Abstract
Background
Recent epidemiological analyses have implicated acute Campylobacter enteritis as a factor that may incite or exacerbate inflammatory bowel disease (IBD) in susceptible individuals. We have demonstrated previously that C. jejuni disrupts the intestinal barrier function by rapidly inducing epithelial translocation of non-invasive commensal bacteria via a transcellular lipid raft-mediated mechanism ('transcytosis'). To further characterize this mechanism, the aim of this current study was to elucidate whether C. jejuni utilizes M cells to facilitate transcytosis of commensal intestinal bacteria.
Results
C. jejuni induced translocation of non-invasive E. coli across confluent Caco-2 epithelial monolayers in the absence of disrupted transepithelial electrical resistance or increased permeability to a 3 kDa dextran probe. C. jejuni-infected monolayers displayed increased numbers of cells expressing the M cell-specific marker, galectin-9, reduced numbers of enterocytes that stained with the absorptive enterocyte marker, Ulex europaeus agglutinin-1, and reduced activities of enzymes typically associated with absorptive enterocytes (namely alkaline phosphatase, lactase, and sucrase). Furthermore, in Campylobacter-infected monolayers, E. coli were observed to be internalized specifically within epithelial cells displaying M-like cell characteristics.
Conclusion
These data indicate that C. jejuni may utilize M cells to promote transcytosis of non-invasive bacteria across the intact intestinal epithelial barrier. This mechanism may contribute to the inflammatory immune responses against commensal intestinal bacteria commonly observed in IBD patients.
Background
Inflammatory bowel diseases (IBD) are chronic T cell-mediated diseases that are thought to result from the loss of immunologic tolerance towards commensal intestinal microorganisms [1]. Intestinal epithelial barrier dysfunction is proposed to be a primary factor contributing to IBD pathogenesis [2,3]. By facilitating the translocation of commensal bacteria across the intestinal barrier, dysfunction of the epithelium may enable the inappropriate activation of T lymphocytes that recognize and respond to constituents of the microbiota. Studies have shown that IBD patients exhibit increased rates of systemic endotoxemia [4], have higher amounts of bacterial DNA in their serum [5], and have exaggerated humoral immune responses to intestinal bacterial antigens [6-8], implying that bacterial antigens are able to translocate across the intestinal epithelium. Although several studies have observed elevated intestinal permeability ("leaky gut") and transcellular uptake of intestinal antigens in IBD patients [9-11], the mechanisms contributing to this barrier dysfunction have yet to be fully elucidated.
An increasing number of clinical studies have indicated that in some patients, IBD onset or reactivation occurs following a bout of acute bacterial enteritis [12-15]. Recently, two controlled cohort studies have implicated that enteritis incited by Campylobacter or Salmonella, which are the leading causes of bacterial enteritis in many countries, is a risk factor for subsequent development of IBD [16,17]. Although the mechanisms involved are unknown, pathogen-mediated intestinal epithelial barrier dysfunction may facilitate the translocation of commensal bacteria across the epithelium. In this regard, we have recently shown that C. jejuni induces translocation of non-invasive commensal bacteria across the intestinal epithelium via an uncharacterized transcellular mechanism involving lipid raft-mediated endocytosis [18,19].
Microfold or M cells are specialized epithelial cells that sample antigens from the mucosal surface and transport them via a transcellular route to the basolateral membrane (herein defined as "transcytosis"). Intestinal M cells are primary found within the follicle-associated epithelium (FAE) of Peyer's patches and isolated lymphoid follicles, but are also occasionally found interspersed amongst the absorptive enterocytes of villar epithelium [20]. While M cells are principally involved in immune surveillance of intestinal antigens, they also represent an important portal by which enteric bacteria can translocate across the intestinal barrier [21]. Studies have suggested that pathogens may exploit M cell function and upregulate the rate of bacterial transcytosis by either stimulating de novo formation of M cells [20,22,23] or increasing the rate of uptake for pre-existing M cells [24]. While controversy exists with respect to which of these mechanisms is responsible for increased bacterial trafficking, it is clear that certain pathogenic microorganisms can alter M cell function and increase the rate of transcytosis.
