Abstract
Heterologous proteins capable of transducing physical or chemical stimuli into electrical signals can be used to control the function of excitable cells in intact tissues or organisms. Restricted genetically to circumscribed populations of cellular targets, these selectively addressable sources of depolarizing current can supply distributed inputs to neural circuits, stimulate secretion, or regulate force and motility. In an initial demonstration of this principle, we have used elements of a G protein coupled signaling system, the phototransduction cascade of the fruit fly, to sensitize generalist vertebrate neurons to light [Zemelman, B. V., Lee, G. A., Ng, M. & Miesenböck, G. (2002) Neuron 33, 15–22]. We now describe the use of ectopically expressed ligand-gated ion channels as transducers of optical or pharmacological stimuli. When either the capsaicin receptor, TRPV1, the menthol receptor, TRPM8, or the ionotropic purinergic receptor P2X2 was introduced into hippocampal neurons, the cells responded to pulsed applications of agonist with characteristic sequences of depolarization, spiking, and repolarization. Responses required cognate matches between receptor and agonist, peaked at firing frequencies of ≈40 Hz, initiated and terminated rapidly, and did not attenuate. Precise dose–response relationships allowed current amplitudes and firing frequencies to be tuned by varying the concentration of ligand. Agonist could be administered either pharmacologically or, in the cases of TRPV1 and P2X2, optically, through photorelease of the active compounds from the respective “caged” precursors, 4,5-dimethoxy-2-nitrobenzyl-capsaicin and P3-[1-(4,5-dimethoxy-2-nitrophenyl)ethyl]-ATP.
Biochemistry has led the way in reducing what once were considered irreducible biological phenomena to fundamental physico-chemical principles. Keys to this accomplishment were the ability to trace the transformations of defined chemical substrates in cellular extracts or intact cells and the reconstitution of biochemical processes with pure components. For neuroscience to follow this reductionist lead, analogous experimental strategies need to be developed: an ability to trace the transformations of defined neuronal activity patterns in explanted neural tissues or intact nervous systems and a capacity to reconstitute neurally encoded information and the behaviors it guides with synthetic neural signals.
The initial hurdle to both of these strategies lies in the difficulty of feeding artificial neural signals into functionally circumscribed but anatomically dispersed populations of neurons. An organic solution to this problem is to harness proteins mediating neuronal excitation as conduits for artificial stimuli and to restrict the expression of these transducers genetically to a predetermined group of target neurons (1, 2). If, for example, a set of generalist neurons could be programmed to express signal transduction machinery that normally is present only in specialized sensory cells, these neurons also might acquire the capacity to respond selectively to the adequate physical or chemical triggers. Not only could all members of a population of neurons then be addressed simultaneously, susceptibility to stimulation rather than the stimulus itself would be localized, and even diffusely broadcast stimuli could elicit precise, patterned responses (1, 2).
Neurons offer two principal routes for the transduction of excitatory signals (3). Metabotropic signaling systems consist of heptahelical receptors that communicate with their effectors through heterotrimeric G proteins. The activation of some of these effectors is coupled to changes in membrane potential (3–7). Ionotropic signaling systems effect changes in membrane potential directly, via chemically or physically gated conductances (3). Previously, we have used components of a metabotropic system, the phototransduction cascade of the fruit fly (7), to sensitize vertebrate neurons to light (2). Expression of what was termed “chARGe” to commemorate the essential elements (arrestin-2, rhodopsin, and a G protein α-subunit) created a light-controlled source of depolarizing current whose activation sparked action potentials (2). We now introduce the use of ionotropic mechanisms for the selective chemical and optical stimulation of genetically designated populations of neurons. In addition to multiple trigger modes, these ionotropic systems possess numerous practical advantages over chARGe, including simplicity, fast kinetics, and broad tunability.
Materials and Methods
Heterologous Expression of Ion Channels.
Candidate ion channels were expressed under the control of the cytomegalovirus promoter in pCI-fluor, a derivative of the mammalian expression vector pCI-neo (Promega). To create pCI-fluor, the aminoglycoside phosphotransferase coding sequence of pCI-neo was replaced with that of enhanced GFP (EGFP) bearing a 20-amino acid N-terminal GAP43 tag (2). Rat TRPV1 (8) and rat TRPM8 (9) were expressed as monomers; rat P2X2 subunits (10) were linked into covalent trimers (11, 12) via tandem repeats of the tripeptide –Ser-Gly-Gly–. Hippocampal neurons, obtained from E19 rats and grown in dissociated cultures, were exposed to 4.2 μg·cm−2 of calcium phosphate-precipitated plasmid DNA (pH 7.08) for 20 min to allow plasmid uptake. Transfections were performed on day 8 after plating; immunocytochemical analyses and electrophysiological recordings were done on days 6–10 after transfection.
