The role of portal blood flow in the metabolism of the liver has been the subject of speculation for many years. In the present study, this controversial question has been re-examined with results which indicate that splanchnic venous blood has a specific beneficial effect upon the liver.
METHODS
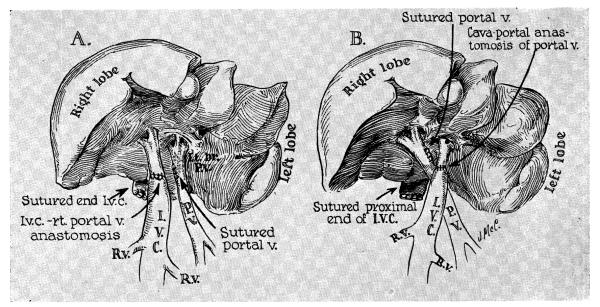
Twenty-two mongrel dogs were used, weighing 9.7 to 26.4 kilograms. Seven of these animals were studied for 70 to 94 days after partial portacaval transposition, which was performed under pentobarbital anesthesia through an upper midline incision (Fig. 1). Either the right (3 experiments) or left (4 experiments) portal vein was detached from the main portal trunk, and revascularized by an end-to-end anastomosis to the supra-adrenal inferior vena cava with No. 6-0 silk. The portal trunk and its other branch were left intact after closure of the lateral defect (Fig. 1) . This procedure divides the liver into two components. One part receives portal blood from the splanchnic venous bed. The other portion receives its portal inflow from systemic sources including the renal and adrenal effluent. Vessel patency was subsequently proved by angiographic and autopsy examinations.
Fig. 1.
Technique of partial portacaval transposition. Note that one portion of the liver receives splanchnic flow but that the other part receives venous inflow from the inferior vena cava. Of 7 chronic animals, 3 were prepared as shown in A and 4 were prepared as shown in B.
Postoperatively these 7 dogs were fed a 1,400 calorie diet, containing 120 Gm. of protein and 35 Gm. of fat. Alkaline phosphatase (Bodansky units), serum glutamic oxalacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), fasting blood sugar, serum bilirubin, and plasma fibrinogen were measured twice a week. Biopsies were obtained under comparable anesthesia just before split transposition and at the time of death from 70 to 94 days later. A specimen was removed from the right and left halves of the liver before placement of any sutures and was fractionated as follows: (1) One gram or more was frozen on dry ice and sent to St. Louis where concentrations2, 10 of glycogen, phosphorylase, glucose-6-phosphatase, acid phosphatase, mutase, and protein were determined. (2) Tissue fractions were fixed in Carnoy’s and formalin solutions for examination by light microscopy. (3) Fragments of the biopsies taken before death were fixed in Palade’s buffered osmium tetroxide solution, embedded in an epoxy resin,* stained with phosphotungstic acid or lead, and examined with a Siemen’s Elmiskop I electron microscope.
At autopsy the liver was dissected free of all attachments and removed. Postmortem angiograms were done to show the absence of cross circulation from one group of lobes to the other. Those lobes supplied by the right branch of the portal vein were then dissected free from those supplied by the left branch and each half weighed separately. The gallbladder was not removed. In order to assess the significance of relative changes in lobar weight, 5 livers from normal dogs were similarly dissected and weighed.
In a previous report,12 evidence was presented that venous inflow to both liver fractions in this preparation was of large volume for at least the first postoperative week. However, the bleed-off method of flow determination then employed was open to some criticism. Accordingly, additional flow measures were done in 8 animals ( 13.6 to 21.3 kilograms) under pentobarbital anesthesia. The two main portal branches were dissected free in the liver hilum. Venous blood flow was measured repeatedly in the individual vessels, and partial transposition was then carried out. One to 4 hours after this had been completed, flow was remeasured in the same locations. A square-wave pulse-field electromagnetic flowmeter* was used with an F-80 X-Y Varian recorder and a snugly fitted flow probe† with a slot cover and intraluminal ground electrode. The system was calibrated‡ in an in vitro model into which a segment of portal vein was incorporated and for which constant blood flow was provided either with a roller pump or controlled gravity. Flow of 5 to 1,000 ml. per minute was measurable with ± 2 percent reproducibility and ± 10 percent of the actual flow; rise time was 10 msec., and electrical zero was stable. The vena caval and aortic pressures were simultaneously monitored with catheters inserted through lumbar vessels.
