Abstract
Dopamine, by activating dopamine D1-type receptors, and adenosine, by activating adenosine A2A receptors, stimulate phosphorylation of DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of Mr 32,000) at Thr-34. In this study, we investigated the effect of metabotropic glutamate (mGlu) receptors on DARPP-32 phosphorylation at Thr-34 in neostriatal slices. A broad-spectrum mGlu receptor agonist, trans-ACPD, and a group I mGlu receptor agonist, DHPG, stimulated DARPP-32 phosphorylation at Thr-34. Studies with mGlu receptor antagonists revealed that the effects of trans-ACPD and DHPG were mediated through activation of mGlu5 receptors. The action of mGlu5 receptors required activation of adenosine A2A receptors by endogenous adenosine. Conversely, the action of adenosine A2A receptors required activation of mGlu5 receptors by endogenous glutamate. Coactivation of mGlu5 and adenosine A2A receptors by exogenous agonists synergistically increased DARPP-32 phosphorylation. mGlu5 receptors did not require activation of dopamine D1-type receptors by endogenous dopamine, nor did dopamine D1-type receptors require activation of mGlu5 receptors by endogenous glutamate. DHPG potentiated the effect of forskolin, but not that of 8-bromo-cAMP, and stimulated DARPP-32 phosphorylation in the presence of the phosphodiesterase inhibitor IBMX, suggesting that mGlu5 receptors stimulate the rate of cAMP formation coupled to adenosine A2A receptors. The action of mGlu5 receptors was attenuated by inhibitors of extracellular signal-regulated kinase, but not by inhibitors of phospholipase C, p38, casein kinase 1, or Cdk5. The results demonstrate that mGlu5 receptors potentiate adenosine A2A/DARPP-32 signaling by stimulating the adenosine A2A receptor-mediated formation of cAMP in an extracellular signal-regulated kinase-dependent manner.
Dopamine- and cAMP-regulated phosphoprotein of Mr 32,000 (DARPP-32) is a signal transduction molecule that is selectively enriched in medium-sized spiny neurons in neostriatum, and plays an obligatory role in dopaminergic signaling. Mice lacking DARPP-32 exhibit profound deficits in their molecular, electrophysiological, and behavioral responses to dopamine, drugs of abuse and antipsychotic medication, demonstrating the importance of DARPP-32 in most of the actions of dopamine (1).
When DARPP-32 is phosphorylated by cAMP-dependent protein kinase (PKA) on Thr-34, it is converted into a potent inhibitor of protein phosphatase-1, and thereby controls the phosphorylation state and activity of many downstream physiological effectors, including various neurotransmitter receptors and voltage-gated ion channels (for review, see ref. 2). Phosphorylation and dephosphorylation of DARPP-32 at Thr-34 are regulated by dopamine, adenosine, glutamate, serotonin, and other neurotransmitters. For example, dopamine, by activating dopamine D1-type receptors (3), and adenosine, by activating adenosine A2A receptors (4), stimulate the phosphorylation of DARPP-32 at Thr-34.
Metabotropic glutamate (mGlu) receptors are subdivided into three groups on the basis of agonist pharmacology, sequence homology, and G-protein effector coupling: group I (mGlu1 and mGlu5 receptors), group II (mGlu2 and mGlu3 receptors), and group III (mGlu4, mGlu6, mGlu7, and mGlu8 receptors; ref. 5). Group I mGlu receptors are coupled to the phospholipase C (PLC) pathway (6), the extracellular signal-regulated kinase (ERK) pathway (7), and the p38 pathway (8); group II and III mGlu receptors are negatively coupled to adenylyl cyclase (9). Individual subtypes of mGlu receptors are assumed to mediate distinct facilitatory (group I) or inhibitory (group II and III) actions on neuronal transmission (10). Recently, mGlu receptors were shown to be involved in cocaine self-administration (11) and methamphetamine-induced neurotoxicity (12). However, the molecular mechanisms underlying the actions of mGlu receptors are not clearly understood. In this study, we investigated the regulation of DARPP-32 phosphorylation at Thr-34 by mGlu receptors by using neostriatal slices. The results obtained indicate that the actions of mGlu5 receptors are linked to A2A adenosine receptors by means of a mechanism involving the ERK cascade.
Materials and Methods
Preparation and Incubation of Neostriatal Slices.
Neostriatal slices were prepared from male C57BL/6 mice (6–8 weeks old), as described (13). Each slice was placed in a polypropylene incubation tube with 2 ml of fresh Krebs-HCO3− buffer containing adenosine deaminase (10 μg/ml). The slices were preincubated at 30°C under constant oxygenation with 95% O2/5% CO2 for 60 min. The buffer was replaced with fresh Krebs-HCO3− buffer, without or with adenosine deaminase as indicated, after 30 min of preincubation. Slices were treated with drugs as specified in each experiment. Drugs were obtained from the following sources: SKF81297, SCH23390, CGS21680, 8-(3-chlorostyryl) caffeine, forskolin, 3-isobutyl-1-methylxanthine (IBMX), MK801 from Sigma; ZM241385, (±)-1-aminocyclopentane-trans-1,3-dicarboxylic acid (trans-ACPD), (RS)-3,5-dihydroxyphenylglycine (DHPG), LY341495, LY367385, 2-methyl-6-(phenylethynyl)pyridine (MPEP), (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG), L(+)-2-amino-4-phosphonobutyric acid (L-AP4), and (RS)-α-cyclopropyl-4-phosphonophenylglycine (CPPG), all from Tocris Cookson (Bristol, U.K.); PD98059 from Alexis (San Diego); SB202190, U0126, and U0124 from Calbiochem; U73122 from Calbiochem and Tocris Cookson; adenosine deaminase from Boehringer-Mannheim (Mannheim, Germany); butyrolactone from Biomol (Plymouth Meeting, PA); tetrodotoxin from Wako Pure Chemical (Osaka, Japan). After drug treatment, slices were transferred to Eppendorf tubes, frozen on dry ice, and stored at −80°C until assayed.
