Abstract
Adenosine receptors modulate dopaminergic function by regulating dopamine release in presynaptic neurons and intracellular signaling in postsynaptic striatal neurons. To investigate how adenosine impinges on the action of dopamine in feeding and locomotion, genetically altered, dopamine-deficient mice were treated with adenosine receptor antagonists. Acute administration of the nonselective adenosine receptor antagonist, caffeine (5–25 mg/kg i.p.), reversed the hypophagia of mutant mice and induced hyperactivity in both control and mutant animals. However, caffeine treatment elicited much less hyperactivity in dopamine-deficient mice than did l-3,4-dihydroxyphenylalanine (l-dopa) administration, which partially restores dopamine content. Caffeine treatment enhanced feeding of l-dopa-treated mutants but, unexpectedly, it reduced their hyperlocomotion. Caffeine administration induced c-Fos expression in the cortex of dopamine-deficient mice but had no effect in the striatum by itself. Caffeine attenuated dopamine agonist-induced striatal c-Fos expression. An antagonist selective for adenosine A2A receptors induced feeding and locomotion in mutants much more effectively than an A1 receptor antagonist. l-dopa-elicited feeding and hyperlocomotion were reduced in mutants treated with an A1 receptor agonist, whereas an A2A receptor agonist decreased l-dopa-induced feeding without affecting locomotion. The observations suggest that the hypophagia and hypoactivity of mutants result not only because of the absence of dopamine but also because of the presence of A2A receptor signaling. This study of a genetic model of dopamine depletion provides evidence that A2A receptor antagonists could ameliorate the hypokinetic symptoms of advanced Parkinson's disease patients without inducing excessive motor activity.
Adenosine accumulates extracellularly from breakdown of ATP released from synaptic vesicles and also diffuses across cell membranes through adenosine transporters (1). It regulates ion channel activity in striatal neurons by activating G protein-coupled receptors. The hypothesis that adenosine and dopamine oppose each other's actions is based on colocalization of their receptors in striatal neurons and coupling of the receptors to opposing G proteins (2). In striatopallidal neurons, adenosine A2A receptors, which couple to Golf proteins, are coexpressed with D2 receptors, which couple to Gi proteins. A1 receptors are expressed in both dopamine D1 receptor-expressing striatonigral and D2 receptor-expressing striatopallidal neurons. In striatonigral neurons, A1 receptors, which couple to Gi proteins, oppose the action of D1 receptors, which couple to Golf proteins. Additionally, adenosine receptors can interact physically with dopamine receptors and lower the affinity of these receptors for dopamine (2). In vitro evidence suggests that glutamate NMDA receptor activity in striatopallidal neurons leads to greater activation of A2A receptors (3).
The model of striatal dopamine/adenosine opposition is further supported by findings that parkinsonism-like motor deficits of D2 receptor-deficient mice can be reversed by A2A receptor antagonists and caffeine (4, 5). Additionally, catalepsy induced by acute D2 receptor antagonist treatment is attenuated in A2A receptor-deficient mice (6). In terms of cAMP-dependent gene expression, enkephalin mRNA is overexpressed in the D2 receptor-deficient striatum but is attenuated in the striatum of mice lacking both D2 and A2A receptors (6). Finally, neurochemical studies have shown that D2 receptor-mediated inhibition of GABA release from striatopallidal neurons is reversed by A2A receptor agonists (7).
In addition to these results showing opposing interactions at the level of motor behavior, gene expression, and neurochemistry, two studies have indicated that adenosine receptor blockade reverses hypophagia in rodents in which dopamine is chronically depleted after intracranial delivery of the neurotoxin, 6-hydroxydopamine (8, 9). Hypophagia in these rodents results from impaired sensorimotor function and motivation (10). One hypothesis is that unopposed adenosine signaling in the striatum of these animals impairs motor and reward-related function. This report addresses how adenosine functions in the striatum of mice in which dopamine production has been genetically inactivated and how it modulates locomotion, feeding behavior, and immediate-early gene expression.
Methods
Behavioral Studies.
All mice were used in accordance with guidelines for animal care and use established by the National Institutes of Health and the University of Washington Animal Care Committee. Dopamine-deficient mice were bred as described (11). Control and Th−/−; DbhTh/+ (tyrosine hydroxylase, dopamine β-hydroxylase) mice used in behavioral experiments were 3- to 10-month-old cagemates, maintained on a mixed C57BL/6 × 129/SvEv genetic background. Control mice included animals that were Th+/+, Th+/−, Dbh+/+; DbhTh/+, and combinations of these genotypes. Locomotion was measured in photo-beam activity cages as described (12). Food pellets (Purina) used were 5015 chow (11% fat, 4.35 kcal/g). Tap water was freely available to mice throughout all experiments. The light cycle was maintained on a 12-h light–dark schedule with lights turning on at 7:00 a.m.
