Abstract
Increasing evidence indicates neuroinflammation is instrumental in the pathogenesis of Parkinson's disease (PD). In PD, there is selective degeneration of neuromelanin (NM)-containing dopamine neurons. Neuromelanin is predominantly cytoprotective within dopaminergic neurons, whereas, NM released from damaged neurons activates microglia. However, the effects of NM on astroglial cells remain largely unknown. Astroglia are essential to neuronal homeostasis and responsive to injury, in part, through secretion of chemokines, including interferon γ inducible protein-10 (CXCL10). Thus, we used an in vitro approach to identify the effects of NM on TNFα-induced CXCL10 expression in human astroglial cells. TNFα-induced CXCL10 expression was inhibited in NM exposed cells. Additionally, TNFα-induced NF-κB activation was inhibited by NM. Given that CXCL10 expression is NF-κB-dependent in human astroglial cells, these findings suggest that NM may inhibit CXCL10 expression, in part, through an NF-κB-dependent mechanism. While the in vivo consequences of NM mediated effects on astroglial CXCL10 expression remain to be fully elucidated, insights obtained in this study further our understanding of the effects of NM on inflammatory signaling in human astroglial cells.
Keywords: Parkinson's disease, chemokine, astrocytes, neuroinflammation, NF-κB
Parkinson's disease (PD) affects the pigmented nigrostriatal dopaminergic neurons and results in a progressive neurodegeneration of these neurons. Parkinson's disease is distinguished as the second most common neurodegenerative disorder with a prevalence of 0.1% of the global population [39]. Clinical manifestations of PD include akinesia, bradykinesia, a rhythmic involuntary tremor at rest (‘pill rolling movement’), postural instability, and extrapyramidal rigidity in which major muscle groups become stiff, collectively referred to as parkinsonism. The appearance of parkinsonism develops when loss of at least 50% of the dopaminergic neurons in the substantia nigra (SN) pars compacta (SNpc) occurs, leading to a reduction of over 80% in dopamine levels in the striatum [39]. Impairment of dopaminergic neurotransmission and parkinsonism are also observed in HIV infected subjects with HIV dementia [24]. Similarly, significant neuronal degeneration is observed in the substantia nigra of HIV infected individuals [21].
The exact mechanism responsible for the neuronal loss in PD and HIV dementia is not completely understood; however, oxidative stress [1, 29], mitochondrial dysfunction [29] and neuroinflammation [16, 20] are involved. In terms of neuroinflammation, levels of the proinflammatory cytokine TNFα are markedly elevated in the SN and cerebral spinal fluid (CSF) of individuals with PD [6, 28] and HIV-1 infection [37, 38]. Likewise, certain chemokines are up-regulated in the CNS of those with neuropathologies associated dopamine neurons, including PD [31], animal models of PD [22] and HIV-1 infection [23]. Importantly, increasing evidence points to activated astrocytes as contributors to the loss of dopaminergic neurons [19, 27, 43]. Elevated TNFα is likely one of several factors which activate astroglia during states of neuroinflammation, resulting in release of proinflammatory and neurotoxic molecules [36, 40]. Chemokines are among the astroglial derived products thought to contribute to neuropathogenesis [20, 31, 43]. We are particularly interested in CXCL10, a chemokine which is known to be neurotoxic [33] and in a murine PD model has been reported to be elevated in the striatum and the ventral midbrain [22]. CXCL10 is also chemoattractant for activated T cells [35], monocytes/macrophages [35], and microglia [17], has antimicrobial activity [11], and induces astrocyte proliferation [17]. Thus, depending on the temporal, regional and magnitude of expression in the brain, CXCL10 may be neuroprotective or neurotoxic. Likewise, while dopamine neurons containing the pigment neuromelanin (NM) selectively degenerate in PD, the extent to which NM contributes to neurodegeneration versus neuroprotection remains unclear. Indeed, there is increasing interest in understanding the role of extracellular NM in neuropathogenesis [5, 42, 49]. Based on these collective insights, the objective of this study was to identify the effects of NM on TNFα-induced CXCL10 expression in human astroglial cells. These findings are expected to further our understanding of the role of astroglia and chemokines in the neuropathology involving dopaminergic neurons.
