Abstract
Respiratory syncytial virus (RSV) is a leading cause of hospitalization in infants. A formalin-inactivated RSV vaccine was used to immunize children in 1966 and elicited non-protective, pathogenic antibody. Two immunized infants died and 80% were hospitalized after subsequent RSV exposure. No vaccine was licensed since.
A widely accepted hypothesis attributed vaccine failure to formalin disruption of protective antigens. Instead, we show that lack of protection was not due to alterations caused by formalin, but to low antibody avidity for protective epitopes. Lack of antibody affinity maturation followed poor Toll-like receptor stimulation. This study explains why the inactivated RSV vaccine failed to protect and consequently led to severe disease, hampering vaccine development for forty-two years. Also, it suggests that inactivated RSV vaccines may be rendered safe and effective by inclusion of TLR-agonists in their formulation. In addition, it identifies affinity maturation as a critical factor for the safe immunization of infants.
Respiratory syncytial virus (RSV) is a leading cause of hospitalization in infants worldwide(1). Approximately 50% of infants are infected with RSV during their first respiratory season(1,2). Hospitalization rates for RSV in the United States have risen by 239% in recent decades(3). There is still no vaccine licensed against the virus.
An important obstacle to vaccine development has been the enhanced respiratory disease(ERD) that affected children immunized with a formalin inactivated vaccine against RSV(FIRSV) in the 1960s(4). The vaccine was immunogenic, but elicited non-protective antibody(4). Immunized children exposed to RSV in the community, and seronegative for the virus before vaccination, experienced an increase in the severity of lung disease(4). Furthermore, two immunized infants died as toddlers upon subsequent RSV infection(4). High titers of RSV were recovered from their lungs(4). The main clinical manifestations in children with ERD were bronchoconstriction and a severe pneumonia(4). Disease was associated with an excess in peribronchiolar eosinophils(4) and non-protective antibody complexed with virus deposited in affected tissue(5).
Even though the immune phenotype of ERD has been extensively characterized(6–12), the most pressing question about the pathogenesis of ERD is why antibodies elicited by FIRSV failed to protect against RSV. A protective response would have prevented ERD. A widely accepted theory ascribes lack of protection to formalin disruption of critical epitopes during vaccine inactivation(7,13,14). But the inability of other non-replicating RSV vaccines to elicit protective antibody and their propensity to trigger aberrant immune manifestations [e.g.:Th2 bias(6,10–12)], suggest that ERD pathogenesis cannot solely be attributed to poor preservation of specific antigens. In fact, high EIA/neutralization ratios in the non-protective antibody response elicited by FIRSV and other non-replicating RSV immunogens(11,12), and the relationship in humanized RSV monoclonal antibodies between better binding strength for the protective F protein and neutralizing capacity(15) suggest that affinity maturation may be important in protection against RSV.
Understanding the mechanisms that led to production of non-protective, pathogenic antibody by FIRSV and the requirements for eliciting protective antibody against RSV is critical for the development of safe vaccines to protect infants. Drawing from the observations described above, we tested the hypothesis that maturation of avidity plays a critical role in protective responses against RSV and that production of antibody of low avidity for the virus was the main cause for vaccine failure and consequent ERD development in affected children.
Results
Non-replicating vaccines against RSV prime for ERD
The manifestations of ERD in animal models include airways hyperresponsiveness(AHR), severe pneumonia with pulmonary eosinophilia, and deficient protective antibody production with recovery of RSV from the lungs(5,9–12). To determine whether different non-replicating vaccines can prime for ERD, we inoculated mice in the footpad with FIRSV, RSV inactivated with ultraviolet light(UVRSV) or purified fusion protein(PFP). The fusion(F) protein is the main neutralizing antigen in RSV(1). Control mice were inoculated with wild type (wt)RSV intranasal(IN), which confers protection against the virus(1). An additional control group received a footpad dose of Hep-2 lysate, as placebo(Fig.1).
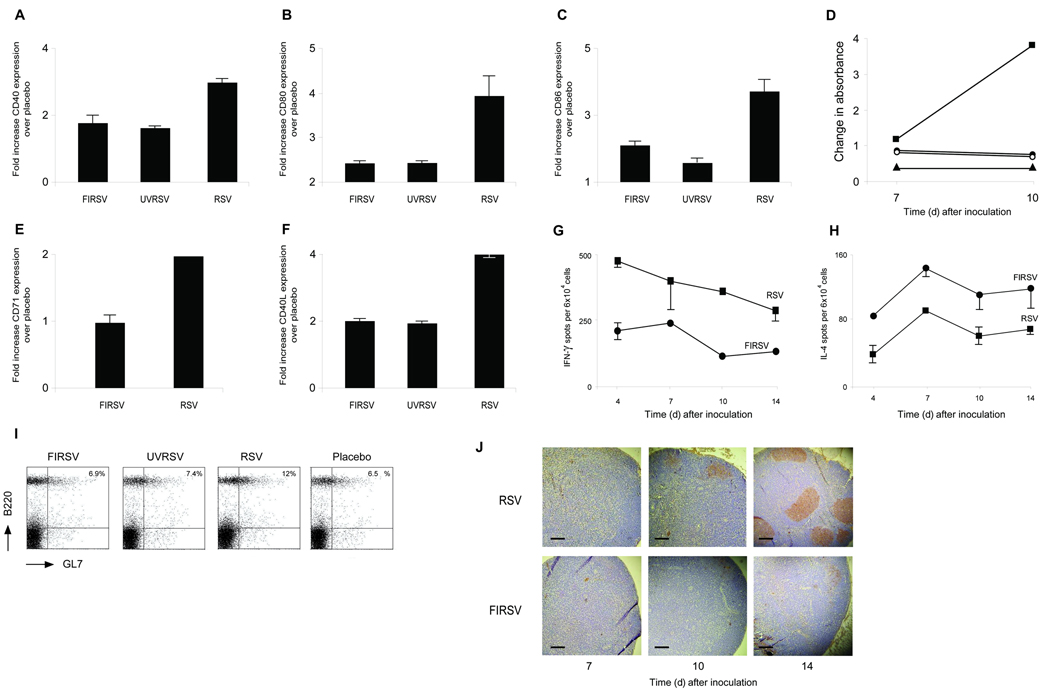
Figure 1. Non-replicating vaccines against RSV prime for ERD.