Immunoblotting.
Frozen tissue samples were sonicated in boiling 1% SDS. Equal amounts of protein (100 μg) were processed by using 12% polyacrylamide gels as described (13). Immunoblotting was carried out by using a phosphorylation state-specific monoclonal antibody raised against a DARPP-32 peptide containing phospho-Thr-34, the site phosphorylated by PKA (mAb-23; 1:750 dilution). A monoclonal antibody (C24-5a; 1:7,500 dilution) generated against DARPP-32, which is not phosphorylation state-specific, was used to determine the total amount of DARPP-32 in samples. None of the experimental manipulations used in the present study altered the total amount of DARPP-32. Antibody binding was detected by the enhanced chemiluminescence (ECL) immunoblotting detection system (Amersham Pharmacia). Phospho-DARPP-32 bands were quantified by densitometry with NIH IMAGE V.1.61 software. For each experiment, values obtained for treated slices were calculated relative to the value for the control slices. Normalized data from multiple experiments were expressed as means ± SEM, and statistical analysis was carried out by using ANOVA and the Newman–Keuls test.
Results
Activation of mGlu5 Receptors Stimulates the Phosphorylation of DARPP-32 at Thr-34.
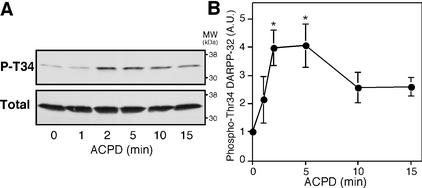
The effect of a broad-spectrum mGlu receptor agonist, trans-ACPD, on DARPP-32 Thr-34 phosphorylation was examined in neostriatal slices by using a phosphorylation state-specific antibody. Phosphorylation of DARPP-32 at Thr-34 was detectable under basal conditions (the stoichiometry of phosphorylation has been estimated to be 0.5–1.0% under basal conditions; ref. 3). Treatment of neostriatal slices with trans-ACPD (100 μM) increased the level of phospho-Thr-34 DARPP-32 by fourfold within 2 min of incubation (Fig. 1). DARPP-32 Thr-34 phosphorylation was maximal at 2–5 min of incubation, and the level of phospho-Thr-34 DARPP-32 subsequently decreased. The total level of DARPP-32 was unchanged by treatment with trans-ACPD.
Figure 1.
Regulation of DARPP-32 phosphorylation at Thr-34 by a broad-spectrum mGlu receptor agonist, trans-ACPD. Neostriatal slices were incubated with trans-ACPD (100 μM) for the indicated times. (A) Phospho-Thr-34 DARPP-32 was detected at a molecular mass of ≈32 kDa by using a monoclonal antibody (mAb-23) against Thr-34-phosphorylated DARPP-32 (Upper); total DARPP-32 was detected in the same membrane by using a monoclonal antibody (C24-5a) against DARPP-32 (Lower). (B) The amounts of phospho-DARPP-32 were quantified by densitometry, and the data were normalized to untreated slices. Data represent means ± SEM for four to seven experiments. *, P < 0.05, compared with control.
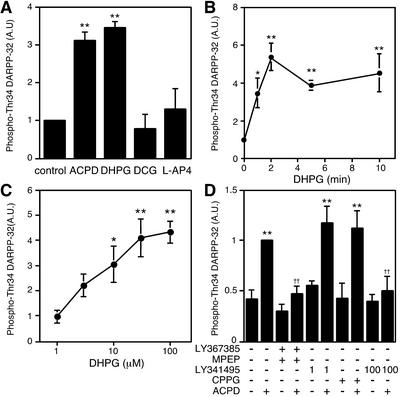
The effects of mGlu receptor agonists, selective for each group, on DARPP-32 Thr-34 phosphorylation were examined (Fig. 2A). Treatment of slices with a group I mGlu receptor agonist, DHPG (100 μM), for 5 min caused an increase in the level of phospho-Thr-34 DARPP-32 comparable to that seen with trans-ACPD. Treatment with a group II mGlu receptor agonist, DCG (10 μM), or a group III mGlu receptor agonist, L-AP4 (50 μM), did not affect the level of phospho-Thr-34 DARPP-32. Treatment of slices with DHPG (100 μM) increased the level of phospho-Thr-34 DARPP-32 with a time course similar to that with trans-ACPD (Figs. 1 and 2B). DHPG stimulated DARPP-32 Thr-34 phosphorylation maximally at a concentration of 100 μM, with a half-maximal effect at ≈5 μM (Fig. 2C).
Figure 2.