M cell trafficking of bacteria has been studied in vitro using polarized Caco-2 monolayers which have been stimulated to differentiate into M-like cells by co-culture with B lymphocytes [25]. Subsequent studies have shown that M-like cells capable of transporting non-invasive Vibrio cholerae are naturally present in Caco-2 monolayers despite the absence of B lymphocytes [26]. The resultant M-like cells display many of the in vivo characteristics of M cells including increased ability to ingest and transport exogenous particles, disorganized microvilli structure, decreased absorptive intestinal epithelial enzyme activities, reduced binding of the lectin, Ulex europaeus agglutinin-1 (UEA-1), and increased expression of the M-cell specific marker, galectin-9 [27]. We utilized Caco-2 monolayers to test the hypothesis that C. jejuni induces trancytosis of non-invasive commensal bacteria across the intestinal epithelium through M-like cells. Specifically, the objectives were to determine if C. jejuni: (1) increases transcytosis of non-invasive E. coli across polarized Caco-2 monolayers; (2) increases the abundance of M-like cells within Caco-2 monolayers; and (3) induces internalization of non-invasive E. coli specifically within M-like cells.
Results and discussion
Our previous findings indicate that C. jejuni 81-176 induces transcytosis of non-invasive E. coli ("commensal bacteria") across polarized human colonic T84 monolayers [18]. Since other studies have shown that certain bacteria can rapidly up-regulate M cell-mediated antigen transcytosis [20,22-24,28], we examined whether C. jejuni utilizes M-like cells to facilitate intestinal epithelial transcytosis of non-invasive bacteria. The T84 cell line has not been characterized with respect to the presence of M-like cells; thus for this current study, we used Caco-2 monolayers in which M-like cells have previously been characterized [25,26] in order to assess whether C. jejuni 81-176 induces translocation of non-invasive E. coli. In agreement with our previous study using T84 monolayers, we observed that C. jejuni induced internalization (~1.4-fold; P = 0.023) and translocation (~7.5-fold; P = 0.023) of E. coli across polarized Caco-2 monolayers. Furthermore, there were no changes in paracellular permeability to a 3 kDa dextran probe (P = 0.13) and transepithelial electrical resistance (TER) remained above 250 Ω × cm2 (P = 0.39; Table 1) which is indicative of intact tight epithelial junctions in this cell type. This supports our previous observations that a transcellular mechanism is responsible for C. jejuni-mediated E. coli translocation. Similarly, a recent study showed that the enteric pathogen, Yersinia pseudotuberculosis, also induces translocation of exogenous particles across both Caco-2 monolayers and human intestinal epithelium by an as yet uncharacterized transcellular mechanism [29]. This novel transcellular mechanism of barrier dysfunction contrasts with the paracellular mechanism described for many enteric pathogens such as enterohemorrhagic E. coli and Salmonella, which increase intestinal permeability by disrupting epithelial tight junctions [30].
Table 1.
E. coli translocation and internalization, and epithelial permeability in Caco-2 monolayers treated with E. coli C25 alone (control) versus monolayers inoculated with E. coli C25 and C. jejuni 81-176
| Control | C. jejuni | P | |
|---|---|---|---|
| Translocated E. coli (log10 CFU/mL) | 0.19 ± 0.19 | 1.42 ± 0.296 | 0.023 |
| Internalized E. coli (log10 CFU/mL) | 3.66 ± 0.36 | 4.97 ± 0.07 | 0.023 |
| Initial TER (Ω × cm2) | 298 ± 4.2 | 311 ± 9.4 | 0.28 |
| Final TER (Ω × cm2) | 354 ± 19.7 | 335 ± 2.1 | 0.39 |
| Permeability (% apical dextran recovered) | 0.95 ± 0.02 | 1.01 ± 0.02 | 0.13 |
Data are expressed as mean ± SEM, n = 3 independent experiments
C. jejuni-treated monolayers displayed an overall reduction in absorptive epithelial enzymatic activities (Table 2). Specifically, alkaline phosphatase was decreased by ~9.2% (P = 0.01), lactase was decreased by ~8.3% (P = 0.001), and sucrase was decreased by 28% (P = 0.046). This is consistent with the previously reported decrease in intestinal sucrase-isomaltase activity that occurs in the Caco-2 monolayers upon differentiation to M-like cells [25]. Notably, intestinal alkaline phosphatase has been recently show to detoxify lipopolysaccharide (a component of Gram negative bacteria cell walls) and prevent bacterial translocation [31], suggesting that reduced alkaline phosphatase activity may be one of the factors contributing to transcytosis of E. coli in C. jejuni-treated monolayers. Also, transient lactose malabsorption has been observed following campylobacteriosis [32], this may warrant further investigation.
Table 2.