The human A172 astroglial cell line (ATCC #CRL-1620; American Type Culture Collection, Manassas, VA) is well documented as a useful model in pharmacology- and neurochemistry-based studies [14]. A172 cells were maintained in Dulbecco's modified Eagle's medium containing 2 mM L-glutamine, 10% fetal bovine serum, 1% nonessential amino acids, 50 U/ml penicillin, 0.05 mg/ml streptomycin and 2 μg/ml amphotericin B. Cell cultures were maintained in a humidified incubator at 37°C, 5% CO2 and 95% air with the medium changed every 48–72 h. Experimental cultures were seeded at a cell density to provide 80–90% confluence at the time of treatment.
Neuromelanin was isolated as previously described [47] from SN samples obtained during autopsies of subjects who died without any known neurological or psychiatric disorders. The purity of the NM was assessed by elemental analysis, amino acid analysis and electron paramagnetic resonance spectroscopy as previously described [44, 45]. Shortly before the experiments were initiated a stock suspension of NM was prepared in sterile water (0.5 mg/ml) and stored at 4°C. Just prior to initiating the experiment, the NM suspension was diluted to desired concentrations (0.02-15 μg/ml) in serum-free culture medium and incubated at room temperature for 2 h before adding to cell cultures. The biological relevance of this concentration range of NM was based on the in situ concentrations found in the SN of normal subjects [46].
A172 cells were cultured in serum free medium for 48 h in the presence or absence of NM (0.02-15 μg/ml). Cells were then co-exposed to human recombinant TNFα (5 ng/ml; Peprotech, Rocky Hill, NJ) for an additional 24 h. While TNFα concentrations in human brain tissue are not well established, 5 ng/ml is within the range typically used for in vitro stimulation of glia and immune cells [41, 50]. Following the treatment period, levels of secreted CXCL10 protein in the culture media were quantitated using a standard dual-antibody solid phase immunoassay (Human CXCL10/IP-10 ELISA Development Kit, Peprotech, cat# 900-K39), according to the manufacturer's instructions as previously described [12]. Absorbance was read at 450 nm (with wavelength correction set at 650 nm) on a BIOTEK HT spectrophotometer.
To assess mitochondrial integrity/cell viability, parallel experiments were performed followed by performance of the MTT assay according to a modified version of the procedure described by [8]. Following, the 72 h treatment period, culture media was replaced with fresh serum free medium containing 0.55 mg/ml 3-[4,5-dimethylthiazol-2-yl]-2,5,-diphenyltetrazolium bromide (MTT) and cultures returned to the humidified incubator (37°C, 5% CO2 and 95% air) for 45 min. Media was removed from the cultures, cells dissolved in 1 ml dimethyl sulfoxide and absorbance measured at 492 nm using a BIO-TEK HT spectrophotometer.
To assess NM effects on NF-κB activation, cells were treated as described above, except the cells were only exposed to TNFα for the final 0.5 h of the 72 h NM exposure period. Nuclear protein fractions were obtained as described in our previous report [15]. NF-κB p65- and NF-κB p50-DNA binding activity was determined using a Transcription Factor kit (Thermo Scientific) according to manufacturer's instructions. Luminescence of the labeled NF-κB-DNA product was measured using a BIO-TEK HT spectrophotometer. Additionally, total cell protein/well was determined using the bicinchoninic acid (BCA) protein assay as previously described [13] in order to normalize data when appropriate.
Prism™ version 4.0 software (GraphPad Inc.) was used for figure presentation, percent transformations and statistical analysis. Analyses included one-way analysis of variance (ANOVA) with Dunnett's comparison post hoc test. Data from 2-5 independent experiments are presented as mean + S.E.M. A probability (p) of < 0.05 was accepted as demonstrating statistically significant differences between groups. The number of replicate measures and independent experiments from which the data were obtained are provided in the individual figure legends.
