Abstract
Background
Formal olfactory testing may be useful as a bedside tool to help differentiate between conditions such as atypical parkinsonism, dementia, and psychiatric conditions. However, the neural basis of olfactory dysfunction, the effect of concurrent cognitive deficits on olfactory testing results, and the exact prevalence of olfactory deficits in populations with corticobasal syndrome (CBS) and the frontal variant of frontotemporal dementia (FTD-FV) are to date unclear.
Objective
To assess the prevalence and the neural basis of olfactory recognition deficits in patients with a clinical diagnosis of CBS or FTD-FV.
Design
Retrospective study of clinical, neuropsychological, and imaging data.
Setting
National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland.
Participants
Twenty-five patients with CBS, 22 with FTD-FV, and 12 age-matched control subjects.
Main Outcome Measures
Results of neuropsychological evaluation, formal olfactory recognition testing (University of Pennsylvania Smell Identification Test [UPSIT]), and voxel-based morphometry analysis of structural magnetic resonance images of the brain.
Results
Mean UPSIT percentile scores were 31.6% for the CBS group and 9.5% for the FTD-FV group. The voxel-based morphometry correlations between local gray matter and UPSIT scores showed a significant volume effect in the right midfrontal gyrus for the FTD-FV patients and in the right insula, right midfrontal gyrus, and bilateral inferior frontal gyrus for the patients with CBS. A linear regression analysis of the UPSIT scores revealed as significant predictors the general memory score of the Wechsler Memory Scale and the Boston Naming Test total score for the patients with FTD-FV and the Mattis Dementia Rating Scale total score for the patients with CBS.
Conclusions
Our data showed a more severe olfactory impairment for CBS patients than previously reported. We also showed a significant relationship between formal olfactory recognition testing scores and specific cognitive domains. These findings could be useful to clinically differentiate FTD-FV and CBS from other dementing illnesses and movement disorders.
Olfactory dysfunction is a frequent finding in healthy aging1 and in neurodegenerative diseases.2 Smell testing is often absent from the neurological examination. However, in recent years, some researchers have advocated for a more complete clinical screening of olfactory capabilities.3 This interest in olfaction was motivated by studies showing early olfactory impairments in patients with Parkinson disease,4 sometimes preceding the development of the motor or cognitive features. In this study, we combined formal olfactory recognition testing and voxel-wise analysis of magnetic resonance images (MRIs) to assess the prevalence and neural basis of olfactory recognition deficits in patients with a clinical diagnosis of corticobasal syndrome (CBS) or the frontal variant of frontotemporal dementia (FTD-FV). It has been proposed that olfactory recognition testing could be useful to distinguish CBS from idiopathic Parkinson disease and the early stages of FTD-FV from primary psychiatric disorders.5 However, few studies, based on a limited number of patients, analyzed the prevalence of olfactory recognition impairments and pathophysiological features in these clinical populations.5–7 In these studies, olfactory recognition deficits were found to be in the mild to moderate range for patients with FTD-FV and to be unusual for patients with CBS.
METHODS
PATIENT SELECTION
We studied 25 patients with a diagnosis of CBS (13 women) and 22 patients with a diagnosis of FTD-FV (10 women). Patients’ diagnoses were reached through a consensus decision between a neurologist with movement disorder expertise and a neuropsychologist (J.G.) based on the clinical history and presentation and on the results of paraclinical studies. The patients were recruited and diagnosed and gave consent as detailed in previous studies from our group.8,9 In 13 patients with CBS, the first motor symptoms were localized to the right side, whereas in 12 patients the motor impairments first presented in the left side. Initial motor impairments in all of the patients with CBS consisted of a rigid/akinetic syndrome resistant to therapeutic doses of levodopa. Patients’ data are summarized in Table 1. All aspects of the study were approved by the institutional review board of the National Institute of Neurological Disorders and Stroke. We also recruited 14 healthy volunteers who were matched by age and education to the patients.
Table 1.
Demographic Data
| Mean (SD) |
||
|---|---|---|
| CBS Patients (n=25) | FTD-FV Patients (n=22) | |
| Age, y | 62.0 (9.0) | 60.3 (8.3) |
| Education, y | 14.4 (3.0) | 15.9 (3.0) |
| Disease duration since symptoms onset, y | 4.0 (1.8) | 4.7 (3.2) |
| UPSIT percentile score, % | 31.6 (22.4) | 9.5 (13.0) |
Abbreviations: CBS, corticobasal syndrome; FTD-FV, frontal variant of frontotemporal dementia; UPSIT, University of Pennsylvania Smell Identification Test.
