Abstract
OBJECTIVES
The development of oral squamous cell carcinoma (OSCC) is a complex, multistep process. To date, numerous oncogenes and tumor-suppressor genes have been implicated in oral carcinogenesis. Of particular interest in this regard are genes involved in cell cycling and apoptosis, such BRAF, KRAS, and PIK3CA genes.
STUDY DESIGN
Mutations of BRAF, KRAS, and PIK3CA were evaluated by direct genomic sequencing of exons 1 of KRAS, 11 and 15 of BRAF, and 9 and 20 of PIK3CA in OSCC specimens.
RESULTS
Both BRAF and KRAS mutations were detected with a mutation frequency of 2% (1/42). PIK3CA mutations were detected at 3% (1/35).
CONCLUSIONS
This is the first report implicating BRAF mutation in OSCC. Our study supports that mutations in the BRAF, KRAS, and PIK3CA genes make at least a minor contribution to OSCC tumorigenesis, and pathway-specific therapies targeting these two pathways should be considered for OSCC in a subset of patients with these mutations.
Keywords: BRAF, KRAS, PIK3CA, oncogene mutation, hot-spot mutation, oral squamous cell carcinoma, OSCC
BACKGROUND
Oral squamous cell carcinoma (OSCC), a subset of head and neck squamous cell carcinoma (HNSCC), is one of the most common human malignancies worldwide, ranking sixth amongst all human cancers 1. The 5-year survival rate for OSCC is a mere 50%, a figure that has remained relatively unchanged for decades 2. Consequently, there has been an increasing focus on identifying key genetic players that may contribute to OSCC pathogenesis, with the overall goal of preventing onset and progression of disease. Furthermore, such knowledge may aid in refining early detection techniques and in developing novel therapeutic approaches. To date, numerous oncogenes and tumor suppressor genes have been implicated in the development of OSCC. Of interest in this regard are mutations in the oncogenes BRAF, KRAS, and PIK3CA.
BRAF is a serine/threonine kinase of the RAF family, which is an integral part of the RAS-RAF-MEK-ERK-MAP kinase pathway. This pathway plays a role in mediating cellular response to cell growth. Somatic point mutations of BRAF, such as those that occur at hot-spot V599E of its kinase domain, can result in elevated kinase function in BRAF 3. Constitutive ERK activation ensues, which then influences the cell cycle at the G1/S transition via cyclins D and E, and also p21 4. KRAS, a protein within the RAS family, functions in the same pathway as BRAF and is located just upstream to it. KRAS appears to be involved in signal transduction and cell cycle regulation 5–8. To date, there has been only limited investigation of both BRAF and KRAS mutations in HNSCCs. Of note, mutations in the RAS gene family (including H-, K-, and N-RAS) have been implicated in upwards of 30% of all human cancers; however, mutation frequencies within OSCCs are varied (5–50%) and appear to be dependent on the specific RAS gene and interestingly, geographic location of the study population 9–15.
Phosphatidylinositide-3-kinases (PI3K) are a family of enzymes that form inositol lipid products; inositol lipid products play key roles in mediating several intracellular pathways 16. PIK3CA—a heterodimeric, Class 1A enzyme—encodes the p110α catalytic subunit of PI3K, which is located at the human chromosomal site 3q26.3 17. “Hot spot” mutations of this enzyme have been shown to be located at E542K, E545K, and H1047R 18, 19, and result in increased cell survival by inhibiting apoptosis 20. Somatic mutations in PIK3CA have been documented in a number of human cancers, including hepatocellular, breast, gastric, lung, esophageal, ovarian, pancreatic, and head and neck cancers 21–25. Three previous studies have shown the presence of PIK3CA amplification or overexpression in OSCCs 26–28.
There appears to be limited literature documenting BRAF, KRAS, and PIK3CA mutations in both HNSCCs and OSCCs. In this paper, we aimed to examine mutational frequencies of all three genes by polymerase chain reaction (PCR) amplification and direct genomic sequencing in a cohort of OSCC specimens.
PATIENTS AND METHODS
Patients and Tissue Samples
Forty-five formalin-fixed, paraffin-embedded OSCC specimens were retrieved from Columbia University’s Oral Diagnostic Biopsy Service. This is a non-overlapping set of specimens from our previous HNSCC studies 29, 30. The histologic diagnosis and grading of each tumor was verified on hematoxylin-eosin stained slides using the criteria established by the World Health Organization 31. A board-certified Oral Pathologist (Dr. Woo), who was blinded from all clinical data, performed this verification of the specimens. Demographic data, tumor location, and tumor differentiation for all samples analyzed are provided in Table 1. Detailed clinicopathologic information (e.g. TNM staging) was often difficult or impossible to analyze owing to the absence of such data in biopsy-obtained specimens. All procedures were performed with approval from the Institutional Review Board (IRB) of Columbia University Medical Center and in accordance with Health Insurance Portability and Accountability Act (HIPAA) regulations.
