Abstract
Two systems for grading soft tissue sarcoma are widely used currently: the National Cancer Institute (NCI) and the Fédération Nationale des Centers de Lutte Contre le Cancer (FNCLCC) systems. Both were developed using cohorts of predominantly adult patients. The Pediatric Oncology Group (POG) system, based on the NCI system, was adapted for grading pediatric non-rhabdomyosarcoma soft tissue sarcoma (NRSTS). The applicability and prognostic utility of the FNCLCC system in pediatric NRSTS has not been assessed or compared to the POG system. Tumors from 130 patients with malignant NRSTS enrolled on three completed multi-institutional clinical trials were assessed. Of 130 tumors, 102 (78%) were localized and 28 (22%) metastatic. Of the localized tumors, 55/102 (54%) were >5cm. The estimated 5-year EFS for the entire group was 47%. As expected, stage and tumor size were predictive of EFS (p<0.001). Both systems were predictive of 5-year event-free survival (EFS) (POG p=0.0095 and FNCLCC p=0.0075). Patients whose tumors received discrepant grades (POG-G3 vs. FNCLCC-G2/G1) (n=44) had an intermediate outcome between those with concordant (G3 (n=44) or G1/G2 (n=42)) grades on both systems (p=0.0018). By multivariate analysis, the mitotic index was predictive of EFS using a cutoff of 10 mitotic figures per 10 high power fields (p<0.001). In conclusion, both the FNCLCC and POG systems provide an adequate prognostic measure of outcome for pediatric NRSTS; albeit, a sizeable subset of cases with apparently intermediate prognosis was graded differently by the two systems. The mitotic index appears to be a key parameter in grading pediatric NRSTS.
Keywords: soft tissue sarcoma, tumor grading, histopathology, childhood cancer, pediatric oncology
INTRODUCTION
The term non-rhabdomyosarcoma soft tissue sarcoma (NRSTS) is commonly utilized to segregate rhabdomyosarcoma from other soft tissue sarcomas arising in children and adolescents, in view of the divergent clinical management of patients in these two categories. Currently, the approach to managing patients with NRSTS takes into consideration the factors that most influence outcome: extent of disease, extent of resection, tumor size, and histologic grade.1
The prognostic role of tumor grading is well established for soft tissue sarcomas arising in both adult2-7 and pediatric8-10 patients. It is endorsed by the World Health Organization classification11 and incorporated into the American Joint Committee on Cancer (AJCC) cancer staging system12. Several systems have been proposed for grading soft tissue sarcomas. The two most utilized systems at present include the National Cancer Institute (NCI) grading system and the French Federation of Cancer Centers (Fédération nationale des centres de lutte contre le cancer; FNCLCC) system.13, 14 Both systems were devised using data derived predominantly from cohorts of adult patients and their prognostic utility has been established primarily in this patient group.2, 13-16 They are similar in terms of their prognostic utility, albeit the FNCLCC system has been shown to be better at predicting distant metastasis development and tumor mortality in adult patients.15
Parham et al proposed a modification of the NCI system for NRSTS arising in children.17 This grading system, commonly referred to as the Pediatric Oncology Group (POG) grading system, was demonstrated to be predictive of clinical outcome.17 Despite the demonstrated prognostic value of the POG grading system, no study has compared its prognostic utility to that of the FNCLCC grading system in children and adolescents with NRSTS. In addition, the applicability of the FNCLCC grading system for pediatric NRSTS has not been evaluated.
In this study, we assess the applicability of the FNCLCC grading system in pediatric NRSTS and compare the prognostic utility of the POG and FNCLCC grading systems in NRSTS arising in this age group. Furthermore, we ask whether the commonly employed histologic variables used for grading soft tissue sarcomas in adults are applicable in NRSTS arising in the pediatric population.
MATERIALS AND METHODS
Study group
This study was approved by the Institutional Review Boards of St. Jude Children’s Research Hospital, University of Arkansas, and University of Utah. The study group included tumor samples from patients with soft tissue sarcomas other than rhabdomyosarcoma enrolled prospectively on three clinical trials (#8653, 8654, and 9553) conducted under the auspices of the Pediatric Oncology Group (POG), a multi-institutional consortium of pediatric cancer centers which is now part of the Children’s Oncology Group (COG). All tumor samples had been worked up at the time of patient enrollment, and data from these three clinical trials have been previously reported.9, 18, 19 The POG-8653 trial9 (June 1986 - May 1992), which included 99 patients (81 eligible), was a randomized comparison of adjuvant chemotherapy (vincristine 1.5 mg/m2, doxorubicin 60 mg/m2, and cyclophosphamide 750 mg/m2 intravenously alternating every 3 weeks with vincristine 1.5 mg/m2, dactinomycin 1.25 mg/m2, and cyclophosphamide 750 mg/m2 intravenously for 52 weeks) or observation alone for patients with tumors locally controlled with surgery (clinical group I) or surgery and radiotherapy (clinical group II/III). The POG-8654 trial19 (June 1986 - March 1994) randomly assigned 75 patients with gross residual or metastatic NRSTS to either VACA chemotherapy (vincristine 1.5 mg/m2, dactinomycin 1 mg/m2, cyclophosphamide 750 mg/m2 intravenously alternating every 3 weeks with vincristine 1.5 mg/m2, doxorubicin 60 mg/m2, cyclophosphamide 750 mg/m2 intravenously for 37 weeks, then vincristine/dactinomycin/cyclophosphamide alone every 3 weeks to week 78) or VACAD chemotherapy (VACA chemotherapy with dacarbazine 500 mg/m2 given with cycles of vincristine/doxorubicin/cyclophosphamide). Radiotherapy was delivered to the primary tumor and sites of metastases at week 6, and second-look surgery was planned for 6 to 12 weeks after completion of radiotherapy. The POG-9553 trial18 (September 1996 - June 2000) was a phase II evaluation of neoadjuvant vincristine (1.5 mg/m2 weekly for 13 doses), ifosfamide (9 g/m2/cycle every 3 weeks for 7 cycles), and doxorubicin (60 mg/m2/cycle every 3 weeks for 6 cycles) administered to 43 patients (39 eligible) with gross residual or metastatic NRSTS. Surgical tumor removal was performed as soon as feasible. Primary site radiotherapy was delivered along with whole lung irradiation for those with pulmonary metastases.