OLFACTORY AND NEUROPSYCHOLOGICAL EVALUATION
Patients’ olfactory recognition capabilities were tested with the University of Pennsylvania Smell Identification Test (UPSIT).10 This test presents suprathreshold concentrations of 40 micro-encapsulated odors in paper strips. For each odorant there are 4 response choices, and the subject is asked to select the response that most accurately describes the perceived odor. All patients also underwent an extensive neuropsychological evaluation; the tests used in this study and the patients’ scores are presented in Table 2. We decided to include in our neuropsychological battery an executive functions test (ie, the Delis-Kaplan Executive Function System sorting test) and some memory measures (ie, the general and associative memory sub-tests of the Wechsler Memory Scale–Third Edition) because both of these cognitive domains have been linked with UPSIT performance in previous studies in clinical populations and in healthy subjects. We also included a naming test (the Boston Naming Test 2) to control for different naming skills, a measure of global cognitive functioning (the Mattis Dementia Rating Scale 2), and a mood disorders self-report questionnaire (the Beck Depression Inventory II). Lezak11 provides a description of the psychometric properties of these tests. The neuropsychological battery was administered in different sessions to take into account possible attentional deficits and to avoid fatigue. Research assistants (including A.L.C.) with extensive experience with patients with FTD and with CBS administered all tests.
Table 2.
Linear Regression Data for Patients With CBS and Patients With FTD-FV
| Mean (SD) | Standardized β Coefficients | t Test | P Value | |
|---|---|---|---|---|
| Patients With CBS | ||||
| Mattis DRS total score | 120.80 (16.90) | 0.49 | 2.24 | .046 |
| Boston Naming Test 2 total correct score | 45.68 (10.50) | −0.01 | −0.05 | .96 |
| WMS general memory score | 44.96 (13.30) | 0.26 | 1.46 | .17 |
| WMS associative memory score | 5.74 (5.22) | −0.21 | −1.39 | .19 |
| Beck Depression Inventory II total score | 12.75 (9.42) | 0.02 | 0.16 | .87 |
| D-KEFS sorting test sorting score | 7.04 (3.15) | 0.05 | 0.27 | .79 |
| Significant VBM clusters gray matter | 0.24 (0.04) | 0.77 | 3.21 | .01 |
| Entorhinal cortex gray matter density | 0.39 (0.05) | 0.64 | 1.11 | .29 |
| Piriform cortex gray matter density | 0.38 (0.05) | −1.19 | −1.92 | .08 |
| Patients With FTD-FV | ||||
| Mattis DRS total score | 111.3 (19.84) | 0.33 | 0.90 | .40 |
| Boston Naming Test 2 total correct score | 16.15 (11.27) | 0.65 | 3.36 | .01 |
| WMS general memory score | 29.75 (12.45) | 0.53 | 3.56 | .01 |
| WMS associative memory score | 6.75 (3.58) | −0.14 | −0.76 | .47 |
| Beck Depression Inventory II total score | 16.15 (11.27) | 0.35 | 1.821 | .11 |
| D-KEFS sorting test sorting score | 4.76 (2.00) | −0.28 | −0.83 | .43 |
| Significant VBM clusters gray matter | 0.12 (0.03) | 0.55 | 2.44 | .04 |
| Entorhinal cortex gray matter density | 0.35 (0.07) | 1.07 | 0.98 | .36 |
| Piriform cortex gray matter density | 0.35 (0.06) | −1.63 | −1.40 | .21 |
Abbreviations: CBS, corticobasal syndrome; D-KEFS, Delis-Kaplan Executive Function System sorting test; DRS, Dementia Rating Scale 2; FTD-FV, frontal variant of frontotemporal dementia; VBM, voxel-based morphometry; WMS, Wechsler Memory Scale–Third Edition.