In 2 final dogs, followed for 56 and 59 days after operation, the right and left portal vein branches, respectively, were detached as described above, and revascularized with an end-to-end autologous jugular vein graft attached to the side of the midabdominal aorta. A similar technique was recently described by Zuidema and associates.19 Total glycogen content of the two sides was measured from biopsies obtained before the animals were put to death. In order to obtain these two test animals with an open arterial anastomosis, 6 dogs were operated upon. The other 4 had thrombosis of the arterial graft, a failure rate similar to that reported by Zuidema and associates.19
RESULTS
Clinical course and biochemical changes after partial transposition
The 7 dogs remained healthy; average weight loss was 0.1 kilogram. Jaundice was not observed. Two animals had increases in SGOT, with a maximum of 105 to 305 SF units between the third and sixth postoperative days. All animals had elevations in SGPT during the first 3 postoperative weeks, ranging from 80 to 1,960 SF units. Four of the 7 dogs also had early rises of alkaline phosphatase levels to 18 to 112 Bodansky units. Serum fibrinogen and blood sugar values were normal.
Gross pathologic observations
In the study by Child and co-workers,3 the 3 lobes supplied by the left portal branch constituted 70 percent of the liver weight in dogs. The 5 normal animals examined in the present study had a similar distribution (Table I). The total weight of the two left lobes and the gallbladder lobe averaged 69 percent (range 64 to 76 percent), compared to 31 percent for the right lobes.
Table I.
Liver weights in normal dogs
| No. | Total liver weight ( T L W ) (Gm.) |
Total body weight (TBW)(Kg.) |
TLW/ TBW* (percent) |
Left lobes (Gm.) |
Right lobes (Gm.) |
||
|---|---|---|---|---|---|---|---|
| Weight (Gm.) |
Percent of total |
Weight (Gm.) |
Percent of total |
||||
| 1 | 314 | 12.2 | 2.6 | 238 | 76 | 76 | 24 |
| 2 | 316 | 12.7 | 2.5 | 202 | 64 | 114 | 36 |
| 3 | 270 | 9.1 | 3.0 | 192 | 71 | 78 | 29 |
| 4 | 454 | 22.5 | 2.0 | 304 | 66 | 150 | 34 |
| 5 | 392 | 12.2 | 3.2 | 258 | 66 | 134 | 34 |
| Mean | 2.7 | 69 | 31 | ||||
The data show the ratio of hepatic to total body weight. In addition, the weight distribution is shown in the liver fractions supplied by the right and left portal branches. Note that the left portal branch normally supplies 69 percent of the liver.
This normal fractionation was markedly altered in the 7 clogs with split transposition. The part of the liver receiving caval blood was shrunk in every case (Table II). This was evident from gross inspection (Fig. 2) and confirmed by weighing. The atrophied portion had lost more than a third of its predicted weight. Conversely, the other portion, supplied with splanchnic venous inflow, weighed more than predicted. Consequently, the total liver weight in relation to total body weight was only slightly reduced.
Table II.
Liver weights in dogs with split transposition
| No. | Total liver weight ( T L W ) (Gm.) |
Total body weight (TBW) (Kg.) |
TLW/TBW (%) |
Left lobes |
Right lobes |
||
|---|---|---|---|---|---|---|---|
| Weight (Gm.) |
Percent of total |
Weight (Gm.) |
Percent of total |
||||
| Left portal branch transposition * | |||||||
| 1 | 385 | 14.5 | 2.7 | 188 | 49 | 197 | 51 |
| 2 | 310 | 12.7 | 2.4 | 124 | 40 | 186 | 60 |
| 3 | 280 | 9.7 | 2.9 | 140 | 50 | 140 | 50 |
| 4 | 253 | 10.9 | 2.4 | 127 | 50 | 126 | 50 |
| Mean | 2.6 | 47.3 | 52.7 | ||||
| Right portal branch transposition | |||||||
| 1 | 436 | 26.4 | 1.7 | 360 | 83 | 76 | 17 |
| 2 | 254 | 9.1 | 2.8 | 194 | 76 | 60 | 24 |
| 3 | 286 | 10.0 | 2.9 | 234 | 82 | 52 | 18 |
| Mean | 2.5 | 80.3 | 19.7 | ||||
The weight distribution in the liver fractions supplied by the right and left portal branches is markedly different from that in normal dogs. Note, however, that the total liver weight is relatively unchanged.