(a) AHR 7 d after RSV challenge in previously immunized BALB/c mice. AHR to acetylcholine challenge is defined by the time-integrated rise in peak airway pressure. Results are means ± SEM (error bars) of 8–11 animals per group and are representative of two independent experiments. For FIRSV, P<0.05 vs. placebo or RSV; and P=NS vs. UVRSV or PFP. (b–d) Pulmonary histopathology 7 days after RSV challenge in mice that had received the indicated preimmunization: Hematoxylin and eosin (b) showing peribronchiolar pneumonia, Hematoxylin and Congo Red (c) showing pulmonary eosinophlia and periacid Schiff (d) showing enhanced bronchiolar mucus production in FIRSV, UVRSV and PFP recipients. Scale bar for b and d: 100 µm; scale bar for c: 25 µm (e) Pulmonary eosinophils per 40X field are increased in recipients of FIRSV, UVRSV and PFP. Results are means ± SEM (error bars) of 6–10 animals per group and are representative of two independent experiments. For FIRSV, P<0.05 vs. RSV, placebo and PFP; P=NS vs. UVRSV. (f) Lung viral titers 4 days after RSV challenge in recipients of inactivated vaccines, RSV or placebo. Results are means ± SEM (error bars) of 6 animals per group. For FIRSV, P<0.05 vs. RSV; P=NS vs. placebo, UVRSV and PFP.
All mice were challenged IN with wtRSV 60 days after vaccination, and those immunized with FIRSV, UVRSV or PFP had increased AHR compared to mice protected by a previous wtRSV infection or mice immunized with placebo(Fig.1a). The three groups immunized with non-replicating vaccines also had severe perivascular and peribronchiolar pneumonia(Fig.1b;score in Suppl. methods), while wtRSV and placebo recipients had mild lung infiltration. In addition, mice primed with non-replicating immunogens had eosinophilc infiltration and more mucus in the lungs than mice from control groups(Fig.1c–e). Lung titers in FIRSV, UVRSV, and PFP vaccinees were similar to those in placebo recipients(Fig.1f). No virus was recovered from the lungs of mice immunized with wtRSV(Fig.1f). These findings demonstrate that different non-replicating vaccines can prime for ERD.
Non-replicating vaccine elicit non-protective, low avidity antibody
The protective role of neutralizing antibody against RSV is well-established(16,17). In fact, unlike cytotoxic T lymphocytes(CTL;18), protective antibodies prevent RSV replication in the lungs upon viral re-challenge(Suppl.Fig.1).
Since inactivated vaccines failed to protect against RSV, we compared the antibody response elicited by inactivated and live vaccines against the neutralizing F protein. Both FIRSV and UVRSV elicited a short-lived antibody response against F that peaked 45 days after inoculation, while wtRSV elicited a long lived anti-F response(Fig.2a).
Figure 2. Non-replicating vaccine elicit non-protective, low avidity antibody.
(a) IgG antibody responses against the RSV F protein determined by immunoassay from sera of FIRSV, UVRSV and RSV recipients. For FIRSV, P=NS compared with UVRSV, and P<0.05 compared with RSV. (b) RSV-specific neutralization as measured by 50% plaque reduction (PRNT50) on day 45 after immunization. Results are means ± SEM (error bars) of 5–8 animals per group and are representative of three independent experiments. For FIRSV, P=NS compared to UVRSV, and P=0.003 compared to RSV. For UVRSV, P=0.002 compared to RSV. (c) Determination of IgG avidity against RSV F after 7M urea wash in sera from pre-immunized BALB/c mice. For FIRSV, P=NS compared to UVRSV and P<0.05 compared to RSV. Results are means ± SEM (error bars) of 6–10 animals per group and are representative of three independent experiments. (d) Determination of IgG avidity against RSV F in sera from pre-immunized mice on day 45 after immunization. 50% avidity for FIRSV, P=NS vs. UVRSV and P<0.001 vs. RSV. For UVRSV, P<0.001 vs. RSV. (e) IgG2a/IgG1 ratio against RSV F in sera from pre-immunized mice.
Subsequently, we compared the neutralizing capacity of antibody elicited by the vaccines. To prevent the confounding effect of steric hindrance in neutralizing antibody responses(19,20), we standardized all sera for anti-F antibody titers. Antibody elicited by inactivated vaccines lacked neutralizing capacity, while wtRSV inoculation generated neutralizing antibody(Fig.2b).
To compare the quality of the polyclonal antibody response we characterized avidities, which play an important role in antibody-mediated protection against other viruses(21–23). Maturation of avidity was absent in the antibody response elicited by FIRSV or UVRSV, while avidity improved over time after wtRSV(Fig2c,d). Comparison of Th bias using IgG2a/IgG1 ratios of anti-F antibody responses revealed low ratios in non-replicating vaccine recipients(consistent with Th2 bias) and high IgG2a/IgG1 ratios in recipients of wtRSV(Fig.2e).
Affinity is critical for protection against RSV
We then studied the relationship between neutralization and avidity in RSV. For this purpose, we established a mouse model of wtRSV infection that, by resection of the ipsilateral popliteal lymph nodes 7 days after footpad inoculation, failed to produce high avidity antibody(Rpn7 mice). In Rpn7 mice, normal levels of antibody against F were detected after wtRSV inoculation(Suppl.Fig.2), but affinity maturation did not occur(Fig.3a;Suppl.Fig.2). Importantly, in generating low avidity antibody, this wtRSV infection was not protective(Fig.3b).
Figure 3. Affinity is critical for protection against RSV.
(a) Anti-F IgG avidity in sera. 50% avidity for FIRSV, P=NS vs.UVRSV and P<0.001 vs. RSV, UVRSV5 and Rpn7. For UVRSV5, P=NS vs. RSV and P<0.05 vs.UVRSV. (b) Correlation between RSV-specific PRNT50 and 50% avidity in sera (pre-standardized for anti-F IgG levels). Black squares=RSV, half-shaded circles=UVRSV5, white circles=UVRSV, black circles=FIRSV, half-shaded squares=Rpn7. (c) Lung viral titers in recipients of passively transferred sera (pre-standardized for anti-F IgG levels) from immunized mice. Results are means ± SEM of 3 animals/group. For UVRSV5, P<0.01 vs. placebo and P=NS vs. RSV. (d) IgG antibody and (e) determination of IgG avidity after 7M urea wash against RSV F422–438peptide from pooled sera of immunized mice. (f) Binding competition between sera (pre-standardized for anti-F IgG levels) from immunized mice and palivizumab for RSV F. For FIRSV, P<0.05 vs. naÏve, UVRSV5 and RSV. FIRSV vs. UVRSV, P=NS. For UVRSV, P<0.05 vs. naÏve, UVRSV5 and RSV. (g) IgG avidity against F after 7M urea wash in mice pre-immunized with FIRSV, FIRSV5 or RSV. (h) Binding of anti-F IgG mAb panel (24,48) to FIRSV (white bars) or RSV (black bars). (i) IgG avidity against FIRSV or RSV after 7M urea wash in sera from mice pre-immunized with FIRSV5. Results are means ± SEM of 7 animals/group. P=0.001. All data is representative of two-three independent experiments.
