Abstract
Infections are a devastating complication of titanium alloy orthopedic implants. Current therapy includes antibiotic-impregnated bone cement, and antibiotic-containing coatings. We hypothesized that daptomycin, a Gram-positive peptide antibiotic, could prevent bacterial colonization on titanium alloy surfaces if covalently bonded via a flexible, hydrophilic spacer. We designed and synthesized a series of daptomycin conjugates for bonding to the surface of 1.0 cm2 Ti6Al4V foils through bisphosphonate groups, reaching a maximum yield of 180 pmol /cm2. Daptomycin-bonded foils killed 53±5% of a high challenge dose of 3×105 cfu Staphylococcus aureus ATCC 29213.
INTRODUCTION
The introduction of medical implants has revolutionized the field of medicine. Orthopedic implants, dental implants, vascular grafts, heart valves and stents have extended life spans and the quality of life (1). However, implant-associated infections are serious complications of all those medical devices (2). The current arthroplasty infection rate ranges from 1-5%, but the infection rate is much higher in trauma patients and in populations at risk for infection (3). Implant removal is usually the only clinical option because bacterial biofilm formation on the surface of the implant protects the microorganisms from the immune system and antibiotic therapy (4, 5). A number of local antibiotic delivery systems, such as antibiotic-containing polymethylmethacrylate cement (6) or biopolymers (7) have been developed to prevent orthopedic implant-associated infections. These approaches suffer from the limitation that the antibiotics can only be released to the surroundings over a short time. In some cases, the porous material holding the antibiotics has to be removed by a second surgery (2).
In order to address the problems faced by local delivery systems, covalent modification of orthopedic implants has been investigated with a variety of antibacterial materials. Quaternary ammonium salts, such as poly(4-vinyl-N-alkylpyridinium bromide) and 3-(trimethoxysilyl)-propyldimethyloctadecyl ammonium chloride, were covalently bonded to glass, metal and polymer surfaces to provide bactericidal activity (8, 9). An antimicrobial peptide LL-37 has also been attached to the surface of titanium alloy implants to prevent infections (10, 11). The peptide has a broad-spectrum activity against bacteria and fungi through peptide-membrane interactions leading to membrane permeation. Ampicillin has been used to modify the surface of polytetrafluoroethylene to effect surface antibiotic activity (12, 13).
Recently, we reported the covalent modification of titanium powder and titanium alloy pins by vancomycin, a Gram-positive peptide antibiotic that blocks peptidoglycan crosslinking (14), via a flexible, hydrophilic spacer (15, 16). The surfaces of the titanium alloy pins were uniformly oxidized with a hydrogen peroxide/sulfuric acid mixture, then functionalized with aminopropyltriethyoxysilane. After amide bonding of two aminoethoxyethoxyacetates to the surface aminopropyl groups, vancomycin was coupled to the titanium alloy surface via a third amide bond. The covalently attached vancomycin prevented Staphylococcus aureus (17) and S. epidermidis (18) colonization and biofilm formation on the titanium alloy surfaces.
This approach should be effective against most Gram-positive infections today, since resistance to vancomycin is relatively rare. Nevertheless, resistance to vancomycin is becoming more prevalent (19, 20), and we must prepare for the time when vancomycin will be much less effective. Treatment with daptomycin, a recently discovered antibacterial cyclic lipopeptide (21), might be a candidate for a second generation antibiotic as there have been no reports yet of resistance. Daptomycin appears to function by penetrating the bacterial cell membrane and causing rapid depolarization, resulting in a loss of membrane potential, leading to inhibition of protein, DNA and RNA synthesis, thereby killing the bacteria (22).
Bisphosphonates bind strongly to metal surfaces and bone tissue (23, 24), and have been successfully integrated into bone-specific drug delivery systems to target protein, peptide, estrogen, antibiotics, antitumor and radioactive pharmaceutics to bone tissue (25). Recently, bisphosphonate has been conjugated with polyethylene glycol or alkanes to directly modify titanium surfaces (26), and an RGD peptide was bound to the titanium implants by conjugating with four phosphonic acid groups (27). Because of the strong and selective affinity of phosphonates to the oxidized surface of titanium (25), we hypothesized that a phosphonate derivative of daptomycin could bond readily to the surface of Ti6Al4V titanium alloy to form an antibiotic monolayer. This approach could reduce the number of chemical synthetic steps required, relative to our vancomycin construct (16), and make sterilization feasible during production.
MATERIALS AND METHODS
Materials
Daptomycin was purchased from Cubist Pharmaceuticals. Fmoc-Lys(εBoc)-OH, HATU were purchased from Novobiochem. Bromoacetic acid, CF3CO2H, CH2Cl2, CH3CN, DCC, Et2O, EtOAc, hexane, H2NEtNH2, iPr2NEt, iPrOH, iPr3SiH, Me2NCHO, and MeOH were purchased from Sigma-Aldrich. Müller-Hinton broth was purchased from Becton-Dickinson. Mal-dPEG®4 NHS ester (#10214) (succinimidyl-TEG-maleimide) was purchased from Quantum Biodesign Ltd. 2-(2-mercaptoethylamino) ethylidene-1,1-bisphosphonic acid (1), prepared as described (28) (Scheme 1) was the generous gift of Dr. Ivan S. Alferiev of the Children’s Hospital of Philadelphia.
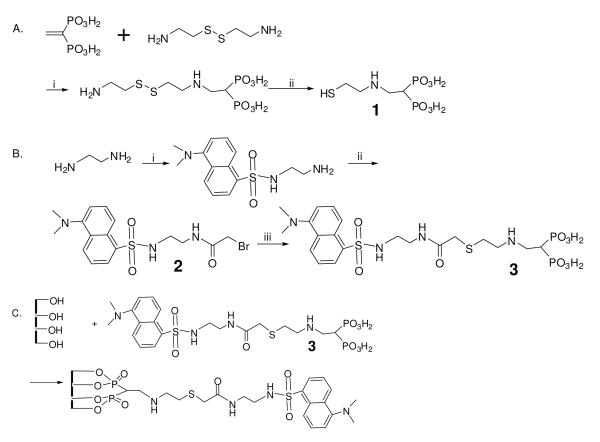
Scheme 1.
Synthesis of dansyl bisphosphonic acid (3) and attachment to the oxidized surface of Ti6Al4V foil. A. 2-(2-mercaptoethylamino) ethylidene-1,1-bisphosphonic acid (1): (i) water, 110°C, 6 hr. (ii) Me3P. B. Dansyl bisphosphonic acid (3): (i) dansyl chloride, rt 2 hr. (ii) bromoacetic acid, DCC, EtOAc. (iii) 2-(2-mercaptoethylamino)ethylidene-1,1-bisphosphonic acid (1), 50% Me2NCHO/50% water. C. Attachment to Ti6Al4V foil.