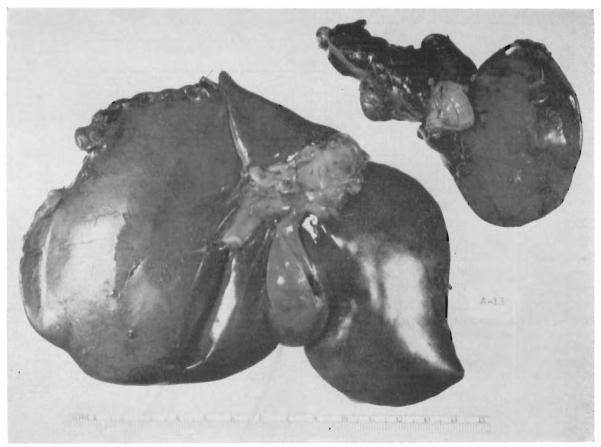
Fig. 2.
Marked atrophy after partial transposition to the right main portal trunk. The autopsy specimen was obtained 73 days after operation. The atrophic right portion weighed 52 grams. The hypertrophied left fraction weighed 234 grams.
Histologic studies
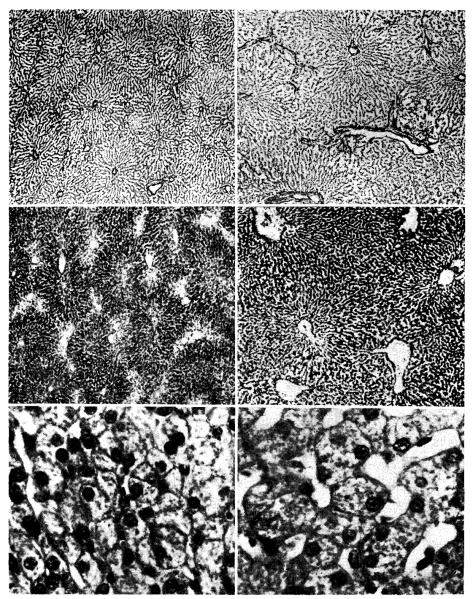
The side of the liver receiving systemic venous blood had undergone striking changes (Fig. 3, right). In the damaged portion there were shrunken lobules with centrizonal atrophy, depletion of glycogen, and irregularity of cell size, shape, ancl staining. There was often central collapse, reticulin condensation, and sinusoidal irregularities. Capsular thickening, increased subcapsular lymphatics, centrizonal cholestasis, and an increased number of littoral cells were present in a few of the animals. The blood vessels and intrahepatic bile ducts were normal. Ultrastructurally many cells showed marked reduction of rough and smooth endoplasmic reticulum; the remaining cisterns were often dilated. Glycogen was scarce and there were moderate numbers of small fat vacuoles. In some cells the electron density of the hyaloplasm was greatly reduced. Mitochrondria were sparse, were often vacuolated, and occasionally contained fibrillar inclusions.
Fig. 3.
Photomicrographs of sections from a liver which had been subjected to partial portacaval transposition. The part of the liver which received vena caval blood (panels on left) shows shrinkage of the liver lobules, depletion of centrilobular glycogen, and atrophy of hepatocytes when compared with the part which received portal blood (panels on right). (Upper, reticulin stain. ×30. Middle, periodic acid–Schiff. ×40. Lower, hematoxylin and eosin. ×250.)
The liver tissue receiving splanchnic venous inflow was well preserved (Fig. 3, left). The gross evidence of hypertrophy in the normally vascularized hepatic fraction was mentioned earlier; microscopically this portion of the liver appeared to have larger lobules and hepatocytes than were present in the pretransposition biopsy. There were also binucleate and trinucleate liver cells, mitoses, and proliferating bile ducts. Ultrastructurally the enlarged cells were essentially normal, although the profiles of endoplasmic reticulum were more irregularly arranged than usual.