Table 1.
Summary of clinicopathologic data and identified mutations in OSCCs
| Case | Gender | Age | Location | Tumor differentiation |
Genes analyzed | Mutation | ||
|---|---|---|---|---|---|---|---|---|
| BRAF | KRAS | PIK3CA | ||||||
| 1 | M | 44 | R buccal mucosa | well | Yes | Yes | Yes | No |
| 2 | F | 82 | Alveolar ridge | well | Yes | Yes | Yes | No |
| 3 | M | 54 | R soft tissue | well | Yes | Yes | Yes | No |
| 4 | F | 73 | Gingiva | moderate | Yes | Yes | Yes | No |
| 5 | F | 57 | R ventrolateral tongue | moderate-poor | Yes | Yes | Yes | No |
| 6 | F | 78 | L maxillary alveolus | moderate-poor | Yes | Yes | Yes | Yes (BRAF) |
| 7 | M | 59 | R lateral tongue | poor | Yes | Yes | Yes | No |
| 8 | M | 71 | R lateral tongue | poor | Yes | Yes | Yes | No |
| 9 | M | 88 | L mandibular mucosa | poor | Yes | Yes | Yes | No |
| 10 | M | 28 | L mandibular alveolar ridge | well | Yes | Yes | Yes | No |
| 11 | F | 62 | R retromolar area | well | No | Yes | No | No |
| 12 | M | 68 | Anterior floor of mouth | well | Yes | Yes | Yes | No |
| 13 | F | 84 | L alveolar ridge | well | Yes | Yes | Yes | No |
| 14 | F | 84 | L buccal mucosa | well-moderate | Yes | Yes | Yes | No |
| 15 | F | 57 | R ventrolateral tongue | well-moderate | Yes | Yes | No | No |
| 16 | F | 62 | R lateral tongue | moderate | Yes | No | No | No |
| 17 | M | 59 | buccal alveolar ridge | moderate | Yes | Yes | Yes | No |
| 18 | M | 75 | R buccal mucosa | moderate | Yes | Yes | No | No |
| 19 | F | 73 | R lateral tongue | moderate | Yes | Yes | No | No |
| 20 | M | 88 | Ventral tongue | moderate-poor | Yes | Yes | Yes | Yes (PIK3CA) |
| 21 | F | 74 | L floor of mouth | poor | Yes | Yes | No | No |
| 22 | M | 60 | L lateral tongue | poor | Yes | Yes | Yes | Yes (KRAS) |
| 23 | F | 91 | L posterior maxilla | well | Yes | Yes | Yes | No |
| 24 | F | 86 | R lateral tongue | moderate | No | Yes | No | No |
| 25 | M | 73 | L floor of mouth | poor | Yes | Yes | Yes | No |
| 26 | M | 75 | R maxillary alveolus | well | Yes | Yes | Yes | No |
| 27 | F | 79 | R mandibular alveolar ridge | well | Yes | Yes | No | No |