The injection volume for all treatments was 10 μl/g i.p., unless otherwise noted. Mice were treated with 0.9% saline, caffeine (Sigma) diluted at various concentrations (0.5, 1.5, 2.5 mg/ml), R-(+)-SCH 23390 hydrochloride (Sigma, 0.02 mg/ml), haloperidol (McNeil Pharmaceutical, 0.2 mg/ml), l-3,4-dihydroxyphenylalanine (l-dopa, Sigma, 33 μl/g i.p., 1.5 mg/ml in 2.5 mg/ml ascorbic acid in PBS), 10% DMSO/10% Cremophor El (Sigma), 1,3-dipropyl-8-cyclopentylxanthine (DPCPX, Sigma, 0.15 mg/ml), 8-(3-chlorostryryl)caffeine (CSC, Sigma, 0.5 mg/ml), CGS 15943 (Sigma, 1 mg/ml), N6-cyclopentyladenosine (CPA, Sigma, 0.03 mg/ml), CGS 21680 hydrochloride (Sigma, 0.05 mg/ml), and (+/−)-SKF 81297 (Sigma, 0.125 mg/ml). For caffeine, SCH 23390, haloperidol, and SKF 81297, the drug vehicle was saline. For DPCPX, CSC, CGS 15943, CPA, and CGS 21680, the drug vehicle was DMSO/Cremophor El. Before feeding and activity were measured, mice were placed in activity cages for a day for acclimatization. On the night before each experimental measurement was taken, food was removed at 11:00 p.m. At 2:00 p.m. on the next day, food was replenished, drugs were administered, and food intake and activity were monitored for 2 or 4 h. Mutants had been injected with their last l-dopa (50 mg/kg) dose 24 h before any experimental treatment was given and were maintained between treatments with the same daily doses of l-dopa.
Immunohistochemistry.
Control and Th−/−; DbhTh/+ mice were fed ad libitum. Mice were treated with drugs and anesthetized with CO2 after 2 h. Mice were transcardially perfused with 10 units/ml heparin sodium (American Pharmaceutical Partners, Los Angeles) in PBS and then 4% paraformaldehyde in PBS. Brains were dissected, immersed in paraformaldehyde, cryoprotected in 30% sucrose, and frozen in isopentane. Free-floating coronal sections (20 μm) were immunostained using rabbit polyclonal antisera directed against c-Fos (1:5,000, Santa Cruz Biotechnology). Immunoreactivity was revealed using a biotinylated goat–anti-rabbit IgG antibody (Vector Laboratories), streptavidin-conjugated horseradish peroxidase (Zymed), and diaminobenzidine chromagen (Zymed). Sections were slide-mounted, coverslipped, and photographed. c-Fos immunoreactive nuclei were quantified from three images of each brain region for each mouse by using scion image (Release 4.02).
Statistical Analysis.
Two-way ANOVA with repeated measures was performed using statistica (Release 6.0, StatSoft, Tulsa, OK).
Results
Feeding and Locomotor Responses to Caffeine.
Dopamine-deficient (Th−/−; DbhTh/+) mice in which Th gene function is inactivated in dopaminergic cells but preserved in noradrenergic and adrenergic cells display parkinsonism-like motor deficits, hypophagia, hypodipsia, and high sensitivity to dopamine receptor agonists (11–13). One possibility is that the phenotypes of mutant mice result not only because of the loss of dopamine (<1% of control levels) but also because of the presence of an opposing neurotransmitter, adenosine. To address this hypothesis, control and mutant mice were treated acutely with the A1 and A2A receptor antagonist, caffeine and food intake and locomotor activity were monitored for 4 h.
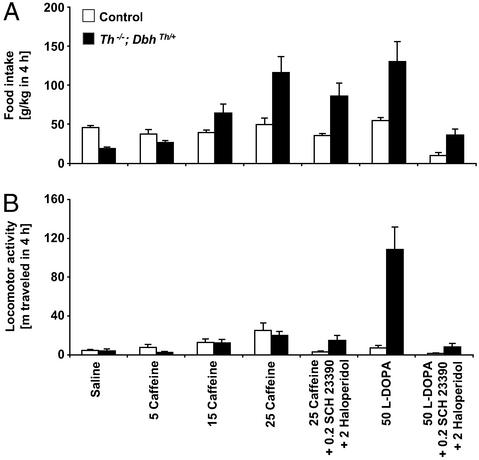
All experiments were performed 24 h after mutants had received their last l-dopa (50 mg/kg) injection, a time when brain dopamine content in mutants is <1% of normal levels (13). Because mutant mice only eat for ≈9 h after routine daily l-dopa (50 mg/kg) injection (13), both control and mutant mice were fasted for 15 h to induce similar hunger levels. In response to a 15-h food restriction and saline injection, control mice ate 46 g/kg in 4 h, whereas mutant mice consumed only 41% as much (Fig. 1A). The feeding of control mice treated with caffeine (5, 15, and 25 mg/kg) was not significantly affected. In contrast, caffeine treatment induced feeding in mutant mice in a dose-dependent manner to levels that were indistinguishable from l-dopa (50 mg/kg) treatment, which restores dopamine production in mutants (refs. 11 and 13; dose-related caffeine effect, P < 0.0001). In response to l-dopa (50 mg/kg) treatment, control mice were unaffected, but mutant mice consumed twice as much food as control mice treated with saline (no genotype difference, P = 0.082; l-dopa effect, P = 0.0003; genotype–l-dopa interaction, P = 0.0012).
Figure 1.
Caffeine- and l-dopa-induced feeding behavior and locomotor activity over 4 h. (A) Food consumption, g/kg body weight (mean ± SEM). (B) Locomotion is reported as distance traveled in meters. Control mice, white bars (n = 8); Th−/−; DbhTh/+ mice, black bars (n = 8). Numbers indicate dose of drug (mg/kg body weight).
The effects of caffeine were most likely mediated by adenosine receptors because caffeine binds to A1 and A2A receptors with affinity constants in the 50 μM range, whereas its IC50 value for other pharmacological targets, such as cyclic nucleotide phosphodiesterases and ryanodine receptors, is in the 500–5,000 μM range (14). In rats treated with caffeine (15 mg/kg), striatal concentrations peak at 13 μM (15). Furthermore, caffeine (25 mg/kg) administration to A2A receptor-deficient mice fails to elicit hyperactivity and, in fact, depresses locomotion (16, 17), suggesting that the activity-inducing effects of this dose of caffeine are mediated by A2A receptors.