Intestinal epithelial enzyme activities for Caco-2 monolayers treated with E. coli C25 alone (control) verses monolayer treated with E. coli C25 and C. jejuni 81-176
| Control | C. jejuni | P | |
|---|---|---|---|
| (units/g protein) | (units/g protein) | ||
| Alkaline Phosphatase | 67.6 ± 1.3 | 61.4 ± 0.5 | 0.01 |
| Lactase | 217.6 ± 1.8 | 199.6 ± 1.3 | 0.001 |
| Sucrase | 77.5 ± 4.5 | 55.8 ± 6.1 | 0.046 |
Data are expressed as mean ± SEM, n = 3 independent experiments
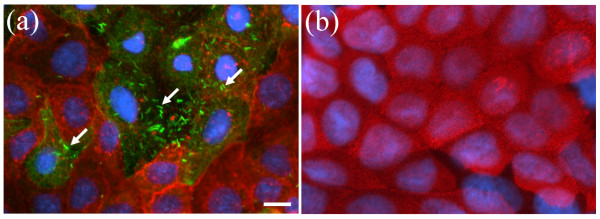
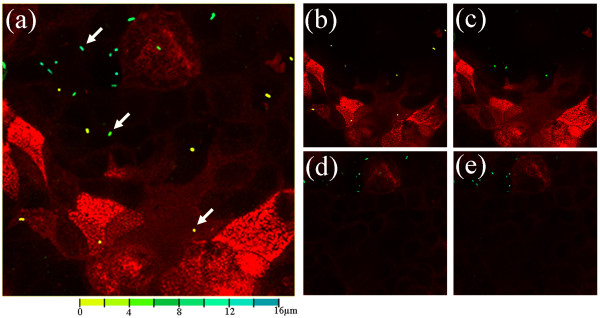
Microscopic analysis revelled an increase (P = 0.002) in the number of cells that stained positive for galectin-9 in C. jejuni-treated (11.6 ± 0.5%) versus control (2.9 ± 0.5%) monolayers. Furthermore, in C. jejuni-treated monolayers, E. coli were primarily associated with Caco-2 cells displaying characteristics of M-like cells, namely those that displayed reduced apical binding of UEA-1 lectin and increased expression of galectin-9 (Figure 1). In contrast, control monolayers exhibited very few galectin-9 positive cells or associated E. coli. Caco-2 monolayers were sectioned using confocal laser analysis, and for each section in the z-axis, E. coli were coloured according to depth from yellow (extracellular) to blue (intracellular). In C. jejuni-treated monolayers, intracellular E. coli were associated with UEA-1-negative Caco-2 cells (Figure 2). The few extracellular bacteria were associated with UEA-1-positive Caco-2 cells (i.e., absorptive enterocytes). In control monolayers, only the occasional extracellular E. coli was observed (not shown). While cautious interpretation of data resulting from M cell staining is necessary owing to lack of a universally accepted human M cell marker, it appears that our collective data indicate that C. jejuni converts Caco-2 cells into cells displaying biochemical, functional, and histological features of M cells. In a similar manner, the differentiation of M-like cells has been shown to occur in response to bacterial infection and inflammation [20,22,33-35].
Figure 1.
Representative epifluorescent micrographs of Caco-2 monolayers stained with the absorptive enterocyte marker Ulex europaeus agglutinin-1 (red), the M-like cell specific marker galectin-9 (green diffuse cellular staining), and Hoechst nuclear stain (blue). (a) Monolayers treated with GFP Escherichia coli C25 (arrows) and C. jejuni 81-176. (b) Monolayers treated with only GFP E. coli C25 (control). Bar = 2 μm.
Figure 2.
Confocal laser sections of Caco-2 monolayers treated with C. jejuni 81-176 plus non-invasive GFP Escherichia coli C25 (arrows) and stained with the absorptive enterocyte marker Ulex europaeus agglutinin-1 (red). E. coli are color-coded according to their respective depth within the monolayer from extracellular (yellow) to intracellular (blue). (a) Merge of all confocal laser sections. (b-e) Representative confocal laser sections starting from the apical surface of the monolayer (E. coli color-coded yellow) to the basolateral surface of the monolayer (E. coli color-coded blue).
An important observation is that the epithelial responses appear to be specific to C. jejuni, since E. coli alone does not cause appreciable formation of M-like cells within Caco-2 monolayers. Although we are unsure of the exact mechanism, current studies have demonstrated that metabolic stress increases internalization and translocation of non-pathogenic E.coli across the intestinal epithelium [36,37]. We and other have previously noted swollen mitochondria (indicative of metabolic stress) in cell culture and animal models of campylobacteriosis [38-42]. It appears that mucosal pathogens often target the mitochondria as part of their common pathogenic strategy [43]. Thus, one possible explanation for our observations could be that increased M cell formation and subsequent transcytosis occur as a consequence of metabolic stress associated with C. jejuni pathogenesis.