MRI ACQUISITION AND STATISTICAL ANALYSIS
Three-dimensional spoiled gradient recalled MRI was performed on a 1.5-T scanner (GE Healthcare, Milwaukee, Wisconsin) (at least 120 contiguous sections; section thickness, 1.5 mm; in-plane resolution, 0.9375×0.9375 mm; flip angle, 20°). Images were preprocessed and analyzed according to the voxel-based morphometry (VBM) protocol12 implemented in Statistical Parametric Mapping 5 (http://www.fil.ion.ucl.ac.uk/spm/) upgraded with the VBM5 toolbox. In each analysis we used modulated, normalized gray matter images smoothed with a 12-mm full-width half-maximum kernel. Differences in local gray matter volumes between the age-matched control group and the 2 patient groups were assessed with 2-group t tests. Significance levels for t statistics were set at P<.0001 uncorrected at the voxel level, and only clusters composed of at least 50 voxels were considered significant.13 All of the subsequent analyses were limited to these areas of significant gray matter volume reduction in patient groups compared with controls. We used a liberal uncorrected threshold in the patient vs control contrasts because we had a priori hypotheses about the UPSIT-based analyses that were the main imaging outcome measures of our study.13
Based on previous research on odor physiology,14,15 we used the following a priori regions of interest for our correlations between UPSIT normalized raw scores and local gray matter: the piriform, entorhinal, and prefrontal cortices; the amygdala; the hippocampus; the insula; the caudate; and the thalamus. Each cluster with a volume effect peak that fell inside these regions of interest was considered significant if it survived a whole brain threshold of P<.005 uncorrected and of P<.001 after the application of a small volume correction over a mask composed by all of the a priori regions of interest. Clusters for which the volume effect peak was not included in these regions of interest were considered significant if they survived a threshold of P<.05 corrected for a false discovery rate (FDR). All of these significance thresholds were at the voxel level. The significant clusters’ gray matter density was extracted with the SPM5 toolbox MarsBaR (http://marsbar.sourceforge.net/) and was then used as a predictor in a linear regression analysis of the UPSIT. The piriform and entorhinal cortices gray matter intensities were also added to the linear regression model because they receive primary olfactory afferents and their involvement has been hypothesized in the reduction of olfactory acuity seen in normal aging.16 We also included in the linear regression the following neuropsychological scores: the Mattis Dementia Rating Scale 2 total score, the Boston Naming Test total correct score, the Beck Depression Inventory II total score, the Delis-Kaplan Executive Function System sorting test score, and the general and associative memory scores of the Wechsler Memory Scale.
RESULTS
SMELL TESTING
The UPSIT mean score for both patient groups is presented in Table 1. In the CBS group, 4 patients were classified as anosmic. Four presented with severe hyposmia; 4 with moderate hyposmia; and 5 with mild hyposmia. The remaining 8 were normosmic. In the FTD-FV group, 10 patients were anosmic. Four patients presented with severe hyposmia, 4 with moderate hyposmia, and 3 with mild hyposmia; only 1 was normosmic.
VBM ANALYSIS
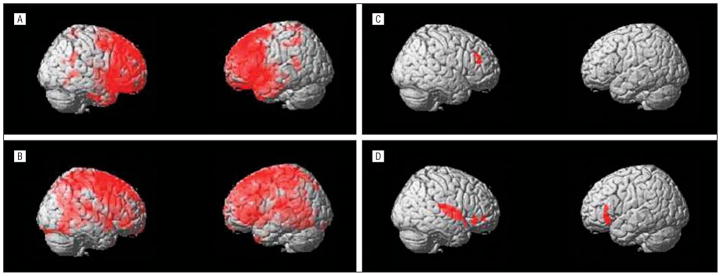
Results of VBM analysis are presented in the Figure and in Table 3. The local maxima of volume loss for the patient vs control contrasts were localized in the right superior and midfrontal gyri and bilaterally in the caudate for the patients with FTD-FV and in the left middle and superior frontal gyri, left postcentral gyrus, and right caudate and thalamus for the patients with CBS.
Figure.
Local volume maxima of volume loss for the voxel-based morphometry analysis contrasts. Areas of local volume reduction in patients with the frontal variant of frontotemporal dementia (FTD-FV) (A) and corticobasal syndrome (CBS) (B) compared with control subjects. Local areas of correlation between local gray matter volume and University of Pennsylvania Smell Identification Test scores in patients with FTD-FV (C) and CBS (D). The statistical thresholds of the individual analysis are described in the “MRI Acquisition and Statistical Analysis” subsection of the “Methods” section.
Table 3.