Immunocytochemistry.
Neurons were fixed in 4% (wt/vol) paraformaldehyde, permeabilized in 0.1% (vol/vol) Triton X-100, exposed to a blocking solution containing 5% (vol/vol) bovine serum and 0.2% (wt/vol) gelatin, and stained with rabbit polyclonal affinity-purified antibodies against GFP (1:250) and guinea pig polyclonal antibodies against TRPV1 (1:1,000; Chemicon). Bound antibodies were detected with AlexaFluor-488 and AlexaFluor-594 conjugates (1:500; Molecular Probes) and visualized by wide-field epifluorescence microscopy.
Electrophysiology.
Transfected neurons were identified by GAP43-EGFP fluorescence and recorded in the whole-cell patch-clamp configuration. Patch pipettes (≈2.5 MΩ) contained 120 mM K-gluconate, 10 mM KCl, 5 mM ATP, 0.3 mM GTP, and 10 mM K-Hepes (pH 7.2). The extracellular recording solution consisted of 119 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 30 mM glucose, 25 mM Na-Hepes (pH 7.4), 50 μM d,l-2-amino-5-phosphonovaleric acid (AP-5), and 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). Membrane potentials and transmembrane currents were recorded with an Axoclamp-2B amplifier (Axon Instruments, Foster City, CA) in bridge and continuous single-electrode voltage-clamp mode, respectively, and digitized at 5 kHz without filtering (Digidata 1200, Axon Instruments). Baseline currents were adjusted after break-in to set the membrane potential to −65 mV in current-clamp recordings; voltage-clamp recordings were performed at a holding potential of −65 mV.
For pharmacological stimulation, a 1-ml bolus of agonist was perfused into an RC-26G recording chamber (Warner Instruments, Hamden, CT) at a laminar flow rate of ≈6 ml/min. In photostimulation experiments, 5 μM 4,5-dimethoxy-2-nitrobenzyl-capsaicin (DMNB-capsaicin; see below) or 1 mM P3-[1-(4,5-dimethoxy-2-nitrophenyl)ethyl]-ATP (DMNPE-ATP; Molecular Probes) present in the extracellular recording solutions were exposed to the unfiltered, collimated output of a mechanically shuttered (Uniblitz, Vincent Associates, Rochester, NY) mercury arc lamp (HBO 100 W/2, Osram, Berlin) attached to the epifluorescence port of a Zeiss Axioskop FS microscope. A 50/50 beamsplitter (Chroma Technology, Brattleboro, VT), necessary to allow visually guided placement of the recording electrode, directed the uncaging beam through a slightly underfilled ×40/0.8 W Zeiss Achroplan objective to the specimen, where it illuminated a disk of ≈500-μm diameter with 5.1 mW of optical power at wavelengths <400 nm (determined subtractively, by using a Chroma E400LP interference filter and a Spectra-Physics 407A power meter).
Caged Agonists.
DMNPE-ATP (Molecular Probes) was used without further purification and was dissolved at 1 mM in extracellular recording solution.
Capsaicin (Fluka) was caged with 4,5-dimethoxy-2-nitrobenzyl chloroformate (Aldrich) according to the reaction scheme in Fig. 1. To a solution of capsaicin (10.7 mg, 0.035 mmol) in methylene chloride (CH2Cl2; 2 ml) at 0°C were added ≈5 equivalents of 4,5-dimethoxy-2-nitrobenzyl chloroformate (46.9 mg, 0.170 mmol), followed by ≈2 equivalents of triethylamine (10 μl, 0.072 mmol). The reaction mixture was stirred in the dark for 2 h at room temperature to allow quantitative conversion of capsaicin to the caged product. The reaction was monitored by thin layer chromatography on silica gel by using 50% ethyl acetate/hexanes as the mobile phase. DMNB-capsaicin migrates at an Rf of 0.10 in this system. The reaction mixture was concentrated to ≈100 μl in the dark under a stream of argon, diluted with 1 ml of 50% ethyl acetate/hexanes, and chromatographed on a silica gel column (230–400 mesh, Merck). The column was developed with a gradient of 50–70% ethyl acetate/hexanes, and fractions at Rf 0.10 were combined and concentrated on a rotary evaporator to yield DMNB-capsaicin quantitatively (18.9 mg, 99%). The compound was dissolved at 100 mM in anhydrous DMSO and stored at −80°C under argon. For photostimulation experiments, a working stock of 5 mM DMNB-capsaicin in DMSO was freshly diluted to 5 μM in extracellular recording solution.