Controversy continues on whether M cells arise from a distinct pre-determined lineage of crypt stem cells, or whether absorptive enterocytes exhibit phenotypic plasticity and can be "converted" to M cells [21,24]. Since immortalized Caco-2 cells are not terminally differentiated but rather resemble crypt stem cells [44,45], they are thus theoretically able to differentiate into different epithelial lineages such as absorptive enterocytes or M-like cells. The rapid timing with which E. coli translocation occurred following C. jejuni infection (i.e., 6 h), suggests that C. jejuni may target progenitor M-like cells within the monolayer rather than convert fully differentiated epithelial cells into M-like cells. Future studies are necessary to understand the precise mechanisms by which M-like cells originate in response to C. jejuni.
Conclusion
Our data indicates that C. jejuni may utilize M cells to promote transcytosis of non-invasive bacteria across the intact intestinal epithelial barrier. Based on previous clinical observations, microscopic aphtoid lesions of the FAE (i.e., regions containing M cells) appear to be the initial site of inflammation in patients with Crohn's disease [46]. Notably, a recent study of patients with Crohn's disease reported increased transmucosal uptake of non-pathogenic E. coli across the ileal FAE despite unaltered epithelial permeability [47], suggesting that trancellular defects in the FAE contribute to the pathophysiology of IBD. M cells may play an important role in IBD pathogenesis by increasing transcytosis of commensal bacteria to the inductive sites of mucosal immune responses [48]. Campylobacter enteritis has been recently identified as a risk factor for IBD [16,17]. By increasing M cell-mediated trancytosis of non-invasive bacteria, C. jejuni may contribute to the activation of T lymphocyte-mediated immune responses against commensal bacteria commonly observed in IBD patients. Future studies will be required to determine whether C. jejuni-induced M cell-mediated transcytosis of commensal bacteria occurs in vivo and whether this causes aberrant T lymphocyte-mediated immune responses.
Methods
Bacteria and growth conditions
C. jejuni 81-176, a reference clinical strain [49], was used throughout this study. For microscopy studies, Escherichia coli C25 transformed with GFP-expressing plasmid pMEK91 was used [50]. Inoculum was prepared by growing C. jejuni or E. coli for 14-16 hours in Columbia broth (37°C, 100 rpm, Difco, Detroit, MI) in microaerobic (10% CO2, 3% H2, 5% O2, balance N2) or aerobic atmosphere, respectively.
Caco-2 M-like cell model
Caco-2 cells (American Type Culture Collection, Manassas, VA) were grown in Advanced Dulbecco's minimal essential medium (DMEM; Gibco Invitrogen Inc., Burlington, ON) supplemented with 10% (v/v) fetal bovine serum, 200 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 80 μg/mL tylosin (all from Sigma-Aldrich, Oakville, ON), and incubated at 37°C and 5% CO2. For E. coli translocation and internalization, epithelial permeability, and enzyme activity assays, cells were seeded onto Transwell filters (3 μm pore size, 1.13 cm2; Costar Corning Inc., Corning, NY) at 1.5 × 105 cells per filter and grown for 21 days. For microscopy, cells were seeded into chamber slides (Nalgene Nunc International, Naperville, IL) at 3 × 105 cells per well.
E. coli translocation and internalization assays
E. coli translocation and internalization were determined as previously described [18]. Briefly, transwell-grown monolayers were washed with Hank's buffered saline (HBS; Gibco) and antibiotic-free DMEM was added to the apical and basal compartments. E. coli inoculum was added to the apical compartment of all monolayers to achieve a multiplicity of infection (MOI) of 100 CFU per enterocyte. Monolayers were then divided in to two groups and half were inoculated with C. jejuni at a MOI of 100, whereas the other half received an equivalent volume of sterile broth (control treatment).
Following incubation, E. coli recovered in the basal compartment (indicating translocation) were enumerated by spreading serial dilutions onto MacConkey agar, incubating the cultures aerobically at 37°C, and enumerating at the dilution yielding 30-300 colony forming units (CFU) per culture. A preliminary experiment confirmed that 6 h was the minimum optimal incubation time for assessing E. coli translocation. To assess E. coli internalization, treated monolayers were washed with HBS and incubated for 1 hour with DMEM containing gentamicin (250 μg/mL; Sigma). Monolayers were then washed, lysed with 0.1% Triton X-100 in PBS (500 μL), and viable bacteria were enumerated as described above. A preliminary experiment confirmed that E. coli were killed by the gentamicin treatment. Transepithelial electrical resistance was monitored at the beginning and end of each experiment with an electrovoltohmeter (World Precision Instruments, Sarasota, FL). Only monolayers with an initial TER > 225 Ω × cm2 were used.