In a few of the animals elongated eosinophilic nuclear inclusions were seen in all the specimens from both liver fractions.
Glycogen, enzyme, and protein changes
The biochemical analyses were done by one of us (B. I. B.) without knowledge of the procedure performed. The glycogen content of the liver fraction receiving splanchnic venous blood was greater in all 7 dogs (p < 0.001) than in the other portion supplied with systemic venous blood (Table III). The enzyme content from the two liver fractions was variable, and in 3 dogs specific changes in protein concentration were not noted (Table III).
Table III.
Glycogen and enzyme comparisons in liver fractions receiving splanchnic versus systemic venous inflow
| No. | Glycogen content (percent) |
μmoles of product formed per minute per gram of liver |
Phosphogluco mutase |
Protein (1 :20 mg./ml homogenate) |
|||
|---|---|---|---|---|---|---|---|
| Phosphorylase | G-6-Phospha tase |
Acid phosphatase |
|||||
| Left portal branch transposition | |||||||
| 1 | L | 1.5 | 39 | 10.7 | 2.4 | 45 | 10.8 |
| R | 2.1 | 27 | 8.0 | 1.9 | 35 | 8.7 | |
| 2 | L | 1.9 | 52 | 11.3 | 3.1 | ||
| R | 2.1 | 49 | 9.2 | 3.0 | |||
| 3 | L | 3.1 | 60 | 11.0 | 2.8 | ||
| R | 4.3 | 61 | 11.3 | 2.6 | |||
| 4 | L | 3.0 | 55 | 10.4 | 3.1 | ||
| R | 3.6 | 7 2 | 11.8 | 3.1 | |||
| Right portal branch transposition | |||||||
| 1 | L | 3.0 | 56 | 9.5 | 3.8 | 71 | 11.2 |
| R | 1.6 | 37 | 4.6 | 3.0 | 58 | 10.7 | |
| 2 | L | 4.4 | 74 | 15.3 | 3.2 | 56 | |
| R | 2.3 | 53 | 17.7 | 3.5 | 31 | ||
| 3 | L | 3.8 | 40 | 10.8 | 3.0 | 50 | 8.5 |
| R | 1.6 | 47 | 10.2 | 1 0 | 81 | 11.3 | |
Blood flow studies
The flow through the left portal vein, which supplies most of the liver mass, was greater than that in the right branch in 7 of 8 unaltered dogs. After partial transposition, the flow in 5 of the portal vein branches remained approximately the same; in the other 11 it was increased. After transposition, the magnitude of the flow changes was not significantly different in the side which received systemic venous inflow from the inferior vena cava compared to that side which now received the entire splanchnic inflow (Table IV).
Table IV.
Venous inflow to liver fractions before and after split transposition*
| No. | Left portal branch |
Right bortal branch |
Weight of dog (Kg.) |
||
|---|---|---|---|---|---|
| Before (ml./min.) |
After (ml./min.) |
Before (ml./min.) |
After (ml./min.) |
||
| Left portacaual transposition | |||||
| 1 | 494 | 458 | 263 | 354 | 13.7 |
| 2 | 485 | 569 | 309 | 678 | 15.6 |
| 3 | 391 | 515 | 277 | 315 | 15.9 |
| 4 | 430 | 822 | 190 | 239 | 21.3 |
| Right portacaval transposition | |||||
| 5 | 339 | 331 | 209 | 214 | 16.1 |
| 6 | 250 | 260 | 142 | 130 | 13.6 |
| 7 | 184 | 279 | 207 | 420 | 15.9 |
| 8 | 306 | 403 | 260 | 329 | 17.7 |
Note that in general the flow to both fractions was increased after the procedure.
Dogs with arterialized portal vein branches
The animals were explored at 30 days, at which time the arterialized portion of the liver was pink and of normal size. After 56 and 59 days, gross atrophy had developed in this portion as proved by the weight of the fractions (Table V). There was also a reduced glycogen content in the arterialized lobes (Table V) as compared to the lobes receiving splanchnic venous inflow.
Table V.