Figure 1.
Synthesis and structure of DMNB-capsaicin. The DMNB chromophore is attached to the phenolic hydroxyl function of capsaicin, a molecular feature important for agonist activity.
DMNB-capsaicin was characterized by 1H NMR and mass spectrometry. 1H NMR spectra were obtained on a Bruker DMX 500 MHz spectrometer and are reported in parts per million (ppm) relative to tetramethylsilane (δ), with coupling constants (J) in Hz. The residual protic solvent in CDCl3 was used as an internal reference. 1H NMR (500 MHz, CDCl3): δ = 7.76 (s, 1H, Ar of DMNB), 7.17 (s, 1H, Ar of DMNB), 7.09 (d, 1H, J = 1.5 Hz, Ar of capsaicin), 6.92 (s, 1H, Ar of capsaicin), 6.86 (d, 1H, J = 1.5 Hz, Ar of capsaicin), 5.71 (s, 2H, Bn of DMNB), 5.70 (m, 1H, vinyl), 5.34 (m, 1H, vinyl), 4.43 (d, 2H, J = 5.5 Hz, Bn of capsaicin), 4.02 (s, 3H, OCH3 of DMNB), 3.98 (s, 3H, OCH3 of DMNB), 3.82 (s, 3H, OCH3 of capsaicin), 2.26 [m, 1H, CH(CH3)2], 2.24 (m, 2H, αH), 2.00 (m, 2H, δH), 1.67 (m, 2H, γH), 1.40 (m, 2H, βH), 1.04 and 0.97 [d, 6H, J = 7.0 Hz, CH(CH3)2]. Atmospheric pressure chemical ionization mass spectra, measured on a JEOL LCmate mass spectrometer, confirmed the predicted molecular mass of DMNB-capsaicin. [C28H36N2O9 + H+]: calculated 545.24; found 545.2.
Results
Candidate Ion Channels.
Candidate ion channels for heterologous expression in central neurons were selected on the basis of seven criteria. The ideal channel would (i) carry a depolarizing sodium or calcium current and (ii) be gated directly by a small, drug-like agonist that is (iii) not used as a neurotransmitter in the central nervous system; (iv) the channel's non- or slowly desensitizing conductance would be formed by (v) a monomeric or homo-oligomeric protein whose (vi) subunits contain an even number of transmembrane segments. This transmembrane topology ensures that both termini of the channel polypeptide emerge on the same side of the plasma membrane, allowing subunits to be linked covalently into multimers to prevent subunit mixing with endogenous channels. Finally, (vii) the channel would be gated by an agonist that can be derivatized with photolabile blocking groups (“cages”) that render the molecule biologically inert. Neurons could then be stimulated either pharmacologically, through direct application of agonist, or optically, through photorelease of agonist from the caged precursor.
TRPV1 and TRPM8, the vanilloid and menthol receptors expressed by nociceptive neurons of the peripheral nervous system (8, 9, 13), match this filter almost perfectly. Both channels and their principal agonists, capsaicin and cooling compounds such as menthol, respectively, are virtually absent from the CNS (refs 8, 9, and 13; but see also ref. 14). Both channels are thought to function as nonselective, sodium- and calcium-permeable homotetramers (8, 9, 13, 15, 16). Capsaicin and some cooling compounds, including menthol and iciclin, contain potential acceptor sites for photoremovable blocking groups.