Subsequently, we promoted maturation of the anti-RSV response by administering consecutive daily doses of UVRSV to mice(UVRSV5 mice; Fig.3a–c). UVRSV5 mice elicited antibodies of better avidity for F than a single dose of UVRSV or FIRSV(Fig.3a), and these high avidity antibodies had enhanced neutralizing capacity against RSV(Fig.3b). We confirmed this correlation of avidity and protection in vivo by comparing passive transfer of immune sera with similar anti-F IgG concentrations to naïve mice challenged with wtRSV(Fig.3c). Protection was observed after administration of sera from wtRSV and UVRSV5-inoculated mice.
To rule out the possibility that non-protective vaccines were eliciting responses against a different repertoire of epitopes than protective immunogens(instead of failing to elicit maturation against critical protective epitopes), we used two complementary strategies. First, we compared antibody responses against a well-characterized protective linear epitope in F encompassing amino acids 422–438(24; F422–438) and found similar levels of anti-F422–438 antibody in recipients of non-protective and protective RSV vaccines(Fig.3d). However, antibodies elicited by inactivated vaccines were of lower avidity than those detected after wtRSV inoculation(Fig.3e). Subsequently, we compared the competitive ability of antibodies elicited by replicating and non-replicating vaccines against the protective monoclonal antibody palivizumab(Fig.3f). As expected, due to higher avidity for F, sera from mice immunized with wtRSV and UVRSV5 were better able to decrease palivizumab binding to F than sera from mice immunized with UVRSV or FIRSV. However, all vaccines were able to elicit competitive antibody. Taken together, these findings confirm that inactivated vaccines can elicit antibody against critical protective epitopes in RSV, but the antibody responses are of lower avidity than those elicited by protective vaccines.
Interestingly, and contrary to our observations in mice immunized with UVRSV5, serial inoculations of FIRSV(FIRSV5) worsened antibody avidity for F(Fig.3g). Therefore, we investigated whether formalin inactivation disrupted RSV epitopes by using a panel of monoclonal antibodies against F, and comparing their recognition of RSV and FIRSV(Fig.3h). Monoclonal antibodies recognized RSV, but binding to FIRSV was comparatively decreased. Furthermore, avidity for FIRSV was enhanced and for RSV decreased in sera from mice immunized with FIRSV5(Fig.3i). These findings suggest that formalin inactivation partially modified RSV epitopes, even though germ-line antibody elicited by FIRSV recognized protective epitopes in RSV(Fig.2;Fig.3d,f) and was associated with ERD pathogenesis(5,25).
Adaptive immunity after inactivated vaccines
Antibody responses to T-dependent antigens are initiated by dendritic cell(DC) maturation followed by Th activation to provide help to B cells, primarily through CD40/CD40 ligand(CD40L) interaction and secretion of cytokines(26,27). Alternatively, B cells can respond directly to T cell dependent antigens upon Toll-like receptor(TLR) stimulation(27). Therefore, we studied DC maturation in mice inoculated with RSV vaccines and found decreased expression of CD40, CD80 and CD86 in recipients of inactivated compared to live RSV vaccine(Fig.4a–c). Similar results were observed in bone marrow-derived DC incubated with inactivated vs. live RSV in vitro, as previously described by other groups (28–31;Suppl.Fig.3).
Figure 4. Adaptive immunity after inactivated vaccines.
Determination of CD40 (a), CD80 (b) and CD86 (c) surface expression in dendritic cells (CD11c+) from regional popliteal lymph nodes (CCR7+) 24 h. after footpad immunization with FIRSV, UVRSV or RSV (mean fold-increase over immunization with placebo of 9–12 animals/group). (d) Lymphoproliferative responses (by BrdU immunoassay) in CD4+ T lymphocytes from regional popliteal nodes after footpad immunization with FIRSV (black circles), UVRSV (white circles), RSV (black squares) or placebo (black triangles). CD4+ T lymphocytes were incubated with PHA (1µg) for 48 h. Representative of three independent experiments. CD71(e) and CD40 ligand (f) surface expression in CD4+ T lymphocytes from regional popliteal nodes by flow cytometry 10 days after FIRSV, UVRSV or RSV footpad immunization (mean fold-increase over immunization with placebo of 6 animals/group). (g) IFN-γ and (h) IL-4 spots/104 mononuclear cells from regional popliteal nodes after footpad immunization as determined by ELISPOT. Representative of two independent experiments. (i) Determination of B cell centrocytes (B220+ and GL7+) from regional popliteal nodes 14 days after footpad immunization. Representative of four independent experiments. (j) Germinal center detection (PNA+) in slides from regional popliteal nodes after footpad immunization. Scale bar: 100 µm. Representative of three independent experiments.
Comparison of Th responses revealed decreased CD4+ T lymphocyte proliferation(Fig.4d), and lower levels of the activation marker CD71(Fig.4e), and the co-stimulatory molecules CD40L(Fig.4f) and ICOS(not shown) in mice immunized with non-replicating vaccines. A Th2 bias characterized by lower interferon-γ and higher interleukin-4 production(Fig.4g,h) was observed in inactivated vaccine recipients.
Upon examination of B cell activation, germinal center formation was similar in recipients of inactivated vaccines or placebo, and decreased when compared to recipients of wtRSV(Fig.4i,j), confirming the absence of affinity maturation.
Deficient activation of TLRs by inactivated vaccines
TLR play a critical role in antibody production(26,27). In fact, TLR signaling is necessary for DC maturation, Th and B cell activation, all responses that are decreased in mice immunized with inactivated vaccines. Furthermore, TLR can directly activate B cells to generate antibody against T cell dependent antigens(27). Therefore we determined whether generation of low avidity, non-protective antibody after immunization with inactivated vaccines was associated with deficient TLR activation.