Chromatography
Normal phase flash chromatography of crude products was carried out on 2.5 × 60 cm silica gel column eluted with 75% hexane/25% EtOAc or 95% CH2Cl2/5% MeOH.
Preparative reversed phase high performance liquid chromatography (HPLC) of intermediates was performed on an Alltech Alltima C18 5μ column, 250×22 mm, coupled to a UV detector at 254 nm, eluted with a linear gradient of 10-50% CH3CN in water containing 0.1% CF3CO2H in 30 min at a flow rate 6 mL/min. The analysis was performed with a Waters 600E system, using Alltech C18 5μ, 250×4.6 mm column coupled to a Waters 486 detector at 254 nm. Sample was eluted with a linear gradient of 20-70% CH3CN in water containing 0.1% CF3CO2H in 30 min with a flow rate of 1mL/min. Thin layer chromatography of intermediates was done on Baker Si250F silica gel TLC plates developed with MeOH/CH2Cl2 solvent system, then visualized with a Mineralight 254 nm lamp.
Apparatus
1H NMR spectra were recorded with a Varian INOVA 400 MHz spectrometer (Palo Alto, CA). Molecular weight analysis was performed on an Ettan MALDI-TOF mass spectrometer (GE Healthcare, Piscataway, NJ). Lyophilization was performed using a Christ Alpha 2–4 freeze-dryer (Martin Christ, Osterode am Harz, Germany) equipped with a Pfeiffer Vacuum Pump (Asslar, Germany). Fluorescence readings were performed on a QuantaMaster double monochromator fluorimeter (Photon Technologies, Birmingham, NJ). Deionized water was produced using a Milli-Q water purification system (Millipore, Bedford, MA).
Synthesis
1-Bromoacetyl-2-dansyl-ethylenediamine (2)
To 20 mL of H2NEtNH2 was added dansyl chloride (1.079 g, 4 mmol) in 30 min under stirring (Scheme 1). The reaction was stirred for 2 hr at room temperature, followed by removal of unreacted H2NEtNH2 under reduced pressure. The residual syrup was dissolved in 20 mL EtOAc, washed with saturated aqueous Na2CO3 solution (3 × 5 mL), and water (3 × 5 mL). The organic phase was dried with Na2SO4, filtered, and the solvent was removed by rotary evaporation. The residual product was redissolved in dry EtOAc, followed by addition of bromoacetic acid (555.6 mg, 4 mmol) and DCC (824 mg, 4 mmol). The mixture was stirred at room temperature for 3 hr, after which the precipitate was removed by filtration. The filtrate was then washed with saturated aqueous Na2CO3 solution (3 × 5 mL), and water (3 × 5 mL). The organic phase was dried with Na2SO4. The desired product (2) was purified by flash chromatography on a silica gel column eluted with 75% hexane/25% EtOAc. Yield: 1.57 g (94.7%). 1H NMR (400 MHz, CDCl3), δ = 8.62 (d, 1H), 8.25-8.30 (m, 2H), 7.55-7.62 (m, 2H), 7.25 (br, 1H), 6.71 ( br, 1H), 5.33 (br, 1H), 3.69 (s, 1H), 3.33-3.37 (m, 2H), 3.07-3.11 (m, 2H), 2.94 (s, 6H). 13C NMR (100 MHz, CDCl3), δ = 166.8, 155.0, 153.6, 134.5, 130.8, 130.0, 129.6, 128.8, 123.7, 118.6, 115.7, 45.8, 43.2, 40.1, 28.8. MALDI-TOF MS, calc. 414.32 Da, found 414.52 Da.
Dansyl-bisphosphonic acid (3)
2-(2-mercaptoethylamino)ethylidene-1,1-bisphosphonic acid (1) (265 mg, 1 mmol) was dissolved in 5 mL of 67% Me2NCHO/33% water and adjusted the pH to 7.0 by NaOH under the protection of argon. Then 1-bromoacetyl-2-dansyl-ethylenediamine (2) (414 mg, 1 mmol) was dissolved in 2 mL Me2NCHO and added dropwise to (1) under the protection of argon (Scheme 1). The mixture was stirred at room temperature for 2 hr. The solvent was then removed by rotary evaporation under vacuum. Finally the residue was dissolved in 10% aqueous CH3CN and purified by preparative reversed phase HPLC. The eluent peak corresponding to the product 2-(9-dansyl-6, 9-diaza-5-oxo-3-thia-nonanyl)-ethylidene-1,1-bisphosphonic acid (3) was pooled, concentrated and lyophilized. 1H NMR (400 MHz, Me2SO), δ =8.48 (d, 1H), 8.29 (d, 1H), 8.13-8.11 (m, 2H), 8.05 (t, 1H), 7.67-7.60 (m, 2H), 7.29 (d, 1H), 3.34-3.27(m, 2H), 3.22 (t, 2H), 3.12-3.09 (m, 4H), 2.85 (s, 6H), 2.85-2.83 (m, 4H), 2.52-2.51 (m, 1H), 2.44-2.36 (m, 1H). 13C NMR (100 MHz, CDCl3), δ = 169.0, 157.9, 151.3, 135.7, 129.5, 129, 128.3, 127.9, 123.7, 119.1, 115,2, 45.6, 45.1, 44.3, 41.7, 40.3, 35.5, 33.5, 27.7. 31P NMR (Me2SO): δ = 17.15 (1H decoupled: s). MALDI-TOF MS, calc. 598.57 Da, found 598.86 Da. Yield: 546 mg (91%).
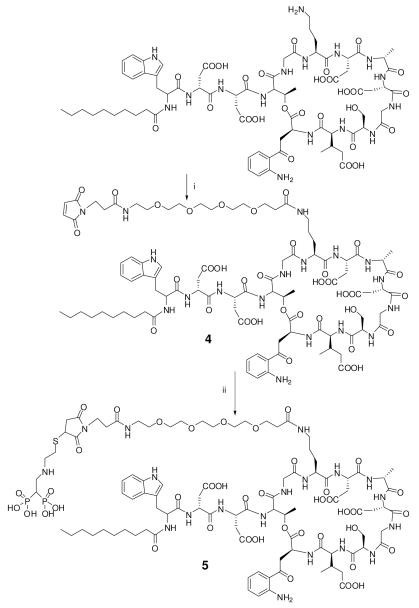
Daptomycin-TEG-maleimide (4)
Daptomycin (100 mg, 0.062 mmol) was dissolved in 1 mL Me2NCHO and added to 1.1 equivalents of succinimidyl-TEG-maleimide (Scheme 2). The mixture was stirred at room temperature overnight. The product was precipitated by Et2O and purified by analytical reversed phase HPLC. The eluent peak corresponding to the product daptomycin-TEG-maleimide (4) was pooled, concentrated and lyophilized. The purified sample was characterized by analytical HPLC (Figure 4B). MALDI-TOF MS, calc. for C90H126N18O35 2020.01 Da, found [M+H]+ 2021.53 Da. Yield: 112 mg (89%).