| 28 | M | 72 | Floor of mouth | well-moderate | No | Yes | Yes | No |
| 29 | F | 88 | R mandibular alveolar ridge | well-moderate | Yes | Yes | Yes | No |
| 30 | M | 63 | R maxillary alveolus | well-moderate | Yes | Yes | Yes | No |
| 31 | F | 89 | R mandibular region | well, focal poor | Yes | Yes | Yes | No |
| 32 | M | 34 | R lateral tongue | well-moderate | Yes | Yes | Yes | No |
| 33 | M | 83 | R lateral tongue | well | Yes | Yes | Yes | No |
| 34 | M | 80 | Posterior hard palate | well | Yes | Yes | Yes | No |
| 35 | M | 60 | R mandibular retromolar pad | well | Yes | Yes | Yes | No |
| 36 | F | 75 | L lateral tongue | well-moderate | Yes | Yes | Yes | No |
| 37 | F | 70 | L posterolateral tongue | moderate | Yes | Yes | Yes | No |
| 38 | M | 67 | L lateral tongue | moderate-poor | Yes | Yes | Yes | No |
| 39 | F | 38 | R mandibular gingiva | well | Yes | Yes | Yes | No |
| 40 | M | 78 | L anterior floor of mouth | poor | Yes | No | No | No |
| 41 | F | 78 | L floor of mouth | well | Yes | Yes | Yes | No |
| 42 | F | 41 | L border (tongue?) | moderate-poor | Yes | No | No | No |
| 43 | F | 68 | L mandibular retromolar pad | poor | Yes | Yes | Yes | No |
| 44 | F | 75 | R maxillary coronal sulcus | well-moderate | Yes | Yes | Yes | No |
| 45 | F | 77 | R buccal vestibule | well | Yes | Yes | Yes | No |
DNA Samples and Mutation Analysis
Each specimen was microdissected and the genomic DNA was extracted using the QIAmp DNA Mini Kit (California, USA), following the manufacturer’s tissue protocol. All DNA concentrations were subsequently measured by spectrophotometer to ensure the presence of adequate amounts of DNA. PCR amplification of genomic DNA was performed and analyzed for mutations in the following genes: BRAF (exons 11 and 15), KRAS (exon 1), and PIK3CA (exons 9 and 20). These regions included the most common KRAS, BRAF, and PIK3CA mutations previously observed in human cancers 18, 32–34. Direct sequencing of each individual PCR product was then carried out. Each sample, consisting of 40ng of genomic DNA, was