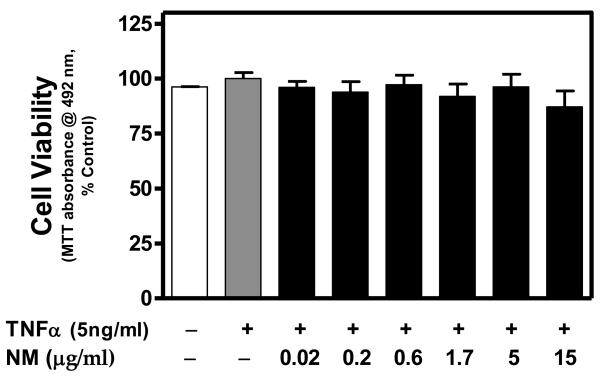
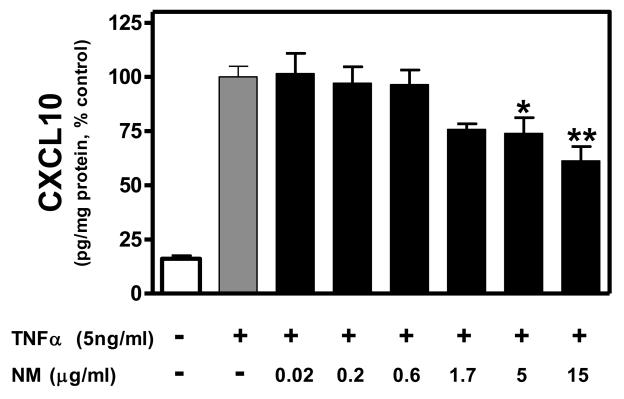
Based on the MTT assay, cell viability was not significantly affected by TNFα or TNFα + NM (Fig. 1). Similarly, NM alone did not alter cell viability (data not shown). Constitutive expression of CXCL10 protein was relatively low, whereas, TNFα significantly induced CXCL10 expression (Fig. 2). NM alone had no effect on CXCL10 expression at any concentration tested (data not shown). However, NM exposure (≥ 5 μg/ml) significantly inhibited TNFα-induced CXCL10 expression in human astroglial cells (Fig. 2).
Figure 1. Neuromelanin is not cytotoxic to TNFα-stimulated human A172 astroglial cells.
A172 cells were cultured in serum free medium for 48 h in the presence or absence of NM (0.02-15 μg/ml), then co-exposed to human recombinant TNFα (5ng/ml) for an additional 24 h. To assess mitochondrial integrity/cell viability, the MTT assay was performed. Data were normalized as percent control (TNFα alone) within each experiment and presented as the mean + S.E.M. of triplicate measures from 2-3 independent experiments. No significant differences (P < 0.05) among treatments were detected by one-way ANOVA.
Figure 2. Neuromelanin inhibits TNFα-induced CXCL10 protein expression in human A172 astroglial cells.
A172 cells were cultured in serum free medium for 48 h in the presence or absence of NM (0.02-15 μg/ml), then co-exposed to human recombinant TNFα (5ng/ml) for an additional 24 h. Secreted CXCL10 protein in the culture media was then quantitated using a standard dual-antibody solid phase immunoassay, according to the manufacturer's instructions as previously described [12]. Absorbance was read at 450 nm (with wavelength correction set at 650 nm) on a BIOTEK HT spectrophotometer. Data were normalized as percent control (TNFα alone) within each experiment and presented as the mean + S.E.M. of triplicate measures from 3-4 independent experiments. Statistical differences determined by one-way ANOVA with Dunnett's post-hoc comparisons. *P < 0.05 vs TNFα; **P < 0.01 vs TNFα.
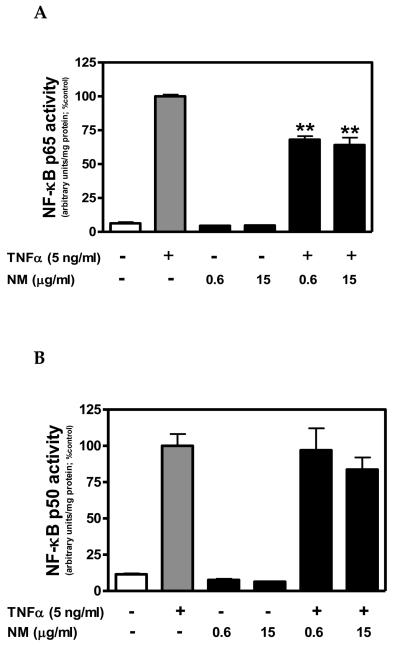
NF-κB activation, as determined by nuclear levels of NF-κB p65 and p50 protein, was relatively low in untreated controls and cells exposed to NM alone (Fig. 3). Conversely, TNFα significantly enhanced nuclear levels of both NF-κB p65 and p50 protein (Fig. 3). TNFα-induced NF-κB p65 activation was inhibited by ≥ 0.6 μg/ml NM, whereas, NF-κB p50 activation was not significantly altered by NM (Fig.3).