Of 217 patients enrolled on these three POG trials, 185 (85%) had available representative hematoxylin-and-eosin-stained slides. These 185 cases were reviewed, and 55 (29%) were excluded either because the available material was suboptimal for microscopic evaluation (n=8) or because the histologic type does not meet the inclusion criteria (see below) (n=47). No outcome data was available on 3 patients. The remaining 130 cases, each from a distinct patient, comprised the study group.
The study group consisted of 74 (57%) male patients and 56 (43%) female patients, with a median age of 11.5 years (range, < 1 to 18 years). Patients were assigned a clinical group designation based on the surgicopathologic staging system developed by the Intergroup Rhabdomyosarcoma Study Group.20 There were 57 patients with clinical group I disease (localized tumor, excised with negative margins and no lymph node involvement), 5 with clinical group II disease (localized tumor, excised with microscopically positive margins and/or lymph node involvement), 40 with clinical group III disease (localized tumor with unresectable or gross residual disease), and 28 with clinical group IV disease (distant metastasis detected at diagnosis). For outcome analysis, five patients with localized resectable tumor and microscopically positive margins were combined with those having localized resectable tumors and negative margins. The median follow up for patients without tumor recurrence was 9.8 years.
Histologic evaluation and tumor grading
Available histologic sections from paraffin-embedded tissues were examined microscopically by three pathologists (DMP, CMC, and JDK). The primary goal of microscopic examination was to assess tumor grade. Corresponding pathology reports from centralized review at the time of clinical trial inclusion were occasionally drawn upon when necessary (data not tallied) to obtain results of pertinent ancillary studies, such as immunohistochemistry for rhabdomyosarcoma-specific markers and molecular diagnostic testing. A working diagnosis was determined for the purposes of this study by consensus among the reviewing pathologists and summarized in Table 1. Cases on which the available slides and/or tissue blocks were qualitatively inadequate or quantitatively insufficient to assess tumor grade were excluded. On cases with adequate histologic material, exclusion criteria included the following: non-malignant neoplasm, rhabdomyosarcoma, small round cell tumors (Ewing sarcoma family tumors, desmoplastic small round cell tumor, neuroblastoma, or Wilms tumor), and tumors of non-soft tissue origin (e.g. gastrointestinal stromal tumor). Tumors that resembled infantile fibrosarcoma histologically but where the patient age at diagnosis was not known were excluded from this study. To further increase inclusion stringency, consensus was not required for cases to be eliminated from this study.
Table 1.
Histologic diagnoses of tumors in study.
| Diagnosis | Number (n) | Frequency (%) |
|---|---|---|
| Synovial sarcoma | 57 | 43.8 |
| Monophasic synovial sarcoma | (26) | n/a |
| Biphasic synovial sarcoma | (16) | n/a |
| Synovial sarcoma, not otherwise specified | (15) | n/a |
| Sarcoma, not otherwise specified | 15 | 11.5 |
| Malignant peripheral nerve sheath tumor | 15 | 11.5 |
| Alveolar soft part sarcoma | 10 | 7.7 |
| Epithelioid sarcoma, distal type | 8 | 6.1 |
| Epithelioid sarcoma, proximal type | 1 | 0.8 |
| Undifferentiated pleomorphic sarcoma | 6 | 4.6 |
| Clear cell sarcoma | 5 | 3.8 |
| Angiosarcoma | 3 | 2.3 |
| Infantile fibrosarcoma | 2 | 1.5 |
| Extraskeletal myxoid chondrosarcoma | 1 | 0.8 |
| Leiomyosarcoma | 1 | 0.8 |
| Liposarcoma, myxoid | 1 | 0.8 |
| Liposarcoma, dedifferentiated | 1 | 0.8 |
| Low grade fibromyxoid sarcoma | 1 | 0.8 |
| Mesenchymal chondrosarcoma | 1 | 0.8 |
| Myofibroblastic sarcoma | 1 | 0.8 |
| Plexiform fibrohistiocytic tumor | 1 | 0.8 |
The mean number of slides reviewed was 4.8 (median 3; range 1-25). The following grading parameters were assessed: cellularity, tumor necrosis, mitotic index, atypical mitotic figures, and nuclear grade. Cellularity was estimated as low, medium, or high, primarily by evaluating the ratio of neoplastic cells to background stroma. The presence and extent of tumor necrosis were assessed on the available glass slides. The mitotic index was determined by counting the number of mitotic figures per 10 high-power microscopic fields (400x magnification; microscopic field area 0.237 mm2) in a minimum of 10 fields (range, 10-50 fields). The presence of atypical mitotic figures, defined as enlarged, hyperchromatic, and/or tripolar or multipolar mitoses, was also noted. Nuclear grade was assigned a score of 1 to 4 (1=no atypia; 2=mild atypia; 3=moderate atypia; 4=severe atypia), per criteria outlined by Fuhrman et al.21
The POG grade was originally determined on the basis of histologic type, necrosis, and mitotic index, as previously described.17 (Table 2) Briefly, tumors that did not qualify for POG grade 1 or grade 3 on the basis of histologic type were assigned POG grade 2 if necrosis was ≤15% of the sampled surface area and the mitotic index was ≤5 mitotic figures per 10 high-power fields. If necrosis was >15% or the mitotic index was >5, a tumor was assigned POG grade 3. It should be noted that POG grade 1 included angiomatoid fibrous histiocytoma (formerly angiomatoid malignant fibrous histiocytoma) and so-called deep-seated dermatofibrosarcoma protuberans, both of which are currently recognized as non-malignant neoplasms and were, thus, excluded from this study.