Local Volume Maxima of Volume Loss for the VBM Analysis Contrastsa
| Cluster Size | Voxel t Value | Voxel z Score | x, y, z Coordinates, mmb | Anatomical Localization |
|---|---|---|---|---|
| CBS Patients vs Healthy Controls | ||||
| 66 233 | 8.55 | 6.35 | −32, 14, 70 | Left midfrontal gyrus, BA 6 |
| 7.81 | 5.99 | −12, −44, 76 | Left postcentral gyrus, BA 3 | |
| 2202 | 7.12 | 5.64 | 8, 10, 16 | Right caudate body |
| 6.46 | 5.27 | 4, −18, 16 | Right thalamus | |
| FTD-FV Patients vs Healthy Controls | ||||
| 31 020 | 6.64 | 5.2 | 58, 40, 22 | Right midfrontal gyrus, BA 46 |
| 6.39 | 5.07 | 34, 28, 58 | Right midfrontal gyrus, BA 8 | |
| 6.39 | 5.06 | 4, 28, 64 | Right midfrontal gyrus, BA 6 | |
| 1391 | 6.4 | 5.07 | 8, 12, 14 | Right caudate body |
| 649 | 5.39 | 4.49 | −4, 0, 14 | Left caudate body |
| Local Gray Matter Volumes and UPSIT Scores in FTD-FV Patients | ||||
| 124 | 3.51 | 3.06 | 54, 36, 32 | Right midfrontal gyrus, BA 46 |
| Local Gray Matter Volumes and UPSIT Scores in CBS Patients | ||||
| 37 | 3.99 | 3.44 | 58, 28, −10 | Right inferior frontal gyrus, BA 47 |
| 604 | 3.95 | 3.42 | 46, 0, 2 | Right insula, BA 13 |
| 3.88 | 3.37 | 52, −26, 16 | Right insula, BA 40 | |
| 3.83 | 3.33 | 40, 16, −16 | Right inferior frontal gyrus, BA 47 | |
| 88 | 3.6 | 3.17 | −54, 26, −8 | Left inferior frontal gyrus, BA 47 |
| 3.39 | 3.02 | −58, 30, 10 | Left inferior frontal gyrus, BA 46 | |
| 31 | 3.34 | 2.98 | 52, 48, −4 | Right midfrontal gyrus, BA 47 |
Abbreviations: BA, Brodmann area; CBS, corticobasal syndrome; FTD-FV, frontal variant of frontotemporal dementia; UPSIT, University of Pennsylvania Smell Identification Test; VBM, voxel-based morphometry.
For all group comparisons and anatomical localizations, the uncorrected P values were .001. The statistical thresholds of the individual analysis are given in the “MRI Acquisition and Statistical Analysis” subsection of the “Methods” section.
All the coordinates reported are according to the Montreal Neurological Institute standard space.
The VBM correlation of local gray matter with normalized UPSIT score revealed for the FTD-FV group a single volume effect in the right midfrontal gyrus, whereas, for the CBS patients, local maxima of volume loss were localized in the right insula, right midfrontal gyrus, and bilateral inferior frontal gyrus.
LINEAR REGRESSION ANALYSIS
The linear regression analysis of the UPSIT raw scores with the predictor variables described in the “Methods” section was significant for the CBS (adjusted R2=0.73; F = 7.123 [P = .02]) and FTD-FV (adjusted R2= 0.74; F=6.143 [P=.01]) groups. The standardized β coefficients for each predictor variable are summarized in Table 2. For the FTD-FV group, the significant predictors of UPSIT scores were the Wechsler Memory Scale general memory score, the Boston Naming Test total correct score, and the gray matter density of the areas found significant at the voxelwise correlation of UPSIT scores and local gray matter values. For the CBS group, the only factors found to be significant predictors of UPSIT scores were the Mattis Dementia Rating Scale 2 total score and the gray matter density of the areas found significant at the voxelwise correlations of UPSIT scores and local gray matter values.
COMMENT
In this study we evaluated the prevalence and the pathophysiological features of olfactory recognition deficits in patients with FTD-FV and patients with CBS. In contrast to other published studies,5–7 we found that half of our patients with CBS presented with olfactory deficits that ranged from moderate hyposmia to complete anosmia. In a published study,6 a raw score of 25 points on the UPSIT has been proposed as an acceptable cutoff choice to help in the differential diagnosis between Parkinson disease and atypical parkinsonism. Moreover, in the same study, the patients with CBS were classified as having normal olfactory recognition capabilities. However, in our study, 6 of 25 patients with CBS presented with an UPSIT raw score below that value. This observed difference could be owing to a larger sample of patients recruited in our study compared with other published studies of CBS.5,6 However, because patients with CBS constitute a pathologically heterogeneous population, another possible source of difference could be the underlying pathological diagnosis. So far, 2 of the patients with CBS in this study received a pathological postmortem diagnosis of cortico-basal degeneration, the most common pathological presentation of CBS. Both were anosmic on the UPSIT. We think that this higher-than-expected olfactory impairment in patients with CBS indicates caution in the use of smell recognition testing for the differential diagnosis of parkinsonism.