Figure 3. Neuromelanin inhibits TNFα-induced NF-κB activation in human A172 astroglial cells.
A172 cells were cultured in serum free medium for 72 h in the presence or absence of NM (0.6 or 15 μg/ml). Cells were co-exposed to human recombinant TNFα (5ng/ml) for the final 0.5 h of the 72 h NM exposure period. Nuclear protein fractions were obtained and NF-κB p65- and NF-κB p50-DNA binding activity was determined using a Transcription Factor kit, according to manufacturer's instructions. Binding activity was determined by luminescence of the labeled NF-κB-DNA product as measured using a BIO-TEK HT spectrophotometer. Binding activity was normalized to total nuclear protein and presented as arbitrary units. Data represent the mean + S.E.M. of duplicate measures from 2 independent experiments. Significant differences were determined by one-way ANOVA with Dunnett's post-hoc comparisons. **P < 0.01 vs TNFα.
Inhibition of astroglial CXCL10 expression by NM may be particularly important in our efforts to elucidate the role of NM in certain neuropathologies, including PD. It is known that in the SN of patients affected by PD a significant amount of extracellular NM is present and around these NM granules was observed an accumulation of activated microglia [4, 25, 26]. While there is increasing evidence that NM is involved in PD neuropathogenesis, it remains unclear exactly to what extent NM contributes to neurodegeneration versus neuroprotection. NM synthesis prevents accumulation of cytosolic catecholamines, thereby providing protection to dopaminergic neurons [34]. Furthermore, NM can be cytoprotective in dopaminergic neurons through chelation of redox active metals, and a variety of other toxic metals and compounds [48]. In contrast to intracellular NM, NM released from damaged dopaminergic neurons results in microglial activation and production of neurotoxic molecules which further damage dopaminergic neurons [42, 47, 49].
Chemokines, including CXCL10, appear to be associated with dopaminergic neurodegeneration. For instance, in the 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP) model of murine PD, CXCL10 mRNA expression was up regulated in the striatum and the ventral midbrain; however, the cellular source of CXCL10 was not determined [22]. Similarly, evidence points to the involvement of CXCL10 in HIV-1 associated neuropathology [10, 32].
The consequences of altered chemokine (i.e, CXCL10) expression in dopaminergic associated neuropathology have not been fully elucidated. Modulation of astroglial CXCL10 expression by NM, could potentially have important implications in neuropathologies associated with dopaminergic neurons. Astroglial chemokine expression is of particular interest given these cells are the most prevalent cell type in the human CNS and are essential for neuronal homeostasis, response to injury, maintenance of the blood-brain barrier and a major source of chemokines in the brain [3, 9, 30].
In terms of direct NM mediated effects, we did not observe NM induced cytotoxicity in A172 cells, but did in human SK-N-SH neuronal cells (data not shown). Together, these findings are consistent with the concept that glial cells in the brain can survive under conditions where neuronal cells degenerate. Also, we provided in vitro evidence that extracellular NM may also exert anti-inflammatory actions. That is, TNF-induced CXCL10 expression in human astroglial cells was inhibited in NM exposed cells. The specific in vivo consequences of NM mediated inhibition of astroglial CXCL10 expression remain to be determined. However, under conditions where CXCL10 is inducing neurotoxicity, NM would potentially be neuroprotective. Conversely, in a microenvironment where CXCL10 is providing neuroprotection (i.e., immune responsiveness or neurotrophic support) inhibition of CXCL10 by NM may be detrimental to neurons in the region. It is also important to note that while this report is focused on a single chemokine, CXCL10, preliminary studies indicate CCL2 expression is also inhibited by NM (data not shown).