To assess whether caffeine induced feeding in mutants in a dopamine receptor-dependent manner, caffeine (25 mg/kg) was coadministered with the dopamine D1- and D2-like receptor antagonists SCH 23390 (0.2 mg/kg) and haloperidol (2 mg/kg), respectively (Fig. 1A). In both groups of mice, the effects of caffeine (25 mg/kg) on feeding were attenuated by the antagonists (genotype difference, P = 0.0056; antagonist effect, P = 0.0061; no genotype–antagonist interaction, P = 0.26). However, whereas combined SCH 23390 and haloperidol treatment reduced feeding after caffeine treatment by 28% in control and 26% and mutant mice, it reduced feeding after l-dopa treatment by 81% in control and 72% in mutant mice, suggesting that the majority of caffeine's effects was independent of dopamine receptor activation (genotype difference, P = 0.0051; antagonist effect, P < 0.0001; genotype–antagonist interaction, P = 0.045).
Chronic administration of caffeine (25 mg/kg, three times daily) supported feeding and survival of the mutants for 4 days but was less efficacious thereafter (data not shown), perhaps reflecting tolerance and desensitization to repeated caffeine exposure. In the absence of daily l-dopa treatment, mutant mice die of starvation and dehydration within 2 days (11).
Locomotor activity was also monitored simultaneously for 4 h. In response to saline injection, mutant mice traveled 3.6 ± 1.8 m (mean ± SEM), and control mice traveled 4.9 ± 0.9 m (Fig. 1B). Because activity was measured during daylight hours and after mice had acclimatized to their environment, the locomotion of control mice was relatively low and equivalent to that of mutants. Caffeine administration induced hyperlocomotion in control and mutant mice in a dose-related manner that reached 5-fold the levels after saline treatment, but the hyperlocomotion of mutants never reached levels observed after l-dopa treatment (dose-related caffeine effect, P < 0.0001). With l-dopa administration, control mice were unaffected, but mutants were 25-fold more active than with saline treatment (genotype difference, P = 0.0006; l-dopa effect, P = 0.0005; genotype–l-dopa interaction, P = 0.0007).
The effects of caffeine (25 mg/kg) were attenuated by coadministration of the D1- and D2-like receptor antagonists in control by 87% and mutant mice by 24% (Fig. 1B; no genotype difference, P = 0.54; antagonist effect, P = 0.016; no genotype–antagonist interaction, P = 0.10). The antagonists reduced locomotor activity after l-dopa treatment by 80% in control and 92% in mutant mice (genotype difference, P = 0.0007; antagonist effect, P = 0.0003; genotype–antagonist interaction, P = 0.0007). Thus, the antagonists were more effective at reducing l-dopa-induced hyperactivity as compared with their effect on caffeine-induced locomotion, suggesting that most of the caffeine-induced activity of mutants was independent of dopamine receptor activation.
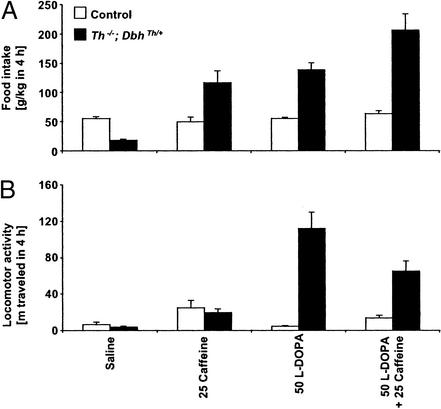
To address the possibility that dopamine receptor activation and adenosine receptor blockade might have additive effects on feeding and locomotion, l-dopa and caffeine were coadministered to control and mutant mice. The prophagic effects of l-dopa on food intake in mutant mice were enhanced by 48% with coadministration of caffeine in a near-additive manner (25 mg/kg, Fig. 2A; caffeine effect, P = 0.0006; l-dopa effect, P = 0.0005; no caffeine–l-dopa interaction, P = 0.22). Interestingly, l-dopa-induced hyperactivity in mutants was reduced 42% by caffeine coadministration (Fig. 2B; no caffeine effect, P = 0.14; l-dopa effect, P = 0.0005; caffeine–l-dopa interaction, P = 0.015).
Figure 2.
Effects of coadministration of l-dopa and caffeine on feeding behavior and locomotor activity over 4 h. (A) Food consumption, g/kg body weight (mean ± SEM). (B) Locomotion is reported as distance traveled in meters. Control mice, white bars (n = 8); Th−/−; DbhTh/+ mice, black bars (n = 8). Numbers indicate dose of drug (mg/kg body weight).
Responses to Selective A1, A2A, and Dual Receptor Antagonists.