P2X2, an ATP-gated nonselective cation channel (10, 17) distinguished by its slow rate of desensitization (18, 19), represents a candidate from a channel family other than the TRP superfamily of ion channels. Although the utility of P2X2 as a selectively addressable source of depolarizing current may currently be limited by the presence of endogenous purinergic receptors at some central synapses (10, 17, 19, 20), P2X2 presents an ideal platform for future chemical genetic efforts aimed at generating engineered channel-ligand combinations that lack natural agonists altogether. ATP-gated ion channels possess one of the simplest known channel architectures (3, 10, 17, 19, 20) and a large extracellular ligand-binding domain (10, 17, 19, 21) whose atomic structure might be determined with relative ease. A high-resolution structure could guide the design of mutations in the channel's ligand-binding domain that abolish sensitivity to ATP (22, 23). “Second-site” substitutions on the nucleotide ligand (22, 23) could complement these mutations and restore functional (but entirely unnatural) receptor-ligand pairs. P2X2 was included in our analysis expressly with this possibility in mind.
Genetic Designation of Targets.
Plasmids carrying two expression cassettes in cis, one encoding TRPV1 (8), TRPM8 (9), or P2X2 (10), and the other membrane-associated GAP43-EGFP as a transfection marker (2), were introduced into rat hippocampal neurons in primary culture. TRPV1 and TRPM8 were synthesized as monomers and expected to associate noncovalently into functional tetramers (15, 16); P2X2 was expressed as a covalently linked trimer (11, 12).
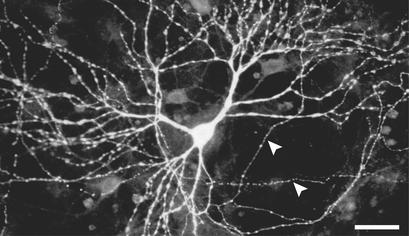
The subcellular distribution of one exogenous channel protein, TRPV1, was examined morphologically. TRPV1 decorated the neuronal plasma membrane in its entirety; it was detected on dendrites, somata, and axons (Fig. 2). This distribution suggested that agonist-induced currents could, at least in part, mimic excitatory synaptic input at dendrites and somata. In addition, opening of TRPV1 channels in spike initiation zones (such as the initial axon segment) would be expected to short-circuit the neuron's spike generator directly.
Figure 2.
Expression of TRPV1 in cultured hippocampal neurons. Dissociated cultures of hippocampal neurons were immunostained with an antibody against TRPV1. TRPV1 protein is detected on the soma and dendrites of a transfected neuron (identified by coexpression of GAP43-EGFP; not shown), as well as on several axons that traverse the field of view (white arrowheads). (Bar = 20 μm.)
Pharmacological Stimulation.
Irrespective of the type of heterologous channel present, the membrane potentials of transfected neurons, recorded under whole-cell current clamp in the presence of glutamate receptor antagonists (50 μM AP-5 plus 10 μM CNQX), remained stably at resting levels in the absence of ligand. The resting potentials of transfected neurons (−50.6 ± 9.6 mV; mean ± SD, n = 30) tended to be more positive than those of untransfected cells (−59.7 ± 2.9 mV; mean ± SD, n = 4), suggesting that the presence of the heterologous channels created small depolarizing leakage currents. The application of a bolus of agonist (50 nM capsaicin/100 μm of menthol/50 μm of ATP) led to a characteristic sequence of depolarization, spiking, and repolarization (Fig. 3) whose time course reflected the “pharmacokinetics” of agonist delivery: the dead volume of the perfusion apparatus, the volume of the agonist-containing bolus, and the exchange time of the recording chamber. The pharmacological specificity of stimulation was absolute; of the nine possible receptor-agonist combinations tested, only the three cognate matches, depicted in the diagonal of Fig. 3, elicited responses. None of the three agonists, including ATP, was able to stimulate hippocampal neurons lacking the cognate exogenous receptor (see the off-diagonal entries in Fig. 3). As intended, responsiveness to the broadly applied pharmacological stimulus was restricted to a genetically delimited population of targets.
Figure 3.
Pharmacological stimulation of genetically designated target neurons. The membrane potentials of hippocampal neurons expressing TRPV1 (Top), TRPM8 (Middle), and P2X2 (Bottom) were recorded in whole-cell current-clamp mode. All neurons were challenged sequentially with 50 nM capsaicin (Left), 100 μM menthol (Center), and 50 μM ATP (Right); approximate periods of agonist application are indicated by horizontal bars. Only cognate matches between ionotropic receptor and agonist (shaded entries in the diagonal) elicit action potentials. Increased baseline noise of the TRPV1-expressing neuron after the application of capsaicin (Top, Center and Right) is caused by residual capsaicin in the perfusion system.