Table 2.
Pediatric Oncology Group (POG) grading system*
Grade 1
|
Grade 2
|
Grade 3
|
From: Parham DM, Webber BL, Jenkins JJ, 3rd, Cantor AB, Maurer HM. Nonrhabdomyosarcomatous soft tissue sarcomas of childhood: formulation of a simplified system for grading. Mod Pathol. 1995;8:705-10
These designations are currently obsolete but were accepted at the time the grading system was proposed. See Discussion for further details.
The FNCLCC grade was determined on the basis of tumor necrosis, mitotic count, and differentiation score, as defined in the original publications.14 (Table 3) These parameters are assigned a score of 1 to 3 for differentiation and mitotic index and a score of 0 to 2 for necrosis. A 3-grade system is obtained by adding the scores obtained for each of these three parameters. Grade 1 is defined as a score sum of 2 or 3; grade 2 as a score sum of 4 or 5; and grade 3 as a score sum of 6 to 8.
Table 3.
Fédération nationale des centres de lutte contre le cancer (FNCLCC) grading system
| Tumor differentiation | |
| Score 1: | Sarcomas closely resembling normal adult mesenchymal tissue (e.g. well-differentiated liposarcoma) |
| Score 2: | Sarcomas for which histologic typing is certain (e.g. myxoid liposarcoma) |
| Score 3: | Embryonal and undifferentiated sarcomas, sarcomas of doubtful type, synovial sarcomas |
| Mitotic count | |
| Score 1: | 0–9 mitoses per 10 HPF |
| Score 2: | 10–19 mitoses per 10 HPF |
| Score 3: | ≥20 mitoses per 10 HPF |
| Tumor necrosis | |
| Score 0: | No necrosis |
| Score 1: | <50% tumor necrosis |
| Score 2: | ≥50% tumor necrosis |
| Histologic grade | |
| Grade 1: | Total score 2, 3 |
| Grade 2: | Total score 4, 5 |
| Grade 3: | Total score 6, 7, 8 |
Statistical analysis
Event-free survival (EFS) (time to the first occurrence of recurrent disease or death from any cause) was calculated using the method of Kaplan and Meier. Time-to-event distributions for different patient subsets were compared using the log-rank test. The independent contribution of factors to the prediction of outcome was assessed using the Cox proportional hazards model.
RESULTS
Of the 130 tumors included in this study, 102 (78%) were from patients who presented with localized disease, and 28 (22%) were from patients who had metastatic disease at presentation. Of the localized tumors, 47 (46%) were ≤5cm in greatest dimension, and 55 (54%) were >5cm in greatest dimension. The estimated 5-year EFS for the entire group was 47%.
Correlation between tumor grade and clinical outcome
Prognostic factors known to be predictive of outcome in NRSTS were confirmed to correlate with clinical outcome in our study group. As illustrated in Figure 1, a significant correlation was found between extent of disease, extent of resection and tumor size and the estimated EFS at 5 years (p<0.001).
Figure 1.
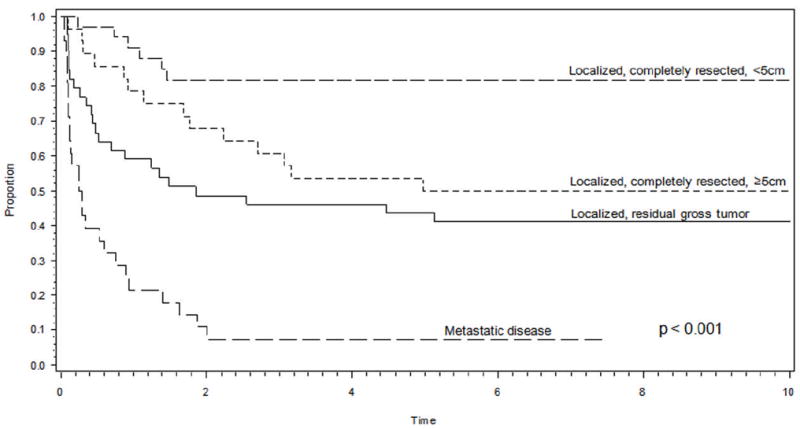
Event-free survival curve demonstrating a significant correlation between tumor localization, size, and resectability and estimated event-free survival at 5 years. The localized completely resected category includes a small number of tumors with microscopically positive margins.
The relationship of tumor grading to EFS is summarized in Tables 4a and 4b. For all patients, there was a significant correlation between both POG grade and FNCLCC grade and EFS (p=0.0095 and p=0.0075, respectively) (Figure 2). This relationship persisted when the comparisons were restricted to patients with localized disease (N=102) (POG grade: p=0.02; FNCLCC grade: p<0.001). The 28 patients with metastatic disease had the following grades (23 POG-G3, 4 POG-G2, 1 POG-G1; 9 FNCLCC-G3; 16 FNCLCC-G2; 3 FNCLCC-G1). However, the POG and FNCLCC grades did not always match. Namely, 45 tumors in the study group were assigned grade 3 by FNCLCC criteria, whereas 90 tumors were assigned grade 3 by POG criteria. Also, while none of the FNCLCC grade 3 tumors received lower grades with the POG system, the converse was not true, as 44 POG grade 3 tumors met criteria for FNCLCC grade 1 (n=3) or 2 (n=41). The histologic types of these 44 tumors that received discrepant grades are listed in Table 5. Of 90 tumors assigned POG grade 3, 84 (93.3%) received their grade independent of histology. The 6 tumors assigned POG grade 3 solely on the basis of histology were alveolar soft part sarcoma, all of which were assigned FNCLCC grade 2.