Scheme 2.
Synthesis of daptomycin-TEG-bisphosphonic acid (5). i) 1.1 equivalent of succinimidyl TEG maleimide, Me2NCHO, r.t. ii) 2-(2-mercaptoethylamino) ethylidene-1,1-bisphosphonic acid (1), 50% Me2NCHO/50% water.
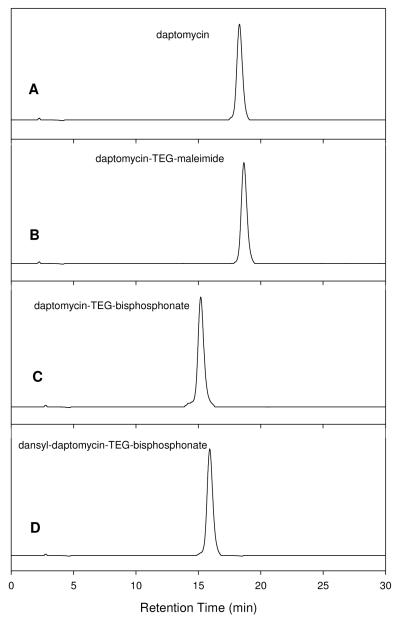
Figure 4.
Analytical HPLC of daptomycin (A), daptomycin-TEG-maleimide (B), daptomycin-TEG-bisphosphonate (C), and dansyl-daptomycin-TEG-bisphosphonate (D). The analysis was performed with a Waters 600E system, using Alltech C18 5μ, 250×4.6 mm column coupled to a Waters 486 detector at 254 nm. Sample was eluted with a linear gradient of 20-70% CH3CN in water containing 0.1% CF3CO2H in 30 min with a flow rate of 1mL/min. The retention times for the peaks in A, B, C, and D were 18.3 min, 18.4 min, 15.5 min and 15.9 min, respectively.
Bisphosphonic acid derivative of daptomycin (5)
To a solution of daptomycin-TEG-maleimide (4) (100 mg, 49.5 μmol) in 1 mL water was added 14.4 mg of 2-(2-mercaptoethylamino)ethylidene-1,1-bisphosphonic acid (1) in 1 mL water (pH 7.0, adjusted by sodium hydroxide) under the protection of argon (Scheme 2). The mixture was stirred at room temperature for 1 hr. The sample was purified by analytical reversed phase HPLC. The eluent peak corresponding to the product daptomycin-TEG-bisphosphonic acid (5) was pooled, concentrated and lyophilized. The purified sample was characterized by analytical HPLC (Figure 4C). MALDI-TOF MS calc. for C94H139N19O41P2S 2285.24Da, found [M+H]+ 2286.13 Da. Yield: 105 mg (93%).
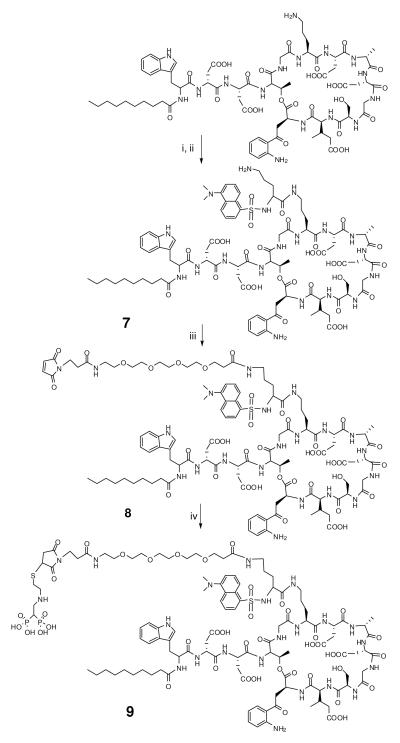
Dansyl-Lys(εBoc)-OH (6)
To a solution of □-H2N-Lys(εBoc)-OH (492.6 mg, 2 mmol) in CH2Cl2 (5 mL) was added 10 mmol of iPr2NEt, followed by addition of 2.1 mmol of dansyl chloride (dissolved in 2 mL CH2Cl2) dropwise over 10 min (Scheme 3). The mixture was stirred at room temperature for 2 hr. The solvent was removed by rotary evaporation, and the residue was dissolved in 10 mL EtOAc, washed with water, then dried with Na2SO4. The solvent was evaporated and the resulting product dansyl-Lys(εBoc)-OH (6) was purified with flash chromatography with 95% CH2Cl2/5% MeOH (Rf = 0.12). 1H NMR (400 MHz, Me2SO), δ = 8.53 (d, 1H), 8.32-8.38 (m, 1H), 8.27 (d, 1H), 7.59-7.49 (m, 2H), 7.20 (d, 1H), 5.93 (s, 1H), 4.43 (s, 1H), 3.97-3.89 (m, 2H) 2.89 (s, 6H), 2.8-2.7 (m, 2H), 1.68-1.6 (m, 2H), 1.38 (s, 9H), 1.22-1.1 (m, 4H). 13C NMR (100 MHz, CDCl3), δ = 174.2, 158.1, 156.2, 151.6, 134.1, 130.7, 129.8, 129.7, 128.4, 123.2, 119.3, 115.3, 81.1, 55.5, 45.8, 39.9, 32, 28.4, 21.7. MALDI-TOF MS calc. for C23H33N3O6S 479.59 Da, found [M+H]+ 480.62 Da. Yield: 765 mg (87%).
Scheme 3.
Synthesis of N-dansyl daptomycin bisphosphonic acid (9). i) Dansyl-Lys (Boc)-NHS ester, Me2NCHO, r.t, overnight. ii) CF3CO2H: water: iPr3SiH (95:2.5:2.5), rt, 1h. iii) succinimidyl-TEG-maleimide, Me2NCHO, rt, overnight. iv) 2-(2-mercaptoethylamino) ethylidene-1, 1-bisphosphonic acid , 50% Me2NCHO/50% water.
Dansyl-Lys-daptomycin (7)
Dansyl-Lys(εBoc)-OH (6) (23.6 mg, 49 μmol), NHS (7.4 mg, 55 μmol), and DCC (11.2 mg 54 μmol), were dissolved in 5 mL anhydrous EtOAc in darkness. After stirring for 2 hr at room temperature, the precipitate was removed by filtration, and the solvent was evaporated. The resultant NHS active ester was dissolved in 1 mL anhydrous Me2NCHO and added to a solution of 100 mg daptomycin in 1 mL Me2NCHO (Scheme 3). The mixture was stirred at room temperature overnight. The product was precipitated by Et2O, sedimented, and dried under vacuum. The residue was incubated with 2 mL 95% CF3CO2H/2.5% water/2.5% iPr3SiH at room temperature for 1 hr to deprotect the Boc group. The deprotected derivative was precipitated by Et2O and purified by analytical reversed phase HPLC. The eluent peak corresponding to the product dansyl-Lys-daptomycin (7) was pooled, concentrated and lyophilized. The purified sample was characterized by analytical HPLC and MALDI-TOF MS. Calc. for C90H125N19O27S, 1937.13 Da, found [M+H]+ 1938.09 Da. Yield: 105 mg (88%).