Liver fraction weights and glycogen content in 2 dogs which had I portal branch arterialized. The other portal branch carried the total splanchnic venous flow
| Dog No. |
Branch arterialized |
Time killed (days) |
Liver fraction |
Glycogen content (percent) |
||||
|---|---|---|---|---|---|---|---|---|
| Left |
Right |
|||||||
| Weight (Gm.) |
Percent of total |
Weight (Gm.) |
Percent of total |
|||||
| Left | Right | |||||||
| 1 | Left | 59 | 251 | 48.4 | 268 | 51.6 | 3.2 | 4.9 |
| 2 | Right | 56 | 477 | 77.4 | 139 | 22.6 | 3.2 | 2.5 |
DISCUSSION
Difficulties in methodology have hampered attempts to either demonstrate or disprove a hepatotrophic effect of splanchnic venous blood. The clinical, biochemical, and histologic abnormalities following Eck fistula were most commonly attributed to a reduction in the quantity rather than the quality of hepatic blood flow. This view was greatly strengthened by the Child’s and colleagues’3 classic studies with portacaval transposition. With this preparation the intestinal venous blood is also completely diverted from the liver, but a normal quantity of total hepatic blood flow is restored by anastomosing the inferior vena cava to the transected main portal vein in the hilum. After transposition, the weight loss, deterioration of hepatic function, and the metabolic derangements so characteristic of Eck fistula were largely eliminated.
Despite these demonstrations of the unquestionable importance of flow volume, several reported observations could be best explained by the additional influence of peculiar qualitative properties of nonhepatic splanchnic venous blood. Seventy years ago, Hahn and co-workers5 noted that meat intoxication did not develop in dogs after Eck fistula if only a single tributary from the splanchnic system entered the liver circulation above the site of the portacaval anastomosis, an observation subsequently confirmed by others.1, 9, 13
Conversely, there has been increasing evidence that the protection provided in the transposition preparation by portal revascularization from systemic venous sources is incomplete. Even in Child’s experiments, the dogs with portacaval transposition did not have completely normal hepatic regenerative capacity; moreover, 4 of his 8 animals had histologic abnormalities in the liver consisting primarily of centrilobular atrophy. Since that time it has been shown that liver glycogen content is sharply diminished in dogs after portacaval transposition.16 Thus, the liver of the dog with complete portacaval transposition is not a completely normal organ.
Finally, the clearest example of a hepatotrophic effect of splanchnic venous blood has been after auxiliary liver transplantation. If a liver homograft is placed in a normal animal and provided with portal inflow from the systemic venous system, it undergoes rapid atrophy.15 Conversely, when the homograft portal vein is provided with splanchnic venous blood, the shrinkage does not occur.11 Apparently, under these competitive conditions, that liver which receives first access to the splanchnic venous blood operates at a physiologic advantage. The capacity of either liver to compete with the other can be reduced by injuring it in a variety of ways.6, 17, 18
The nature of the hepatotrophic influence in splanchnic venous blood remains speculative. There is no evidence that the atrophy in the nonportalized liver fraction is due to ischemia. The flow studies in the present experiments indicate that the volume of portal venous inflow is increased equivalently in both liver fractions after split transposition. Furthermore, studies by Herrmann and colleagues7 have shown that the oxygen content of these two inflow sources is comparable and that, if anything, the venous saturation is slightly higher in the systemic venous blood. In this connection, it is important to note that the two dogs with arterialization of one portal branch had marked atrophy on that side; here the supernormal portal venous oxygen content19 and a presumably augmented flow4, 14 did not prevent either the atrophy or deglycogenation.
Instead, the available evidence points more to a “starvation” sequence which is greatly exaggerated either when an extra liver is provided as in an auxiliary liver transplantation or under the peculiar circumstances of the presently reported experiments. The fact that profound changes occurred in glycogen concentration but not in proteins or various enzymes raises two specific possibilities. First, the failure of primary hepatic perfusion by the glucose-rich blood returning from the intestine may be the critical adverse factor. Alternatively, the diversion of insulin around the liver may retard glycogen storage.
In the present study the principal emphasis has been on the atrophy which afflicts the liver fraction receiving systemic venous or arterial portal inflow rather than venous inflow from the splanchnic bed. It is also of interest, however, that the other liver portion which did receive splanchnic inflow appeared to have undergone hypertrophy. In reports from the literature8 and in the control studies in the present publication, the relation of liver weight to total body weight was quite constant in normal dogs. After split transposition total predicted liver weight was almost normal; the atrophy affecting one portion of the liver was balanced by hypertrophy of the other portion. This was evident not only from the maintenance of total liver weight but also by the histologic characteristics of the hypertrophied lobes.