Table 4.
|
a. Correlation between tumor grade and outcome (all patients). | |||
|---|---|---|---|
| POG Grade | Estimated 5-year EFS | FNCLCC grade | Estimated 5-year EFS |
| 1 (n=04) | 75% | 1 (n=12) | 58% |
| 2 (n=38) | 68% | 2 (n=75) | 57% |
| 3 (n=90) | 37% | 3 (n=45) | 26% |
| p=0.0095 | p=0.0075 | ||
|
b. Correlation between tumor grade and outcome (patients with localized disease only). | |||
|---|---|---|---|
| POG Grade | Estimated 5-year EFS | FNCLCC grade | Estimated 5-year EFS |
| 1 (n=03) | 100% | 1 (n=09) | 78% |
| 2 (n=34) | 76% | 2 (n=58) | 72% |
| 3 (n=65) | 47% | 3 (n=35) | 29% |
| p=0.020 | p<0.001 | ||
Figure 2.
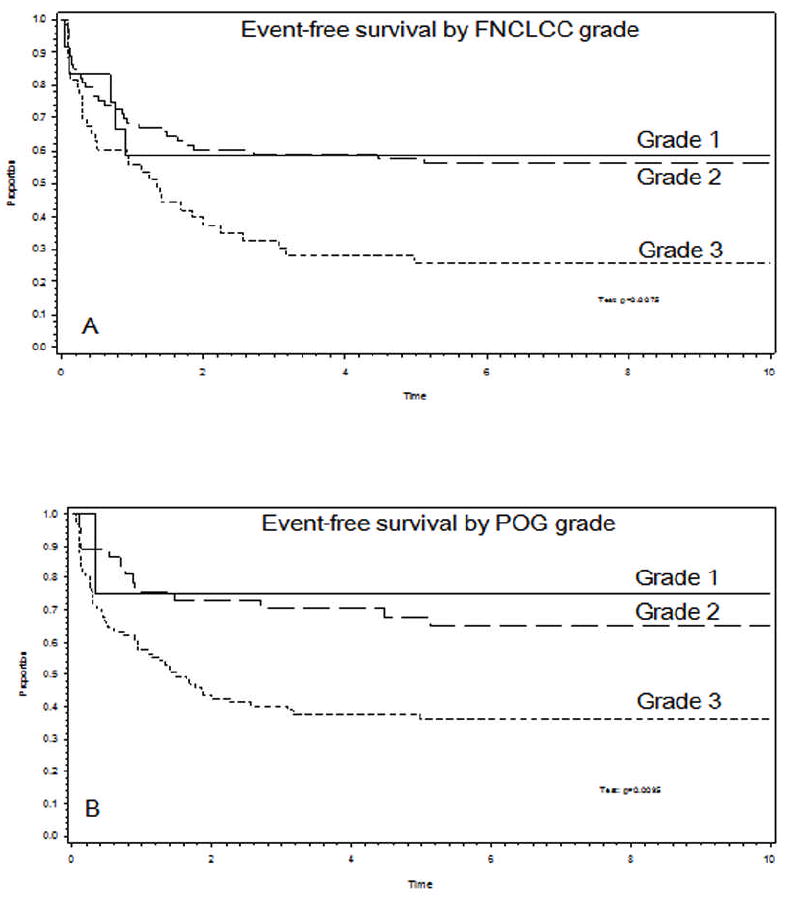
Event-free survival curve demonstrating that both the FNCLCC grading system (A) and POG grading system (B) are predictive of event-free survival at 5 years.
Table 5.
Histologic type of tumors with discrepant grading on the POG and FNCLCC systems (POG grade 3 / FNCLCC grade 1 or 2)
| Histologic Type | N | FNCLCC grade | POG grade |
|---|---|---|---|
| Synovial sarcoma | 15 | 2 | 3 |
| Sarcoma, not otherwise specified | 9 | 2 | 3 |
| Alveolar soft part sarcoma | 8 | 2 | 3 |
| Malignant peripheral nerve sheath tumor | 4 | 2 | 3 |
| Epithelioid sarcoma, distal type | 3 | 2 | 3 |
| Epithelioid sarcoma, proximal type | 1 | 1 | 3 |
| Liposarcoma, myxoid | 1 | 1 | 3 |
| Sarcoma, not otherwise specified | 1 | 1 | 3 |
| Clear cell sarcoma | 1 | 2 | 3 |
| Extraskeletal myxoid chondrosarcoma | 1 | 2 | 3 |
The estimated 5-year EFS was reassessed after regrouping patients into three categories as follows: 1) patients whose tumors were assigned grade 3 by both the POG and FNCLCC systems; 2) patients whose tumors were assigned grade 1 or 2 by both the POG and FNCLCC systems; and 3) patients whose tumors were graded discrepantly. When regrouped in this manner, the 44 patients whose tumors were graded discrepantly had a prognosis that was intermediate between those whose tumors were assigned grade 1 or 2 on both systems and those assigned grade 3 on both systems (p=0.0018) (Figure 3). Similar results were observed when the analysis was restricted to patients who presented with localized disease (p<0.001). Grade continued to be associated with EFS after accounting for extent of disease by using the Cox model and stratifying patients by extent of disease (as defined in Figure 1) (see Table 6). This remained true when the analysis was restricted to patients with localized disease (Table 6).
Figure 3.