Dansyl-Lys-daptomycin-TEG-maleimide (8)
Dansyl-Lys-daptomycin (7) (120 mg, 0.062 mmol) was dissolved in 1 mL Me2NCHO and added to 1.1 equivalents of succinimidyl-TEG-maleimide in 1 mL Me2NCHO (Scheme 3). The mixture was stirred at room temperature overnight. The product was precipitated by Et2O and purified by analytical reversed phase HPLC. The eluent peak corresponding to the product dansyl-Lys-daptomycin-TEG-maleimide (8) was pooled, concentrated and lyophilized. The purified sample was characterized by analytical HPLC and MALDI-TOF MS. Calc for C108H149N21O38S, 2381.52 Da, found [M+H]+ 2383.10 Da. Yield: 128 mg (87%).
Dansyl-Lys-daptomycin-TEG-bisphosphonic acid (9)
The conjugation of 2-(2-mercaptoethylamino) - ethylidene-1,1-bisphosphonic acid (1) with 8 to give the dansyl-Lys-daptomycin-TEG-bisphosphonic acid derivative (9) (Scheme 3) was carried out according to the procedure described above for preparing (5). The purified dansyl-Lys-daptomycin-TEG-bisphosphonic acid (9) was characterized by analytical HPLC (Fig. 4D). MALDI-TOF MS: calc. for C112H162N22O44P2S2 2646.68 Da, found [M+H]+ 2648.02 Da.
Methods
Oxidation of Ti6Al4V foil surface
Ti6Al4V foil (0.6mm thickness ) was cut into 1×1cm squares and washed twice with acetone, then once each with double-deionized water, saturated aqueous Alconox, and 0.1 M NaOH. Finally, the foils were washed with 50% concentrated HCl/50% MeOH at room temperature for 30 min with sonication every 5 minutes, followed by three washes with double-deionized water. The cleaned foil surfaces were then oxidized in 50% H2O2/50% concentrated H2SO4 at 4°C for 4 hr. The oxidized foils were washed four times with water, Me2NCHO, and water, dried overnight under vacuum and placed in the argon atmosphere chamber of an MO-20M glove box (29).
Attachment of dansyl-bisphosphonic acid (3) to Ti6Al4V foils
2-(9-Dansyl-6,9-diaza-5-oxo-3-thia-nonanyl)-ethylidene-1,1-bisphosphonic acid (3) (5 mg/mL) was incubated with oxidized Ti6Al4V foils in water (pH was adjusted to 7.0 using 1 M NaOH) at room temperature for 16 hr (Scheme 1) with intermittent shaking.
Stability of dansyl-bisphosphonic acid (3) on the surface of titanium foil
After washing with water and PBS, the dansyl-bisphosphonate foils were immersed in PBS, pH 7.4 and kept at 37°C to investigate the stability of the linkage between the titanium and dansyl. The dansyl-bisphosphonate residues released into the solution were measured by total fluorescence (λex 337 nm and λem 520 nm) (30) at several time points over one month.
After one month of incubation at pH 7.4, 1 M NaOH was added to the PBS buffer to adjust the pH to 12, and the foils were incubated with the basic solution for 4 hr at room temperature, with intermittent sonication, to release all the remaining dansyl moieties from the surface of the foils, as described (30). Dansyl moieties in solution were measured by fluorescence as before. In each batch of foils, the total fluorescence stripped from the foil was treated as 100%.
Attachment of daptomycin-TEG-bisphosphonic acid (5) to Ti6Al4V foils
Daptomycin-TEG-bisphosphonic acid (5) (2 mg, 0.88 □mol) was dissolved in 1 mL water and the pH was adjusted to 7.0 with 1 M NaOH. Six foils (1×1×0.06 cm) were immersed in the solution and kept at room temperature for 16 hr with intermittent gentle shaking, avoiding foil to foil contact. The solution was removed and the foils were washed with water (3×1.5 mL), Me2NCHO (3×1.5 mL), and water (3×2 mL). The foils were dried under vacuum at room temperature.
Fluorescent quantitation of daptomycin derivatives on the titanium foil surface
Dansyl-Lys-daptomycin-TEG-bisphosphonic acid (9) (5 mg/mL) was incubated with 20 Ti6Al4V foils at pH 7 at room temperature for 16 hr, followed by washing with water, Me2NCHO, and water. The modified foils were then incubated with 0.01 M NaOH at room temperature for 4 hr. The fluorescence was recorded with λex 337 nm and λem 520 nm (30), quantitated against a standard curve of known dansyl concentrations, as before (16).
Minimum inhibitory concentrations
Minimum inhibitory concentrations (MIC) were determined by broth microdilution according to NCCLS guidelines (31) except that the Müller-Hinton Broth was supplemented to 50 mg/L Ca2+ and all assays were performed at 37°C with S. aureus ATCC 29213.
Antibacterial activity assay on daptomycin-TEG-bisphosphonate-Ti6Al4V foils
Each dried foil was adhered in place in an individual well of a 24-well plate by low melting point wax with the upper surface uncovered (Figure 1). The upper surfaces of the foils were sterilized with 70% iPrOH for 30 min at room temperature, followed by rinsing with sterile broth three times. Then 200 μL of Müller-Hinton broth (complemented with Ca2+) containing 3×105 cfu were transferred into each well containing a foil, and incubated at 37°C for 16 hr (same time point with that used in MIC test). The broth in the wells was diluted with 800 μL of fresh Müller-Hinton broth and the optical density at 600 nm was recorded. The experiment was carried out with non-immobilized daptomycin-modified foils and immobilized blank foils as controls.
Figure 1.

Each dried Ti6Al4V foil was adhered in place in an individual well of a 24-well plate by low melting point wax for antibiotic activity evaluation with the upper surface uncovered. Left: empty well; right: one piece of Ti6Al4V foil adhered in the well using low melting point wax.
Statistical Analysis
Data were expressed as mean±SE. The significance of differences between means of experimental groups was determined by using Student’s t test.
RESULTS
Design and synthesis of dansyl-bisphosphonic acid (3) as a fluorescent model for Ti6Al4V bonding
The reaction of dansyl chloride with a large excess of H2NEtNH2 provided dansylamidoethylamine as the main product, and the residual H2NEtNH2 could be collected for further use (Scheme 1). The reaction of dansylamidoethylamine and bromoacetic acid gave 1-bromoacetyl-2-dansyl-ethylenediamine (2). The dansyl-bisphosphonic acid derivative (3) was prepared by selective reaction of 1-bromoacetyl-2-dansyl-ethylenediamine (2) with 2-(2-mercaptoethylamino) ethylidene-1,1-bisphosphonic acid (1) under basic conditions (Scheme 1).