To determine dose–response relationships, the peak amplitudes as well as the integrated charges carried by depolarizing currents at different agonist concentrations were measured under whole-cell voltage clamp, at a constant holding potential of −65 mV. As illustrated for TRPV1 in Fig. 4A, current amplitudes and charge transfers saturated: half-maximal responses were seen at ≈150 nM capsaicin; the pseudolinear response range extended from 70 to 200 nM. TRPM8 showed a qualitatively similar dose–response curve, with a half-maximal response at 150 μm of menthol (results not shown). The maximal agonist-induced currents varied considerably from neuron to neuron (range: 360–2,457 nA, n = 8), presumably as a result of differences in neuronal surface areas and channel densities caused by variable copy numbers of transfected plasmid. Under the assumptions of a 35-pS single-channel conductance at −65 mV (8) and linear summation of current, we estimate that transfected neurons expressed between 160,000 and 1,000,000 functional TRPV1 channels.
Figure 4.
Dose dependence of the capsaicin response in TRPV1-expressing neurons. (A) Transmembrane currents of a TRPV1-expressing hippocampal neuron were recorded in whole-cell voltage-clamp mode, at a constant holding potential of −65 mV and variable concentrations of capsaicin. Charge transfers as a function of capsaicin concentration were calculated by integrating the agonist-evoked currents over time (Inset: approximate periods of agonist presence are indicated by a horizontal bar). Depolarizing currents are depicted as downward deflections from baseline; charge transfers and corresponding current traces are drawn in identical shades of gray. (B) Membrane potentials of a TRPV1-expressing hippocampal neuron were recorded in whole-cell current-clamp mode, at the indicated concentrations of capsaicin. Capsaicin was perfused into the recording chamber between 200 and 400 ms and remained present thereafter.
Because the amount of depolarizing current injected into a neuron could be controlled by titrating the concentration of agonist (Fig. 4A), the frequency of action potentials should be tunable as well. Current-clamp recordings displayed in Fig. 4B demonstrate that this was indeed the case. Spike rates, evaluated in sliding 200-ms windows, rose as a function of increasing concentration of agonist, peaking at a frequency of 40 Hz in our data set (382 agonist applications to 60 neurons). In contrast to the simple sigmoidal dose–response relationship that characterized peak and integrated channel currents (Fig. 4A), the relationship between agonist concentration and firing frequency was complicated by nonlinearities intrinsic to the mechanism of spike generation. Departures from sigmoidal behavior were particularly evident at elevated agonist concentrations (Fig. 4B, 500 and 5,000 nM capsaicin). Rapid depolarization rates led to a brief burst of spikes at high frequency that was followed by a long-lasting plateau, during which the membrane remained depolarized but action potentials were absent (Fig. 4B Bottom). In all likelihood, the lack of excitability during the plateau phase reflects the accumulation of voltage-gated sodium channels in the inactivated state, in which they remain trapped until the membrane is repolarized. Intermittent delivery of agonist may help to remove inactivation and sustain high firing rates over extended periods of time.
Photostimulation.
A particularly attractive way of delivering pulses of agonist is to photorelease the active compound from an inactive, photolabile precursor (24–28). One such precursor, the DMNPE ester of ATP (18, 24), was available from a commercial source; another was synthesized by reacting capsaicin with DMNB chloroformate to attach a DMNB blocking group in carbonate ester linkage to the phenolic hydroxyl function of capsaicin (Fig. 1), a molecular feature important for agonist activity (29, 30). Absorption of photons in the near-UV wavelength range (absorption maximum ≈ 355 nm) by the DMNB and DMNPE chromophores is expected to produce reactive aci-nitro intermediates, which, in a series of rate-limiting dark reactions, liberate the free agonists (26).
The presence of the DMNB or DMNPE blocking groups rendered capsaicin and ATP biologically inert, presumably by sterically preventing the caged ligands from binding to their respective receptors. The membrane potentials and transmembrane conductances of neurons expressing TRPV1 or P2X2, recorded in whole-cell current- or voltage-clamp mode, respectively, were unaffected by the presence of 5 μM DMNB-capsaicin and 1 mM DMNPE-ATP, concentrations that exceeded the saturating levels of the free agonists (determined in Fig. 4A), 10- to 20-fold. No trace of biological activity caused by chemical or enzymatic decomposition of the caged compounds was detected in recordings lasting for >30 min.