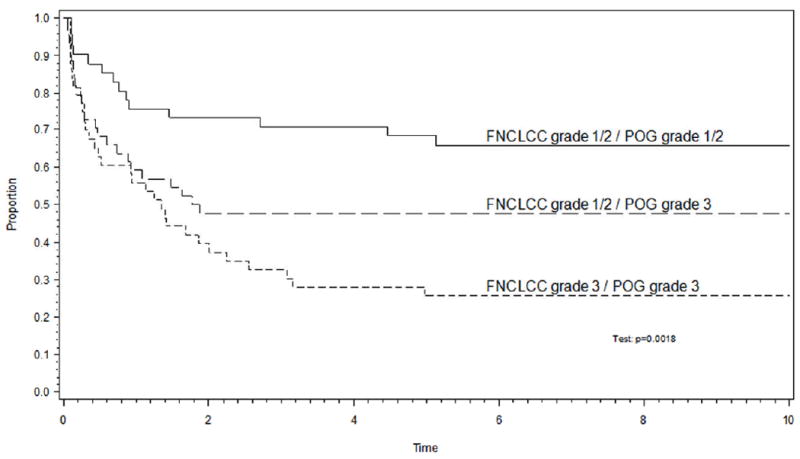
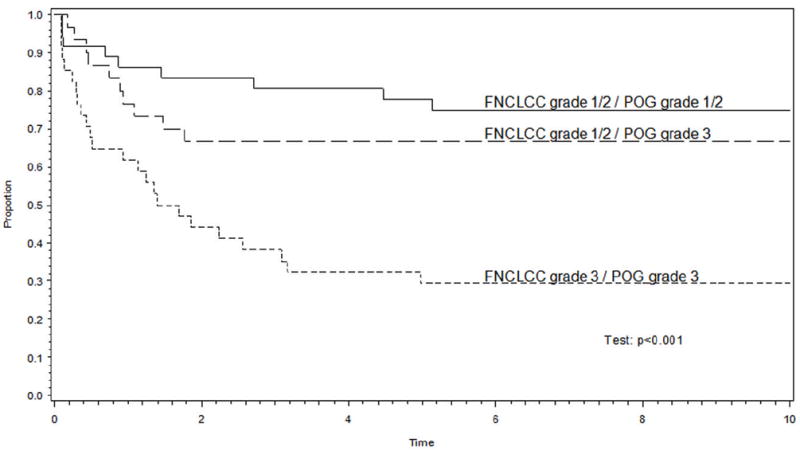
(a) The 44 patients whose tumors were discrepantly assigned grade 3 on the POG system and grade 1 or 2 on the FNCLCC system had an event-free survival experience that was intermediate between those whose tumors were assigned grade 1/2 or grade 3 on both systems. (b) The analysis remained valid in the subset of patients with non-metastatic disease.
Table 6.
Comparison of outcome to combined POG and FNCLCC grades
| Relative increase in risk of failure |
|||
|---|---|---|---|
| Category | Estimated 5-year EFS | All patients (N=130) | Patients with localized disease (N=102) |
| FNCLCC grade 1 or 2 / POG grade 1 or 2 (n=42) | 68% | 1 | 1 |
| FNCLCC grade 1 or 2 / POG grade 3 (n=44) | 48% | 1.7 | 1.7 |
| FNCLCC grade 3 / POG grade 3 (n=44) | 26% | 2.5 | 4.0 |
| p=0.0018 | P=0.01* | P=0.0005* | |
P-values refer to effect of grade after stratification by extent of disease (resected, size < 5 cm; resected, size ≥5 cm; unresected; metastatic disease)
Prognostic utility of individual grading parameters
Most grading systems for NRSTS, including the POG and FNCLCC systems, are based on a number of histologic parameters and differ primarily by the extent to which each of the parameters is weighted. When the prognostic significance of individual histologic grading parameters (mitotic index, necrosis, FNCLCC tumor differentiation score, nucleoli, atypical mitotic figures, and nuclear grade) was assessed using univariate, analysis, only the mitotic index emerged as a significant prognostic feature (p<0.001). (Table 7) The difference in EFS by mitotic index appeared to occur naturally at a breakpoint of 10 mitotic figures per 10 high-power fields (p<0.001) (Figure 4). As expected, mitotic index was highly correlated with grade, with all patients graded 1/2 having a mitotic index < 10 and 42/44 patients graded 3 having a mitotic index ≥10. For those 44 patients whose tumors were graded discrepantly on the POG and FNCLCC systems, mitotic index was highly predictive of EFS (p=0.0055) (Figure 5). The correlation between mitotic index and EFT remained significant when assessed in patients with clinically intermediate risk disease. The clinically intermediate risk group consisted of 69 patients (29 patients in clinical group II and tumor >5cm; 40 patients in clinical group III) who exhibited a 71% EFS when the mitotic index was <10 and 19% when the mitotic index was ≥10 (p<0.001).
Table 7.
Histologic parameters versus 5-year event-free survival.
| Parameter | p-value |
|---|---|
| Necrosis | 0.20 |
| Nucleoli | 0.97 |
| Nuclear grade | 0.46 |
| Differentiation score | 0.73 |
| Mitotic count | <0.001 |
Figure 4.
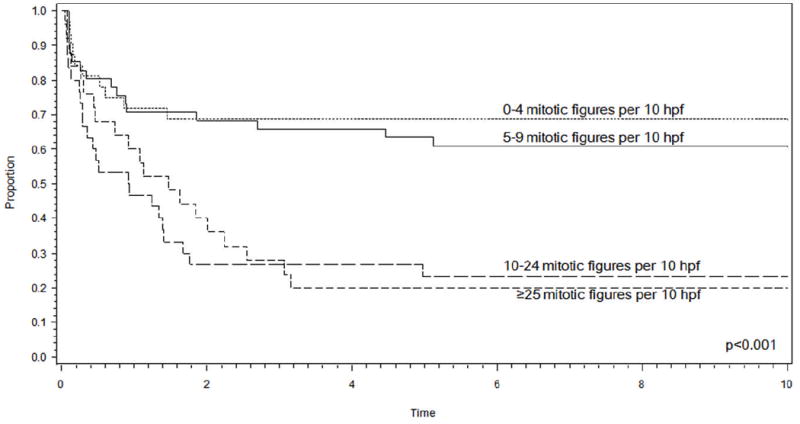
Event-free survival curve demonstrating that the number of mitotic figures alone is predictive of event-free survival at 5 years, and a notable difference in outcome appears to occur naturally at a breakpoint of 10 mitotic figures per 10 high-power fields. (hpf: high-power field)
Figure 5.