Attachment of dansyl-bisphosphonic acid (3) to Ti6Al4V foils
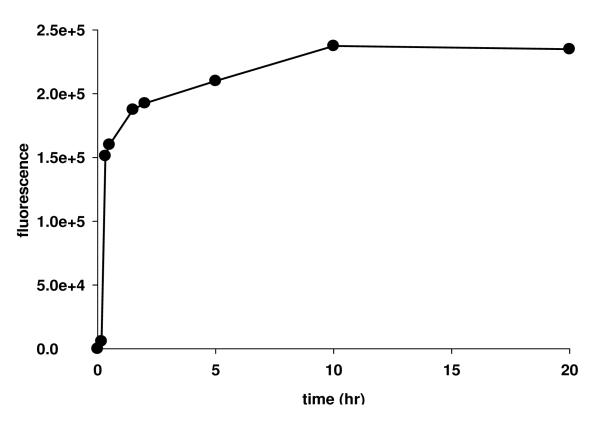
2-(9-Dansyl-6,9-diaza-5-oxo-3-thia-nonanyl)-ethylidene-1,1-bisphosphonic acid (3) (5 mg/mL) was incubated with oxidized Ti6Al4V foils in water (pH was adjusted to 7.0 using 1 M NaOH) at room temperature for 16 hr (Scheme 1) with intermittent shaking. The time course of attachment is shown in Figure 2. Coupling was largely complete by 1 hr, and leveled off by 10 hr.
Figure 2.
Time course of attachment of dansyl bisphosphonic acid bound to the surface of Ti6Al4V foils at different time points. The passivated foils were incubated with dansyl bisphosphonic acid (pH 7.4, 2.5 mg/mL) at room temperature. The foils were removed from the solution at a series of time points, washed with Me2NCHO and water, followed by incubating with 1 mL 0.01 M NaOH at room temperature for 3 hr. The fluorescence in the NaOH solution was recorded with λex 337 nm and λem 520 nm (30).
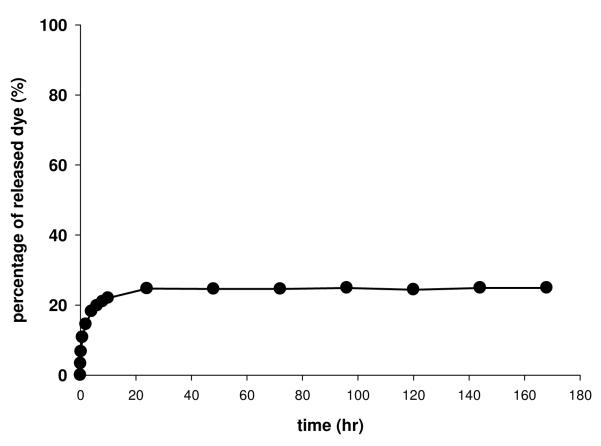
Stability of dansyl-bisphosphonic acid (3) on the surface of titanium foil
The dansyl-bisphosphonate residues released into the, pH 7.4 PBS solution at 37°C were measured by total fluorescence (λex 337 nm and λem 520 nm) (30) at several time points over one month (Figure 3). In each batch of foils, the total fluorescence stripped from the foil was treated as 100%. The data showed that about 25% percent of the dye was released in the first 10 hr of incubation in pH 7.4 phosphate saline buffer. No more dansyl was released to the buffer in one month. It seems likely that the rapidly eluted dansyl moieties were only adsorbed, not covalently bonded. The results imply that the covalently attached dansyl-bisphosphonic acid was stable on the surface of titanium foils in PBS at 37°C.
Figure 3.
Release kinetics of dansyl bisphosphonic acid on the surface of Ti6Al4V foil. The foil was incubated in 1 mL phosphate buffer (pH 7.4) at 37°C. Dansyl fluorescence was recorded with with λex 337 nm and λem 520 nm. The data was expressed as release percentage = (Fsample-F0)/(Ffinal-F0). Fsample: the fluorescence at a certain time point; F0: background of phosphate buffer; Ffinal: fluorescence of the loading dansyl bisphosphonic acid.
Design and synthesis of of bisphosphonic acid derivative of daptomycin (5) for bonding to Ti6Al4V foils
Daptomycin is a cyclic lipopeptide antibiotic with one primary amino group, four carboxylic acid groups and one sensitive indole ring (21). A study of N-acylated ornithine analogs of daptomycin indicated that acylation of the ε-amino group on ornithine did not significantly influence the antibacterial activity (32, 33). Only the ε-amino group on ornithine reacted with NHS active esters. Electron density on the aromatic amine of the daptomycin anthraniloyl residue is more delocalized than usual by the neighboring carbonyl function. The resulting low nucleophilicity greatly disfavors reaction with NHS esters.
In order to increase the flexibility of attached daptomycin on the surface of titanium foil, bifunctional TEG with maleimide and NHS groups was selected to conjugate with daptomycin to form daptomycin-TEG-maleimide (4) overnight in Me2NCHO, after which the maleimide specifically reacted with sulfhydryl group of 2-(2-mercaptoethylamino)ethylidene-1,1-bisphosphonic acid daptomycin-TEG-maleimide (1) at pH 7.0 with a yield of 93% (Scheme 2).
Dansyl-Lys-daptomycin-TEG-bisphosphonic acid (9)
The antimicrobial activity of daptomycin prompted us to prepare dansyl-daptomycin bisphosphonates to enable fluorescent monitoring of foil bonding. In order to introduce the dansyl group, the ε-amine of lysine was coupled with dansyl while the ε-amine side chain of lysine was protected with Boc (Scheme 3). To prepare dansyl-Lys-daptomycin (7), the ε-dansyl-Lys(εBoc(6) was converted to a succinimidyl active ester, and used to acylate the ornithine residue of daptomycin (Scheme 3). The deprotected lysine ε-amino side chain in (7) was reacted with succinimidyl-TEG-maleimide to give dansyl-Lys-daptomycin-TEG-maleimide (8). The conjugation of 2-(2-mercaptoethylamino)ethylidene-1,1-bisphosphonic acid (1) with 8 to give the dansyl-Lys-daptomycin-TEG-bisphosphonic acid derivative (9) (Scheme 3) was carried out according to the procedure described above for preparing (5). The purified dansyl-Lys-daptomycin-TEG-bisphosphonic acid (9) was characterized by analytical HPLC (Fig. 4D)
Fluorescent quantitation of daptomycin derivatives on the titanium foil surface
After confirming the antibacterial activity of attached daptomycin on the surface of titanium foils, dansyl-Lys-daptomycin-TEG-bisphosphonic acid (9) was synthesized, and was used as a model to investigate how much daptomycin was coupled to the solid surface. The daptomycin loading fell in the range of 0.06-0.18 nmol/cm2, below the theoretical maximum of adducts that could be coupled to the surface of titanium foil, 0.22 nmol/cm2 (34).