Epithelial permeability assay
Transwell-grown monolayers were inoculated with E. coli ± C. jejuni as described above, and monolayers were washed with Ringer's buffer 6 hours after inoculation. A 3 kDa FITC-dextran probe (100 mM in Ringer's buffer; 500 μL per well; Molecular Probes, Eugene, OR) was added to the apical compartment and 1 mL of Ringer's buffer was added to the basal compartment, and the monolayers were incubated for 3 hours at 37°C as described previously [18]. Samples (200 μl) were collected from the basal compartment and the absorbance at 485 nm was measured. Data were expressed as % apical dextran recovered in the basal compartment.
Epithelial enzyme activity assays
Transwell-grown monolayers were inoculated with E. coli ± C. jejuni as described above. After 6 hours, monolayers were washed with PBS and lysed with 0.2% Triton X-100 in PBS (500 μL per well) on ice for 20 minutes. Cell lysates were clarified by centrifugation (2 minutes, 16,000 (g). Total protein concentration of the lysates was determined using the Bradford protein assay (Bio-Rad, Mississauga, ON) and were calculated by interpolation of a standard curve generated with known concentrations of bovine serum albumin (BSA).
Alkaline phosphatase activity was measured by hydrolysis of p-nitrophenyl phosphate (pNPP; Sigma) reagent according to the manufacturer's instructions. Briefly, cell lysates (20 μL) were incubated for 30 minutes at 37°C with pNPP reagent (500 μL). The reaction was stopped with 2 M NaOH (150 μL) and measured spectrophotometrically at A410 nm. Enzyme activity was calculated by interpolation of a standard curve generated with alkaline phophatase of defined activity. Enzyme activity was expressed as units/g protein, where 1 unit is defined as the amount of enzyme that hydrolyzed 1 μM of pNPP per minute at 37°C.
Sucrase and lactase activities were measured according to the method of Dahlqvist [51]. In brief, cell lysates (50 μL) were incubated with sucrose or lactose (50 μL of 100 mM disaccharide in 0.1 M maleate buffer, pH 6; Sigma) in each of two separate tubes. One set of tubes, used to measure background endogenous glucose in the sample, was heated at 95°C for 2 minutes. The second set of tubes was placed in a 37°C water bath for 4 hours followed by 95°C for 2 minutes. Glucose oxidase reagent (200 μL; Sigma) was added to each tube and then incubated at 37°C for 1 hour. A coloured product of O-dianisidine, which is produced based on the amount of glucose liberated in the samples, was measured spectrophotometrically at A420 nm. Glucose in each lysate was calculated by interpolation of a glucose standard curve. Enzyme activity was expressed as units/g protein, where 1 unit is defined as the amount of enzyme that liberated 1 μM of glucose in 1 hour.
M cell staining, epifluorescent and confocal microscopy
Confluent Caco-2 monolayers grown on chamber slides were inoculated with E. coli ± C. jejuni as described above. Caco-2 monolayers were washed with PBS 6 hours after inoculation, and fixed in paraformaldehyde (2%). Galectin-9 staining was performed as previously described [27]. Briefly, slides were washed with PBS, incubated with glycine (1% in PBS) for 15 minutes, and washed with PBS. Cells were permeabilized for 10 minutes with Triton X-100 (0.5% in PBS), blocked with BSA (2% in PBS), incubated with goat anti-human Galectin-9 antibodies (1% in PBS; R&D Systems Inc., Minneapolis, MN) followed by Alexa-488 conjugated anti-goat IgG (0.2% in PBS; Molecular Probes). Monolayers were then washed with PBS and incubated for 30 minutes with TRITC-conjugated UEA-1 (5 μg/mL in PBS; Sigma). After washing with PBS, slides were stained for 15 minutes with Hoechst nuclear stain (1 μM; Molecular Probes) and washed with PBS. Coverslips were mounted with Aqua-Mount (Lerner Laboratories, Pittsburgh, PA) according to the manufacturer's instructions. Slides were examined by epifluorescent microscopy using appropriate filters. Slides were blinded by taping the labels, and a grid was drawn on the back of each slide. The same section of the grid was examined by epifluorescent microscopy and scored for total number of cells and number of M cells (i.e., galectin-9 positive, UEA negative). Confocal optical sectioning was carried out using an inverted microscope equipped with a confocal laser scanning imaging system (Leica Microsystems, Wetzler, Germany).