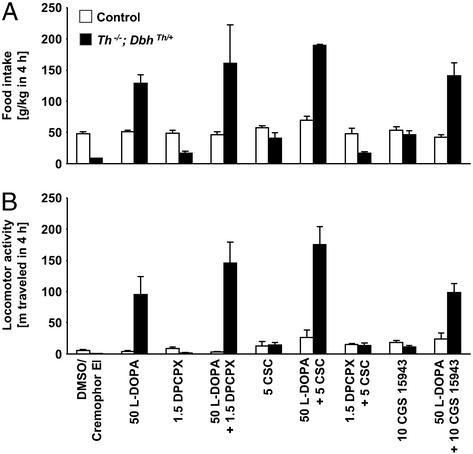
The effects of selective A1 and A2A receptor antagonists on feeding and locomotion were also assessed. The selective A1 receptor antagonist, DPCPX (1.5 mg/kg), did not increase food intake in mutant mice as compared with vehicle treatment (Fig. 3A). Coadministration of DPCPX with l-dopa did not significantly enhance l-dopa-induced feeding. DPCPX radioligand binding is abolished in the brain of A1 receptor-deficient mice, suggesting that this drug blocks A1 receptors preferentially (18). In contrast, the selective A2A receptor antagonist, CSC (5 mg/kg), increased feeding in mutants by 494% as compared with vehicle treatment but had no effect in controls (genotype difference, P < 0.0001; CSC effect, P = 0.0003; genotype–CSC interaction, P = 0.021). Coadministration of CSC with l-dopa augmented l-dopa-induced feeding by 36% in control mice and by 49% in mutant mice (genotype difference, P = 0.0002; CSC effect, P = 0.0027; no genotype–CSC interaction, P = 0.067). As with caffeine, the stimulatory effect of CSC on l-dopa-elicited feeding in mutant mice was nearly additive (l-dopa effect, P = 0.0002; CSC effect, P = 0.011; no l-dopa–CSC interaction, P = 0.13). CSC (5 mg/kg) was reported to be without any activity-inducing effects in A2A receptor-deficient mice (6), suggesting that it blocks A2A receptors specifically. Coadministration of DPCPX (1.5 mg/kg) and CSC (5 mg/kg) did not increase the feeding response of control and mutant mice to CSC alone.
Figure 3.
Selective adenosine A1, A2A, and dual antagonist effects on feeding behavior and locomotor activity over 4 h. (A) Food consumption, g/kg body weight (mean ± SEM). (B) Locomotion is reported as distance traveled in meters. Control mice, white bars (n = 4–8); Th−/−; DbhTh/+ mice, black bars (n = 4–8). Numbers indicate dose of drug (mg/kg body weight).
In terms of locomotor activity, A1 receptor blockade with DPCPX induced activity in control mice by 60% and 110% in mutant mice over responses to vehicle treatment (Fig. 3B; genotype difference, P = 0.0021; DPCPX effect, P = 0.0044; no genotype–DPCPX interaction, P = 0.062). But DPCPX treatment did not significantly affect l-dopa-induced hyperlocomotion in mutants. The A2A receptor antagonist, CSC, induced locomotor activity in control mice by 129% and 1,952% in mutant mice over responses to vehicle treatment (no genotype difference, P = 0.77; CSC effect, P = 0.020; no genotype–CSC interaction, P = 0.40). CSC administration augmented l-dopa-induced hyperactivity of mutants by 85% (genotype difference, P = 0.0006; CSC effect, P < 0.0001; genotype–CSC interaction, P = 0.0033). The stimulatory effect of CSC on l-dopa-elicited hyperlocomotion in mutant mice appeared to be synergistic (l-dopa effect, P = 0.0022; CSC effect, P < 0.0001; l-dopa–CSC interaction, P = 0.0016). Coadministration of DPCPX (1.5 mg/kg) and CSC (5 mg/kg) did not augment the locomotor response of mutant mice to CSC alone.
The acute effects of CGS 15943, a nonmethylxanthine, dual adenosine receptor antagonist that does not inhibit phosphodiesterases, were also determined. CGS 15943 has been reported to inhibit adenosine receptors in the 3- to 20-nM range, whereas it has no effect on phosphodiesterases at concentrations as high as 10 μM (19). Treatment with CGS 15943 (10 mg/kg) had no effect on ingestive behavior of control mice, but it induced feeding in mutant mice by 457% over vehicle treatment (genotype difference, P = 0.0002; CGS 15943 effect, P < 0.0001; genotype–CGS 15943 interaction, P = 0.0005). CGS 15943 did not enhance the l-dopa-induced prophagic response of mutants, unlike caffeine or CSC. A higher dose of CGS 15943 (16 mg/kg) failed to induce more feeding in mutants (data not shown).
CGS 15943 treatment induced locomotion in control mice by 237% and mutant mice by 1,394% over vehicle treatment (genotype difference, P = 0.029; CGS 15943 effect, P < 0.0001; no genotype–CGS 15943 interaction, P = 0.47). CGS 15943 did not enhance the l-dopa-induced hyperlocomotion of mutants.
Responses to Coadministration of l-Dopa and Selective A1 and A2A Agonists.