Antibacterial activity of surface tethered-daptomycin
The MIC of DAP-TEG-bisphosphonic acid (5) was 8.0 μg/mL with S. aureus ATCC 29213 as the model bacterium in calcium-complemented Müller-Hinton broth, compared with an MIC of 0.8 μg/mL for daptomycin (See Supplemental Material). The mass of daptomycin attached to a single 1.0 cm2 Ti6Al4V foil at the maximum observed yield of 0.18 nmol/cm2 is 0.83 μg. If that amount of (5) was released into the 200 μL of broth applied to each foil, the concentration of (5) in solution would only be 4.2 μg/mL, lower than 8.0 μg/mL (MIC of daptomycin-TEG-bisphosphonic acid).
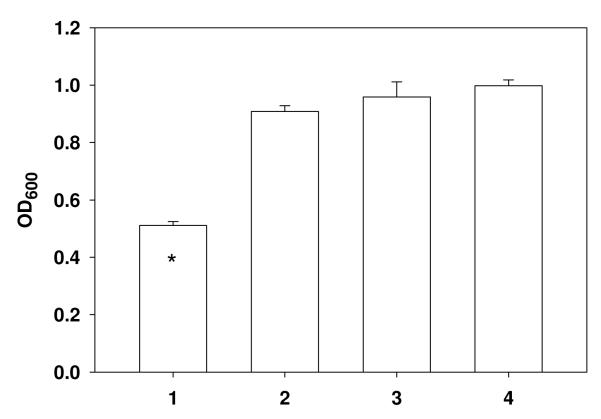
However, the covalently bound daptomycin on the solid phase foil surfaces killed 53±5% of the high challenge dose of 3×105 cfu In the 200 μL of broth applied to each foil (Figure 5). The data showed that there was a significant difference (p=0.00001) between the bacterial growth inhibition activities of the DAP-TEG-bisphosphonate surface and the non-modified control surfaces. No significant antibacterial activity difference (p=0.20, 0.49, 0.20) was observed among the non-modified or non-adhered control surfaces.
Figure 5.
Histogram of antibacterial activity of daptomycin bisphosphonic acid covalently bound to the surface of Ti6Al4V foils. 1: daptomycin modified foils adhered by wax, covered by 200 μL of broth containing 3×105 cfu S. aureus; 2: non-adhered daptomycin modified foils floating in 200 μL of broth containing 3×105 cfu S. aureus; 3: adhered blank foils incubated with 200 μL of broth containing 3×105 cfu S. aureus; 4: 200 μL of broth containing 3×105 cfu S. aureus. Symbol * indicates statistically significant differences (p<0.05) from the other groups. The y-axis is the optical density of the bacteria-containing broth at 600 nm.
DISCUSSION
The gold standard for implants is titanium because of its biocompatibility and osseointegrating ability (35). Much effort has been paid to the surface modification of titanium implants to enhance osseointegration. And biofuctionalization of the titanium implants surface by attaching therapeutic proteins, peptides, or antibiotics have attracted the attention of scientists from metallurgy and medicinal chemistry (36). We have linked vancomycin to titanium powder and pins by aminopropylation of the surface with aminopropyltriethoxysilane and attaching the antibiotics to the functionalized surface via a pair of diethyleneglycol spacers to extend the drug away from the metal surface. The attached antibiotics significantly inhibited the growth of bacteria on the metal surface (15, 16). The extensive chemical manipulations and the incompatibility of metallurgy and organic chemistry prompted us to investigate a more convenient and compatible method. In fact, functionalization of titanium alloy surfaces using a “dip and rinse” protocol that combines the goals of efficacy and convenience is being sought by those working in this area (27).
Bisphosphonate binds strongly to metal surfaces and bone tissue (23, 24), so it became our first choice in the design and preparation of a daptomycin conjugate to be attached to Ti6Al4V alloy through the “dip and rinse” process. The preparation reported here of dansyl-TEG-bisphosphonic acid and its stable attachment to the surface of oxidized Ti6Al4V demonstrates the practicality of “dip and rinse” modification by daptomycin-bisphosphonate conjugates. The bioactivity and stability results indicated that bisphosphonate derivatives of daptomycin could effectively attach to Ti6Al4V foils and provide significant antibacterial activity. It is inevitable that some daptomycin bisphosphonates will dissociate from the metal surfaces over time. However, the dissociated daptomycin bisphosphonates are likely to be trapped by the surrounding bone tissue, thereby reducing systemic distribution, because of the bone-targeting activity of bisphosphonates after systemic administration of drug-bisphosphonate conjugates (25). By changing the number of phosphonates linked with the drugs, the stability might be adjustable in order to meet different requirements.
The selection of antibiotics to be covalently attached to the surface of implants is key to surface antibacterial activity. Current strategies to prevent implant-related infections include antibiotic-eluting bioceramics, drug-impregnated bone cements, and natural and synthetic polymers loaded with antimicrobials (37). Erythromycin, tobramycin, gentamicin, cephalexin, norfloxacin, sulbactam-cefoperazone, sulbactam-ampicillin and antimicrobial peptides have been used in the delivery system. These materials deliver active drugs only from the time of implantation, lasting at most only through the immediate postoperative period, and deliver a relatively low solution concentration.
Covalent attachment of antibiotics to the surface of implants is the new direction in the development of self-protecting bactericidal implants. Amipicillin (38), vancomycin (15) and antimicrobial peptides (11) have been reported to be attached to the surface of implant materials such as polytetrafluoroethylene and titanium alloys. There are several factors that should be taken into consideration to select the antibiotics to be attached to the surface of an implant. The antimicrobial should ideally act at the bacterial cell surface, such as beta-lactams, vancomycin, quaternary ammonium, or antimicrobial peptides. If the site of action is inside the bacterium, permanent bonding would significantly reduce bioactivity. We observed that phenomenon previously when we explored the attachment of minocycline to the surface of Ti6Al4V foils through an acid-labile linkage that could be broken in response to the lower pH caused by a local bacterial infection (39). It will also be valuable to investigate the effect of longer or shorter spacers.
The second important characteristic is that the interaction of the antimicrobial with the bacteria should be non-covalent, so that the antibiotic remains unchanged and active after each bacterial challenge. Even though ampicillin and other beta-lactam antibiotics were effective against Gram-positive and some Gram-negative bacteria, the beta-lactam interaction with bacterial transpeptidases opens the lactam ring to covalently modify the enzyme, so that the antibiotics can only be used once. Daptomycin, however, acts in the bacterial membrane, leading to a change in cell polarity and permeability that kills the bacteria (22). The interaction of daptomycin with bacteria depends only on physical binding. As a result, daptomycin could retain its structural integrity and bactericidal activity against successive bacterial challenges, as we observed with vancomycin bound to titanium alloy surfaces (15, 17, 18).