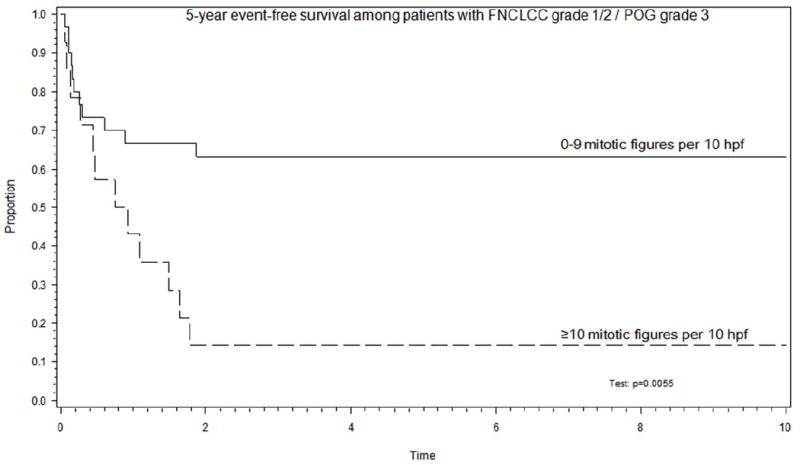
Mitotic count using a cutoff of 10 mitotic figures per 10 high power fields is a significant event-free survival outcome discriminator in the subset of 44 patients whose tumors were graded discrepantly on the POG and FNCLCC systems. (hpf: high-power field)
Further multivariate analysis using a Cox model demonstrated that, after accounting for the effect of extent of disease and mitotic index on EFS, grading was no longer predictive of EFS (p=0.65).
DISCUSSION
Although histologic grading remains widely applied for adult soft tissue sarcoma22, grading of pediatric NRSTS has been less consistently utilized. A grading system based on a modification of the NCI grading system was tested in a cohort of pediatric patients that were prospectively treated using standardized POG protocols. Experience with this POG grading system indicated that histologic grading of pediatric NRSTS has predictive value in determining clinical outcome, largely independent of factors other than stage.8-10 In recent years, the FNCLCC grading system has become one of the most common sarcoma grading systems in use.11, 23 Despite its wide recognition as a useful grading system for adult soft tissue sarcomas, the applicability of the FNCLCC system in pediatric NRSTS has not been investigated systematically. This retrospective comparison of the FNCLCC and POG grading systems was designed to assess the utility of grading parameters and cutoffs used in both systems in a relatively large cohort of pediatric NRSTS with available long term outcome data. Our findings demonstrate that the POG and FNCLCC grading systems correlate with outcome in pediatric NRSTS. Additionally, results from this study suggest that mitotic index shows highly significant correlation with EFS in patients with NRSTS.
The fact that both the FNCLCC and POG grading systems are equally effective predictors of EFS is not unexpected in view of their inherent similarities. In our experience, both systems were easy to apply. The FNCLCC system is a three-tiered system based on assessment of tumor necrosis, mitotic count, and a differentiation score. Necrosis is weighted slightly less than the other two parameters in the FNCLCC system. The differentiation score is a somewhat arbitrary value intended to place weight on the histopathologic features of a neoplasm by attempting to estimate its degree of histologic resemblance to mature soft tissue components. Similarly, the POG grading system is a three-tiered system that uses histopathology as an a priori criterion to determine whether a neoplasm should be assigned POG grade 1 or 3. For neoplasms that cannot be graded solely on the basis of their histopathologic type, necrosis and mitotic count are used to complete the grading process and assign cases to either POG grade 2 or 3. Although both the FNCLCC and POG systems have in common the reliance on assessment of necrosis and mitotic index, each utilizes different cut-off points for these two parameters. In the present study group, the POG system appeared to up-grade tumors in comparison to the FNCLCC system. We postulate that the etiology of this observed discrepancy stems from a low cutoff for the mitotic index in the POG system, leading to a skewed upward allocation of FNCLCC grade 2 cases to POG grade 3. This is further supported by the fact that none of the FNCLCC grade 3 tumors received lower grades on the POG system. In this regard, the FNCLCC system is superior to the POG system for tumors of intermediate grade. Assessing the outcome of discrepantly graded tumors (POG grade 3/FNCLCC grades 1/2) with the aid of a new mitotic index cutoff appears to provide a practical means to better stratify patients with intermediate grade tumors. Thus, mitotic index emerged in our study group as a highly relevant grading parameter in pediatric NRSTS using a cutoff of 10 mitotic figures per 10 high-power fields. The latter observation needs to be further investigated in a larger prospective study.
Histologic type is an arguably indispensable parameter for grading pediatric NRSTS because it provides the biologic context for any grading scheme. The reliance on a single, albeit apparently powerful, transcendent parameter such as the mitotic index, cannot be sufficient to adequately assess prognosis in all types of pediatric NRSTS. This was the basic premise behind assigning cases to either grade 1 or grade 3 solely on the basis of histopathologic type in the POG system. This principle was indirectly validated in our study group and by Parham et al17 through demonstration of a significant correlation between POG grade and EFS. A similar conclusion may be drawn regarding the FNCLCC system. However, limitations exist in both the POG and FNCLCC grading systems with regards to histologic type. For example, the POG system is outdated when it comes to histologic types defined to warrant an a priori low grade status. Thus, the entities “angiomatoid malignant fibrous histiocytoma”, “deep-seated dermatofibrosarcoma protuberans” and “infantile hemangiopericytoma”, all originally regarded as POG grade 1 tumors, are currently either regarded as obsolete or non-malignant entities. Similarly, the tumor differentiation score of the FNCLCC grading system is difficult to apply and does not lend itself to scrupulous evidence-based validation.