Our voxel-wise analysis for the CBS group showed significant correlations between gray matter loss and UPSIT scores in the right insula, right midfrontal gyrus, and bilateral inferior frontal gyrus; these areas have been linked with olfactory processing in different functional MRI studies in healthy controls15 and in subjects with Parkinson disease.17
Our linear regression analysis also showed the Mattis Dementia Rating Scale 2 total score to be a significant predictor of UPSIT scores in patients with CBS; a correlation between global cognitive measures and olfactory function has been described in older individuals with the olfactory deficits associated with a quicker rate of cognitive decline and the development of cognitive dysfunction.18 In line with our VBM results, olfactory deficits have also been linked with the emergence of frontal lobe impairments in healthy aging adults.18 More studies are needed to verify whether olfactory deficits could represent a useful marker to predict the rate of cognitive decline in patients with CBS.
The patients with FTD-FV showed significant olfactory recognition deficits, with more than half of them presenting with severe hyposmia or anosmia. Early diagnosis of FTD is often difficult, and some patients are initially diagnosed as having a mood disorder. It has been shown that smell testing could be useful to distinguish adult-onset depression from neurodegenerative conditions such as Alzheimer disease because of the statistically significant better olfactory function in subjects with adult-onset major depressive disorder.19 Future studies are needed to compare the olfactory impairment between FTD and major depressive disorder and to evaluate the usefulness of olfactory recognition testing in early recognition of FTD-FV.
The only significant correlation between gray matter and UPSIT scores in our FTD-FV group was in the right midfrontal gyrus. The midfrontal gyrus has been linked with olfactory recognition memory in a recent functional MRI study in healthy volunteers16; this observation is consistent with results of our regression analysis, which pointed to a role for memory in UPSIT performance for the FTD-FV cohort. Memory deficits seem to be more severe in patients with FTD-FV than previously thought, with at least 8% of patients with pathologically confirmed FTD-FV presenting with memory deficits in the initial phase of the disease.20 Moreover, memory impairments have been linked with poor olfactory recognition performance both in healthy subjects17,21 and in brain-damaged patients.22 These findings suggest that memory deficits should be formally evaluated when patients with FTD undergo testing for olfactory deficits. We also found a relationship between the Boston Naming Test 2 and UPSIT scores in the FTD-FV group; a correlation between object-naming deficits and olfactory recognition impairments has been shown in healthy aging adults23 and in patients with semantic dementia.5 A recent study5 failed to show a relationship between semantic deficits and olfaction in patients with FTD-FV; compared with that study, however, our patients with FTD-FV presented with a longer disease history and more severe object-naming deficits. Further studies are needed to clarify the role of semantic deficits in olfactory processing in patients with FTD.
None of the local maxima of volume loss reported in our VBM analysis were located in primary olfactory areas such as the entorhinal and piriform cortices, but they were instead included in areas involved in higher-order processing of olfactory information.13,14 These findings indicate that at least some of the olfactory recognition impairments identified in our patient groups are secondary to other cognitive deficits, and thus a thorough neuropsychological evaluation should always accompany olfactory assessment in these patients.
Acknowledgments
Funding/Support: This study was supported by the intramural program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke.
Footnotes
Financial Disclosure: None reported.
Author Contributions: Drs Pardini and Grafman had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Grafman. Acquisition of data: Cavanagh. Analysis and interpretation of data: Pardini and Huey. Drafting of the manuscript: Pardini and Huey. Critical revision of the manuscript for important intellectual content: Pardini, Cavanagh, and Grafman. Statistical analysis: Huey. Administrative, technical, and material support: Cavanagh. Study supervision: Grafman.