Statistical analysis
Each assay was conducted at least three times on separate occasions. For each replicate, observations were conducted at least in triplicate, and mean values were used for analysis. Data are expressed as means ± SEM. Unpaired Student's t-test were used to compare means of control versus C. jejuni-treated samples (GraphPad InStat software, GraphPad Software Inc., San Diego, CA). P ≤ 0.05 was considered significant.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LKT participated in the design of the study, performed experiments, conducted data analysis, and drafted the manuscript. FL conducted confocal microscopy and image analysis. GDI participated in the design of the study and edited the manuscript. All authors read and approved the final manuscript.
Contributor Information
Lisa D Kalischuk, Email: lisa.kalischuk-tymensen@agr.gc.ca.
Frances Leggett, Email: Frances.Leggett@agr.gc.ca.
G Douglas Inglis, Email: Douglas.Inglis@agr.gc.ca.
Acknowledgements
We thank Dr. ME Konkel, Washington State University, for the gift of pMEK91. This work was supported by an AAFC Peer Review Grant to GDI and LKT.
References
- Maul J, Duchmann R. Can loss of immune tolerance cause IBD? Inflamm Bowel Dis. 2008;14(Suppl 2):S115–116. doi: 10.1002/ibd.20679. [DOI] [PubMed] [Google Scholar]
- Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, Abraham C, Turner JR. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551–563. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2008;14(3):401–407. doi: 10.3748/wjg.14.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner KR, Halliday MI, Barclay GR, Milne L, Brown D, Stephens S, Maxwell RJ, Rowlands BJ. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut. 1995;36:897–901. doi: 10.1136/gut.36.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A, Frances R, Amoros A, Zapater P, Garmendia M, Ndongo M, Cano R, Jover R, Such J, Perez-Mateo M. Cytokine association with bacterial DNA in serum of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:508–514. doi: 10.1002/ibd.20806. [DOI] [PubMed] [Google Scholar]
- Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer Buschenfelde KH zum. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RJ, Heazlewood SP, Gilshenan KS, O'Brien M, McGuckin MA, Florin TH. IgG antibodies against common gut bacteria are more diagnostic for Crohn's disease than IgG against mannan or flagellin. Am J Gastroenterol. 2008;103:386–396. doi: 10.1111/j.1572-0241.2007.01577.x. [DOI] [PubMed] [Google Scholar]
- Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut. 1996;38:365–375. doi: 10.1136/gut.38.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderholm JD, Streutker C, Yang PC, Paterson C, Singh PK, McKay DM, Sherman PM, Croitoru K, Perdue MH. Increased epithelial uptake of protein antigens in the ileum of Crohn's disease mediated by tumour necrosis factor alpha. Gut. 2004;53:1817–1824. doi: 10.1136/gut.2004.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurmann G, Bruwer M, Klotz A, Schmid KW, Senninger N, Zimmer KP. Transepithelial transport processes at the intestinal mucosa in inflammatory bowel disease. Int J Colorectal Dis. 1999;14:41–46. doi: 10.1007/s003840050181. [DOI] [PubMed] [Google Scholar]
- Porras M, Martin MT, Yang PC, Jury J, Perdue MH, Vergara P. Correlation between cyclical epithelial barrier dysfunction and bacterial translocation in the relapses of intestinal inflammation. Inflamm Bowel Dis. 2006;12:843–852. doi: 10.1097/01.mib.0000231571.88806.62. [DOI] [PubMed] [Google Scholar]
- Halfvarson J, Jess T, Magnuson A, Montgomery SM, Orholm M, Tysk C, Binder V, Jarnerot G. Environmental factors in inflammatory bowel disease: a co-twin control study of a Swedish-Danish twin population. Inflamm Bowel Dis. 2006;12:925–933. doi: 10.1097/01.mib.0000228998.29466.ac. [DOI] [PubMed] [Google Scholar]
- Mylonaki M, Langmead L, Pantes A, Johnson F, Rampton DS. Enteric infection in relapse of inflammatory bowel disease: importance of microbiological examination of stool. Eur J Gastroenterol Hepatol. 2004;16:775–778. doi: 10.1097/01.meg.0000131040.38607.09. [DOI] [PubMed] [Google Scholar]
- Stallmach A, Carstens O. Role of infections in the manifestation or reactivation of inflammatory bowel diseases. Inflamm Bowel Dis. 2002;8:213–218. doi: 10.1097/00054725-200205000-00009. [DOI] [PubMed] [Google Scholar]