amplified with primers that covered the entire coding region and the exon/intron boundaries of the exon to be analyzed (E11F/ E11R and E15F/E15R of BRAF; E1F/E1R of KRAS; E9F/E9R and E20F/E20R of PIK3CA) as per previous studies 29, 35, 36. The genomic sequencing was performed with ABI’s 3100 capillary automated sequencers at the DNA Core Facility of Columbia University Medical Center. Upon analysis of the sequencing results, all mutations were verified by independent PCR analysis and successive reverse-sequencing of the PCR product. PCR primers were also utilized as the sequencing primers. Corresponding normal tissues derived from surrounding nontumorous tissue or from a tumor-free block (as determined by Dr. Woo) served as the normal control for each patient.
RESULTS
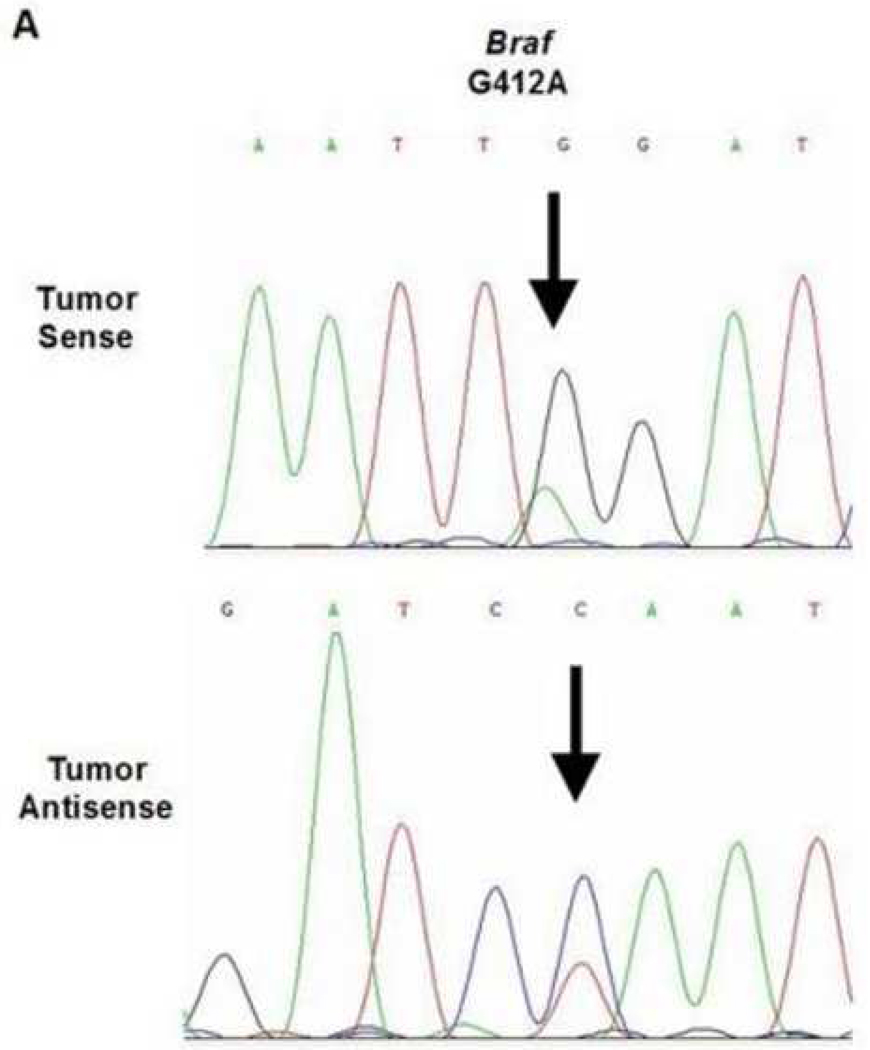
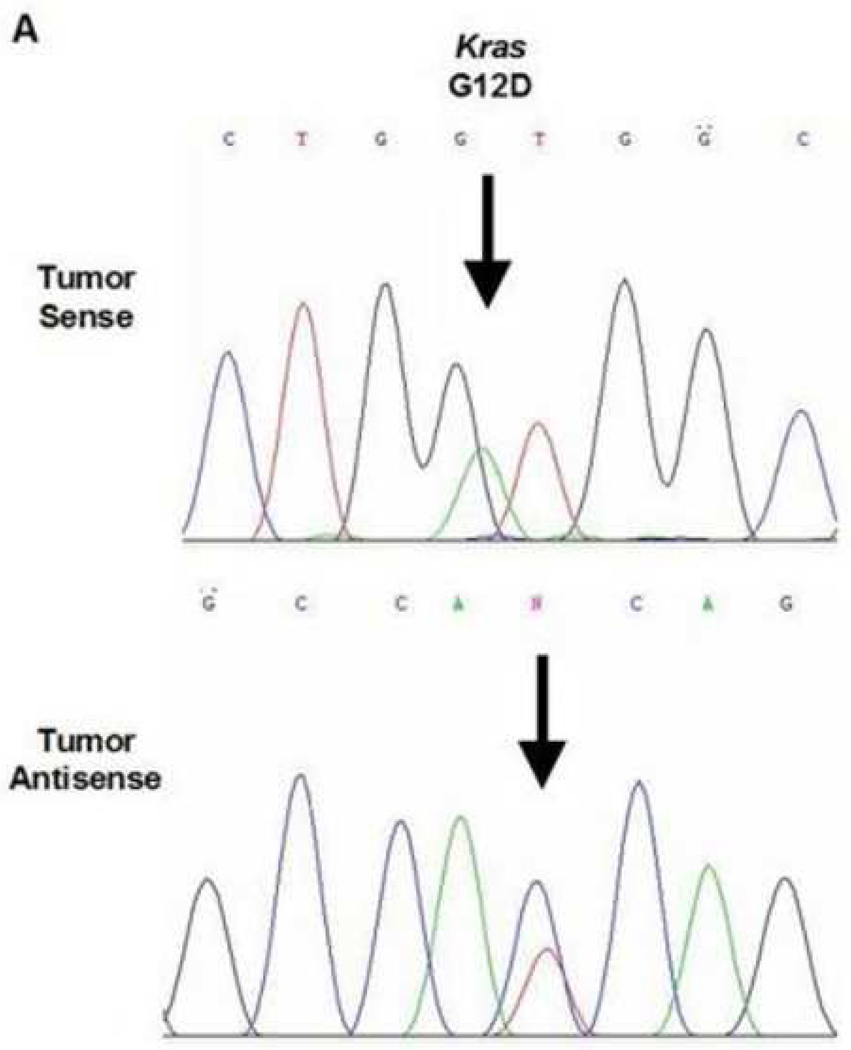
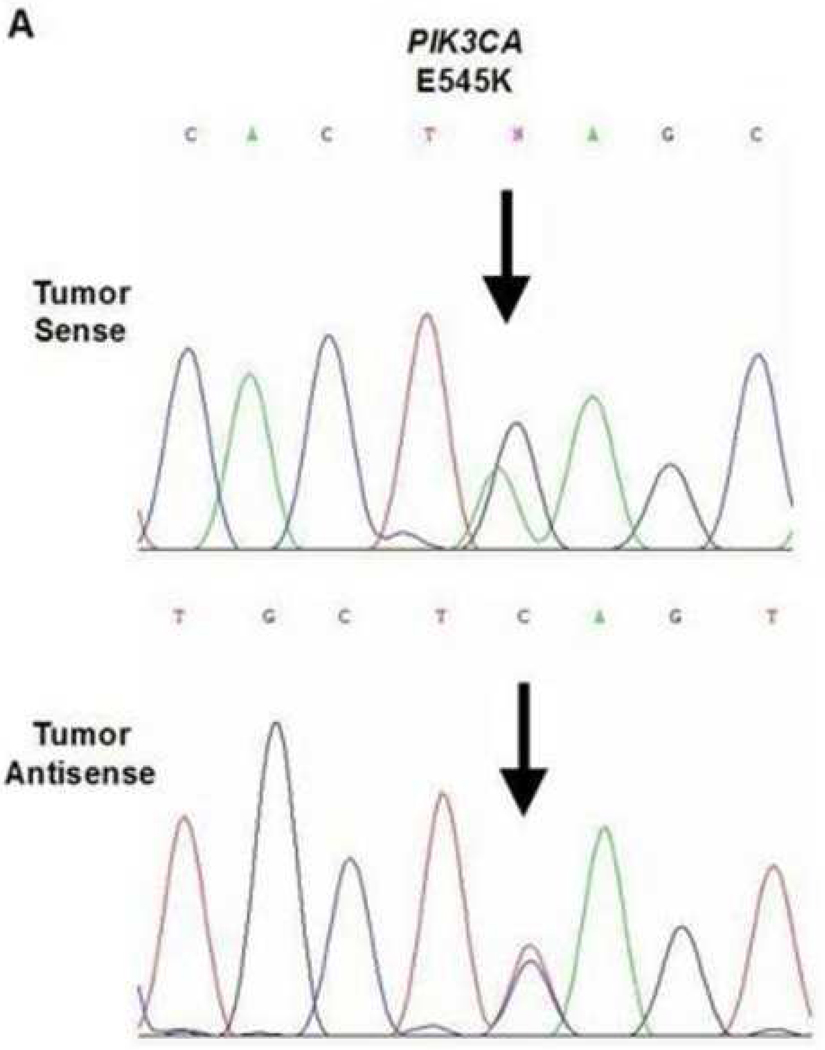
A total of three mutations were found within the 45 cases of OSCC. Due to varying concentrations of DNA in the specimens and varying sensitivities of the primers, each gene that was analyzed had a different sample size. One of the 42 samples analyzed demonstrated a BRAF mutation (2.4%, exon 11, G412A). The specimen containing the mutation was a moderate-to-poorly differentiated OSCC obtained from the maxillary alveolar mucosa (Figure 1). One of the 42 samples analyzed demonstrated a KRAS mutation (2.4%, exon 1, G12D). This specimen was a moderately differentiated OSCC obtained from lateral tongue (Figure 2). Lastly, one of the 35 samples analyzed demonstrated a PIK3CA mutation (3%, exon 9, E545K). This specimen was a moderateto-poorly differentiated OSCC obtained from the ventral tongue (Figure 3).
Figure 1.
BRAF mutation in a maxillary alveolus OSCC. A. Direct genomic sequencing result. All mutations within the nucleotide sequences are indicated by the black arrows. All mutations were verified by a second independent sequencing analysis and anti-sense sequencing.
Figure 2.
KRAS mutation in a lateral tongue OSCC. A. Direct genomic sequencing result. All mutations were verified by a second independent sequencing analysis and anti-sense sequencing.