Activation of the transcription factor, NF-κB, is associated with dopaminergic associated neuropathology. For instance, within the ventral midbrain of PD patients, NF-κB p65 levels are elevated in microglia and astrocytes as compared to age-matched controls [18]. Similarly, in a mouse model of PD, MPTP significantly induced NF-κB activation in astrocytes of the SN [2]. Numerous inflammatory responses in astrocytes, including chemokine expression, are transcriptionally regulated by NF-κB [7, 36]. In particular, we previously utilized functionally distinct inhibitors of NF-κB activation to demonstrate that TNFα-induced CXCL10 expression in human A172 astroglial cells is NF-κB-dependent [12]. Interestingly, the data presented in this manuscript suggest that NM may inhibit TNF-induced CXCL10 expression in human astroglial cells, in part, through an NF-κB p65 dependent mechanism. The importance of NM mediated inhibition of NF-κB activation likely extends beyond just inhibition of CXCL10 expression. That is, the expression of many inflammatory molecules in astrocytes is transcriptionally regulated by NF-κB, thus, NM may inhibit transcription of multiple inflammatory molecules in astroglia. Seemingly in contrast, in rat microglia, NM induces inflammatory signaling including NF-κB activation, and expression of inducible nitric oxide synthase and TNFα [42, 49]. In previous work [49], NM injected into the rat SN induced microgliosis and astrogliosis. It was speculated that astrocytosis was a protective response consequent to the neurodegeneration induced by activated microglia. However, based on the present data we can suggest also a direct effect of NM on astrocytes which would counteract the neurodegenerative effect caused by microglia. Together, these findings suggest that NM modulation of inflammatory signaling may differ among glial cell types. However, to our knowledge, this is the first report of NM effects on NF-κB activation and CXCL10 expression in human astroglial cells.
Therefore, this study provides novel insights into the effects of NM on inflammatory signaling in human astroglial cells. Further investigation is needed to fully elucidate the mechanism by which NM inhibits inflammatory signaling in astroglial cells and determine the in vivo consequences of these NM mediated effects in astroglia.
Acknowledgements
This work was supported in part by NIH grants AA 014955 and NS 062664 (RLD); Oklahoma State University Center for Health Sciences Intramural grant CHS-0809 (RLD); and LZ was supported by the MIUR-PRIN project 2005035582 on Chemical Processes and Structural Modifications in Neurodegeneration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agrawal L, Louboutin JP, Marusich E, Reyes BA, Van Bockstaele EJ, Strayer DS. Dopaminergic neurotoxicity of HIV-1 gp120: reactive oxygen species as signaling intermediates. Brain Res. 2010;1306:116–130. doi: 10.1016/j.brainres.2009.09.113. [DOI] [PubMed] [Google Scholar]
- 2.Aoki E, Yano R, Yokoyama H, Kato H, Araki T. Role of nuclear transcription factor kappa B (NF-kappaB) for MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine)-induced apoptosis in nigral neurons of mice. Exp Mol Pathol. 2009;86:57–64. doi: 10.1016/j.yexmp.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banati RB, Daniel SE, Blunt SB. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson's disease. Mov Disord. 1998;13:221–227. doi: 10.1002/mds.870130205. [DOI] [PubMed] [Google Scholar]
- 5.Beach TG, Sue LI, Walker DG, Lue LF, Connor DJ, Caviness JN, Sabbagh MN, Adler CH. Marked microglial reaction in normal aging human substantia nigra: correlation with extraneuronal neuromelanin pigment deposits. Acta Neuropathol. 2007;114:419–424. doi: 10.1007/s00401-007-0250-5. [DOI] [PubMed] [Google Scholar]
- 6.Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci Lett. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- 7.Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Research. 1987;47:936–942. [PubMed] [Google Scholar]