- Ruigomez A, Garcia LA Rodriguez, Panes J. Risk of irritable bowel syndrome after an episode of bacterial gastroenteritis in general practice: influence of comorbidities. Clin Gastroenterol Hepatol. 2007;5:465–469. doi: 10.1016/j.cgh.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Garcia LA Rodriguez, Ruigomez A, Panes J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology. 2006;130:1588–1594. doi: 10.1053/j.gastro.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Gradel KO, Nielsen HL, Schonheyder HC, Ejlertsen T, Kristensen B, Nielsen H. Increased short- and long-term risk of inflammatory bowel disease after Salmonella or Campylobacter gastroenteritis. Gastroenterology. 2009;137:495–501. doi: 10.1053/j.gastro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Kalischuk LD, Inglis GD, Buret AG. Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid rafts. Gut Pathog. 2009;1:2. doi: 10.1186/1757-4749-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalischuk LD, Buret AG. A role for Campylobacter jejuni-induced enteritis in inflammatory bowel disease? Am J Physiol Gastrointest Liver Physiol. 2009;298:G1–9. doi: 10.1152/ajpgi.00193.2009. [DOI] [PubMed] [Google Scholar]
- Jang MH, Kweon MN, Iwatani K, Yamamoto M, Terahara K, Sasakawa C, Suzuki T, Nochi T, Yokota Y, Rennert PD. et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci USA. 2004;101:6110–6115. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerneis S, Pringault E. Plasticity of the gastrointestinal epithelium: the M cell paradigm and opportunism of pathogenic microorganisms. Semin Immunol. 1999;11:205–215. doi: 10.1006/smim.1999.0176. [DOI] [PubMed] [Google Scholar]
- Borghesi C, Taussig MJ, Nicoletti C. Rapid appearance of M cells after microbial challenge is restricted at the periphery of the follicle-associated epithelium of Peyer's patch. Lab Invest. 1999;79:1393–1401. [PubMed] [Google Scholar]
- Meynell HM, Thomas NW, James PS, Holland J, Taussig MJ, Nicoletti C. Up-regulation of microsphere transport across the follicle-associated epithelium of Peyer's patch by exposure to Streptococcus pneumoniae R36a. Faseb J. 1999;13:611–619. doi: 10.1096/fasebj.13.6.611. [DOI] [PubMed] [Google Scholar]
- Gebert A, Steinmetz I, Fassbender S, Wendlandt KH. Antigen transport into Peyer's patches: increased uptake by constant numbers of M cells. Am J Pathol. 2004;164:65–72. doi: 10.1016/S0002-9440(10)63097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerneis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- Blanco LP, DiRita VJ. Bacterial-associated cholera toxin and GM1 binding are required for transcytosis of classical biotype Vibrio cholerae through an in vitro M cell model system. Cell Microbiol. 2006;8:982–998. doi: 10.1111/j.1462-5822.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- Pielage JF, Cichon C, Greune L, Hirashima M, Kucharzik T, Schmidt MA. Reversible differentiation of Caco-2 cells reveals galectin-9 as a surface marker molecule for human follicle-associated epithelia and M cell-like cells. Int J Biochem Cell Biol. 2007;39:1886–1901. doi: 10.1016/j.biocel.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Man AL, Lodi F, Bertelli E, Regoli M, Pin C, Mulholland F, Satoskar AR, Taussig MJ, Nicoletti C. Macrophage migration inhibitory factor plays a role in the regulation of microfold (M) cell-mediated transport in the gut. J Immunol. 2008;181:5673–5680. doi: 10.4049/jimmunol.181.8.5673. [DOI] [PubMed] [Google Scholar]
- Ragnarsson EG, Schoultz I, Gullberg E, Carlsson AH, Tafazoli F, Lerm M, Magnusson KE, Soderholm JD, Artursson P. Yersinia pseudotuberculosis induces transcytosis of nanoparticles across human intestinal villus epithelium via invasin-dependent macropinocytosis. Lab Invest. 2008;88:1215–1226. doi: 10.1038/labinvest.2008.86. [DOI] [PubMed] [Google Scholar]
- Sears CL. Molecular physiology and pathophysiology of tight junctions V. assault of the tight junction by enteric pathogens. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1129–1134. doi: 10.1152/ajpgi.2000.279.6.G1129. [DOI] [PubMed] [Google Scholar]
- Goldberg RF, Austen WG Jr, Zhang X, Munene G, Mostafa G, Biswas S, McCormack M, Eberlin KR, Nguyen JT, Tatlidede HS. et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci USA. 2008;105:3551–3556. doi: 10.1073/pnas.0712140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro A, Selles H, Calvo MA, Olivares JL, Castillo J, Gomez-Lus F, Bueno M. [Digestive complications of Campylobacter enteritis] An Esp Pediatr. 1985;22:275–279. [PubMed] [Google Scholar]