Figure 3.
PIK3CA mutation in a ventral tongue OSCC. A. Direct genomic sequencing result. All mutations were verified by a second independent sequencing analysis and anti-sense sequencing.
Normal tissue from the specimens containing the PIK3CA, BRAF and KRAS mutations were examined by sequencing analysis. No mutations were detected in the corresponding normal tissues, suggesting that these mutations were somatic in nature.
DISCUSSION
The results of our study show a mutation frequency of 2.4% in BRAF; 2.4% in KRAS; and 2.9% in PIK3CA, respectively. It should be noted that the sample size was variable between the three genes (see Table 1). We believe that this was due to either the variability in tumor cellularity of each specimen or the sensitivity of the primers. Although all three of these genes have been previously implicated in HNSCCs, there is little data regarding their involvement in OSCCs. Shelly et al examined BRAF mutations occurring in exon region 15 in canine oral cancer specimens and found no mutations in their cohort of samples 37. Weber et al investigated both BRAF and KRAS mutations via PCR analysis of genomic DNA in HNSCC of various sites 14. This group demonstrated a 3% mutation frequency of BRAF, also in the exon 15 region, in their pharynx and hypopharynx specimens but none in oral specimens. They also identified a 6% mutation frequency of KRAS, which were found in specimens deriving from the pharynx and floor of mouth. Hoa et al noted overexpression of the KRAS protein by reverse transcriptase-PCR (RT-PCR) in their HNSCC cell lines 38. Also, oncogenic activation of KRAS was previously shown to have a causal role in the development of oral cancer in mice and humans; this was demonstrated via mouse modeling and subsequent RT-PCR 39 and cell transfection assays 40. It is interesting to note that variability in the frequency of KRAS mutations has been linked to ethnicity and certain environmental factors, such as use of chewing tobacco 11, 13, 41–43.