Whole-field illumination with the collimated, unfiltered output of a mercury arc lamp delivered 26 mW·mm−2 of optical power at wavelengths <400 nm. In the presence of 5 μM DMNB-capsaicin, TRPV1-positive neurons (but not untransfected neurons) within the illuminated field responded to the optical stimulus with a flurry of activity (Fig. 5A). After a single light pulse lasting for 1 s, action potentials were fired at frequencies of 15–40 Hz. Activity was confined to a sharply delimited window in time whose onset lagged behind that of the optical stimulus by a predictable interval (5,035 ± 2,061 ms; mean ± SD, n = 10), and whose duration (2,651 ± 383 ms; mean ± SD, n = 10) slightly exceeded that of the light exposure. Repeated photostimulation of the same neuron was followed by stereotyped responses that did not attenuate (Fig. 5A).
Figure 5.
Photostimulation of genetically designated target neurons. Membrane potentials of hippocampal neurons expressing TRPV1 (A, black trace) or P2X2 (B, black trace), or of untransfected control neurons (A and B, gray traces), were recorded in whole-cell current-clamp mode in the presence of 50 μM DMNB-capsaicin (A) or 1 mM DMNPE-ATP (B), under safelight conditions (shaded backgrounds). The neurons were illuminated twice for 1 s (white backgrounds) with the unfiltered output of a mercury arc lamp, which delivered an optical energy of 5.1 mJ in the spectral band <400 nm. Optical stimuli were followed by stereotyped electrical responses in neurons that expressed the cognate ionotropic receptor.
P2X2-positive neurons exposed to a 1-s light pulse in the presence of 1 mM DMNPE-ATP exhibited light-evoked responses that were qualitatively similar to those of TRPV1-positive neurons in the presence of DMNB-capsaicin but showed distinctive temporal characteristics (Fig. 5B). The window of activity followed the optical stimulus after a shorter lag period (1,136 ± 96 ms; mean ± SD, n = 16), lasted for a slightly shorter span of time (2,456 ± 1,273 ms; mean ± SD, n = 16), and returned to baseline more gradually than the TRPV1 response. Different uncaging and channel-gating kinetics are likely to underlie these characteristic temporal response patterns. The mechanistic basis of the observed response latencies is currently unknown, but comparatively low illumination intensities may play a role. The light pulses used in our experiments carried an optical energy of 26 mJ mm−2 in the spectral band <400 nm during a 1-s exposure; high-intensity flash lamps or UV lasers compress comparable energies into a few hundred microseconds (28, 31) and may thus elicit instant, precisely timed responses.
Discussion
Photochemical stimulation of neurons through the selective activation of heterologously expressed ionotropic receptors retains the fundamental innovation introduced by chARGe: susceptibility to stimulation rather than the stimulus itself is localized, genetically (2). But instead of relying on a metabotropic cascade triggered by light, the excitatory signal is transduced by directly gated ion channels. Agonists, which are supplied either pharmacologically or optically through photorelease from the caged precursors, induce a depolarizing current and/or short-circuit the neuron's spike generator directly.
This simple excitatory engine possesses five main advantages over the more elaborate mechanism of chARGe. First, expression of a single heterologous gene (as opposed to three genes in the case of chARGe) is sufficient to sensitize a cell to stimulation. The dependence on only one transgene eliminates the need to balance relative expression levels and, as the complexity of genetic manipulations rises sharply with the number of genes involved, will simplify the creation of genetically modified cells, tissues, and organisms.
Second, “on” and “off” kinetics of the neuronal response are tightly and reproducibly coupled to those of the stimulus (Figs. 3 and 5), in favorable contrast with the somewhat unpredictable kinetics of chARGe. When chARGe is expressed in cultured hippocampal neurons, the latency of the first spike after the onset of the optical stimulus varies, ranging from a few hundred milliseconds to several tens of seconds (2). After cessation of the light stimulus, the settling time of the chARGed neuron again varies considerably; not infrequently, a tail of low-level activity persists for several tens of seconds after the illuminating beam is shuttered (2). Poor coupling between chARGe and the “light-activated” conductance that serves as its effector probably lies at the root of these phenomena. The use of a directly gated ion channel sidesteps the issue, by eliminating the inefficiency and jitter of signal transduction through a heterologous metabotropic cascade. Remaining imprecisions in the timing of the response most likely reflect the crude modes of agonist delivery in our validating experiments rather than noise intrinsic to the biological transduction mechanism. Technical refinements, such as high-intensity flash photolysis (31) of DMNB-capsaicin or DMNPE-ATP, are expected to afford millisecond control over spike times.