The daptomycin bonded to the Ti6Al4V surfaces killed 53±5% of the high challenge dose of bacteria suspended in a drop of broth applied to each foil. Under the condition of our experiments, the surface was exposed to 1.5 × 106 cfu/mL, many more than in clinical practice, where most circulating bacteria will be suppressed by systemic antibiotics, and only a limited number of opportunistic bacteria first attack an implant.
In conclusion, bisphosphonic acid derivatives of daptomycin were synthesized. The conjugates were stably attached to Ti6Al4V surfaces and provided antibacterial activity against high doses of S. aureus. The antibiotics can stay bonded to the alloy for an extended period to prevent infections on the surfaces of implants. The integration of bisphosphonates with antibiotics opens a new way for covalent protection of implants.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Richard Wassell and Mr. Zhixian Lu of the Thomas Jefferson University Proteomics Center for assistance in mass spectrometry, and Dr. Ivan S. Alferiev of Division of Cardiology, the Children‘s Hospital of Philadelphia, for his generous gift of 2-(2-mercaptoethylamino) ethylidene-1,1-bisphosphonic acid. This work was supported by a grant from Cubist Pharmaceuticals to E.W., and a grant from the National Institutes of Health (AR051303) to Dr. Irving Shapiro.
Abbreviations
- Boc
t-butyloxycarbonyl
- CF3CO2H
trifluoroacetic acid
- CH2Cl2
dichloromethane
- CH3CN
acetonitrile
- DCC
N,N’-dicyclohexylcarbodiimide
- EtOAc
ethyl acetate
- Et2O
diethyl ether
- Fmoc
fluorenylmethoxycarbonyl
- HATU
2-(7-aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyl-uronium hexa-fluorophosphate
- H2NEtNH2
ethylenediamine
- H2O2
hydrogen peroxide
- H2SO4
sulfuric acid
- HPLC
high performance liquid chromatography
- iPrOH
isopropanol
- iPr2NEt
diisopropylethylamine
- iPr3SiH
triisopropylsilane
- MALDI-TOF
matrix-assisted laser desorption-ionization time-of-flight
- MeOH
methanol
- Me2NCHO
N,N-dimethylformamide
- Me2SO
dimethylsulfoxide
- MIC
minimum inhibitory concentration
- mp
melting point
- Na2CO3
sodium carbonate
- Na2SO4
sodium sulfate
- NHS
N-hydroxysuccinimide
- RGD
arginine-glycine-aspartate
- rt
room temperature
- TEG
tetraethyleneglycol
Footnotes
Conflicts of interest: E.W. is a cofounder of SecureImplant LLC, which might ultimately benefit from the results of this investigation, but did not support the work.
SUPPORTING INFORMATION Supporting Information is available concerning determination of minimum inhbiitory concentrations. This information is available free of charge via the Internet at http://pubs.acs.org/.
REFERENCES
- (1).Moss AJ, Hamburger S, Moore RM, Jr., Jeng LL, Howie LJ. Use of selected medical device implants in the United States, 1988. Adv Data. 1991:1–24. [PubMed] [Google Scholar]
- (2).Garvin KL, Hanssen AD. Infection after total hip arthroplasty: Past, present, and future. J. Bone Joint Surg. 1995;77A:1576–1588. doi: 10.2106/00004623-199510000-00015. [DOI] [PubMed] [Google Scholar]
- (3).Mahan J, Seligson D, Henry SL, Hynes P, Dobbins J. Factors in pin tract infections. Orthopedics. 1991;14:305–308. [PubMed] [Google Scholar]
- (4).Duggan JM, Georgiadis GM, Kleshinski JF. Management of prosthetic joint infections. Infect. Med. 2001;18:534–541. [Google Scholar]
- (5).Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–54. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- (6).Huang YY, Chung TW. Microencapsulation of gentamicin in biodegradable PLA and/or PLA/PEG copolymer. Journal of Microencapsulation. 2001;18:457–465. doi: 10.1080/02652040010019479. [DOI] [PubMed] [Google Scholar]
- (7).Mi FL, Shyu SS, Lin YM, Wu YB, Peng CK, Tsai YH. Chitin/PLGA blend microspheres as a biodegradable drug delivery system: a new delivery system for protein. Biomaterials. 2003;24:5023–5036. doi: 10.1016/s0142-9612(03)00413-7. [DOI] [PubMed] [Google Scholar]
- (8).Isquith AJ, Abbott EA, Walters PA. Surface-bonded antimicrobial activity of an organosilicon quaternary ammonium chloride. Applied and Environmental Microbiology. 1972;24:859–863. doi: 10.1128/am.24.6.859-863.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Tiller JC, Liao CJ, Lewis K, Klibanov AM. Designing surfaces that kill bacteria on contact. Proceedings of the National Academy of Sciences. 2001;98:5981. doi: 10.1073/pnas.111143098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Boman HG. Antibacterial peptides: basic facts and emerging concepts. Journal of Internal Medicine. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- (11).Gabriel M, Nazmi K, Veerman EC, NieuwAmerongen AV, Zentner A. Preparation of LL-37-grafted titanium surfaces with bactericidal activity. Bioconjugate Chem. 2006;17:548–550. doi: 10.1021/bc050091v. [DOI] [PubMed] [Google Scholar]
- (12).Aumsuwan N, Heinhorst S, Urban MW. The effectiveness of antibiotic activity of penicillin attached to expanded poly(tetrafluoroethylene) (ePTFE) surfaces: A quantitative assessment. Biomacromolecules. 2007;8:3525–3530. doi: 10.1021/bm700803e. [DOI] [PubMed] [Google Scholar]
- (13).Aumsuwan N, Danyus RC, Heinhorst S, Urban MW. Attachment of ampicillin to expanded poly(tetrafluoroethylene): surface reactions leading to inhibition of microbial growth. Biomacromolecules. 2008;9:1712–1718. doi: 10.1021/bm800176t. [DOI] [PubMed] [Google Scholar]
- (14).Kahne D, Leimkuhler C, Lu W, Walsh C. Glycopeptide and lipoglycopeptide antibiotics. Chem Rev. 2005;105:425–48. doi: 10.1021/cr030103a. [DOI] [PubMed] [Google Scholar]
- (15).Jose B, Antoci J,V, Zeiger AR, Wickstrom E, Hickok NJ. Vancomycin covalently bonded to titanium beads kills Staphylococcus aureus. Chemistry & Biology. 2005;12:1041–1048. doi: 10.1016/j.chembiol.2005.06.013. [DOI] [PubMed] [Google Scholar]