Regulation of DARPP-32 phosphorylation at Thr-34 by group I mGlu receptors. (A) Neostriatal slices were incubated for 5 min with the following mGlu receptor agonists: trans-ACPD (100 μM); a group I agonist, DHPG (100 μM); a group II agonist, DCG (10 μM); a group III agonist, L-AP4 (50 μM). (B and C) Neostriatal slices were incubated with DHPG (100 μM) for the indicated times (B), or the indicated concentrations of DHPG for 5 min (C). (D) Neostriatal slices were incubated for a total of 15 min in the absence or presence of the following mGlu receptor antagonists: a combination of group I mGlu receptor antagonists, an mGlu1 antagonist LY367385 (100 μM) plus an mGlu5 antagonist MPEP (10 μM); LY341495 (1 μM), a group II mGlu receptor antagonist at the concentration used; CPPG (100 μM), a group III mGlu receptor antagonist; and LY341495 (100 μM), a nonselective mGlu receptor antagonist at the concentration used. LY367385 plus MPEP, LY341495, or CPPG were added at 0 min and trans-ACPD (100 μM) was added at 10 min of incubation. *, P < 0.05; **, P < 0.01, compared with control; ††, P < 0.01, compared with trans-ACPD alone. (n = 4–9.)
To identify the particular group of mGlu receptors involved in the regulation of DARPP-32 Thr-34 phosphorylation, slices were treated with trans-ACPD (100 μM) in the presence of selective antagonists for each group (Fig. 2D). Treatment of slices with a combination of group I antagonists, an mGlu1 antagonist LY367385 (100 μM) plus an mGlu5 antagonist MPEP (10 μM), did not affect the basal level of phospho-Thr-34 DARPP-32, but did abolish the stimulatory effect of trans-ACPD on DARPP-32 Thr-34 phosphorylation. Treatment with LY341495 at 1 μM, a selective group II mGlu receptor antagonist at this low concentration (IC50 0.014–0.030 μM in vitro; ref. 5), or CPPG (100 μM), a group III mGlu receptor antagonist, did not affect either the basal level or trans-ACPD-stimulated level of phospho-Thr-34 DARPP-32. The effect of trans-ACPD on DARPP-32 Thr-34 phosphorylation was abolished by treatment with LY341495 at 100 μM, a nonselective antagonist of all groups of mGlu receptors at this high concentration (IC50 6.8–22 μM in vitro; ref. 5). These results with mGlu receptor agonists and antagonists indicate that activation of group I mGlu receptors, but not that of group II or group III mGlu receptors, stimulates the phosphorylation of DARPP-32 at Thr-34 in neostriatal neurons.
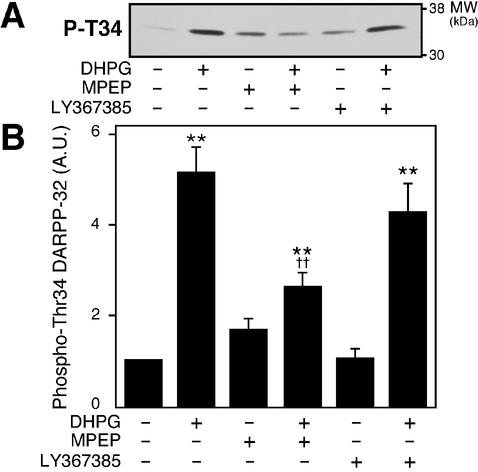
To identify the subtype of group I mGlu receptors involved in this regulation, the effect of DHPG was examined in the presence of the mGlu5 antagonist, MPEP (10 μM), or the mGlu1 antagonist, LY367385 (100 μM) (Fig. 3). The effect of DHPG on DARPP-32 Thr-34 phosphorylation was antagonized by MPEP but not by LY367385, indicating that mGlu5 receptors play a central role in the regulation of DARPP-32 Thr-34 phosphorylation in neostriatal neurons.
Figure 3.
Regulation of DARPP-32 phosphorylation at Thr-34 by mGlu5 receptors. Neostriatal slices were incubated for a total of 12 min in the absence or presence of the mGlu1 antagonist, LY367385 (100 μM), or the mGlu5 antagonist, MPEP (10 μM). LY367385 or MPEP was added at 0 min, and DHPG (100 μM) was added at 10 min of incubation. An immunoblot for detection of phospho-Thr-34 DARPP-32 is shown in A, and the data quantitated are shown in B. **, P < 0.01, compared with control; ††, P < 0.01, compared with DHPG alone. (n = 4–13.)
Interdependence of the Actions of mGlu5 Receptors and A2A Adenosine Receptors in the Phosphorylation of DARPP-32 at Thr-34.
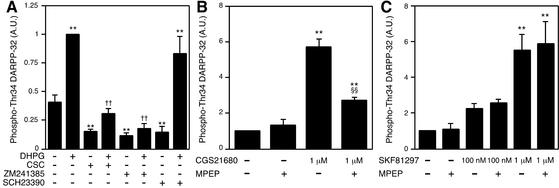
Activation of group I mGlu receptors has been shown to potentiate cAMP formation induced by activation of receptors positively coupled to adenylyl cyclase, such as adenosine A2A receptors and dopamine D1-type receptors, and by forskolin (14–16). The possible role of adenosine A2A and dopamine D1-type receptors in mGlu5 receptor-stimulated DARPP-32 Thr-34 phosphorylation was examined (Fig. 4A). Treatment of slices with adenosine A2A antagonists, 8-(3-chlorostyryl) caffeine (1 μM) or ZM241385 (1 μM), or a dopamine D1 antagonist SCH23390 (1 μM) decreased the basal level of phospho-Thr-34 DARPP-32, suggesting the presence of endogenous adenosine and dopamine in neostriatal slices. 8-(3-Chlorostyryl) caffeine attenuated the ability of DHPG (100 μM) to stimulate DARPP-32 Thr-34 phosphorylation, whereas ZM241385 abolished the effect of DHPG. However, SCH23390 had no effect on the stimulation of DARPP-32 Thr-34 phosphorylation caused by DHPG. These results suggest that the actions of mGlu5 receptors depend on the activation of A2A adenosine receptors by endogenous adenosine.