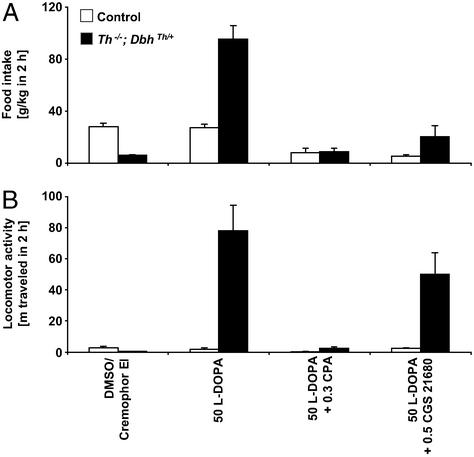
The possibility that selective adenosine receptor agonists might reduce the feeding and locomotor responses elicited by l-dopa treatment was examined. Preliminary studies indicated that A1 and A2A agonists were shorter acting than drugs used in the above experiments, so data from 2-h experiments are reported. The selective A1 receptor agonist, CPA (0.3 mg/kg), attenuated feeding responses to l-dopa of control mice by 70% and mutant mice by 91% (Fig. 4A; genotype difference, P < 0.0001; CPA effect, P < 0.0001; genotype–CPA interaction, P < 0.0001). It also diminished the locomotor responses to l-dopa of control mice by 84% and mutant mice by 97% (Fig. 4B; genotype difference, P < 0.0001; CPA effect, P < 0.0001; genotype–CPA interaction, P < 0.0001). CPA has been reported to have 500 times selectivity for the A1 receptor over A2A receptors (20). Similarly, the selective A2A receptor agonist, CGS 21680 (0.5 mg/kg), reduced feeding in l-dopa-administered control mice by 81%, and it diminished the response of mutant mice by 78% (genotype difference, P < 0.0001; CGS 21680 effect, P < 0.0001; genotype–CGS 21680 interaction, P = 0.0006). At this dose, l-dopa-induced hyperlocomotion of mutants was unaffected. CGS 21680 (0.5 mg/kg) fails to depress motor function in A2A receptor-deficient mice (16), substantiating the specificity of this dose of the drug for A2A receptors.
Figure 4.
Selective adenosine A1 and A2A agonist effects on feeding behavior and locomotor activity over 2 h. (A) Food consumption, g/kg body weight (mean ± SEM). (B) Locomotion is reported as distance traveled in meters. Control mice, white bars (n = 8); Th−/−; DbhTh/+ mice, black bars (n = 8). Numbers indicate dose of drug (mg/kg body weight).
Brain c-Fos Induction.
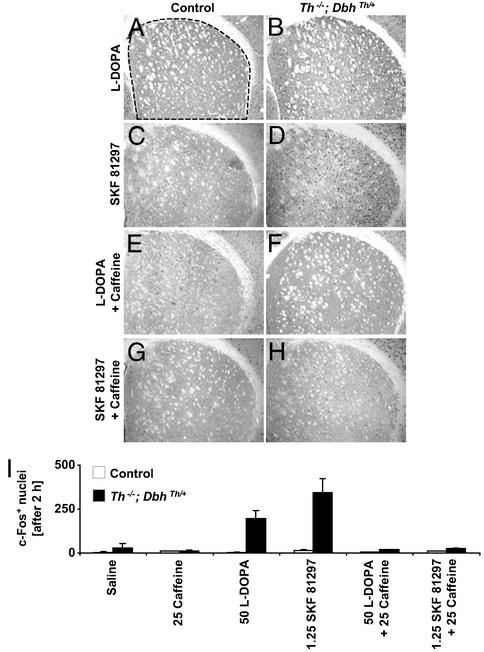
To address the possibility that the feeding and locomotor responses to caffeine might be caused by changes in striatal intracellular signaling, induction of the immediate-early gene, c-fos, was assessed as a marker of acute elevations in cAMP and Ca2+ in striatal neurons. Control and mutant mice were injected with saline, animals were killed after 2 h, and induction of nuclear c-Fos immunoreactivity was assessed. Few immunoreactive nuclei were observed in saline-treated control or mutant mice (Fig. 5I). Control and mutant mice failed to exhibit much nuclear immunoreactivity after caffeine (25 mg/kg) treatment in either the caudate putamen (Fig. 5I) or nucleus accumbens (data not shown). This drug and dose were chosen for these experiments because of their efficacy and relevance in the induction of feeding and activity in mutants. l-dopa (50 mg/kg) treatment led to enhanced c-Fos expression in the dorsal striatum of mutant (Fig. 5B), but not in control mice (Fig. 5A). Administration of the direct D1-like receptor agonist, SKF 81297 (1.25 mg/kg), had little effect on c-Fos expression in control striatum (Fig. 5C), but it induced abundant c-Fos immunoreactivity in mutant striatum (Fig. 5D). The l-dopa-induced c-Fos response of mutants was attenuated by coadministration of caffeine (Fig. 5F). The dual treatment had no effect on control mice (Fig. 5E). To test whether caffeine's effects on immediate-early gene expression were independent of its effects on dopamine release, control and mutants were treated with caffeine and SKF 81297. SKF 81297-induced, striatal c-Fos expression in mutants was reduced markedly by caffeine coadministration (Fig. 5H). Treatment with caffeine and SKF 81297 had little effect on control mice (Fig. 5G). Quantitation of the number of c-Fos-positive nuclei in the caudate putamen after various treatments is shown in Fig. 5I.
Figure 5.
Induction of c-Fos immunoreactivity in the striatum after 2 h. (A, C, E, and G) Representative control coronal sections showing the dorsal caudate putamen (top, dorsal; right, lateral). (B, D, F, and H) Representative Th−/−; DbhTh/+ sections. (A and B) Sections after l-dopa (50 mg/kg) treatment. (C and D) Sections after SKF 81297 (1.25 mg/kg) treatment. (E and F) Sections after l-dopa (50 mg/kg) treatment + caffeine (25 mg/kg) treatment. (G and H) Sections after SKF 81297 (1.25 mg/kg) treatment + caffeine (25 mg/kg) treatment. (I) c-Fos-positive nuclei in dorsal CPu (mean ± range). Control mice, white bars (n = 2 mice, three images per mouse); Th−/−; DbhTh/+ mice, black bars (n = 2 mice, three images per mouse). Dashed line indicates region where nuclei were counted. Numbers indicate dose of drug (mg/kg body weight).