In this retrospective study, morphology was by necessity used as the primary means to assess histologic type. This does not reflect the current diagnostic approach to pediatric soft tissue tumors, which are frequently evaluated using ancillary methods such as immunohistochemistry, fluorescence in situ hybridization, and PCR-based methods, and it is regarded as a limitation of this analysis. However, the authors contend that this limitation, which was inevitable in this retrospective analysis using referral material, is mitigated by the fact that all cases included in this study were subjected to rigorous diagnostic workup at the time the patients were enrolled on clinical trials that have been extensively described in several publications.9, 18, 19 It can be also argued that therapeutic protocols grouping all histologic types of NRSTS under a single rubric, such as the POG protocols in which patients in this study were enrolled, limit the statistical value of analyzing the clinicopathologic features of rare sarcoma types and might indirectly constitute a limitation of this study. However, a ‘blanket’ grouping approach to pediatric NRSTS also has practical merits, which can be summarized by the following: 1) Since few active agents have been identified to date, clinical management of pediatric NRSTS at present depends solely on histologic grade and a small number of clinicosurgical prognostic parameters, not on specific histologic types; 2) The rarity of many types of pediatric NRSTS prohibits devising large-scale clinical trials for each histologic type individually, so that virtually all prior and current large clinical trials address pediatric NRSTS as a single group; 3) Grading is a parameter that transcends histologic type in many NRSTS by reflecting the integrated impact of multiple biologic parameters, making it a universally useful tool to assess prognosis.24
In conclusion, grading of NRSTS has prognostic value that currently transcends considerations of individual histologic types and allows incorporation of rare lesions into multi-institutional therapeutic research. A two-tiered low grade/high grade system that relies primarily on mitotic index and histologic type appears to be a suitable scheme to grade pediatric NRSTS. The mitotic index appears to be a crucial parameter for grading pediatric NRSTS when using a cutoff of 10 mitotic figures per 10 high power fields. Because histologic type plays a critical role in assigning a priori grade to certain types of pediatric NRSTS, this parameter is also indispensable in any grading system. A preliminary grading scheme based on data from this retrospective analysis is proposed (Table 8). In addition, a decision tree is proposed for patients with intermediate clinical risk disease, incorporating clinical group stratification and mitotic index (Figure 6). Data gained from collection of large scale controlled studies such as those conducted by the Children’s Oncology Group will be important to validate the findings of this study, the applicability and utility of the novel proposed grading scheme, and in determining the impact of individual histologic types on outcome as data accrues.
Table 8.
Proposed new grading system for pediatric nonrhabdomyosarcoma soft tissue sarcoma.
Low grade
|
| Or |
|
High grade
|
| Or |
|
Figure 6.
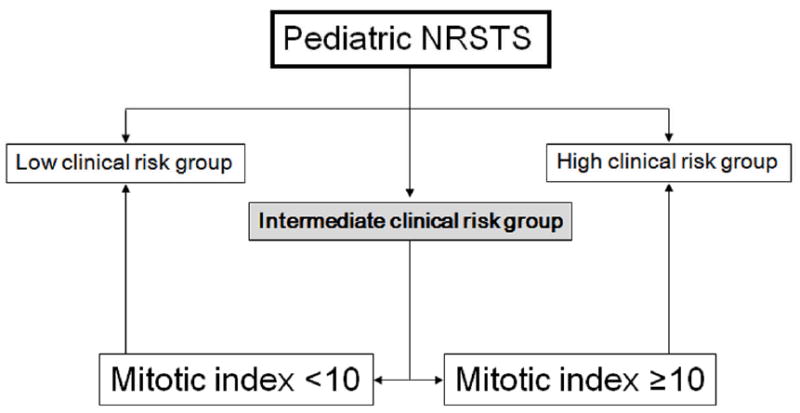
Proposed decision scheme for clinical risk assessment of pediatric non-rhabdomyosarcoma soft tissue sarcoma incorporating mitotic figures as a discriminating feature in patients within the intermediate risk clinical group. The latter is defined as clinical group II patients with tumor size >5cm or clinical group III. This scheme does not apply to tumors whose grade is assigned a priori (see Table 8).
Acknowledgments
This study was supported primarily by the Children’s Oncology Group (COG), and in part by the National Institutes of Health (NIH) grant CA023099 (JDK, SLS) and the American Lebanese Syrian Associated Charities (ALSAC). Support was also provided by NIH grants U10 CA98543 and U10 CA98413 (COG). The authors would like to thank Kimberly Lombardi for assistance with material procurement.
Footnotes
This study was presented in part as a platform paper at the 2007 United States and Canadian Academy of Pathology meeting, San Diego, California.
DISCLOSURE/CONFLICT OF INTEREST
None of the authors have financial conflicts of interest to disclose. SLS receives funding from the Children’s Oncology Group/National Childhood Cancer Foundation.