Figure 4.
Interdependence of mGlu5 receptors and adenosine A2A receptors. (A) Neostriatal slices were incubated for a total of 15 min in the absence or presence of adenosine A2A receptor antagonists, 8-(3-chlorostyryl) caffeine (CSC, 1 μM), ZM241385 (1 μM), or a dopamine D1 receptor antagonist, SCH23390 (1 μM). 8-(3-Chlorostyryl) caffeine, ZM241385, or SCH23390 was added at 0 min, and DHPG (100 μM) was added at 10 min of incubation. (B) Neostriatal slices were pretreated with adenosine deaminase (10 μg/ml) for 60 min to decrease the content of endogenous adenosine. Neostriatal slices were incubated for a total of 15 min in the absence or presence of the mGlu5 antagonist, MPEP (10 μM), in the continued presence of adenosine deaminase. MPEP was added at 0 min, and an adenosine A2A agonist, CGS21680 (1 μM), was added at 10 min of incubation. (C) Neostriatal slices were incubated for a total of 15 min in the absence or presence of MPEP (10 μM). MPEP was added at 0 min, and a dopamine D1 agonist, SKF81297 (100 nM or 1 μM), was added at 10 min of incubation. **, P < 0.01, compared with control; ††, P < 0.01, compared with DHPG alone; §§, P < 0.01, compared with CGS21680 alone. (n = 5–7.)
Next, we examined whether the effects of adenosine A2A receptor activation might depend on tonic activation of mGlu5 receptors by endogenous glutamate (Fig. 4B). For these studies, adenosine deaminase was added throughout the experiments to remove the endogenous adenosine. Treatment of slices with an adenosine A2A agonist, CGS21680 (1 μM), increased the level of phospho-Thr-34 DARPP-32 by ≈5-fold. The mGlu5 antagonist, MPEP (10 μM), had no effect on the basal level of phospho-Thr-34 DARPP-32, but attenuated the stimulatory effect of CGS21680. Thus, the actions of mGlu5 receptors and adenosine A2A receptors are interdependent and require endogenous adenosine for the actions of the mGlu5 receptor agonist and endogenous glutamate for the actions of the adenosine A2A receptor agonist.
We examined whether dopamine D1 receptors require coactivation of mGlu5 receptors by endogenous glutamate (Fig. 4C). Treatment of slices with MPEP did not affect the basal or dopamine D1 agonist (100 nM and 1 μM) SKF81297-stimulated levels of phospho-Thr-34 DARPP-32. The results (Fig. 4 A and C) suggest that mGlu5 receptors do not require coactivation of dopamine D1-type receptors by endogenous dopamine, and that dopamine D1-type receptors do not require coactivation of mGlu5 receptors by endogenous glutamate, to exert their effects on DARPP-32 Thr-34 phosphorylation.
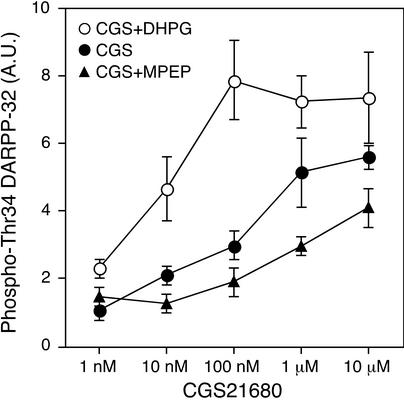
To investigate further the interaction between adenosine A2A receptors and mGlu5 receptors, the effect of different concentrations of CGS21680 on DARPP-32 Thr-34 phosphorylation was examined in the presence of DHPG or MPEP (Fig. 5). In these studies, adenosine deaminase was present throughout the experiments. Treatment with CGS21680 alone increased the level of phospho-Thr-34 DARPP-32 maximally at a concentration of 10 μM, with a half-maximal effect at ≈200 nM. When slices were coincubated with CGS21680 and DHPG, DHPG induced a leftward shift of the dose–response curve for CGS21680, with a half-maximal effect at ≈10 nM. In contrast, when slices were coincubated with CGS21680 and MPEP, MPEP induced a rightward shift of the dose–response curve for CGS21680, with a half-maximal effect at ≈1 μM. The maximal increase in DARPP-32 Thr-34 phosphorylation in the presence of CGS21680 (10 μM) and DHPG was significantly higher than that in the presence of CGS21680 (10 μM) and MPEP (P < 0.05). These results indicate that activation of mGlu5 receptors either by endogenous glutamate or an exogenous agonist results in the potentiation of the effects of adenosine A2A receptors on DARPP-32 phosphorylation at Thr-34.
Figure 5.
Activation of mGlu5 receptor potentiates the effect of adenosine A2A receptors on DARPP-32 phosphorylation at Thr-34. Neostriatal slices were pretreated with adenosine deaminase (10 μg/ml) for 60 min. Neostriatal slices were incubated with CGS21680 alone for 5 min (●), CGS21680 plus DHPG (100 μM) for 5 min (○), or CGS21680 plus MPEP (10 μM) (▴) in the continued presence of adenosine deaminase. In experiments with CGS21680 plus MPEP, neostriatal slices were incubated for a total of 15 min in the presence of MPEP (10 μM). MPEP was added at 0 min, and CGS21680 was added at 10 min of incubation. Data represent means ± SEM for five to seven experiments.