Since promoting antibody affinity maturation with UVRSV5 led to protective responses(Fig.3b,c), while maturation using FIRSV5 polarized responses towards epitopes altered by formalin inactivation(Fig.3g–i), and the effects of single doses of both vaccines on the adaptive immune response were similar(Fig.1–Fig.4), we conducted all subsequent experiments using UVRSV as a model of inactivated vaccine immunization.
First, we examined TLR activation in DC by comparing Iκ-Bα degradation in CD11c+ cells from regional draining lymph nodes(Fig.5a). Degradation of Iκ-Bα, a consequence of TLR activation, was decreased in mice inoculated with UVRSV vs. wtRSV. Second, to determine whether TLR activation is critical for maturation of avidity and protection against RSV, we compared responses elicited by wtRSV in wt and Myd88+/− mice(heterozygous mice were chosen to reflect decreased TLR activation, Fig.5b,c). MyD88 is a critical downstream adaptor of most TLR, including TLR4 and TLR7, which are activated by the F protein and the RSV genome, respectively(32–35). After wtRSV IN inoculation, both avidity for F and neutralizing capacity against RSV were decreased in Myd88+/− compared to wt mice(Fig.5b,c).
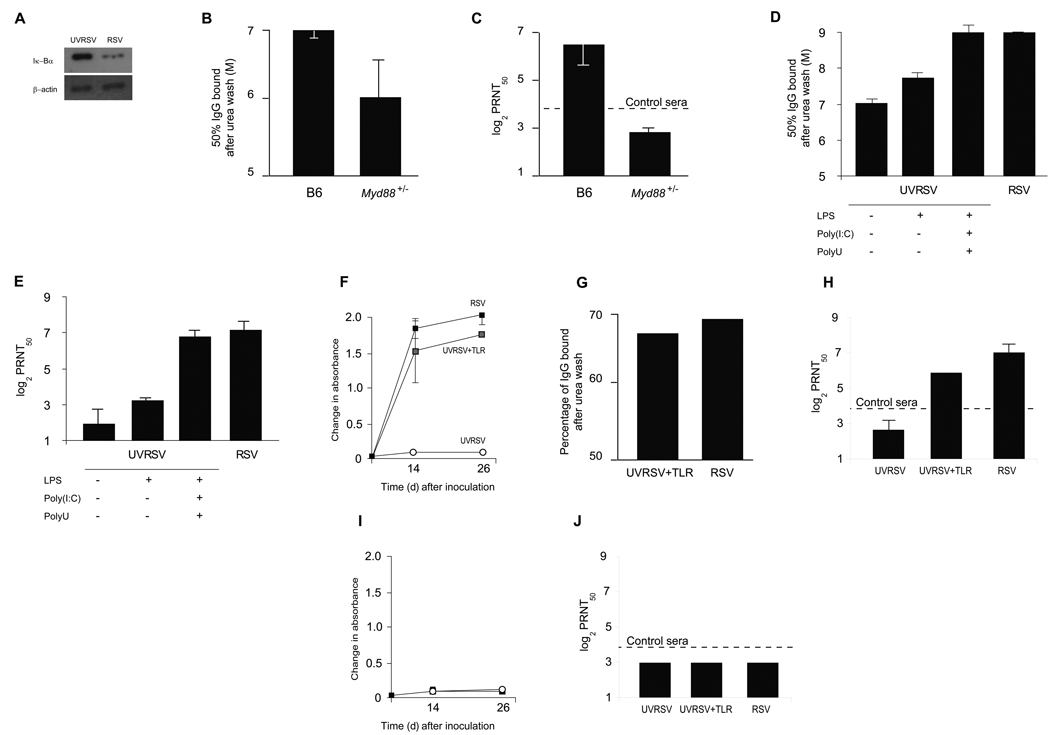
Figure 5. Deficient activation of TLRs by inactivated vaccines.
(a) Iκ-Bα expression by Western Blot in dendritic cells (CD11c+) isolated from regional popliteal lymph nodes 24 h. after footpad inoculation of UVRSV or RSV. Representative of two independent experiments. (b) Determination of 50% avidity against RSV F and (c) RSV-specific neutralization (PRNT50) in sera from wt B6 and Myd88+/− mice inoculated intranasally with RSV. Results are means ± SEM (error bars) of 6–8 animals per group and representative of two independent experiments. P < 0.05 for both comparisons. (d) Determination of 50% avidity against RSV F and (e) RSV-specific neutralization (PRNT50) in sera from pre-immunized BALB/c mice with RSV, UVRSV, UVRSV+LPS, and UVRSV+LPS+Poly(I:C)+PolyU. For UVRSV vs. UVRSV+LPS+Poly(I:C)+PolyU P<0.05, vs. RSV P<0.01. For RSV vs. UVRSV+LPS+Poly(I:C)+PolyU P =NS. Representative of two independent experiments. (f-h) Anti-F IgG responses, IgG avidity and RSV-specific neutralization in Myd88−/− mice recipients of wt B cells and (i,j) anti-F IgG responses and neutralization in µMT mice recipients of Myd88−/− B cells. Mice were inoculated with wtRSV (black squares), UVRSV+TLR (gray squares) or UVRSV (white circles). In F and H, P<0.05 for UVRSV vs. wtRSV and vs. UVRSV+TLR.
Then, we determined whether remedial TLR stimulation in mice immunized with UVRSV elicited protective antibody against the virus(Suppl.Fig.4). Supplementation of UVRSV with the TLR4 agonist LPS increased CD40, CD80 and CD86 cell surface expression in CD11c+, CCR7+ cells(Suppl.Fig.4). Moreover, LPS supplementation enhanced germinal center formation in regional lymph nodes(Suppl.Fig.4), promoted maturation of avidity against F(Fig.5d) and consequently protective antibody against RSV(Fig.5e). Importantly, simultaneous remedial stimulation of TLR4, TLR3 and TLR7 using LPS, poly(I:C) and polyU(UVRSV+TLR) further improved affinity maturation and neutralizing capacity in UVRSV recipients(Fig.5d,e). This positive modulation of protective antibody responses contrasted with the lack of effect observed after adding the TLR-independent adjuvant alum to UVRSV(ref.36,37;Suppl.Fig.5). Taken together, these findings confirm the importance of TLR stimulation in generating affinity maturation and protective antibody responses against RSV.