The missense mutation detected in PIK3CA exon 9 in our study was a previously reported “hot spot” mutation for squamous cell carcinoma 44, 45. Our results are in agreement with Samuels et al who confirmed that an increasing number of mutations (>75%) were found to be located in the helical and kinase domains of PIK3CA, which includes exons 9 and 20 18. PIK3CA mutants were noted to have increased lipid kinase activity, seemingly due to alterations in the p110α catalytic subunit, with a subsequent downstream constitutive activation of Akt signaling 18, 19. Mutations—such the E545K mutation—were shown to promote aberrant cell growth in vitro and induce tumorigenesis at a rate of 50% in newly hatched chicks 19. The mutation frequency for PIK3CA for our study is slightly lower than those reported in other SCC studies: namely, 11% in a HNSCC series, and 7.4 and 21.4% in OSCC clinical specimens and cell lines, respectively 29, 44. The reason for this disparity remains unclear to us; although we propose that sample size and geographic differences in the study populations (Asian vs. North American) may play roles.
We do recognize that there are several limitations to this study, including the restricted sample size and lack of clincopathologic data. Investigating the mutational frequencies of these three genes in OSCC resection specimens will be desirable in the near future, where factors of prognostic significance, such as patient-related factors (e.g. ethnicity, tobacco history, etc.) and tumor-related factors (e.g. tumor thickness, perineural invasion, etc.), are more easily accessible. Of particular interest is exploring a possible correlation with histologic differentiation, as the three mutations identified in this study occurred in moderately and poorly differentiated tumors.
Although somatic mutations of KRAS, BRAF, and PIK3CA are not frequent events in OSCC, as suggested by our study, the detection of these mutations is important to support the notion that the RAS-RAF-MEK-ERK-MAP kinase and PIK3CA-PTEN-AKT pathways are involved in OSCC tumorigenesis. The oncogenic activations of these pathways may include additional mechanisms other than small mutations, such as amplification and overexpression. For example, it has been shown previously that PIK3CA is frequently amplified in OSCC 26–28, 44. Although PIK3CA amplification has not been shown to be a useful prognosis marker in OSCC, PIK3CA mutation and amplification have been associated with advanced stages of OSCC and metastasis 28, 44. Future studies should investigate possible roles of PIK3CA in metastasis. Our findings advocate that pathway-specific therapies targeting these two pathways should be pursued in OSCC.
Acknowledgments
This work was supported by the NCI R01CA109525 and the Irving Scholar Award of Columbia University. Dr. Karl C. Bruckman was also supported by the Columbia University College of Dental Medicine Research Assistant fellowship for the time period of 09/2006-05/2008.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kademani D. Oral cancer. [erratum appears in Mayo Clin Proc. 2007; 82:1017] Mayo Clinic Proceedings. 2007;82:878–887. doi: 10.4065/82.7.878. [DOI] [PubMed] [Google Scholar]
- 2.Brunin F, Mosseri V, Jaulerry C, Point D, Cosset JM, Rodriguez J. Cancer of the base of the tongue: past and future. Head & Neck. 1999;21:751–759. doi: 10.1002/(sici)1097-0347(199912)21:8<751::aid-hed11>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Mercer KE, Pritchard CA. Raf proteins and cancer: B-Raf is identified as a mutational target. Biochimica et Biophysica Acta. 2003;1653:25–40. doi: 10.1016/s0304-419x(03)00016-7. [DOI] [PubMed] [Google Scholar]
- 4.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Molecular & Cellular Biology. 1997;17:5598–55611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egan SE, Weinberg RA. The pathway to signal achievement. Nature. 1993;365:781–783. doi: 10.1038/365781a0. [DOI] [PubMed] [Google Scholar]
- 6.Khosravi-Far R, White MA, Westwick JK, Solski PA, Chrzanowska-Wodnicka M, Van Aelst L, et al. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Molecular & Cellular Biology. 1996;16:3923–3933. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khosravi-Far R, Der CJ. The Ras signal transduction pathway. Cancer & Metastasis Reviews. 1994;13:67–89. doi: 10.1007/BF00690419. [DOI] [PubMed] [Google Scholar]