Activation of Intracellular Signaling by mGlu5 Receptors Involves Postsynaptic Potentiation of cAMP Formation.
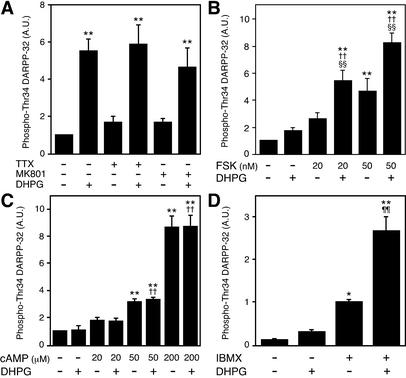
To examine further the mechanism by which activation of mGlu5 receptors increases DARPP-32 Thr-34 phosphorylation, slices were pretreated with TTX (1 μM) or an N-methyl-d-aspartate (NMDA) receptor antagonist MK801 (100 μM; Fig. 6A). Pretreatment with TTX or MK801 did not attenuate the effect of DHPG on DARPP-32 Thr-34 phosphorylation, suggesting that DHPG is acting at postsynaptic medium spiny neurons using a mechanism other than NMDA-receptor activation.
Figure 6.
DHPG stimulates DARPP-32 phosphorylation at Thr-34 by potentiating the formation of cAMP. (A) Neostriatal slices were incubated for a total of 15 min in the absence or presence of TTX (1 μM) or an NMDA receptor antagonist, MK801 (100 μM). TTX or MK801 was added at 0 min, and DHPG (100 μM) was added at 10 min of incubation. (B and C) Neostriatal slices were incubated for a total of 15 min in the presence of the adenosine A2A receptor antagonist, ZM241385 (1 μM), to minimize the contribution of endogenous adenosine. ZM241385 was added at 0 min, and forskolin (FSK; 20 nM or 50 nM) (B), 8-bromo-cAMP (cAMP; 20, 50, or 200 μM) (C), and/or DHPG (100 μM) at 10 min of incubation. (D) Neostriatal slices were incubated for a total of 15 min in the absence or presence of a phosphodiesterase inhibitor, IBMX (100 μM). IBMX was added at 0 min, and DHPG (100 μM) was added at 10 min of incubation. *, P < 0.05; **, P < 0.01, compared with control; ††, P < 0.01, compared with DHPG alone; §§, P < 0.01, compared with forskolin alone; ¶¶, P < 0.01, compared with IBMX alone. (n = 5–7.)
To determine whether activation of mGlu5 receptors stimulates PKA activity by increasing the efficacy of cAMP formation, the effect of DHPG on DARPP-32 Thr-34 phosphorylation was examined in the presence of forskolin (Fig. 6B) or the cAMP analogue, 8-bromo-cAMP (Fig. 6C). Treatment of slices with DHPG (100 μM) alone did not affect the level of phospho-Thr-34 DARPP-32, because slices were pretreated with the adenosine A2A receptor antagonist, ZM241385. DHPG enhanced the effect of forskolin, but not that of 8-bromo-cAMP, on DARPP-32 Thr-34 phosphorylation. These results suggest that activation of mGlu5 receptors stimulates DARPP-32 phosphorylation by increasing cAMP content, not by regulating the efficacy of activation of PKA by cAMP. To examine whether the effect of mGlu5 receptors on DARPP-32 phosphorylation is mediated through the activation of cAMP formation or the inhibition of cAMP degradation, the effect of DHPG (100 μM) was examined in the presence of a phosphodiesterase inhibitor, IBMX (100 μM). Treatment with IBMX alone increased the level of phospho-Thr-34 DARPP-32 by ≈13-fold. DHPG increased DARPP-32 Thr-34 phosphorylation ≈34-fold over control values and 2.7-fold over IBMX-stimulated values. Therefore, the ability of mGlu5 receptors to potentiate adenosine A2A receptor-mediated DARPP-32 Thr-34 phosphorylation is likely attributable to an effect on cAMP formation.
Activation of Intracellular Signaling by mGlu5 Receptors Involves the ERK Pathway.
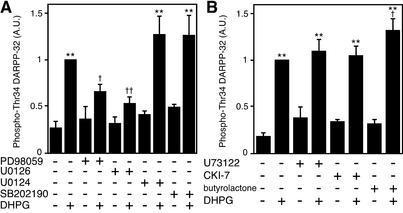
To elucidate further the intracellular signaling mechanism by which mGlu5 receptors regulate DARPP-32 Thr-34 phosphorylation, the effect of DHPG was examined in the presence of specific inhibitors of ERK or p38 (Fig. 7A). Treatment of slices with an ERK inhibitor, PD98059 (50 μM), did not affect the basal level of phospho-Thr-34 DARPP-32. However, PD98059 significantly attenuated the stimulatory effect of DHPG on DARPP-32 Thr-34 phosphorylation. Similarly, treatment of slices with a structurally unrelated ERK inhibitor U0126 (40 μM), but not with its inactive analogue U0124 (40 μM), attenuated the stimulatory effect of DHPG. Treatment of slices with a p38 inhibitor, SB202190 (10 μM), did not affect the basal level of phospho-Thr-34 DARPP-32 or the increase in DARPP-32 Thr-34 phosphorylation caused by DHPG. In control experiments, treatment of slices with DHPG (100 μM) for 2 min slightly but significantly increased the level of phospho-p42 (1.39 ± 0.13-fold, n = 4). DHPG also slightly increased the level of phospho-p44, but this effect was not statistically significant.