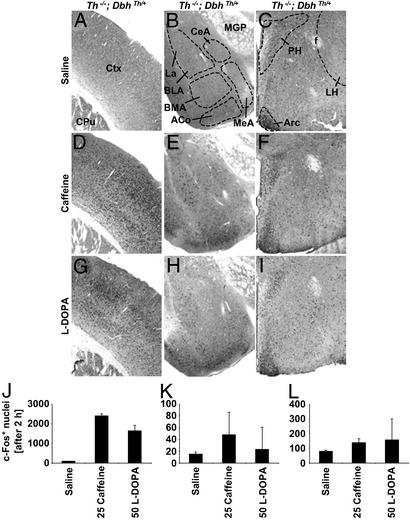
Although there was no effect of caffeine alone on c-Fos expression in the striatum (Fig. 5), there was a large induction of c-Fos in somatosensory cortex (Fig. 6D), similar to that observed with l-dopa (Fig. 6G). There were no dramatic effects of either caffeine or l-dopa treatment on c-Fos expression in the amygdala (Fig. 6 E and H) or hypothalamus of mutant mice (Fig. 6 F and I). The number of immunoreactive nuclei after various treatments for cortex, amygdala, and hypothalamus is shown in Fig. 6 J, K, and L, respectively.
Figure 6.
c-Fos expression in the cortex, amygdala, and hypothalamus of Th−/−; DbhTh/+ mice after 2 h. (A, D, and G) Representative coronal sections showing the cortex (top, dorsal; right, lateral). (B, E, and H) Representative coronal sections showing the amygdala (top, dorsal; right, medial; La, lateral amygdala; BLA, basolateral amygdala; BMA, basomedial amygdala, ACo, cortical amygdaloid nucleus; CeA, central amygdala; MeA, medial amygdala; MGP, medial globus pallidus). (C, F, and I) Representative coronal sections showing the hypothalamus (top, dorsal; right, lateral; PH, paraventricular hypothalamic nucleus; Arc, arcuate; LH, lateral hypothalamus, f, fornix). (A–C) Sections after 0.9% saline treatment. (D–F) Sections after caffeine (25 mg/kg) treatment. (G–I) Sections after l-dopa (50 mg/kg) treatment. (J–L) Number of c-Fos-positive nuclei in cortex, amygdala, and hypothalamus, respectively (mean ± range; n = 2 mice, three images per mouse). Numbers indicate dose of drug (mg/kg body weight).
Discussion
Previous investigators have postulated that a link exists between caffeine's effects on motor function and its antagonism of adenosine receptors in the striatum based primarily on the highly enriched striatal expression of A2A receptors and the long-standing notion that striatal neurons modulate voluntary movement and sensorimotor function (2). Induction of locomotor activity by relatively low doses of caffeine (5–25 mg/kg) in control and Th−/−; DbhTh/+ mice may reflect regional effects of the drug in the striatum. Additionally, induction of feeding by caffeine may also be driven by striatal adenosine receptor blockade. Localized viral gene transfer experiments, in which dopamine production was reintroduced into the caudate putamen of mutants, underscore the importance of striatal neuronal activity in facilitating feeding behavior (21). The high sensitivity of mutants to caffeine relative to that of control mice in terms of feeding also suggests that the drug's effects are mediated by parts of a neuronal circuit where dopaminergic function is normally present, like the striatum. Feeding induced by caffeine may also reflect activity changes in other brain regions of mutants. The observation that caffeine induced c-Fos expression in the cortex, but not the striatum, amygdala, or hypothalamus, may reflect direct effects on cortical adenosine receptors (22). Alternatively, cortical c-Fos expression may result from enhanced cAMP signaling caused by caffeine's actions elsewhere in the brain. Delivery of caffeine to specific brain regions may help to define regions where it can elicit feeding in mutants and thus discriminate among these possibilities.
In the striatum, A1 receptors are expressed in postsynaptic neurons as well as in dopaminergic, glutamatergic, and cholinergic terminals (2). Blockade of A1 receptors, which are coupled to inhibitory G proteins, in presynaptic inputs could lead to enhanced release of dopamine, glutamate, and acetylcholine. But A1 receptor antagonism with DPCPX was largely ineffective at inducing feeding and locomotion in mutants and did not augment the response to the A2A antagonist, suggesting that the prophagic and activity-inducing effects of caffeine are mediated by inhibition of postsynaptic A2A receptors. Indeed, with low caffeine doses (25 mg/kg or lower) in wild-type animals, it has been hypothesized that A2A receptors instead of A1 receptors are preferentially blocked, and hyperlocomotion is induced by net elevation of efficacy of D2 receptor signaling and depression in Golf activity in striatopallidal neurons (17, 23). Higher caffeine doses (75–100 mg/kg) elicit different responses that involve other pharmacological targets in addition to adenosine receptors, and these higher doses induce striatal immediate-early gene expression (23–30). Finally, the effects of caffeine in mutants were only marginally inhibited by a combination of dopamine receptor antagonists that substantially reduced l-dopa-activated locomotor activity and feeding, suggesting that most of caffeine's effects are not mediated by release of residual dopamine. Previous investigators have observed that caffeine-induced feeding in 6-hydroxydopamine-lesioned rats can be blocked by either D1 or D2 receptor antagonists and have concluded that caffeine does enhance presynaptic dopamine release (9). The amount of residual dopamine present in the brains of Th−/−; DbhTh/+ mice is much less than that remaining in 6-hydroxydopamine-lesioned rats (11, 13) and may account for the difference between the mutant mice and lesioned rats.