Finally, to define the requirements for antibody production against RSV and examine the role of B cells, we passively transferred B cells from wt into Myd88−/− mice and inoculated them with live RSV, UVRSV+TLR or UVRSV(Fig.5f–j). Interestingly, both live RSV and UVRSV+TLR elicited protective antibody of similar avidity for F from wt B cells in Myd88−/− recipients. Inoculation of UVRSV failed to elicit a response. Then, we passively transferred B cells from Myd88−/− to µMT mice(lacking mature B cells) and repeated the inoculation strategies(Fig.5i,j). In this case, Myd88−/− B cells failed to produce anti-RSV IgG in µMT mice despite the presence of wt DC. These findings suggest that stimulation of TLR in B cells is critical to elicit protective antibody against RSV.
UVRSV plus TLR agonists protects against ERD
Finally, we examined whether UVRSV+TLR prevented ERD by immunizing mice with UVRSV+TLR, UVRSV, protective live RSV or placebo. After challenge, recipients of UVRSV+TLR had lower airways resistance, milder peribronchiolar and perivascular cellular infiltration(score: mild) and lower eosinophil pulmonary counts and mucus production than UVRSV-immunized mice with ERD(Fig.6a–d;Fig.1b–d). RSV lung titers in mice protected by UVRSV+TLR were significantly lower than in mice inoculated with UVRSV(Fig.6e). In fact, manifestations in UVRSV+TLR recipients were similar to those observed in mice protected by a previous dose of wtRSV.
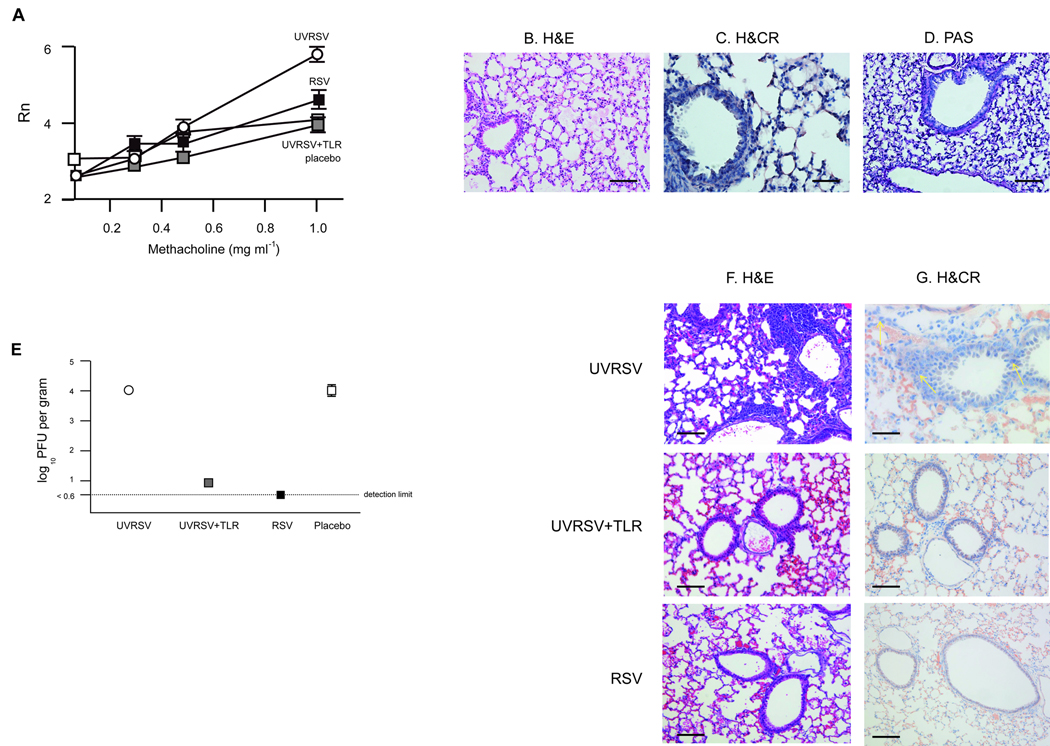
Figure 6. UVRSV plus TLR agonists protects against ERD.
(a) Airways resistance 7 d after RSV challenge in previously immunized BALB/c mice. Results are means ± SEM (error bars) of 4–6 animals per group and are representative of two independent experiments. Mice were inoculated with wtRSV (black squares), UVRSV+TLR (gray squares), UVRSV (white circles) or placebo (white squares). For UVRSV+TLR, P<0.05 vs. UVRSV; and P=NS vs. wtRSV or placebo. (b–d) Pulmonary histopathology 7 days after RSV challenge in mice that had received UVRSV+TLR preimmunization: Hematoxylin and eosin (b), Hematoxylin and Congo Red (c) and periacid Schiff (d) showing absence of pneumonia, eosinophilia and mucus production. Scale bar for b and d: 100 µm; scale bar for c: 25 µm (e) Lung viral titers 4 days after RSV challenge in recipients of UVRSV+TLR, UVRSV, RSV or placebo. Results are means ± SEM (error bars) of 4–5 animals per group. For UVRSV+TLR, P<0.05 vs. UVRSV and placebo; P=NS vs. wtRSV. (f,g) Pulmonary histopathology in µMT mice immunized with FIRSV and passively transfused with sera obtained from UVRSV+TLR, UVRSV or wtRSV inoculated mice. µMT mice were subsequently challenged with wtRSV (day 7 post-challenge): Hematoxylin and eosin (f), Hematoxylin and Congo Red (g). Scale bar for f: 100 µm; scale bar for g: 25 µm Yellow arrows showing eosinophils.
These observations were confirmed in FIRSV-immunized µMT mice receiving sera from UVRSV+TLR, UVRSV or wtRSV vaccinees(Fig.6f,g). Upon RSV challenge, characteristic lung infiltration and eosinophila were present only in recipients of UVRSV sera.
Discussion
The mechanism responsible for lack of protection in the antibody response elicited by FIRSV against RSV remained unclear for decades, hampering development of new vaccines against the virus. This study demonstrates that non-protective antibody elicited by inactivated RSV immunogens, and ultimately associated with the development of ERD(4,5), results from lack of affinity maturation due to deficient TLR activation in B cells. These findings modify the previous paradigm, ascribing poor antibody function to formalin disruption of protective epitopes(7,13,14). Also, they open the possibility that inactivated RSV vaccines may be rendered safe and effective by inclusion of TLR-agonists in their formulation.