Figure 7.
ERK inhibitors attenuate the effect of DHPG on DARPP-32 phosphorylation at Thr-34. (A) Neostriatal slices were incubated in the presence of ERK inhibitors PD98059 (50 μM) or U0126 (40 μM), an inactive analogue of ERK inhibitor U0124 (40 μM), or a p38 inhibitor SB202190 (10 μM) for 60 min, followed by 2 min in the absence or presence of DHPG (100 μM). (B) Neostriatal slices were incubated in the presence of a PLC inhibitor U73122 (25 μM), a CK1 inhibitor CKI-7 (100 μM), or a Cdk5 inhibitor butyrolactone (10 μM) for 60 min, followed by 2 min in the absence or presence of DHPG (100 μM). **, P < 0.01, compared with control; †, P < 0.05; ††, P < 0.01, compared with DHPG alone. (n = 4–9.)
We have recently reported that, by activating casein kinase 1 (CK1) in a PLC-dependent manner, group I mGlu receptors cause an increase in the phosphorylation of DARPP-32 at Ser-137 (17, 18). Moreover, by activating cyclin-dependent kinase 5 (Cdk5), this activation of CK1 causes an increase in the phosphorylation of DARPP-32 at Thr-75 (17). A possible role for PLC, CK1, and Cdk5 in the regulation of DARPP-32 Thr-34 phosphorylation by mGlu5 receptors was examined by using specific inhibitors (Fig. 7B). Treatment of slices with PLC inhibitor U73122 (25 μM), CK1 inhibitor CKI-7 (25 μM), or Cdk5 inhibitor butyrolactone (10 μM) did not affect the basal level of phospho-Thr-34 DARPP-32 or diminish the increase in DARPP-32 Thr-34 phosphorylation caused by DHPG.
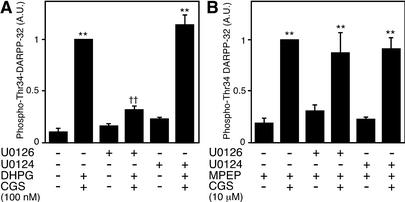
To elucidate the role of ERK in the regulation of DARPP-32 Thr-34 phosphorylation by mGlu5 and adenosine A2A receptors, the effect of ERK inhibitor U0126 was examined under two conditions (see Fig. 5): (i) CGS21680 (100 nM) plus DHPG (100 μM), in which condition the small effect of 100 nM CGS21680 alone is greatly potentiated by 100 μM DHPG, and (ii) CGS21680 (10 μM) plus MPEP (10 μM), in which condition the effect of CGS21680 (10 μM) is largely independent of the mGlu5 receptor pathway. Pretreatment with U0126 (40 μM), but not with its inactive analogue U0124 (40 μM), attenuated the effect of CGS21680 (100 nM) plus DHPG on DARPP-32 Thr-34 phosphorylation (Fig. 8A). In contrast, U0126 as well as U0124 did not affect the increase in DARPP-32 Thr-34 phosphorylation induced by CGS21680 (10 μM) plus MPEP (Fig. 8B). These results indicate that ERK is involved in the regulation by the mGlu5 receptor signaling pathway of the adenosine A2A receptor signaling pathway, not in the adenosine A2A receptor signaling pathway per se.
Figure 8.
ERK is involved in mGlu5 receptor signaling but not in adenosine A2A receptor signaling. Neostriatal slices were preincubated with an ERK inhibitor, U0126 (40 μM), or an inactive analogue of U0126, U0124 (40 μM), for 60 min. (A) Neostriatal slices were incubated with CGS21680 (100 nM) plus DHPG (100 μM) for 5 min. (B) Neostriatal slices were incubated for a total of 15 min in the presence of MPEP (10 μM). MPEP was added at 0 min, and CGS21680 (10 μM) was added at 10 min of incubation. **, P < 0.01, compared with control; ††, P < 0.01, compared with DHPG alone. (n = 4–7.)
Discussion
In this study, we demonstrated that activation of metabotropic glutamate receptors, specifically group I mGlu5 receptors, increases the state of phosphorylation of DARPP-32 at Thr-34 (the PKA-site). This action of mGlu5 receptors requires activation of adenosine A2A receptors by endogenous adenosine, and the action of adenosine A2A receptors requires activation of mGlu5 receptors by endogenous glutamate to increase DARPP-32 Thr-34 phosphorylation. Furthermore, coactivation of mGlu5 and adenosine A2A receptors by their agonists synergistically stimulates the phosphorylation of DARPP-32 at Thr-34, suggesting an interdependence of mGlu5 and adenosine A2A receptor signaling. In contrast, mGlu5 receptors do not require activation of dopamine D1-type receptors by endogenous dopamine, and dopamine D1-type receptors do not require activation of mGlu5 receptors by endogenous glutamate, suggesting that mGlu5 and dopamine D1-type receptors can function independently.