Unexpectedly, caffeine attenuated dopaminergic agonist-induced hyperlocomotion and c-Fos expression in the mutants, which contrasts with previous observations that A2A receptor antagonists potentiate D1 receptor agonist-induced motor behavior and cAMP-dependent transcription in rats unilaterally lesioned with 6-hydroxydopamine by an indirect neuronal mechanism (31, 32). l-dopa and D1 receptor agonists induce c-Fos expression in dopamine-depleted rodents by increasing cAMP in striatonigral neurons (12, 33). Caffeine could affect striatal cAMP-dependent transcription in a variety of ways. First, low-dose caffeine treatment could block A2A receptors preferentially, which would reduce cAMP signaling in striatopallidal neurons. However, detection of depressions in cAMP-coupled gene expression is difficult because c-Fos expression is basally low in these cells, and even if this depression were detectable, c-Fos is induced in striatonigral cells with dopamine agonist treatment. Second, caffeine could block A1 receptors on D1 receptor-expressing striatonigral neurons, which would inhibit a Gi-coupled receptor and enhance net cAMP signaling. However, dopaminergic agonist-induced c-Fos expression was reduced by caffeine. Third, low-dose caffeine treatment could indirectly dampen hyperlocomotion and c-Fos expression in striatonigral cells by blockade of A1 receptors on cholinergic interneurons, promotion of local acetylcholine release, and activation of muscarinic receptors, which is known to oppose D1 receptor signaling in this type of readout (34, 35). Finally, it is possible that caffeine's dampening of dopamine agonist-induced activity and gene expression reflects effects independent of A1 and A2A receptors, as the A1 receptor blocker DPCPX; the A2A receptor inhibitor CSC; and the nonmethylxanthine, dual antagonist CGS 15943 did not reduce l-dopa-induced hyperlocomotion.
Because the depletion of dopamine signaling in Th−/−; DbhTh/+ mice is so severe and dopamine production can be restored with l-dopa administration, these animals serve as effective models of late-stage Parkinson's disease and l-dopa-induced dyskinesia (i.e., excessive and often involuntary movement caused by repeated l-dopa treatment of Parkinson's disease patients) in a manner that complements other systems in which reductions of dopaminergic function have been studied. For example, previous reports showed that adenosine receptor antagonists reversed hypoactivity in D2 receptor-deficient mice with partial efficacy (4–6). In Th−/−; DbhTh/+ mice, however, a comparison could be made between the effects of adenosine receptor antagonism and dopamine restoration. Although adenosine receptor blockade induced locomotion in both control and Th−/−; DbhTh/+ mice, the activity levels of mutants never reached those obtained with l-dopa treatment. The differences in the effects of adenosine antagonists and dopamine agonists in Th−/−; DbhTh/+ mice suggest that adenosine antagonist treatments for Parkinson's disease patients could be developed that generate less dyskinetic side effects than dopamine replacement therapy, a notion that is supported by results from recent studies using monkeys (36, 37). Additionally, other investigators have shown that excessive motor function and increased D1 receptor-dependent gene expression induced by repeated injections of l-dopa into 6-hydroxydopamine-lesioned mice are reduced in A2A receptor-deficient mutants (38). The attenuation by caffeine of l-dopa-elicited hyperlocomotion and striatal c-Fos expression in Th−/−; DbhTh/+ mice is consistent with this finding and also suggests that adenosine antagonists could be used to block l-dopa-induced dyskinesias.
Acknowledgments
We thank G. Froelick for histological assistance, V. Denenberg for statistical analysis, and B. Willis for helpful discussion. D.S.K. was supported by a graduate research fellowship from the National Science Foundation.
Abbreviations
- CPA
N6-cyclopentyladenosine
- CSC
8-(3-chlorostryryl) caffeine
- Dbh
dopamine β-hydroxylase
- DPCPX
1,3-dipropyl-8-cyclopentylxanthine
- Th
tyrosine hydroxylase
- l-dopa
l-3,4-dihydroxyphenylalanine
References
- 1.Zimmermann H. Trends Neurosci. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 2.Ferré S, Fredholm B B, Morelli M, Popoli P, Fuxe K. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- 3.Nash J E, Brotchie J M. J Neurosci. 2000;20:7782–7789. doi: 10.1523/JNEUROSCI.20-20-07782.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoyama S, Kase H, Borrelli E. J Neurosci. 2000;20:5848–5852. doi: 10.1523/JNEUROSCI.20-15-05848.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zahniser N R, Simosky J K, Mayfield R D, Negri C A, Hanania T, Larson G A, Kelly M A, Grandy D K, Rubinstein M, Low M J, Fredholm B B. J Neurosci. 2000;20:5949–5957. doi: 10.1523/JNEUROSCI.20-16-05949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J F, Moratalla R, Impagnatiello F, Grandy D K, Cuellar B, Rubinstein M, Beilstein M A, Hackett E, Fink J S, Low M J, et al. Proc Natl Acad Sci USA. 2001;98:1970–1975. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferré S, O'Connor W T, Fuxe K, Ungerstedt U. J Neurosci. 1993;13:5402–5406. doi: 10.1523/JNEUROSCI.13-12-05402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stricker E M, Zimmerman M B, Friedman M I, Zigmond M J. Nature. 1977;267:174–175. doi: 10.1038/267174a0. [DOI] [PubMed] [Google Scholar]