Third, the intensity of the neuronal response, which is quantified as the amount of depolarizing current injected or the firing rate attained, can be graded by varying the concentration of agonist (Fig. 4). Although the dose of photons incident on a chARGed neuron can control the intensity of the electrical response as well, the dose–response relationship is rather loose: firing patterns and firing frequencies of chARGed neurons at identical illumination intensities vary widely (2). And with peak spike frequencies of 7.5 Hz under sustained illumination (2), the dynamic range of a chARGed neuron is much narrower than that of neurons expressing TRPV1 or P2X2, both of which can be driven at frequencies of at least 40 Hz at comparable optical power.
Fourth, the lack of crosstalk between the three receptor-ligand pairs we currently use (Fig. 3), along with additional natural or engineered receptor-ligand combinations that will undoubtedly become available in the future, should allow multiple distinct populations of neurons to be addressed simultaneously and independently. Spectral variants of the rhodopsin component of chARGe can, in principle, also endow different groups of neurons with their own specific communication frequencies (2), but overlap between absorption bands (32) places a low practical limit on their number.
Fifth, multiple trigger modes are available to gate the heterologously expressed channels. Agonist can be supplied pharmacologically or optically; TRPV1 and TRPM8 may in addition be controlled by temperature shifts (8, 9, 13). Although the speed, added spatial resolution, and “action at a distance” of photolytic uncaging will make light the trigger of choice for most experiments in vitro and many applications in vivo, there are situations where it may be impractical or unnecessary to direct a light beam or an optical waveguide to a target region. In these circumstances (in behaving animals, or if exquisite temporal control over the response is not essential), pharmacological stimulation offers a powerful alternative. Capsaicin acting on TRPV1 expressed ectopically in nociceptor neurons of Caenorhabditis elegans, for example, has elicited “synthetic” avoidance behaviors that wild-type animals lack (33). Because agonist will be effective throughout its pharmacological distribution volume, its anatomical sites of action need not be known in advance and genetic shotgun searches for the cellular substrates of specific behaviors become possible: groups of neurons in their normal operational context could be genetically sensitized to stimulation and implicated as carriers of behaviorally relevant information if their ligand-controlled activation generates characteristic phenotypes. Ultimately, the principle of targeted pharmacological stimulation may even find therapeutic uses, by providing selective molecular control over the function of tissues or implants containing genetically engineered neuronal, endocrine, secretory, or contractile cells.
Acknowledgments
We thank David Julius for cDNAs encoding TRPV1 and TRPM8 and Alan North for a cDNA encoding P2X2. G.M. is an Alfred P. Sloan and Klingenstein Fellow, a Beckman Young Investigator, and a Searle Scholar. This work is dedicated to Dr. Gottfried Miesenböck on the occasion of his 65th birthday.