The foregoing observations may have application in several areas. First, the importance of splanchnic flow has already been demonstrated in auxiliary liver homotransplantation. Second, it seems likely that some of the findings in various clinical portoprival states can be explained by the mechanisms herein described. Finally, these observations may help to clarify conflicting data, particularly those in the field of carbohydrate metabolism, in which investigators who have employed animals with altered vascularity of the liver have obtained results different from those who have worked with normal animals.
SUMMARY
The influence of nonhepatic splanchnic venous blood on dog liver morphology and biochemical content was investigated by performing partial portacaval transposition, anastomosing the supra-adrenal inferior vena cava to either the right or left branch of the main portal vein. In the resulting preparation, nonhepatic splanchnic venous blood supplies one portion of the liver and systemic venous blood perfuses the remaining fraction.
Seven dogs were studied for 70 to 94 days, 3 with right and 4 with left transposition. No clinical abnormalities were noted. Transient enzyme elevations were seen early after operation but reverted to normal. The most striking feature was the gross and microscopic atrophy and deglycogenation which occurred in the part of the liver receiving systemic venous blood.
Blood flow studies were performed in 8 additional dogs with an electromagnetic square wave flowmeter. Flow was measured in both right and left portal vein branches before and 1 to 4 hours after partial transposition to either the right (4 dogs) or left (4 dogs) main branch. Flow rates were increased in 11 instances and remained essentially the same in 5.
In 2 more dogs, a jugular venous autograft was placed between the abdominal aorta and the right or left main portal vein branch. Atrophy and deglycogenation in the portion receiving arterial blood was comparable to that described above in liver fractions perfused with systemic venous blood.
The evidence from these and earlier experiments that splanchnic venous blood contains a hepatotrophic substance is discussed.
Acknowledgments
Aided by grants AM 07772, AM 06344, HE 07735, AM 06283, A1 04152, FR 05357, FR 00051, and FR 00069 from the United States Public Health Service and by a gtant from the Medical Research Council of Great Britain.
Footnotes
Araldite M supplied by Ciba (A.R.L.) Ltd., Duxford, Cambridge, England.
Model 0-5000, Statham Instruments, Inc., 12401 West Olympic Boulevard, Los Angeles, Calif.
In Vivo Metric Systems, 5339 Agnes Avenue, North Hollywood, Calif.
Flow probe calibrator 0-5900, Statham Instruments, Inc.
ADDENDUM Since submission of this manuscript, absence of differential hepatic atrophy was reported in 5 dogs submitted to partial portacaval transposition by Welborn, Lanier, and Foster of Vanderbilt University (S. Forum 17: 381, 1966). Consequently, 8 new experiments were performed, anastornosing the vena cava to the left portal branch in 4 of the dogs and to the right main trunk in the others. The liver portion receiving systemic venous inflow underwent definite atrophy in every case after 48 to 56 days. When the transposition was to the left lobes which normally constitute 69 percent of the liver mass, these now accounted for only 32 to 52 percent of the total weight. When the transposition was to the right lobes which normally make up 31 percent of the total liver weight, this percentage fell to 14 to 26. Furthermore, the lobar complexes receiving splanchnic venous inflow hypertrophied in every instance with the result that total liver weights were relatively unchanged. These more recent data, as well as those in the original 7 dogs described in the text, indicate that differential liver atrophy occurs in this preparation without exception at some time before the seventh week. Failure of Welborn, Lanier, and Foster to observe this effect is not easily explicable but may have been due to some unrecognized difference in experimental design.