The molecular basis for the difference in the pattern of interdependence of mGlu5 and A2A receptors compared with that of mGlu5 and D1-type receptors is not clear at the present time. This difference might be explained by the distinct expression patterns of adenosine A2A receptors and dopamine D1-type receptors in neostriatum (19, 20). Adenosine A2A receptors are predominantly expressed in striatopallidal medium spiny neurons, where dopamine D2-type receptors are also largely expressed (20). In contrast, dopamine D1-type receptors are predominantly expressed in striatonigral medium spiny neurons. The results obtained in this study suggest that functional coupling of mGlu5 receptors is likely to be different in the two types of medium spiny neurons. As a result, the availability of Golfα, which couples dopamine D1-type receptors and adenosine A2A receptors to adenylyl cyclase in neostriatum (21, 22), may be different in the two types of medium spiny neuron. Alternatively, the actions of mGlu5 receptors may be regulated in different ways by other G protein-coupled receptors, such as dopamine D2-type receptors and opioid receptors, within the two types of medium-sized spiny neurons (23, 24).
Activation of mGlu receptors has been found to activate the classical mitogen-activated protein kinase (ERK) pathway (7), as well as the p38 pathway (8). The effect of DHPG on DARPP-32 Thr-34 phosphorylation was attenuated by the ERK inhibitors, PD98059 and U0126, but not by the p38 inhibitor, SB202190, suggesting that activation of mGlu5 receptors stimulates DARPP-32 Thr-34 phosphorylation via an ERK-dependent pathway. Furthermore, ERK was involved in the regulation by mGlu5 receptor signaling of adenosine A2A receptor signaling, not in adenosine A2A receptor signaling per se. It has been reported that ERK signaling is more active in striatopallidal medium spiny neurons than in striatonigral medium spiny neurons (25), which might explain why mGlu5 and adenosine A2A receptors, but not mGlu5 and dopamine D1-type receptors, are interdependent. Activation of ERK might occur through a mechanism involving Ca2+/calmodulin-dependent kinase II (CaMKII; ref. 26) or Src-family tyrosine kinase (6). Molecular mechanisms by which ERK is activated by mGlu5 receptors and by which ERK stimulates cAMP formation coupled to adenosine A2A receptors remain to be clarified.
We have recently reported that group I mGlu receptors activate CK1 and Cdk5 in a PLC-dependent manner, leading to the enhanced phosphorylation of DARPP-32 at Ser-137 (the CK1-site) and Thr-75 (the Cdk5-site; refs. 17 and 18). Interestingly, the effect of DHPG on DARPP-32 Thr-34 phosphorylation was not antagonized by inhibitors of PLC, CK1, or Cdk5, indicating that the effect of mGlu5 receptors on Thr-34 is not mediated through the activation of PLC/CK1/Cdk5 signaling. Together with our previous studies (17, 18), the present results indicate that group I mGlu receptors activate both ERK and PLC/CK1/Cdk5 signaling pathways in neostriatal neurons, and that ERK and PLC/CK1/Cdk5 selectively regulate different sites of DARPP-32 phosphorylation.
In striatum, mGlu receptors have been shown to contribute to a variety of processes including neural plasticity (27), motor behavior (28), and glutamate excitotoxicity (29). Behavioral effects observed in response to perturbing signaling through the mGlu5 receptor pathway can be explained by the results of the present study. For instance, intrastriatal injection of nonselective or group I mGlu receptor agonists induced delayed contralateral turning behavior, which is possibly mediated by the potentiation of action of adenosine A2A receptors in striatopallidal neurons followed by the disinhibition of subthalamic nucleus neurons, with the consequent increase in output of dopaminergic nigrostriatal neurons (28). In 6-hydroxydopamine-lesioned rats, the intracerebroventricular injection of an mGlu5 agonist counteracted the contralateral turning behavior elicited by a dopamine D2 agonist, which could be explained by the potentiation of reciprocal interaction between adenosine A2A and dopamine D2 receptors (30). Recently, Chiamulera et al. (11) reported that mice lacking the mGlu5 receptor do not self-administer cocaine and show no increased locomotor activity after cocaine treatment, despite the increase in dopamine release in nucleus accumbens. In another study, methamphetamine-induced neurotoxicity was found to be antagonized by mGlu5 antagonists (12). These latter results suggest important roles for activation of mGlu5 receptors in the actions of cocaine and methamphetamine. In our previous studies, it was found that cocaine-induced locomotor activity and methamphetamine-induced neurotoxicity are both attenuated in mice lacking DARPP-32 (1). The results of the present study suggest that the dependence of the actions of cocaine and methamphetamine on mGlu5 agonists may be attributable in part to the synergistic interactions of mGlu5 and A2A adenosine receptors and the consequent phosphorylation of DARPP-32 at Thr-34 in neostriatal neurons.
Acknowledgments
We thank Y. Terasaki for excellent technical assistance. This research was supported by grants-in-aid for scientific research from the Japan Society for the Promotion of Science (to A.N.), Grant-in-Aid for Scientific Research on Priority Area from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to A.N.), and U.S. Public Health Service Grants MH-40899 and DA10044 (to A.C.N and P.G.).