- 9.Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- 10.Cinque P, Bestetti A, Marenzi R, Sala S, Gisslen M, Hagberg L, Price RW. Cerebrospinal fluid interferon-gamma-inducible protein 10 (IP-10, CXCL10) in HIV-1 infection. J Neuroimmunol. 2005;168:154–163. doi: 10.1016/j.jneuroim.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167:623–627. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- 12.Davis RL, Buck DJ, Saffarian N, Stevens CW. The opioid antagonist, beta-funaltrexamine, inhibits chemokine expression in human astroglial cells. J Neuroimmunol. 2007;186:141–149. doi: 10.1016/j.jneuroim.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis RL, Dertien J, Syapin PJ. Ethanol-induced modulation of inducible nitric-oxide synthase activity in human A172 astrocytoma cells. Alcohol Clin Exp Res. 2002;26:1404–1411. doi: 10.1097/01.ALC.0000030841.92766.80. [DOI] [PubMed] [Google Scholar]
- 14.Davis RL, Syapin PJ. Chronic ethanol inhibits CXC chemokine ligand 10 production in human A172 astroglia and astroglial-mediated leukocyte chemotaxis. Neurosci Lett. 2004;362:220–225. doi: 10.1016/j.neulet.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Davis RL, Syapin PJ. Ethanol increases nuclear factor-kappa B activity in human astroglial cells. Neurosci Lett. 2004;371:128–132. doi: 10.1016/j.neulet.2004.08.051. [DOI] [PubMed] [Google Scholar]
- 16.Deshpande M, Zheng J, Borgmann K, Persidsky R, Wu L, Schellpeper C, Ghorpade A. Role of activated astrocytes in neuronal damage: potential links to HIV-1-associated dementia. Neurotox Res. 2005;7:183–192. doi: 10.1007/BF03036448. [DOI] [PubMed] [Google Scholar]
- 17.Flynn G, Maru S, Loughlin J, Romero IA, Male D. Regulation of chemokine receptor expression in human microglia and astrocytes. J Neuroimmunol. 2003;136:84–93. doi: 10.1016/s0165-5728(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh A, Roy A, Matras J, Brahmachari S, Gendelman HE, Pahan K. Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson's disease. J Neurosci. 2009;29:13543–13556. doi: 10.1523/JNEUROSCI.4144-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henning J, Strauss U, Wree A, Gimsa J, Rolfs A, Benecke R, Gimsa U. Differential astroglial activation in 6-hydroxydopamine models of Parkinson's disease. Neurosci Res. 2008;62:246–253. doi: 10.1016/j.neures.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 21.Itoh K, Mehraein P, Weis S. Neuronal damage of the substantia nigra in HIV-1 infected brains. Acta Neuropathol. 2000;99:376–384. doi: 10.1007/s004010051139. [DOI] [PubMed] [Google Scholar]
- 22.Kalkonde YV, Morgan WW, Sigala J, Maffi SK, Condello C, Kuziel W, Ahuja SS, Ahuja SK. Chemokines in the MPTP model of Parkinson's disease: absence of CCL2 and its receptor CCR2 does not protect against striatal neurodegeneration. Brain Res. 2007;1128:1–11. doi: 10.1016/j.brainres.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 23.Kolb SA, Sporer B, Lahrtz F, Koedel U, Pfister HW, Fontana A. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol. 1999;93:172–181. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- 24.Koutsilieri E, Sopper S, Scheller C, ter Meulen V, Riederer P. Parkinsonism in HIV dementia. J Neural Transm. 2002;109:767–775. doi: 10.1007/s007020200063. [DOI] [PubMed] [Google Scholar]
- 25.Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 26.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 27.McGeer PL, McGeer EG. Glial reactions in Parkinson's disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 28.Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson's disease. J Neural Transm Suppl. 2000:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- 29.Onyango IG. Mitochondrial dysfunction and oxidative stress in Parkinson's disease. Neurochem Res. 2008;33:589–597. doi: 10.1007/s11064-007-9482-y. [DOI] [PubMed] [Google Scholar]
- 30.Prat A, Biernacki K, Wosik K, Antel JP. Glial cell influence on the human blood-brain barrier. Glia. 2001;36:145–155. doi: 10.1002/glia.1104. [DOI] [PubMed] [Google Scholar]
- 31.Shimoji M, Pagan F, Healton EB, Mocchetti I. CXCR4 and CXCL12 expression is increased in the nigro-striatal system of Parkinson's disease. Neurotox Res. 2009;16:318–328. doi: 10.1007/s12640-009-9076-3. [DOI] [PubMed] [Google Scholar]
- 32.Sui Y, Potula R, Dhillon N, Pinson D, Li S, Nath A, Anderson C, Turchan J, Kolson D, Narayan O, Buch S. Neuronal apoptosis is mediated by CXCL10 overexpression in simian human immunodeficiency virus encephalitis. Am J Pathol. 2004;164:1557–1566. doi: 10.1016/S0002-9440(10)63714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sui Y, Stehno-Bittel L, Li S, Loganathan R, Dhillon NK, Pinson D, Nath A, Kolson D, Narayan O, Buch S. CXCL10-induced cell death in neurons: role of calcium dysregulation. Eur J Neurosci. 2006;23:957–964. doi: 10.1111/j.1460-9568.2006.04631.x. [DOI] [PubMed] [Google Scholar]