References
- 1.Spunt SL, Skapek SX, Coffin CM. Pediatric nonrhabdomyosarcoma soft tissue sarcomas. Oncologist. 2008;13(6):668–78. doi: 10.1634/theoncologist.2007-0182. [DOI] [PubMed] [Google Scholar]
- 2.Coindre JM, Terrier P, Bui NB, Bonichon F, Collin F, Le Doussal V, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. 1996;14(3):869–77. doi: 10.1200/JCO.1996.14.3.869. [DOI] [PubMed] [Google Scholar]
- 3.Coindre JM, Terrier P, Guillou L, Le Doussal V, Collin F, Ranchere D, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. 2001;91(10):1914–26. doi: 10.1002/1097-0142(20010515)91:10<1914::aid-cncr1214>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Gaynor JJ, Tan CC, Casper ES, Collin CF, Friedrich C, Shiu M, et al. Refinement of clinicopathologic staging for localized soft tissue sarcoma of the extremity: a study of 423 adults. J Clin Oncol. 1992;10(8):1317–29. doi: 10.1200/JCO.1992.10.8.1317. [DOI] [PubMed] [Google Scholar]
- 5.Heise HW, Myers MH, Russell WO, Suit HD, Enzinger FM, Edmonson JH, et al. Recurrence-free survival time for surgically treated soft tissue sarcoma patients. Multivariate analysis of five prognostic factors. Cancer. 1986;57(1):172–7. doi: 10.1002/1097-0142(19860101)57:1<172::aid-cncr2820570133>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 6.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14(5):1679–89. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 7.Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 225 patients. Cancer. 2003;97(10):2530–43. doi: 10.1002/cncr.11365. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari A, Casanova M, Collini P, Meazza C, Luksch R, Massimino M, et al. Adult-type soft tissue sarcomas in pediatric-age patients: experience at the Istituto Nazionale Tumori in Milan. J Clin Oncol. 2005;23(18):4021–30. doi: 10.1200/JCO.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 9.Pratt CB, Pappo AS, Gieser P, Jenkins JJ, Salzbergdagger A, Neff J, et al. Role of adjuvant chemotherapy in the treatment of surgically resected pediatric nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J Clin Oncol. 1999;17(4):1219–26. doi: 10.1200/JCO.1999.17.4.1219. [DOI] [PubMed] [Google Scholar]
- 10.Spunt SL, Poquette CA, Hurt YS, Cain AM, Rao BN, Merchant TE, et al. Prognostic factors for children and adolescents with surgically resected nonrhabdomyosarcoma soft tissue sarcoma: an analysis of 121 patients treated at St Jude Children’s Research Hospital. J Clin Oncol. 1999;17(12):3697–705. doi: 10.1200/JCO.1999.17.12.3697. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher CDM, Unni KK, Mertens F. Pathology and Genetics: Tumors of Soft Tissue and Bone World Health Organization Classification of Tumours Lyon. France: IARC Press; 2002. [Google Scholar]
- 12.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al. AJCC Cancer Staging Manual. 6. St. Louis; Springer: 2002. [Google Scholar]
- 13.Costa J, Wesley RA, Glatstein E, Rosenberg SA. The grading of soft tissue sarcomas. Results of a clinicohistopathologic correlation in a series of 163 cases. Cancer. 1984;53(3):530–41. doi: 10.1002/1097-0142(19840201)53:3<530::aid-cncr2820530327>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 14.Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33(1):37–42. doi: 10.1002/ijc.2910330108. [DOI] [PubMed] [Google Scholar]
- 15.Guillou L, Coindre JM, Bonichon F, Nguyen BB, Terrier P, Collin F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol. 1997;15(1):350–62. doi: 10.1200/JCO.1997.15.1.350. [DOI] [PubMed] [Google Scholar]
- 16.Mandard AM, Petiot JF, Marnay J, Mandard JC, Chasle J, de Ranieri E, et al. Prognostic factors in soft tissue sarcomas. A multivariate analysis of 109 cases. Cancer. 1989;63(7):1437–51. doi: 10.1002/1097-0142(19890401)63:7<1437::aid-cncr2820630735>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Parham DM, Webber BL, Jenkins JJ, 3rd, Cantor AB, Maurer HM. Nonrhabdomyosarcomatous soft tissue sarcomas of childhood: formulation of a simplified system for grading. Mod Pathol. 1995;8(7):705–10. [PubMed] [Google Scholar]
- 18.Pappo AS, Devidas M, Jenkins J, Rao B, Marcus R, Thomas P, et al. Phase II Trial of Neoadjuvant Vincristine, Ifosfamide, and Doxorubicin With Granulocyte Colony-Stimulating Factor Support in Children and Adolescents With Advanced-Stage Nonrhabdomyosarcomatous Soft Tissue Sarcomas: A Pediatric Oncology Group Study. J Clin Oncol. 2005;23(18):4031–8. doi: 10.1200/JCO.2005.03.209. [DOI] [PubMed] [Google Scholar]
- 19.Pratt CB, Maurer HM, Gieser P, Salzberg A, Rao BN, Parham D, et al. Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: a Pediatric Oncology Group study. Med Pediatr Oncol. 1998;30(4):201–9. doi: 10.1002/(sici)1096-911x(199804)30:4<201::aid-mpo1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 20.Maurer HM, Beltangady M, Gehan EA, Crist W, Hammond D, Hays DM, et al. The Intergroup Rhabdomyosarcoma Study-I. A final report. Cancer. 1988;61(2):209–20. doi: 10.1002/1097-0142(19880115)61:2<209::aid-cncr2820610202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6(7):655–63. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors. 5. St. Louis; Mosby: 2007. [Google Scholar]
- 23.Deyrup AT, Weiss SW. Grading of soft tissue sarcomas: the challenge of providing precise information in an imprecise world. Histopathology. 2006;48(1):42–50. doi: 10.1111/j.1365-2559.2005.02288.x. [DOI] [PubMed] [Google Scholar]
- 24.Rosai J. Why microscopy will remain a cornerstone of surgical pathology. Lab Invest. 2007;87(5):403–8. doi: 10.1038/labinvest.3700551. [DOI] [PubMed] [Google Scholar]


