance with the use of a prophylactic strategy.
Figure 2.
Impact of different ganciclovir regimens on reactivation of CMV (A) and pulmonary fibrosis (B) in a murine model of CMV reactivation and lung injury induced by cecal ligation and puncture [51].
Since the ability of a pilot study to assess the impact on true clinical endpoints is limited, the incorporation of a surrogate endpoint (biomarker) should be strongly considered, with the use of various clinical events (mortality, length of stay, duration of mechanical ventilation, safety) as secondary endpoints. Although a single consensus biomarker for trials of acute lung injury has not been well-defined, IL-6 and IL-8 have previously been hypothesized to be important in the pathogenesis of ALI/ARDS, shown to be important in predicting clinical outcomes in ICU patients, and have also been linked to CMV in vitro and in vivo [25-33]. Furthermore, current interventional clinical trials in patients with acute lung injury/ARDS have used these cytokines as primary endpoints [NCT00351533, NCT00559130]. Thus, levels of these cytokines (or changes over time) are appropriate to consider as surrogate endpoints for initial “proof of concept” pilot studies of CMV prevention in the ICU setting.
Acknowledgements
We gratefully acknowledge Linda Norkool's secretarial assistance and Sherry Dodson's assistance with literature searches.
ABBREVIATIONS
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- BAL
bronchoalveolar lavage fluid
- ICU
Intensive care unit
References
- 1.Kalil AC, Florescu DF. Prevalence and mortality associated with cytomegalovirus infection in nonimmunosuppressed patients in the intensive care unit. Crit Care Med. 2009 Aug;37(8):2350–2358. doi: 10.1097/CCM.0b013e3181a3aa43. [DOI] [PubMed] [Google Scholar]
- 2.Hamprecht K, Baumeister A, Beck R, Haeberle H, Heininger A. The lung as a central compartment of active CMV infection. Inflammation Research Supplement 2. 2007:S242. (Abstract A383) [Google Scholar]
- 3.Limaye AP, Kirby KA, Rubenfeld GD, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008 Jul 23;300(4):413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domart Y, Trouillet JL, Fagon JY, Chastre J, Brun-Vezinet F, Gibert C. Incidence and morbidity of cytomegaloviral infection in patients with mediastinitis following cardiac surgery. Chest. 1990 Jan;97(1):18–22. doi: 10.1378/chest.97.1.18. [DOI] [PubMed] [Google Scholar]
- 5.Papazian L, Fraisse A, Garbe L, et al. Cytomegalovirus. An unexpected cause of ventilator-associated pneumonia. Anesthesiology. 1996 Feb;84(2):280–287. doi: 10.1097/00000542-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Stephan F, Meharzi D, Ricci S, Fajac A, Clergue F, Bernaudin JF. Evaluation by polymerase chain reaction of cytomegalovirus reactivation in intensive care patients under mechanical ventilation. Intensive Care Med. 1996 Nov;22(11):1244–1249. doi: 10.1007/BF01709343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kutza AS, Muhl E, Hackstein H, Kirchner H, Bein G. High incidence of active cytomegalovirus infection among septic patients. Clin Infect Dis. 1998 May;26(5):1076–1082. doi: 10.1086/520307. [DOI] [PubMed] [Google Scholar]
- 8.Cook CH, Yenchar JK, Kraner TO, Davies EA, Ferguson RM. Occult herpes family viruses may increase mortality in critically ill surgical patients. Am J Surg. 1998 Oct;176(4):357–360. doi: 10.1016/s0002-9610(98)00205-0. [DOI] [PubMed] [Google Scholar]
- 9.Desachy A, Ranger-Rogez S, Francois B, et al. Reactivation of human herpesvirus type 6 in multiple organ failure syndrome. Clin Infect Dis. 2001 Jan 15;32(2):197–203. doi: 10.1086/318474. [DOI] [PubMed] [Google Scholar]
- 10.Heininger A, Jahn G, Engel C, Notheisen T, Unertl K, Hamprecht K. Human cytomegalovirus infections in nonimmunosuppressed critically ill patients. Crit Care Med. 2001 Mar;29(3):541–547. doi: 10.1097/00003246-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Razonable RR, Fanning C, Brown RA, et al. Selective reactivation of human herpesvirus 6 variant a occurs in critically ill immunocompetent hosts. J Infect Dis. 2002 Jan 1;185(1):110–113. doi: 10.1086/324772. [DOI] [PubMed] [Google Scholar]
- 12.Cook CH, Martin LC, Yenchar JK, et al. Occult herpes family viral infections are endemic in critically ill surgical patients. Crit Care Med. 2003 Jul;31(7):1923–1929. doi: 10.1097/01.CCM.0000070222.11325.C4. [DOI] [PubMed] [Google Scholar]
- 13.Jaber S, Chanques G, Borry J, et al. Cytomegalovirus infection in critically ill patients: associated factors and consequences. Chest. 2005 Jan;127(1):233–241. doi: 10.1378/chest.127.1.233. [DOI] [PubMed] [Google Scholar]