- 8.Moodie SA, Wolfman A. The 3Rs of life: Ras, Raf and growth regulation. Trends in Genetics. 1994;10:44–48. doi: 10.1016/0168-9525(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JA, Irish JC, McLachlin CM, Ngan BY. H-ras oncogene mutation and human papillomavirus infection in oral carcinomas. Archives of Otolaryngology -- Head & Neck Surgery. 1994;120:755–760. doi: 10.1001/archotol.1994.01880310059011. [DOI] [PubMed] [Google Scholar]
- 10.Barbacid M. ras genes. Annual Review of Biochemistry. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 11.Das N, Majumder J, DasGupta UB. ras gene mutations in oral cancer in eastern India. Oral Oncology. 2000;36:76–80. doi: 10.1016/s1368-8375(99)00058-5. [DOI] [PubMed] [Google Scholar]
- 12.Nunez F, Dominguez O, Coto E, Suarez-Nieto C, Perez P, Lopez-Larrea C. Analysis of ras oncogene mutations in human squamous cell carcinoma of the head and neck. Surgical Oncology. 1992;1:405–411. doi: 10.1016/0960-7404(92)90043-k. [DOI] [PubMed] [Google Scholar]
- 13.Saranath D, Chang SE, Bhoite LT, Panchal RG, Kerr IB, Mehta AR, et al. High frequency mutation in codons 12 and 61 of H-ras oncogene in chewing tobacco-related human oral carcinoma in India. British Journal of Cancer. 1991;63:573–578. doi: 10.1038/bjc.1991.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber A, Langhanki L, Sommerer F, Markwarth A, Wittekind C, Tannapfel A. Mutations of the BRAF gene in squamous cell carcinoma of the head and neck. Oncogene. 2003;22:4757–4759. doi: 10.1038/sj.onc.1206705. [DOI] [PubMed] [Google Scholar]
- 15.Williams HK. Molecular pathogenesis of oral squamous carcinoma. Mol Pathol. 2000;53:165–172. doi: 10.1136/mp.53.4.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–254. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 17.Volinia S, Hiles I, Ormondroyd E, Nizetic D, Antonacci R, Rocchi M, et al. Molecular cloning, cDNA sequence, and chromosomal localization of the human phosphatidylinositol 3-kinase p110 alpha (PIK3CA) gene. Genomics. 1994;24:472–477. doi: 10.1006/geno.1994.1655. [DOI] [PubMed] [Google Scholar]
- 18.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 19.Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci U S A. 2005 Jan;102:802–807. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. Journal of Biological Chemistry. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 21.Lee JW, Soung YH, Kim SY, Lee HW, Park WS, Nam SW, et al. PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. Oncogene. 2005;24:1477–1480. doi: 10.1038/sj.onc.1208304. [DOI] [PubMed] [Google Scholar]
- 22.Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, et al. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res. 2005;11:2875–2878. doi: 10.1158/1078-0432.CCR-04-2142. [DOI] [PubMed] [Google Scholar]
- 23.Phillips WA, Russell SE, Ciavarella ML, Choong DY, Montgomery KG, Smith K, et al. Mutation analysis of PIK3CA and PIK3CB in esophageal cancer and Barrett's esophagus. Int J Cancer. 2006;118:2644–2646. doi: 10.1002/ijc.21706. [DOI] [PubMed] [Google Scholar]
- 24.Schonleben F, Qiu W, Ciau NT, Ho DJ, Li X, Allendorf JD, et al. PIK3CA mutations in intraductal papillary mucinous neoplasm/carcinoma of the pancreas. Clin Cancer Res. 2006;12:3851–3855. doi: 10.1158/1078-0432.CCR-06-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu G, Mambo E, Guo Z, Hu S, Huang X, Gollin SM, et al. Uncommon Mutation, but Common Amplifications, of the PIK3CA Gene in Thyroid Tumors. J Clin Endocrinol Metab. 2005;90:4688–4693. doi: 10.1210/jc.2004-2281. [DOI] [PubMed] [Google Scholar]
- 26.Estilo CL, P OC, Ngai I, Patel SG, Reddy PG, Dao S, et al. The role of novel oncogenes squamous cell carcinoma-related oncogene and phosphatidylinositol 3-kinase p110alpha in squamous cell carcinoma of the oral tongue. Clin Cancer Res. 2003;9:2300–2306. [PubMed] [Google Scholar]