Several observations support the importance of antibody avidity for protection against respiratory viruses, including measles virus(MV) with its risk for atypical disease(21,38). A formalin-inactivated vaccine against MV elicited low avidity, non-protective antibody associated with serious clinical manifestations in individuals exposed to wtMV(21). In this case, low avidity antibody neutralized viral infection through the CD46 low affinity MV receptor, but did not through the CD150 high affinity wtMV receptor and promoted immune complex-mediated illness(21). However, low avidity polyclonal responses against other viruses may suffice to confer protection. For example, disease enhancement was never described for influenza virus despite wide use of protective non-replicating vaccines that do not promote affinity maturation(39). The low affinity interaction between the binding site for the receptor in the influenza hemagglutinin and the cellular receptor for the virus (KD=2×10−3;40) may facilitate neutralization by antibodies of relatively low avidity. In the case of RSV, differences in affinity affect the neutralizing capacity of anti-RSV monoclonal antibodies(15) and our study provides evidence for their critical role in natural protection against wt infection. The wt receptor for RSV has not been identified. Perhaps, differences in affinity of the interaction between viral attachment proteins and their receptors or in the mechanisms of neutralization among viruses may explain the discrepancies in the efficacy of inactivated vaccines against RSV and influenza(23,41,42).
This report highlights the importance of TLR activation in B cells for protection against RSV and prevention of ERD. RSV F interacts with TLR4 initiating membrane fusion and MyD88-dependent and independent pathways leading to activation of several transcription factors, notably NF-κB. Once RSV begins transcribing and replicating its genome, TLR-mediated detection appears to depend upon autophagy-associated mechanisms that engage TLR7 and TLR3 in endosomic compartments(cytosolic viral dsRNA also activates CARD-helicases and PKR)(33–35,43–45). Our data suggest that efficient detection of RSV by TLR, which converge on a redundant set of transcription factors, is necessary for antibody production and affinity maturation. Non-replicating RSV vaccines(e.g.:UVRSV) stimulate only a subset of these receptors in a weak fashion(like TLR4; ref.31), becoming protective only after incorporation of exogenous TLR ligands. Since most TLR share the same downstream effectors, different agonists may achieve similar effects provided that the intensity of the signal is appropriate. This conclusion is supported by the synergistic effect of adjuvants containing mixed-TLR ligands. Remediation of UVRSV with TLR agonists leads to dendritic cell maturation, T helper activation and B cell affinity maturation. Specifically, our B cell transfer experiments and those using Myd88+/− mice suggest the existence of a threshold of activation below which affinity maturation does not occur and protective antibody is not elicited. Moreover, for wtRSV the threshold can only be surpassed if MyD88-dependent pathways in B cells are engaged. This does not imply that MyD88-independent pathways do not play a role, but rather that their activation alone is insufficient.
Perhaps a consequence of choosing formalin as the chemical for vaccine inactivation in 1966 was to bias the T cell response in ERD towards Th2(7). Partial alteration of RSV epitopes during formalin inactivation likely contributed to the generation of non-protective antibody. But epitope disruption played a secondary role in disease priming, as the low avidity antibodies elicited by FIRSV recognized protective epitopes, and other non-replicating RSV vaccines not treated with formalin in this and other publications showed evidence of priming for aberrant immune manifestations(6,11,12). Other studies associated the Th2 bias in ERD to the absence of Th-modulatory CTL in FIRSV recipients(8,46) or postulated a role for alum in T cell polarization(47). However, ERD phenotypes did not differ with or without alum (5,6). Whether deficient TLR activation contributed to the Th2 bias in ERD remains to be determined.
Interestingly, our findings may also contribute to clarify why none of the children who were seropositive for RSV before immunization with FIRSV developed ERD(4), even though wtRSV infections confer only partial protection against subsequent exposures(1,2). The pre-existing high avidity antibody elicited by wtRSV before immunization, likely “outcompeted” the low avidity clones elicited by FIRSV and prevented non-protective, pathogenic B cell priming against the virus. Our study also may explain why no child ever developed ERD twice, as the B cells elicited by RSV infection during ERD also eventually “outcompeted” pathogenic B cells primed by FIRSV and reestablished a normal response against future re-infections.
In summary, we demonstrate a critical role for antibody avidity in protective responses against RSV and in the pathogenesis of ERD. Poor TLR stimulation by inactivated RSV vaccines was associated with lack of maturation and led to production of non-protective antibodies. These antibodies were critical for ERD pathogenesis, as they failed to neutralize RSV allowing unrestricted replication and secondary stimulation of FIRSV-primed Th2 cells. Further, low avidity antibody contributed to disease severity through immune complex formation and deposition in affected tissue(5). Our findings indicate that safe and effective RSV vaccines for infants require neutralizing antibody with similar avidity for protective antigens to that elicited by live virus inoculation. (3000 words)
Methods
Viruses and vaccines
We prepared FIRSV vaccines using the RSV A2 strain grown in Hep-2 cells (5), and achieved UV-inactivation of RSV A2 via 3 cycles of irradiation at 3600 × 100 µJ/cm2 dose of ultraviolet light. Purified fusion protein (PFP) obtained from RSV A2-infected Hep-2 cells.
Mice and immunizations
4–8-wk-old BALB/c, C57BL/6 mice wt and µMT mice (The Jackson Laboratory, Bal Harbor, ME) and Myd88+/−and Myd88−/− mice were housed under laminar flow hoods in an environmentally controlled specific pathogen-free animal facility. We performed footpad immunizations using 50µl containing 105 plaque-forming units (pfu) of RSV, FIRSV or UVRSV, or 1µg of either PFP or Hep-2 lysates (placebo). We immunized intranasally (IN) with RSV using 105 pfu. RSV IN challenge of pre-immunized mice used 106 pfu of live RSV. We administered TLR agonists by footpad inoculation as follows: LPS (Sigma) 1 µg, Poly(I:C) (Invivogen) 2.5 µg, and PolyU (Invivogen) 0.1 µg.