Abbreviations
- DARPP-32
dopamine- and cAMP-regulated phosphoprotein of Mr 32,000
- PKA
cAMP-dependent protein kinase
- mGlu
metabotropic glutamate
- PLC
phospholipase C
- IBMX
3-isobutyl-1-methylxanthine
- trans-ACPD
(±)-1-aminocyclopentane-trans-1,3-dicarboxylic acid
- DHPG
(RS)-3,5-dihydroxyphenylglycine
- MPEP
2-methyl-6-(phenylethynyl)pyridine
- ERK
extracellular signal-regulated kinase
- CK1
casein kinase 1
- Cdk5
cyclin-dependent kinase 5
References
- 1.Fienberg A A, Hiroi N, Mermelstein P, Song W-J, Snyder G L, Nishi A, Cheramy A, O'Callaghan J P, Miller D, Cole D, et al. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 2.Greengard P, Allen P B, Nairn A C. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 3.Nishi A, Snyder G L, Greengard P. J Neurosci. 1997;17:8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Svenningsson P, Lindskog M, Rognoni F, Fredholm B B, Greengard P, Fisone G. Neuroscience. 1998;84:223–228. doi: 10.1016/s0306-4522(97)00510-1. [DOI] [PubMed] [Google Scholar]
- 5.Schoepp D D, Jane D E, Monn J A. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- 6.Hermans E, Challiss R A. Biochem J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peavy R D, Conn P J. J Neurochem. 1998;71:603–612. doi: 10.1046/j.1471-4159.1998.71020603.x. [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee P K, DeCoster M A, Campbell F Z, Davis R J, Bazan N G. J Biol Chem. 1999;274:6493–6498. doi: 10.1074/jbc.274.10.6493. [DOI] [PubMed] [Google Scholar]
- 9.Nakanishi S. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- 10.Nicoletti F, Bruno V, Copani A, Casabona G, Knopfel T. Trends Neurosci. 1996;19:267–271. doi: 10.1016/S0166-2236(96)20019-0. [DOI] [PubMed] [Google Scholar]
- 11.Chiamulera C, Epping-Jordan M P, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 12.Battaglia G, Fornai F, Busceti C L, Aloisi G, Cerrito F, De Blasi A, Melchiorri D, Nicoletti F. J Neurosci. 2002;22:2135–2141. doi: 10.1523/JNEUROSCI.22-06-02135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishi A, Bibb J A, Matsuyama S, Hamada M, Higashi H, Nairn A C, Greengard P. J Neurochem. 2002;81:832–841. doi: 10.1046/j.1471-4159.2002.00876.x. [DOI] [PubMed] [Google Scholar]
- 14.Cartmell J, Schaffhauser H, Wichmann J, Mutel V. Br J Pharmacol. 1997;121:1263–1268. doi: 10.1038/sj.bjp.0701266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balazs R, Miller S, Chun Y, O'Toole J, Cotman C W. J Neurochem. 1998;70:2446–2458. doi: 10.1046/j.1471-4159.1998.70062446.x. [DOI] [PubMed] [Google Scholar]
- 16.Paolillo M, Montecucco A, Zanassi P, Schinelli S. Eur J Neurosci. 1998;10:1937–1945. doi: 10.1046/j.1460-9568.1998.00203.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu F, Ma X H, Ule J, Bibb J A, Nishi A, DeMaggio A J, Yan Z, Nairn A C, Greengard P. Proc Natl Acad Sci USA. 2001;98:11062–11068. doi: 10.1073/pnas.191353898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F, Virshup D M, Nairn A C, Greengard P. J Biol Chem. 2002;277:45393–45399. doi: 10.1074/jbc.M204499200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surmeier D J, Song W J, Yan Z. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svenningsson P, Le Moine C, Fisone G, Fredholm B B. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 21.Herve D, Levi-Strauss M, Marey-Semper I, Verney C, Tassin J P, Glowinski J, Girault J-A. J Neurosci. 1993;13:2237–2248. doi: 10.1523/JNEUROSCI.13-05-02237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kull B, Svenningsson P, Fredholm B B. Mol Pharmacol. 2000;58:771–777. doi: 10.1124/mol.58.4.771. [DOI] [PubMed] [Google Scholar]
- 23.Yao L, Arolfo M P, Dohrman D P, Jiang Z, Fan P, Fuchs S, Janak P H, Gordon A S, Diamond I. Cell. 2002;109:733–743. doi: 10.1016/s0092-8674(02)00763-8. [DOI] [PubMed] [Google Scholar]
- 24.Lindskog M, Svenningsson P, Fredholm B, Greengard P, Fisone G. Eur J Neurosci. 1999;11:2182–2186. doi: 10.1046/j.1460-9568.1999.00597.x. [DOI] [PubMed] [Google Scholar]
- 25.Gerfen C R, Miyachi S, Paletzki R, Brown P. J Neurosci. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choe E S, Wang J Q. Neurosci Lett. 2001;313:129–132. doi: 10.1016/s0304-3940(01)02258-3. [DOI] [PubMed] [Google Scholar]
- 27.Pisani A, Calabresi P, Centonze D, Bernardi G. Br J Pharmacol. 1997;120:1007–1014. doi: 10.1038/sj.bjp.0700999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kearney J A, Frey K A, Albin R L. J Neurosci. 1997;17:4415–4425. doi: 10.1523/JNEUROSCI.17-11-04415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calabresi P, Centonze D, Pisani A, Bernardi G. Exp Neurol. 1999;158:97–108. doi: 10.1006/exnr.1999.7092. [DOI] [PubMed] [Google Scholar]
- 30.Popoli P, Pezzola A, Torvinen M, Reggio R, Pintor A, Scarchilli L, Fuxe K, Ferre S. Neuropsychopharmacology. 2001;25:505–513. doi: 10.1016/S0893-133X(01)00256-1. [DOI] [PubMed] [Google Scholar]