Abbreviations
- chARGe
genetically encoded phototrigger consisting of Drosophila rhodopsin (NinaE), arrestin-2, and the cognate G protein α-subunit (Dgq)
- DMNB
4,5-dimethoxy-2-nitrobenzyl
- DMNPE
P3-[1-(4,5-dimethoxy-2-nitrophenyl)ethyl]
References
- 1.Zemelman B V, Miesenböck G. Curr Opin Neurobiol. 2001;11:409–414. doi: 10.1016/s0959-4388(00)00227-0. [DOI] [PubMed] [Google Scholar]
- 2.Zemelman B V, Lee G A, Ng M, Miesenböck G. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- 3.Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 4.Stryer L. J Biol Chem. 1991;266:10711–10714. [PubMed] [Google Scholar]
- 5.Wickman K, Clapham D E. Physiol Rev. 1995;75:865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- 6.Prasad B C, Reed R R. Trends Genet. 1999;15:150–153. doi: 10.1016/s0168-9525(99)01695-9. [DOI] [PubMed] [Google Scholar]
- 7.Hardie R C, Raghu P. Nature. 2001;413:186–193. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- 8.Caterina M J, Schumacher M A, Tominaga M, Rosen T A, Levine J D, Julius D. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 9.McKemy D D, Neuhausser W M, Julius D. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 10.Valera S, Hussy N, Evans R J, Adami N, North R A, Surprenant A, Buell G. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 11.Nicke A, Baumert H G, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G. EMBO J. 1998;17:3016–3028. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoop R, Thomas S, Rassendren F, Kawashima E, Buell G, Surprenant A, North R A. Mol Pharmacol. 1999;56:973–981. doi: 10.1124/mol.56.5.973. [DOI] [PubMed] [Google Scholar]
- 13.Peier A M, Moqrich A, Hergarden A C, Reeve A J, Andersson D A, Story G M, Earley T J, Dragoni I, McIntyre P, Bevan S, Patapoutian A. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 14.Mezey E, Toth Z E, Cortright D N, Arzubi M K, Krause J E, Elde R, Guo A, Blumberg P M, Szallasi A. Proc Natl Acad Sci USA. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clapham D E, Runnels L W, Strübing C. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 16.Montell C, Birnbaumer L, Flockerzi V. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 17.Brake A J, Wagenbach M J, Julius D. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- 18.Ding S, Sachs F. J Physiol (London) 2000;522:199–214. doi: 10.1111/j.1469-7793.2000.t01-1-00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.North R A. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 20.Brake A J, Julius D. Annu Rev Cell Dev Biol. 1996;12:519–541. doi: 10.1146/annurev.cellbio.12.1.519. [DOI] [PubMed] [Google Scholar]
- 21.Newbolt A, Stoop R, Virginio C, Surprenant A, North R A, Buell G, Rassendren F. J Biol Chem. 1998;273:15177–15182. doi: 10.1074/jbc.273.24.15177. [DOI] [PubMed] [Google Scholar]
- 22.Shah K, Liu Y, Deirmengian C, Shokat K M. Proc Natl Acad Sci USA. 1997;94:3565–3570. doi: 10.1073/pnas.94.8.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bishop A, Buzko O, Heyeck-Dumas S, Jung I, Kraybill B, Liu Y, Shah K, Ulrich S, Witucki L, Yang F, et al. Annu Rev Biophys Biomol Struct. 2000;29:577–606. doi: 10.1146/annurev.biophys.29.1.577. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan J H, Forbush B, III, Hoffman J F. Biochemistry. 1978;17:1929–1935. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- 25.Walker J W, McCray J A, Hess G P. Biochemistry. 1986;25:1799–1805. doi: 10.1021/bi00355a052. [DOI] [PubMed] [Google Scholar]
- 26.McCray J A, Trentham D R. Annu Rev Biophys Biophys Chem. 1989;18:239–270. doi: 10.1146/annurev.bb.18.060189.001323. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox M, Viola R W, Johnson K W, Billington A P, Carpenter B K, McCray J A, Guzikowski A P, Hess G P. J Org Chem. 1990;55:1585–1589. [Google Scholar]
- 28.Callaway E M, Katz L C. Proc Natl Acad Sci USA. 1993;90:7661–7665. doi: 10.1073/pnas.90.16.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walpole C S, Wrigglesworth R, Bevan S, Campbell E A, Dray A, James I F, Perkins M N, Reid D J, Winter J. J Med Chem. 1993;36:2362–2372. doi: 10.1021/jm00068a014. [DOI] [PubMed] [Google Scholar]
- 30.Walpole C S, Bevan S, Bloomfield G, Breckenridge R, James I F, Ritchie T, Szallasi A, Winter J, Wrigglesworth R. J Med Chem. 1996;39:2939–2952. doi: 10.1021/jm960139d. [DOI] [PubMed] [Google Scholar]
- 31.Rapp G. Methods Enzymol. 1998;291:202–222. doi: 10.1016/s0076-6879(98)91014-x. [DOI] [PubMed] [Google Scholar]
- 32.Hardie R C. In: Progress in Sensory Physiology. Ottoson D, editor. Vol. 5. Berlin: Springer; 1983. pp. 1–79. [Google Scholar]
- 33.Tobin D M, Madsen D M, Kahn-Kirby A, Peckol E L, Moulder G, Barstead R, Maricq A V, Bargmann C I. Neuron. 2002;35:307–318. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]