Airways hyperresponsiveness
Seven days after challenge mice were anesthetized with 20 ml kg−1 of Avertin (2, 2, 2 tribromoethanol, Fluka + 2-methyl-2-butanol, Fisher), intubated, and ventilated at a rate of 120 breaths/min with a constant tidal volume of air(0.2 ml). Animals were then paralyzed with decamethonium bromide(25 µg kg−1) and after establishing a stable airway pressure, acetylcholine was given intravenously(50 µg kg−1). We measured the dynamic airway pressure for 5 minutes. APTI determinations were confirmed by analysis of lung resistance to aerosolized methacholine (0.01– 1 mg kg−1).
Histopathology
We stained formalin-fixed lung sections (5–7 µm) using either periodic acid-Schiff (PAS), hematoxylin + Congo Red or hematoxylin and eosin (H&E). We scored eosinophils as previously described (25) and pneumonia according to Suppl. Methods. We removed popliteal lymph nodes aseptically from mice and sliced them in 5–7 µm sections (after fixation) for immunohistochemistry. A lectin from Arachis hypogaea (PNA) conjugated with biotin (Sigma) was used to detect centrocytes.
Virus Titration in Lung Tissue
We removed lungs from mice aseptically 4 days after RSV challenge and obtained titers as described elsewhere (5). Results are expressed as pfu g−1 of lung tissue.
Antibody assays
We measured avidity using 6, 7, 8 or 9 M urea washes, as described (21). Sera for avidity/PRNT correlations, competition binding assays, and passive transfer experiments were standardized for anti-F antibody levels prior to use. For competition assays, we used palivizumab at a dose of 1ng per well. RSV-specific neutralization was performed using volumes of 200 µl per sample. Sera from uninfected mice were assayed as controls.
Passive transfer experiments
BALB/c mice received via tail vein injection a maximum of 200µl of sera containing equal anti-F antibody levels. The control group received 200µl of naïve sera. One day later, we inoculated all animals IN with 106 pfu of wt RSV. Four days after infection, we removed the lungs and performed virus titration as described above. For B cell transfer experiments, we injected 7 × 106 purified B cells from C57BL/6 or Myd88−/− mice i.v. into Myd88−/− or µMT mice, respectively.
Flow cytometry
We stained cells for flow cytometry via standard procedures, and used antibody to mouse CD11c (BD Biosciences), CCR7 (BioLegend), CD40, CD80 and CD86 (all from BD Biosciences) to characterize DC activation. Antibody to mouse CD4 and CD154 (BD Biosciences) were used for T cell analyses. Germinal center cells were detected using antibody to mouse CD45R/B220 and GL7 (BD Biosciences). Cells were evaluated using FACScalibur (BD Biosciences) and analyzed using CELLQuest software (BD Biosciences).
Lymphoproliferation assay
We isolated CD4+ T lymphocytes from popliteal nodes and incubated them for 48h in the presence of phytohemagglutinin (PHA). Then, we added a pulse of BrdU (10 µM, Roche) to each well for the last 18 h of the assay and detected proliferation by ELISA (Roche).
ELISPOT assays
We performed ELISPOT assays in freshly obtained lymph node cells as previously described (25).
Western Blots
We obtained purified CD11c+ cells (Miltenyi Biotec) from popliteal nodes of mice belonging to various experimental groups, and detected IκBα with a rabbit anti- IκBα antibody (Santa Cruz Biotechnology), followed by a HRP-conjugated anti-rabbit IgG (Santa Cruz Biotechnology) and SuperSignal Chemiluminescent Substrate (Pierce).
CD8+ T lymphocyte depletion
We depleted CD8+ T lymphocytes by administration of Gk2.43 monoclonal antibody, as previously described (18). An irrelevant monoclonal antibody was used as control.
Statistical analysis
Data were analyzed with statistical software (GraphPad Prism). Comparisons among airway responses were made using analysis of variance for parametric cases. The Kruskal-Wallis test was used for nonparametric comparisons where appropriate. Maturation of avidity after immunization was analyzed by regression analysis. Data are expressed as mean ± SEM. A P<0.05 was considered significant. All experiments were approved by and performed according to guidelines of the Johns Hopkins Medical Institutions and the Infant Foundation (790 words).
Supplementary Material
Acknowledgements
Purified fusion protein (PFP) was a generous gift from V. Randolph, Wyeth Lederle., Gk2.43 monoclonal antibody for CD8+ T lymphocyte depletion was generously provided by B.Graham and T. Johnson, National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank Maria del Carmen Puggioli for excellent technical assistance.
Supported by AI-054952 (FPP) and the Thomas and Carol McCann Innovative Research Fund for Asthma and Respiratory Diseases (FPP). MFD, ACM, JPB and SC are recipients of Doctoral Awards from CONICET, Argentina. GAM is a recipient of the Thrasher Research Fund Early Career Award.