- 34.Sulzer D, Bogulavsky J, Larsen KE, Behr G, Karatekin E, Kleinman MH, Turro N, Krantz D, Edwards RH, Greene LA, Zecca L. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci U S A. 2000;97:11869–11874. doi: 10.1073/pnas.97.22.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson WL, Van Eldik LJ. Inflammatory cytokines stimulate the chemokines CCL2/MCP-1 and CCL7/MCP-3 through NFkB and MAPK dependent pathways in rat astrocytes [corrected] Brain Res. 2009;1287:47–57. doi: 10.1016/j.brainres.2009.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- 38.Wesselingh SL, Takahashi K, Glass JD, McArthur JC, Griffin JW, Griffin DE. Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. J Neuroimmunol. 1997;74:1–8. doi: 10.1016/s0165-5728(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 39.Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson's disease. Br J Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams R, Dhillon NK, Hegde ST, Yao H, Peng F, Callen S, Chebloune Y, Davis RL, Buch SJ. Proinflammatory cytokines and HIV-1 synergistically enhance CXCL10 expression in human astrocytes. Glia. 2009;57:734–743. doi: 10.1002/glia.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams R, Yao H, Peng F, Yang Y, Bethel-Brown C, Buch S. Cooperative induction of CXCL10 involves NADPH oxidase: Implications for HIV dementia. Glia. 58:611–621. doi: 10.1002/glia.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L, Lucius R. Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson's disease. Faseb J. 2003;17:500–502. doi: 10.1096/fj.02-0314fje. [DOI] [PubMed] [Google Scholar]
- 43.Yasuda Y, Shimoda T, Uno K, Tateishi N, Furuya S, Yagi K, Suzuki K, Fujita S. The effects of MPTP on the activation of microglia/astrocytes and cytokine/chemokine levels in different mice strains. J Neuroimmunol. 2008;204:43–51. doi: 10.1016/j.jneuroim.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Zecca L, Costi P, Mecacci C, Ito S, Terreni M, Sonnino S. Interaction of human substantia nigra neuromelanin with lipids and peptides. J Neurochem. 2000;74:1758–1765. doi: 10.1046/j.1471-4159.2000.0741758.x. [DOI] [PubMed] [Google Scholar]
- 45.Zecca L, Fariello R, Riederer P, Sulzer D, Gatti A, Tampellini D. The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson's disease. FEBS Lett. 2002;510:216–220. doi: 10.1016/s0014-5793(01)03269-0. [DOI] [PubMed] [Google Scholar]
- 46.Zecca L, Gallorini M, Schunemann V, Trautwein AX, Gerlach M, Riederer P, Vezzoni P, Tampellini D. Iron, neuromelanin and ferritin content in the substantia nigra of normal subjects at different ages: consequences for iron storage and neurodegenerative processes. J Neurochem. 2001;76:1766–1773. doi: 10.1046/j.1471-4159.2001.00186.x. [DOI] [PubMed] [Google Scholar]
- 47.Zecca L, Wilms H, Geick S, Claasen JH, Brandenburg LO, Holzknecht C, Panizza ML, Zucca FA, Deuschl G, Sievers J, Lucius R. Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: implications for Parkinson's disease. Acta Neuropathol. 2008;116:47–55. doi: 10.1007/s00401-008-0361-7. [DOI] [PubMed] [Google Scholar]
- 48.Zecca L, Zucca FA, Wilms H, Sulzer D. Neuromelanin of the substantia nigra: a neuronal black hole with protective and toxic characteristics. Trends Neurosci. 2003;26:578–580. doi: 10.1016/j.tins.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W, Phillips K, Wielgus AR, Liu J, Albertini A, Zucca FA, Faust R, Qian SY, Miller DS, Chignell CF, Wilson B, Jackson-Lewis V, Przedborski S, Joset D, Loike J, Hong JS, Sulzer D, Zecca L. Neuromelanin Activates Microglia and Induces Degeneration of Dopaminergic Neurons: Implications for Progression of Parkinson's Disease. Neurotox Res. 2009 doi: 10.1007/s12640-009-9140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng JC, Huang Y, Tang K, Cui M, Niemann D, Lopez A, Morgello S, Chen S. HIV-1-infected and/or immune-activated macrophages regulate astrocyte CXCL8 production through IL-1beta and TNF-alpha: involvement of mitogen-activated protein kinases and protein kinase R. J Neuroimmunol. 2008;200:100–110. doi: 10.1016/j.jneuroim.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]