- Lugering A, Floer M, Lugering N, Cichon C, Schmidt MA, Domschke W, Kucharzik T. Characterization of M cell formation and associated mononuclear cells during indomethacin-induced intestinal inflammation. Clin Exp Immunol. 2004;136:232–238. doi: 10.1111/j.1365-2249.2004.02438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvelier CA, Quatacker J, Mielants H, De Vos M, Veys E, Roels HJ. M-cells are damaged and increased in number in inflamed human ileal mucosa. Histopathology. 1994;24:417–426. doi: 10.1111/j.1365-2559.1994.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Rhee KJ, Sethupathi P, Driks A, Lanning DK, Knight KL. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J Immunol. 2004;172:1118–1124. doi: 10.4049/jimmunol.172.2.1118. [DOI] [PubMed] [Google Scholar]
- Nazli A, Yang PC, Jury J, Howe K, Watson JL, Soderholm JD, Sherman PM, Perdue MH, McKay DM. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am J Pathol. 2004;164:947–957. doi: 10.1016/S0002-9440(10)63182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K, Caldwell J, Phan V, Prescott D, Nazli A, Wang A, Soderholm JD, Perdue MH, Sherman PM, McKay DM. Decreased epithelial barrier function evoked by exposure to metabolic stress and nonpathogenic E. coli is enhanced by TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2008;294:G669–678. doi: 10.1152/ajpgi.00382.2007. [DOI] [PubMed] [Google Scholar]
- De Melo MA, Gabbiani G, Pechere JC. Cellular events and intracellular survival of Campylobacter jejuni during infection of HEp-2 cells. Infect Immun. 1989;57:2214–2222. doi: 10.1128/iai.57.7.2214-2222.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey CD, Montag DM, Pittman FE. Morphologic observations of experimental Campylobacter jejuni infection in the hamster intestinal tract. Am J Pathol. 1986;122:152–159. [PMC free article] [PubMed] [Google Scholar]
- Kalischuk LD, Inglis GD, Buret AG. Strain-dependent induction of epithelial cell oncosis by Campylobacter jejuni is correlated with invasion ability and is independent of cytolethal distending toxin. Microbiology. 2007;153:2952–2963. doi: 10.1099/mic.0.2006/003962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JX, Ma BL, Xie YL, Huang DS. Electron microscopic appearance of the chronic Campylobacter jejuni enteritis of mice. Chin Med J (Engl) 1991;104:1005–1010. [PubMed] [Google Scholar]
- Newell DG, Pearson A. The invasion of epithelial cell lines and the intestinal epithelium of infant mice by Campylobacter jejuni/coli. J Diarrhoeal Dis Res. 1984;2:19–26. [PubMed] [Google Scholar]
- Blanke SR. Micro-managing the executioner: pathogen targeting of mitochondria. Trends Microbiol. 2005;13:64–71. doi: 10.1016/j.tim.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Engle MJ, Goetz GS, Alpers DH. Caco-2 cells express a combination of colonocyte and enterocyte phenotypes. J Cell Physiol. 1998;174:362–369. doi: 10.1002/(SICI)1097-4652(199803)174:3<362::AID-JCP10>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Owen RL. Mid-life crisis for M cells. Gut. 1998;42:11–12. doi: 10.1136/gut.42.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura Y, Kamoi R, Iida M. Pathogenesis of aphthoid ulcers in Crohn's disease: correlative findings by magnifying colonoscopy, electron microscopy, and immunohistochemistry. Gut. 1996;38:724–732. doi: 10.1136/gut.38.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keita AV, Salim SY, Jiang T, Yang PC, Franzen L, Soderkvist P, Magnusson KE, Soderholm JD. Increased uptake of non-pathogenic E. coli via the follicle-associated epithelium in longstanding ileal Crohn's disease. J Pathol. 2008;215:135–144. doi: 10.1002/path.2337. [DOI] [PubMed] [Google Scholar]
- Gullberg E, Soderholm JD. Peyer's patches and M cells as potential sites of the inflammatory onset in Crohn's disease. Ann N Y Acad Sci. 2006;1072:218–232. doi: 10.1196/annals.1326.028. [DOI] [PubMed] [Google Scholar]
- Korlath JA, Osterholm MT, Judy LA, Forfang JC, Robinson RA. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J Infect Dis. 1985;152:592–596. doi: 10.1093/infdis/152.3.592. [DOI] [PubMed] [Google Scholar]
- Mixter PF, Klena JD, Flom GA, Siegesmund AM, Konkel ME. In vivo tracking of Campylobacter jejuni by using a novel recombinant expressing green fluorescent protein. Appl Environ Microbiol. 2003;69:2864–2874. doi: 10.1128/AEM.69.5.2864-2874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist A. Method for assay of intestinal disaccharidases. Anal Biochem. 1964;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]