References
- 1.Collins PL, Chanock RM, et al. In: Fields Virology. [fouth edition] Knipe DM, Howley PM, editors. NY, USA: Raven Press; 2001. pp. 1443–1486. [Google Scholar]
- 2.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory Syncytial virus. Am. J. Dis. Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 3.Shay DK, et al. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 4.Kim HW, et al. Respiratory Syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 5.Polack FP, et al. A role for immune complexes in enhanced respiratory Syncytial virus disease. J. Exp. Med. 2002;196:859–865. doi: 10.1084/jem.20020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham BS, et al. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory Syncytial virus. J. Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 7.Moghaddam A, et al. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat. Med. 2006;12:905–907. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- 8.Srikiatkhachorn A, Braciale TJ. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory Syncytial virus infection. J. Exp. Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connors M, et al. Pulmonary histopathology induced by respiratory Syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J. Virol. 1992;66:7444–7451. doi: 10.1128/jvi.66.12.7444-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connors M, et al. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J. Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connors M, et al. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia--RSV recombinants or RSV. Vaccine. 1992;10:475–484. doi: 10.1016/0264-410x(92)90397-3. [DOI] [PubMed] [Google Scholar]
- 12.Murphy BR, Sotnikov AV, Lawrence LA, Banks SM, Prince GA. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3–6 months after immunization. Vaccine. 1990;8:497–502. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- 13.Murphy BR, Walsh EE. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J Clin Microbiol. 1988;26:1595–1597. doi: 10.1128/jcm.26.8.1595-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prince GA, et al. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactivated virus. J Virol. 1986;57:721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu H, et al. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol. Biol. 2007;368:652–665. doi: 10.1016/j.jmb.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 16.Johnson S, et al. A direct comparison of the activities of two humanized respiratory syncytial virus monoclonal antibodies: MEDI-493 and RSHZl9. J Infect Dis. 1999;180:35–40. doi: 10.1086/314846. [DOI] [PubMed] [Google Scholar]
- 17.American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn, Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics. 2003;112:1442–1446. [PubMed] [Google Scholar]
- 18.Graham BS, et al. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy BR, et al. Immunization of cotton rats with the fusion (F) and large (G) glycoproteins of respiratory syncytial virus (RSV) protects against RSV challenge without potentiating RSV disease. Vaccine. 1989;7:533–540. doi: 10.1016/0264-410x(89)90278-8. [DOI] [PubMed] [Google Scholar]
- 20.Mapletoft JW, et al. Intranasal immunization of mice with a formalin-inactivated bovine respiratory syncytial virus vaccine co-formulated with CpG oligodeoxynucleotides and polyphosphazenes results in enhanced protection. J Gen Virol. 2008;89:250–260. doi: 10.1099/vir.0.83300-0. [DOI] [PubMed] [Google Scholar]
- 21.Polack FP, Hoffman SJ, Crujeiras G, Griffin DE. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nature Med. 2003;9:1209–1213. doi: 10.1038/nm918. [DOI] [PubMed] [Google Scholar]
- 22.Bachmann MF, et al. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2027. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 23.Fleury D, et al. A complex of influenza hemagglutinin with a neutralizing antibody that binds outside the virus receptor binding site. Nat. Struct. Biol. 1999;6:530–534. doi: 10.1038/9299. [DOI] [PubMed] [Google Scholar]
- 24.Arbiza J, et al. Characterization of two antigenic sites recognized by neutralizing monoclonal antibodies directed against the fusion glycoprotein of human respiratory syncytial virus. J. Gen. Virol. 1992;73:2225–2234. doi: 10.1099/0022-1317-73-9-2225. [DOI] [PubMed] [Google Scholar]
- 25.Melendi GA, et al. C5 modulates airway hyperreactivity and pulmonary eosinophilia during enhanced respiratory syncytial virus disease by decreasing C3a receptor expression. J. Virol. 2007;81:991–999. doi: 10.1128/JVI.01783-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 27.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 28.Rudd BD, et al. Type I interferon regulates respiratory virus infected dendritic cell maturation and cytokine production. Viral Immunol. 2007;20:531–540. doi: 10.1089/vim.2007.0057. [DOI] [PubMed] [Google Scholar]
- 29.Rudd BD, et al. MyD88-mediated instructive signals in dendritic cells regulate pulmonary immune responses during respiratory virus infection. J Immunol. 2007;178:5820–5827. doi: 10.4049/jimmunol.178.9.5820. [DOI] [PubMed] [Google Scholar]
- 30.Smit JJ, et al. The balance between plasmacytoid DC versus conventional DC determines pulmonary immunity to virus infections. PLoS ONE. 2008;3:e1720. doi: 10.1371/journal.pone.0001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polack FP, et al. The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proc Natl Acad Sci USA. 2005;102:8996–9001. doi: 10.1073/pnas.0409478102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 33.Kurt-Jones EA, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 34.Rudd BD, et al. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J. Immunol. 2006;176:1937–1942. doi: 10.4049/jimmunol.176.3.1937. [DOI] [PubMed] [Google Scholar]
- 35.Lindemans CA, et al. Respiratory syncytial virus inhibits granulocyte apoptosis through a phosphatidylinositol 3-kinase and NF-kappaB-dependent mechanism. J. Immunol. 2006;176 doi: 10.4049/jimmunol.176.9.5529. 5529–2237. [DOI] [PubMed] [Google Scholar]
- 36.Gavin AL, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kool M, et al. Cutting Edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 38.Polack FP, et al. Production of atypical measles in rhesus macaques: evidence for disease mediated by immune complex formation and eosinophils in the presence of fusion-inhibiting antibody. Nature Med. 1999;5:629–634. doi: 10.1038/9473. [DOI] [PubMed] [Google Scholar]
- 39.Brokstad KA, et al. Cross-reaction but no avidity change of the serum antibody response after influenza vaccination. Vaccine. 1995;13:1522–1528. doi: 10.1016/0264-410x(95)00095-i. [DOI] [PubMed] [Google Scholar]
- 40.Knossow M, et al. Mechanism of neutralization of influenza virus infectivity by antibodies. Virology. 2002;302:294–298. doi: 10.1006/viro.2002.1625. [DOI] [PubMed] [Google Scholar]
- 41.Barbey-Martin C, et al. An antibody that prevents the hemagglutinin low pH fusogenic transition. Virology. 2002;294:70–74. doi: 10.1006/viro.2001.1320. [DOI] [PubMed] [Google Scholar]
- 42.Sauter NK, et al. Hemagglutinins from two influenza virus variants bind to sialic acid derivatives with millimolar dissociation constants: a 500-MHz proton nuclear magnetic resonance study. Biochemistry. 1989;28:8388–8396. doi: 10.1021/bi00447a018. [DOI] [PubMed] [Google Scholar]
- 43.Kolokoltsov AA, et al. Small interfering RNA profiling reveals key role of clathrin-mediated endocytosis and early endosome formation for infection by respiratory syncytial virus. J Virol. 2007;81:7786. doi: 10.1128/JVI.02780-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu P, et al. Retinoic acid-inducible gene I mediates early antiviral response and toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol. 2007;81:1401. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhoj VG, et al. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc Natl Acad Sci USA. 2008;105:14046–14051. doi: 10.1073/pnas.0804717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hussell T, Baldwin CJ, O’Garra A, Openshaw PJ. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur. J. Immunol. 1997;27:3341–3349. doi: 10.1002/eji.1830271233. [DOI] [PubMed] [Google Scholar]
- 47.Neuzil KM, et al. Adjuvants influence the quantitative and qualitative immune response in BALB/c mice immunized with respiratory syncytial virus FG subunit vaccine. Vaccine. 1997;15:525–532. doi: 10.1016/s0264-410x(97)00218-1. [DOI] [PubMed] [Google Scholar]
- 48.García-Barreno B, et al. Marked differences in the antigenic structure of human respiratory syncytial virus F and G glycoproteins. J Virol. 1989;63:925–932. doi: 10.1128/jvi.63.2.925-932.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.