- 14.von Muller L, Klemm A, Weiss M, et al. Active cytomegalovirus infection in patients with septic shock. Emerg Infect Dis. 2006 Oct;12(10):1517–1522. doi: 10.3201/eid1210.060411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziemann M, Sedemund-Adib B, Reiland P, Schmucker P, Hennig H. Increased mortality in long-term intensive care patients with active cytomegalovirus infection. Crit Care Med. 2008 Dec;36(12):3145–3150. doi: 10.1097/CCM.0b013e31818f3fc4. [DOI] [PubMed] [Google Scholar]
- 16.Chiche L, Forel JM, Roch A, et al. Active cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients. Crit Care Med. 2009 Jun;37(6):1850–1857. doi: 10.1097/CCM.0b013e31819ffea6. [DOI] [PubMed] [Google Scholar]
- 17.Osawa R, Singh N. Cytomegalovirus infection in critically ill patients: a systematic review. Crit Care. 2009;13(3):R68. doi: 10.1186/cc7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papazian L, Doddoli C, Chetaille B, et al. A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Crit Care Med. 2007 Mar;35(3):755–762. doi: 10.1097/01.CCM.0000257325.88144.30. [DOI] [PubMed] [Google Scholar]
- 19.Papazian L, Thomas P, Bregeon F, et al. Open-lung biopsy in patients with acute respiratory distress syndrome. Anesthesiology. 1998 Apr;88(4):935–944. doi: 10.1097/00000542-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000 May 4;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 21.Levitt JE, Gould MK, Ware LB, Matthay MA. The pathogenetic and prognostic value of biologic markers in acute lung injury. J Intensive Care Med. 2009 May-Jun;24(3):151–167. doi: 10.1177/0885066609332603. [DOI] [PubMed] [Google Scholar]
- 22.Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, Brower RG, Standiford TJ, Martin TR, Matthay MA. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest. 2010 Feb;137(2):288–296. doi: 10.1378/chest.09-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005 Jan;33(1):1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230-232. [DOI] [PubMed] [Google Scholar]
- 24.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000 May 4;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 25.Carlquist JF, Edelman L, Bennion DW, Anderson JL. Cytomegalovirus induction of interleukin-6 in lung fibroblasts occurs independently of active infection and involves a G protein and the transcription factor, NF-kappaB. J Infect Dis. 1999 May;179(5):1094–1100. doi: 10.1086/314734. [DOI] [PubMed] [Google Scholar]
- 26.Compton T, Kurt-Jones EA, Boehme KW, et al. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003 Apr;77(8):4588–4596. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murayama T, Ohara Y, Obuchi M, et al. Human cytomegalovirus induces interleukin-8 production by a human monocytic cell line, THP-1, through acting concurrently on AP-1- and NF-kappaB-binding sites of the interleukin-8 gene. J Virol. 1997 Jul;71(7):5692–5695. doi: 10.1128/jvi.71.7.5692-5695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craigen JL, Yong KL, Jordan NJ, et al. Human cytomegalovirus infection up-regulates interleukin-8 gene expression and stimulates neutrophil transendothelial migration. Immunology. 1997 Sep;92(1):138–145. doi: 10.1046/j.1365-2567.1997.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwamoto GK, Konicek SA. Cytomegalovirus immediate early genes upregulate interleukin-6 gene expression. J Investig Med. 1997 Apr;45(4):175–182. [PubMed] [Google Scholar]
- 30.Tong CY, Bakran A, Williams H, Cuevas LE, Peiris JS, Hart CA. Association of tumour necrosis factor alpha and interleukin 6 levels with cytomegalovirus DNA detection and disease after renal transplantation. J Med Virol. 2001 May;64(1):29–34. doi: 10.1002/jmv.1013. [DOI] [PubMed] [Google Scholar]
- 31.Humar A, St Louis P, Mazzulli T, et al. Elevated serum cytokines are associated with cytomegalovirus infection and disease in bone marrow transplant recipients. J Infect Dis. 1999 Feb;179(2):484–488. doi: 10.1086/314602. [DOI] [PubMed] [Google Scholar]
- 32.Humbert M, Devergne O, Cerrina J, et al. Activation of macrophages and cytotoxic cells during cytomegalovirus pneumonia complicating lung transplantations. Am Rev Respir Dis. 1992 May;145(5):1178–1184. doi: 10.1164/ajrccm/145.5.1178. [DOI] [PubMed] [Google Scholar]
- 33.Humbert M, Delattre RM, Fattal S, et al. In situ production of interleukin-6 within human lung allografts displaying rejection or cytomegalovirus pneumonia. Transplantation. 1993 Sep;56(3):623–627. doi: 10.1097/00007890-199309000-00024. [DOI] [PubMed] [Google Scholar]