- (16).Edupuganti OP, Antoci V, Jr., King SB, Jose B, Adams CS, Parvizi J, Shapiro IM, Zeiger AR, Hickok NJ, Wickstrom E. Vancomycin covalently bound to Ti6Al4V pins provides longterm inhibition of Staphylococcus aureus colonization. Bioorganic & Medicinal Chemistry Letters. 2007;17:2692–2696. doi: 10.1016/j.bmcl.2007.03.005. [DOI] [PubMed] [Google Scholar]
- (17).Antoci V, Jr., King SB, Jose B, Parvizi J, Zeiger AR, Wickstrom E, Freeman TA, Composto RJ, Ducheyne P, Shapiro IM, Hickok NJ, Adams CS. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. Journal of Orthopaedic Research. 2007;25:858–866. doi: 10.1002/jor.20348. [DOI] [PubMed] [Google Scholar]
- (18).Antoci V, Jr., Adams CS, Parvizi J, Davidson HM, Composto RJ, Freeman TA, Wickstrom E, Ducheyne P, Jungkind D, Shapiro IM, Hickok NJ. The inhibition of Staphylococcus epidermidis biofilm formation by vancomycin-modified titanium alloy and implications for the treatment of periprosthetic infection. Biomaterials. 2008;29:4684–4690. doi: 10.1016/j.biomaterials.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, Flannagan SE, Kolonay JF, Shetty J, Killgore GE, Tenover FC. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science. 2003;302:1569–71. doi: 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- (20).Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455:804–7. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hancock REW. Mechanisms of action of newer antibiotics for Gram-positive pathogens. The Lancet Infectious Diseases. 2005;5:209–218. doi: 10.1016/S1473-3099(05)70051-7. [DOI] [PubMed] [Google Scholar]
- (22).Steenbergen JN, Alder J, Thorne GM, Tally FP. Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections. J Antimicrob Chemother. 2005;55:283–8. doi: 10.1093/jac/dkh546. [DOI] [PubMed] [Google Scholar]
- (23).Ehrick RS, Capaccio M, Puleo DA, Bachas LG. Ligand-modified aminobisphosphonate for linking proteins to hydroxyapatite and bone surfaces. Bioconjugate Chem. 2008;19:315–321. doi: 10.1021/bc700196q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Auernheimer J, Kessler H. Benzylprotected aromatic phosphonic acids for anchoring peptides on titanium. Bioorganic & Medicinal Chemistry Letters. 2006;16:271–273. doi: 10.1016/j.bmcl.2005.10.018. [DOI] [PubMed] [Google Scholar]
- (25).Zhang S, Gangal G, Uludag H. ‘Magic bullets’ for bone diseases: progress in rational design of bone-seeking medicinal agents. Chemical Society Reviews. 2007;36:507–531. doi: 10.1039/b512310k. [DOI] [PubMed] [Google Scholar]
- (26).Gawalt ES, Avaltroni MJ, Koch N, Schwartz J. Self-assembly and bonding of alkanephosphonic acids on the native oxide surface of titanium. Langmuir. 2001;17:5736–5738. [Google Scholar]
- (27).Auernheimer J, Zukowski D, Dahmen C, Kantlehner M, Enderle A, Goodman SL, Kessler H. Titanium implant materials with improved biocompatibility through coating with phosphonate-anchored cyclic RGD peptides. Chembiochem. 2005;6:2034–2040. doi: 10.1002/cbic.200500031. [DOI] [PubMed] [Google Scholar]
- (28).Alferiev IS, Connolly JM, Levy RJ. A novel mercapto-bisphosphonate as an efficient anticalcification agent for bioprosthetic tissues. Journal of Organometallic Chemistry. 2005;690:2543–2547. [Google Scholar]
- (29).Antoci V, Jr., Adams CS, Hickok NJ, Shapiro IM, Parvizi J. Vancomycin bound to Ti rods reduces periprosthetic infection: preliminary study. Clin Orthop Relat Res. 2007;461:88–95. doi: 10.1097/BLO.0b013e318073c2b2. [DOI] [PubMed] [Google Scholar]
- (30).Silverman BM, Wieghaus KA, Schwartz J. Comparative properties of siloxane vs phosphonate monolayers on a key titanium alloy. Langmuir. 2005;21:225–228. doi: 10.1021/la048227l. [DOI] [PubMed] [Google Scholar]
- (31).Jorgensen JH, Cleeland WA, Craig G, Doern M, Ferraro J, Finegold CM, Hansen SL, Jenkins SG, Novick WJ, Pfaller MA. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd approved standard. National Committee for Clinical Laboratory Standards. 1993;13:1–12. [Google Scholar]
- (32).Hill J, Siedlecki J, Parr I, Morytko M, Yu X, Zhang Y, Silverman J, Controneo N, Laganas V, Li T. Synthesis and biological activity of N-acylated ornithine analogues of daptomycin. Bioorganic & Medicinal Chemistry Letters. 2003;13:4187–4191. doi: 10.1016/j.bmcl.2003.07.019. [DOI] [PubMed] [Google Scholar]
- (33).Siedlecki J, Hill J, Parr I, Yu X, Morytko M, Zhang Y, Silverman J, Controneo N, Laganas V, Li T. Array synthesis of novel lipodepsipeptide. Bioorganic & Medicinal Chemistry Letters. 2003;13:4245–4249. doi: 10.1016/j.bmcl.2003.07.025. [DOI] [PubMed] [Google Scholar]
- (34).Xiao SJ, Textor M, Spencer ND, Wieland M, Keller B, Sigrist H. Immobilization of the cell-adhesive peptide Arg-Gly-Asp-Cys (RGDC) on titanium surfaces by covalent chemical attachment. J Mater Sci Mater Med. 1997;8:867–72. doi: 10.1023/a:1018501804943. [DOI] [PubMed] [Google Scholar]
- (35).Liu X, Chu PK, Ding C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Materials Science and Engineering R: Reports. 2004;47:49–121. [Google Scholar]
- (36).Schliephake H, Scharnweber D. Chemical and biological functionalization of titanium for dental implants. Journal of Materials Chemistry. 2008;18:2404–2414. [Google Scholar]
- (37).Wu P, Grainger DW. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials. 2006;27:2450–2467. doi: 10.1016/j.biomaterials.2005.11.031. [DOI] [PubMed] [Google Scholar]
- (38).Aumsuwan N, Heinhorst S, Urban MW. Antibacterial surfaces on expanded polytetrafluoroethylene; penicillin attachment. Biomacromolecules. 2007;8:713–718. doi: 10.1021/bm061050k. [DOI] [PubMed] [Google Scholar]
- (39).Chen C-P, Zeiger AR, Wickstrom E. Bactericidal activity of extended 9-glycyl-minocycline derivatives. Bioorganic & Medicinal Chemistry Letters. 2007;17:6558–6562. doi: 10.1016/j.bmcl.2007.09.077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.