References
- 1.Kovács T. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res Rev. 2004;3(2):215–232. doi: 10.1016/j.arr.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Arch Neurol. 1998;55(1):84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- 3.Suchowersky O, Reich S, Perlmutter J, Zesiewicz T, Gronseth G, Weiner WJ Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis and prognosis of new onset Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66(7):968–975. doi: 10.1212/01.wnl.0000215437.80053.d0. [DOI] [PubMed] [Google Scholar]
- 4.Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38(8):1237–1244. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- 5.Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA. Distinct patterns of olfactory impairment in Alzheimer’s disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45(8):1823–1831. doi: 10.1016/j.neuropsychologia.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Wenning GK, Shephard B, Hawkes C, Petruckevitch A, Lees A, Quinn N. Olfactory function in atypical parkinsonian syndromes. Acta Neurol Scand. 1995;91(4):247–250. doi: 10.1111/j.1600-0404.1995.tb06998.x. [DOI] [PubMed] [Google Scholar]
- 7.Müller A, Reichmann H, Livermore A, Hummel T. Olfactory function in idiopathic Parkinson’s disease (IPD): results from cross-sectional studies in IPD patients and long-term follow-up of de-novo IPD patients. J Neural Transm. 2002;109(5–6):805–811. doi: 10.1007/s007020200067. [DOI] [PubMed] [Google Scholar]
- 8.Huey ED, Grafman J, Wassermann EM, et al. Characteristics of frontotemporal dementia patients with a Progranulin mutation. Ann Neurol. 2006;60(3):374–380. doi: 10.1002/ana.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pharr V, Uttl B, Stark M, Litvan I, Fantie B, Grafman J. Comparison of apraxia in corticobasal degeneration and progressive supranuclear palsy. Neurology. 2001;56(7):957–963. doi: 10.1212/wnl.56.7.957. [DOI] [PubMed] [Google Scholar]
- 10.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94(2 pt 1):176–178. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Lezak M. Neuropsychological Assessment. 3. New York, NY: Oxford University Press Inc; 1995. [Google Scholar]
- 12.Ridgway GR, Henley SM, Rohrer JD, Scahill RI, Warren JD, Fox NC. Ten simple rules for reporting voxel-based morphometry studies. Neuroimage. 2008;40 (4):1429–1435. doi: 10.1016/j.neuroimage.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Grossman M, McMillan C, Moore P, et al. What’s in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127(pt 3):628–649. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- 14.Savic I, Gulyas B, Larsson M, Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26(3):735–745. doi: 10.1016/s0896-6273(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 15.Savic I. Imaging of brain activation by odorants in humans. Curr Opin Neurobiol. 2002;12(4):455–461. doi: 10.1016/s0959-4388(02)00346-x. [DOI] [PubMed] [Google Scholar]
- 16.Cerf-Ducastel B, Murphy C. FMRI brain activation in response to odors is reduced in primary olfactory areas of elderly subjects. Brain Res. 2003;986(1–2):39–53. doi: 10.1016/s0006-8993(03)03168-8. [DOI] [PubMed] [Google Scholar]
- 17.Westermann B, Wattendorf E, Schwerdtfeger U, et al. Functional imaging of the cerebral olfactory system in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2008;79(1):19–24. doi: 10.1136/jnnp.2006.113860. [DOI] [PubMed] [Google Scholar]
- 18.Royall DR, Chiodo LK, Polk MS, Jaramillo CJ. Severe dysosmia is specifically associated with Alzheimer-like memory deficits in nondemented elderly retirees. Neuroepidemiology. 2002;21(2):68–73. doi: 10.1159/000048619. [DOI] [PubMed] [Google Scholar]
- 19.Duff K, McCaffrey RJ, Solomon GS. The Pocket Smell Test: successfully discriminating probable Alzheimer’s dementia from vascular dementia and major depression. J Neuropsychiatry Clin Neurosci. 2002;14(2):197–201. doi: 10.1176/jnp.14.2.197. [DOI] [PubMed] [Google Scholar]
- 20.Hodges JR, Davies RR, Xuereb JH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56(3):399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- 21.Economou A. Olfactory identification in elderly Greek people in relation to memory and attention measures. Arch Gerontol Geriatr. 2003;37(2):119–130. doi: 10.1016/s0167-4943(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 22.Moberg PJ, Arnold SE, Doty RL, et al. Olfactory functioning in schizophrenia: relationship to clinical, neuropsychological, and volumetric MRI measures. J Clin Exp Neuropsychol. 2006;28(8):1444–1461. doi: 10.1080/13803390500434409. [DOI] [PubMed] [Google Scholar]
- 23.Westervelt HJ, Ruffolo JS, Tremont G. Assessing olfaction in the neuropsychological exam: the relationship between odor identification and cognition in older adults. Arch Clin Neuropsychol. 2005;20(6):761–769. doi: 10.1016/j.acn.2005.04.010. [DOI] [PubMed] [Google Scholar]