- 27.Freier K, Schwaenen C, Sticht C, Flechtenmacher C, Muhling J, Hofele C, et al. Recurrent FGFR1 amplification and high FGFR1 protein expression in oral squamous cell carcinoma (OSCC) Oral Oncol. 2007;43:60–66. doi: 10.1016/j.oraloncology.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Liu CJ, Lin SC, Chen YJ, Chang KM, Chang KW. Array-comparative genomic hybridization to detect genomewide changes in microdissected primary and metastatic oral squamous cell carcinomas. Mol Carcinog. 2006;45:721–731. doi: 10.1002/mc.20213. [DOI] [PubMed] [Google Scholar]
- 29.Qiu W, Schonleben F, Li X, Ho DJ, Close LG, Manolidis S, et al. PIK3CA mutations in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:1441–1446. doi: 10.1158/1078-0432.CCR-05-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu W, Tong G-X, Spiros M, Close LG, Assaad AM, Su GH. Novel mutant-enriched sequencing identified high frequency of PIK3CA mutations in pharyngeal cancer. International Journal of Cancer. 2008;122:1189–1194. doi: 10.1002/ijc.23217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnes L, Eveson JW, Reichart PA, Sidransky D. Pathology and Genetics of Head and Neck Tumours. Lyon: IARC; 2005. Tumors of the oral cavity and oropharynx; pp. 163–175. [Google Scholar]
- 32.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 33.Naoki K, Chen TH, Richards WG, Sugarbaker DJ, Meyerson M. Missense mutations of the BRAF gene in human lung adenocarcinoma. Cancer Res. 2002;62:7001–7003. [PubMed] [Google Scholar]
- 34.Yuen ST, Davies H, Chan TL, Ho JW, Bignell GR, Cox C, et al. Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Cancer Res. 2002;62:6451–6455. [PubMed] [Google Scholar]
- 35.Rumsby G, Carter RL, Gusterson BA. Low incidence of ras oncogene activation in human squamous cell carcinomas. British Journal of Cancer. 1990;61:365–368. doi: 10.1038/bjc.1990.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schonleben F, Qiu W, Bruckman KC, Ciau NT, Li X, Lauerman MH, et al. BRAF and KRAS gene mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/IPMC) of the pancreas. Cancer Lett. 2006;8:8. doi: 10.1016/j.canlet.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shelly S, Chien MB, Yip B, Kent MS, Theon AP, McCallan JL, et al. Exon 15 BRAF mutations are uncommon in canine oral malignant melanomas. Mammalian Genome. 2005;16:211–217. doi: 10.1007/s00335-004-2441-x. [DOI] [PubMed] [Google Scholar]
- 38.Hoa M, Davis SL, Ames SJ, Spanjaard RA. Amplification of wild-type K-ras promotes growth of head and neck squamous cell carcinoma. Cancer Res. 2002;62:7154–7156. [PubMed] [Google Scholar]
- 39.Caulin C, Nguyen T, Longley MA, Zhou Z, Wang XJ, Roop DR. Inducible activation of oncogenic K-ras results in tumor formation in the oral cavity. Cancer Research. 2004;64:5054–5058. doi: 10.1158/0008-5472.CAN-04-1488. [DOI] [PubMed] [Google Scholar]
- 40.Howell RE, Wong FS, Fenwick RG. A transforming Kirsten ras oncogene in an oral squamous carcinoma. Journal of Oral Pathology & Medicine. 1990;19:301–305. doi: 10.1111/j.1600-0714.1990.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 41.Chang SE, Bhatia P, Johnson NW, Morgan PR, McCormick F, Young B, et al. Ras mutations in United Kingdom examples of oral malignancies are infrequent. International Journal of Cancer. 1991;48:409–412. doi: 10.1002/ijc.2910480318. [DOI] [PubMed] [Google Scholar]
- 42.Paterson IC, Eveson JW, Prime SS. Molecular changes in oral cancer may reflect aetiology and ethnic origin. European Journal of Cancer Part B, Oral Oncology. 1996;32B:150–153. doi: 10.1016/0964-1955(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 43.Yarbrough WG, Shores C, Witsell DL, Weissler MC, Fidler ME, Gilmer TM. ras mutations and expression in head and neck squamous cell carcinomas. Laryngoscope. 1994;104:1337–1347. doi: 10.1288/00005537-199411000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Kozaki K, Imoto I, Pimkhaokham A, Hasegawa S, Tsuda H, Omura K, et al. PIK3CA mutation is an oncogenic aberration at advanced stages of oral squamous cell carcinoma. Cancer Sci. 2006;97:1351–1358. doi: 10.1111/j.1349-7006.2006.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scully C, Bagan JV. Recent advances in Oral Oncology. Oral Oncology. 2007;43:107–115. doi: 10.1016/j.oraloncology.2006.12.007. [DOI] [PubMed] [Google Scholar]