- 9.Casas M, Prat G, Robledo P, Barbanoj M, Kulisevsky J, Jané F. Pharmacol Biochem Behav. 2000;66:257–263. doi: 10.1016/s0091-3057(00)00189-1. [DOI] [PubMed] [Google Scholar]
- 10.Marshall J F, Richardson J S, Teitelbaum P. J Comp Physiol Psychol. 1974;87:808–830. doi: 10.1037/h0037223. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q Y, Palmiter R D. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 12.Kim D S, Szczypka M S, Palmiter R D. J Neurosci. 2000;20:4405–4413. doi: 10.1523/JNEUROSCI.20-12-04405.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szczypka M S, Rainey M A, Kim D S, Alaynick W A, Marck B T, Matsumoto A M, Palmiter R D. Proc Natl Acad Sci USA. 1999;96:12138–12143. doi: 10.1073/pnas.96.21.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi O H, Shamim M T, Padgett W L, Daly J W. Life Sci. 1988;43:387–398. doi: 10.1016/0024-3205(88)90517-6. [DOI] [PubMed] [Google Scholar]
- 15.Svenningsson P, Nomikos G G, Fredholm B B. J Neurosci. 1999;19:4011–4022. doi: 10.1523/JNEUROSCI.19-10-04011.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ledent C, Vaugeois J M, Schiffmann S N, Pedrazzini T, El Yacoubi M, Vanderhaeghen J J, Costentin J, Heath J K, Vassart G, Parmentier M. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- 17.El Yacoubi M, Ledent C, Ménard J F, Parmentier M, Costentin J, Vaugeois J M. Br J Pharmacol. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson B, Halldner L, Dunwiddie T V, Masino S A, Poelchen W, Giménez-Llort L, Escorihuela R M, Fernández-Teruel A, Wiesenfeld-Hallin Z, Xu X J, et al. Proc Natl Acad Sci USA. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams M, Francis J, Ghai G, Braunwalder A, Psychoyos S, Stone G A, Cash W D. J Pharmacol Exp Ther. 1987;241:415–420. [PubMed] [Google Scholar]
- 20.Lohse M J, Klotz K N, Schwabe U, Cristalli G, Vittori S, Grifantini M. Naunyn-Schmiedeberg's Arch Pharmacol. 1988;337:687–689. doi: 10.1007/BF00175797. [DOI] [PubMed] [Google Scholar]
- 21.Szczypka M S, Kwok K, Brot M D, Marck B T, Matsumoto A M, Donahue B A, Palmiter R D. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 22.Moreau J L, Huber G. Brain Res Rev. 1999;31:65–82. doi: 10.1016/s0165-0173(99)00059-4. [DOI] [PubMed] [Google Scholar]
- 23.Dassesse D, Ledent C, Parmentier M, Schiffmann S N. Synapse. 2001;42:63–76. doi: 10.1002/syn.1100. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima T, Daval J L, Morgan P F, Post R M, Marangos P J. Brain Res. 1989;501:307–314. doi: 10.1016/0006-8993(89)90647-1. [DOI] [PubMed] [Google Scholar]
- 25.Johansson B, Lindstrom K, Fredholm B B. Neuroscience. 1994;59:837–849. doi: 10.1016/0306-4522(94)90288-7. [DOI] [PubMed] [Google Scholar]
- 26.Svenningsson P, Ström A, Johansson B, Fredholm B B. J Neurosci. 1995;15:3583–3593. doi: 10.1523/JNEUROSCI.15-05-03583.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svenningsson P, Nomikos G G, Fredholm B B. J Neurosci. 1995;15:7612–7624. doi: 10.1523/JNEUROSCI.15-11-07612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svenningsson P, Georgieva J, Kontny E, Heilig M, Fredholm B B. Eur J Neurosci. 1997;9:2135–2141. doi: 10.1111/j.1460-9568.1997.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 29.Bennett H J, Semba K. J Comp Neurol. 1998;401:89–108. doi: 10.1002/(sici)1096-9861(19981109)401:1<89::aid-cne6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 30.Dassesse D, Vanderwinden J M, Goldberg I, Vanderhaeghen J J, Schiffmann S N. Eur J Neurosci. 1999;11:3101–3114. doi: 10.1046/j.1460-9568.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 31.Pinna A, di Chiara G, Wardas J, Morelli M. Eur J Neurosci. 1996;8:1176–1181. doi: 10.1111/j.1460-9568.1996.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 32.Fenu S, Pinna A, Ongini E, Morelli M. Eur J Pharmacol. 1997;321:143–147. doi: 10.1016/s0014-2999(96)00944-2. [DOI] [PubMed] [Google Scholar]
- 33.Robertson G S, Vincent S R, Fibiger H C. Neuroscience. 1992;49:285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- 34.Morelli M, Fenu S, Cozzolino A, Pinna A, Carta A, Di Chiara G. Neuroscience. 1993;53:673–678. doi: 10.1016/0306-4522(93)90615-m. [DOI] [PubMed] [Google Scholar]
- 35.Di Chiara G, Morelli M, Consolo S. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 36.Kanda T, Jackson M J, Smith L A, Pearce R K, Nakamura J, Kase H, Kuwana Y, Jenner P. Ann Neurol. 1998;43:507–513. doi: 10.1002/ana.410430415. [DOI] [PubMed] [Google Scholar]
- 37.Grondin R, Bedard P J, Hadj Tahar A, Gregoire L, Mori A, Kase H. Neurology. 1999;52:1673–1677. doi: 10.1212/wnl.52.8.1673. [DOI] [PubMed] [Google Scholar]
- 38.Fredduzzi S, Moratalla R, Monopoli A, Cuellar B, Xu K, Ongini E, Impagnatiello F, Schwarzschild M A, Chen J F. J Neurosci. 2002;22:1054–1062. doi: 10.1523/JNEUROSCI.22-03-01054.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]