REFERENCES
- 1.Bollman JL. The animal with an Eck fistula. Physiol. Rev. 1961;41:607. [Google Scholar]
- 2.Brown BI, Brown DH. The subcellular distribution of enzymes in type II glycogenosis and the occurrence of an oligo-a-l,4-glucan glucohydrolase in human tissues. Biochim. et biophys. acta. 1965;110:124. doi: 10.1016/s0926-6593(65)80101-1. [DOI] [PubMed] [Google Scholar]
- 3.Child CG, III, Barr D, Holswade GR, Harrison CS. Liver regeneration following portacaval transposition in dogs. Ann. Surg. 1953;138:600. doi: 10.1097/00000658-195310000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher B, Russ C, Updegraff H, Fisher ER. Effect of increased hepatic blood flow upon liver regeneration. Arch. Surg. 1954;69:263. doi: 10.1001/archsurg.1954.01270020129015. [DOI] [PubMed] [Google Scholar]
- 5.Hahn M, Massen O, Nencki M, Pavlov J. Die Eck’sche Fistel zwischen der unteren Hohlvene und der Pfortader und ihre Folgen fur den Organismus. Arch. exper. Path. Leipzig. 1893;32:161. [Google Scholar]
- 6.Halgrimson CG, Marchioro TL, Faris TD, Porter KA, Peters GN, Starzl TE. Experimental and clinical auxiliary hepatic homotransplantation with emphasis on host portacaval shunt. Arch. Surg. 1966;93:107. doi: 10.1001/archsurg.1966.01330010109014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrmann TJ, Taylor PD, Marchioro TL, Starzl TE. Oxygen and C02 content in the splanchnic and nonsplanchnic blood of dogs with portacaval transposition. Surgery. 1966;60:1229. [PMC free article] [PubMed] [Google Scholar]
- 8.Hollenberg M, Dougherty J. Liver blood flow measured by portal venous and hepatic arterial routes with Kr85. Am. J. Physiol. 1966;210:926. doi: 10.1152/ajplegacy.1966.210.5.926. [DOI] [PubMed] [Google Scholar]
- 9.Jolly PC, Younger RK, Foster JH. Hepatic metabolic response to complete and partial portal diversion. Surg. Gynec. & Obst. 1965;121:795. [PubMed] [Google Scholar]
- 10.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265. [PubMed] [Google Scholar]
- 11.Marchioro TL, Porter KA, Dickinson TC, Faris T, Starzl TE. Physiologic requirements for auxiliary liver homotransplantation. Surg. Gynec. & Obst. 1965;121:17. [PMC free article] [PubMed] [Google Scholar]
- 12.Marchioro TL, Porter KA, Illingworth B, Faris TD, Herrmann TJ, Sudweeks A, Starzl TE. The specific influence of nonhepatic splanchnic venous blood flow upon the liver. S. Forum. 1965;16:280. [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds VH, Wilson RE. Absence of recurrent ammonia intoxication following right hemicolectomy with anastomosis between the superior mesenteric vein and the inferior vena cava. Ann. Surg. 1961;154:826. doi: 10.1097/00000658-196111000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siderys H, Judd D, Herendeen TL, Kilman JW, Waldhausen JA. The experimental production of elevated portal pressure by increasing portal flow. Surg. Gynec. & Obst. 1965;120:514. [PubMed] [Google Scholar]
- 15.Starzl TE, Marchioro TL, Rowlands DT, Jr., Kirkpatrick CH, Wilson WEC, Rifkind D, Waddell WR. Immunosuppression after experimental and clinical homotransplantation of the liver. Ann. Surg. 1964;160:41–1. doi: 10.1097/00000658-196409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starzl TE, Marchioro TL, Sexton A, Illingworth B, Waddell WR, Faris T, Herrmann TJ. The effect of portacaval transposition upon carbohydrate metabolism. Experimental and clinical observations. Surgery. 1965;57:687. [PMC free article] [PubMed] [Google Scholar]
- 17.Thomford NR, Shorter RG, Hallenbeck GA. Homotransplantation of the canine liver: Survival and histology with and without azathioprine. Arch. Surg. 1965;90:527. doi: 10.1001/archsurg.1965.01320100071012. [DOI] [PubMed] [Google Scholar]
- 18.Tretbar LL, Beven EG, Hermann RE. Homotransplantation of an auxiliary dog liver into the pelvis—Effect of portacaval shunt in the prevention of liver atrophy. S. Forum. 1965;16:219. [PubMed] [Google Scholar]
- 19.Zuidema GD, Gaisford WD, Abell MR, Brody TM, Neill SA, Child CG. Segmental portal arterialization of canine liver. Surgery. 1953;53:689. [PubMed] [Google Scholar]