- 34.Boeckh M, Nichols WG. Immunosuppressive effects of beta-herpesviruses. Herpes. 2003 May;10(1):12–16. [PubMed] [Google Scholar]
- 35.Falagas ME, Snydman DR, Griffith J, Werner BG. Exposure to cytomegalovirus from the donated organ is a risk factor for bacteremia in orthotopic liver transplant recipients. Boston Center for Liver Transplantation CMVIG Study Group. Clin Infect Dis. 1996 Sep;23(3):468–474. doi: 10.1093/clinids/23.3.468. [DOI] [PubMed] [Google Scholar]
- 36.George MJ, Snydman DR, Werner BG, et al. The independent role of cytomegalovirus as a risk factor for invasive fungal disease in orthotopic liver transplant recipients. Boston Center for Liver Transplantation CMVIG-Study Group. Cytogam, MedImmune, Inc. Gaithersburg, Maryland. Am J Med Aug. 1997;103(2):106–113. doi: 10.1016/s0002-9343(97)80021-6. [DOI] [PubMed] [Google Scholar]
- 37.Rand KH, Pollard RB, Merigan TC. Increased pulmonary superinfections in cardiac-transplant patients undergoing primary cytomegalovirus infection. N Engl J Med. 1978 Apr 27;298(17):951–953. doi: 10.1056/NEJM197804272981705. [DOI] [PubMed] [Google Scholar]
- 38.Chatterjee SN, Fiala M, Weiner J, Stewart JA, Stacey B, Warmer N. Primary cytomegalovirus and opportunistic infections. Incidence in renal transplant recipients. JAMA. 1978 Nov 24;240(22):2446–2449. [PubMed] [Google Scholar]
- 39.Limaye AP, Bakthavatsalam R, Kim HW, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. 2006 Jun 27;81(12):1645–1652. doi: 10.1097/01.tp.0000226071.12562.1a. [DOI] [PubMed] [Google Scholar]
- 40.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis. 2002 Feb 1;185(3):273–282. doi: 10.1086/338624. [DOI] [PubMed] [Google Scholar]
- 41.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002 Dec 15;100(13):4358–4366. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 42.Winston DJ, Busuttil RW. Randomized controlled trial of oral ganciclovir versus oral acyclovir after induction with intravenous ganciclovir for long-term prophylaxis of cytomegalovirus disease in cytomegalovirus-seropositive liver transplant recipients. Transplantation. 2003 Jan 27;75(2):229–233. doi: 10.1097/01.TP.0000040601.60276.96. [DOI] [PubMed] [Google Scholar]
- 43.Rubin RH, Kemmerly SA, Conti D, et al. Prevention of primary cytomegalovirus disease in organ transplant recipients with oral ganciclovir or oral acyclovir prophylaxis. Transpl Infect Dis. 2000 Sep;2(3):112–117. [PubMed] [Google Scholar]
- 44.Flechner SM, Avery RK, Fisher R, et al. A randomized prospective controlled trial of oral acyclovir versus oral ganciclovir for cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Transplantation. 1998 Dec 27;66(12):1682–1688. doi: 10.1097/00007890-199812270-00019. [DOI] [PubMed] [Google Scholar]
- 45.Winston DJ. Prevention of cytomegalovirus disease in transplant recipients. Lancet. 1995 Nov 25;346(8987):1380–1381. doi: 10.1016/s0140-6736(95)92401-9. [DOI] [PubMed] [Google Scholar]
- 46.Duncan SR, Grgurich WF, Iacono AT, et al. A comparison of ganciclovir and acyclovir to prevent cytomegalovirus after lung transplantation. Am J Respir Crit Care Med. 1994 Jul;150(1):146–152. doi: 10.1164/ajrccm.150.1.8025741. [DOI] [PubMed] [Google Scholar]
- 47.Hodson EM, Jones CA, Webster AC, et al. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet. 2005 Jun 18-24;365(9477):2105–2115. doi: 10.1016/S0140-6736(05)66553-1. [DOI] [PubMed] [Google Scholar]
- 48.Strippoli GF, Hodson EM, Jones C, Craig JC. Preemptive treatment for cytomegalovirus viremia to prevent cytomegalovirus disease in solid organ transplant recipients. Transplantation. 2006 Jan 27;81(2):139–145. doi: 10.1097/01.tp.0000183970.71366.da. [DOI] [PubMed] [Google Scholar]
- 49.Reischig T, Jindra P, Hes O, Svecova M, Klaboch J, Treska V. Valacyclovir prophylaxis versus preemptive valganciclovir therapy to prevent cytomegalovirus disease after renal transplantation. Am J Transplant. 2008 Jan;8(1):69–77. doi: 10.1111/j.1600-6143.2007.02031.x. [DOI] [PubMed] [Google Scholar]
- 50.Kliem V, Fricke L, Wollbrink T, Burg M, Radermacher J, Rohde F. Improvement in long-term renal graft survival due to CMV prophylaxis with oral ganciclovir: results of a randomized clinical trial. Am J Transplant. 2008 May;8(5):975–983. doi: 10.1111/j.1600-6143.2007.02133.x. [DOI] [PubMed] [Google Scholar]
- 51.Forster MR, Trgovcich J, Zimmerman P, Chang A, Miller C, Klenerman P, Cook CH. Antiviral Res. 2009 Dec 11; doi: 10.1016/j.antiviral.2009.12.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]