Abstract
Metallothioneins (MTs) are small, cysteine-rich polypeptides, but the role of MTs in inducing the formation of adaptive response is still largely unknown. We investigated the roles of metallothionein genes (mtl-1 and mtl-2) in the formation of cross-adaptation response to neurobehavioral toxicity from metal exposure in Caenorhabditis elegans. Pre-treatment with mild heat-shock at L2-larva stage effectively prevented the formation of the neurobehavioral defects and the activation of severe stress response in metal exposed nematodes at concentrations of 50 and 100 µM, but pre-treatment with mild heat-shock did not prevent the formation of neurobehavioral defects in 200 µM of metal exposed nematodes. During the formation of cross-adaptation response, the induction of mtl-1 and mtl-2 promoter activity and subsequent GFP gene expression were sharply increased in 50 µM or 100 µM of metal exposed Pmtl-1::GFP and Pmtl-2::GFP transgenic adult animals after mild heat-shock treatment compared with those treated with mild heat-shock or metal exposure alone. Moreover, after pre-treatment with mild heat-shock, no noticeable increase of locomotion behaviors could be observed in metal exposed mtl-1 or mtl-2 mutant nematodes compared to those without mild heat-shock pre-treatment. The defects of adaptive response to neurobehavioral toxicity induced by metal exposure formed in mtl-1 and mtl-2 mutants could be completely rescued by the expression of mtl-1 and mtl-2 with the aid of their native promoters. Furthermore, over-expression of MTL-1 and MTL-2 at the L2-larval stage significantly suppressed the toxicity on locomotion behaviors from metal exposure at all examined concentrations. Therefore, the normal formation of cross-adaptation response to neurobehavioral toxicity induced by metal exposure may need the enough accumulation of MTs protein in animal tissues.
Introduction
An adaptive response is a phenomenon in which a sub-lethal or non-lethal pre-treatment causes an increased resistance when an organism is challenged with higher doses or concentrations of that particular agent [1]. Such an adaptive response to oxidative damage occurs in a variety of organisms [2]–[4]. In addition to conferring protection against the same agent, cross adaptation can usually occur. Cross-adaptation response is defined as the capacity of cells or organisms to become resistant to a lethal agent when pretreated with a different lethal substance [1]. In particular, since organisms live in an environment where the threat of oxidative damage is continual, cellular and molecular mechanisms may have evolved to avoid and repair this damage and pre-exposure to mild stress may confer resistance to agents [5]–[6].
In nematode Caenorhabditis elegans, non-lethal stress such as mild heat shock can also have beneficial effects on stress resistance and longevity [7]–[8]. Lithgow et al. (1995) investigated the relationship between increased thermotolerance and life-span by developing conditions for environmental induction of thermotolerance, and found that the pretreatment at sub-lethal temperature induces increased thermotolerance and small but statistically highly significant increase in life expectancy [9]. Pre-exposure of wild-type nematodes to oxygen can confer a protective effect against the lethality imposed by subsequent X-irradiation [1]. Short-term exposure to hyperoxia can further extend the life span of age-1, and age-1 mutant also show resistance to paraquat and heat shock [10]. Moreover, young nematodes adapted the oxidative stress induced by the quinine plumbagin or hyperoxia treatment by increasing their content of superoxide dismutase (SOD) and they survived; whereas older nematodes did not induce SOD and suffered loss of viability, suggesting the adaptation to oxidative stress in young, but not in mature or old C. elegans [11]. Furthermore, pre-treatment with mild UV irradiation suppresses reproductive toxicity induced by subsequent cadmium in nematodes [12].
C. elegans, a free-living soil nematode, is an excellent model organism because of its short lifespan, ease of manipulation, and low cost. It has been found favor as a valuable bioindicator organism in toxicological study and ecological assessment for its best-characterized properties at the genetic, physiological, molecular, and developmental levels [13]–[14]. So far, the heavy metal toxicity and contamination can be effectively detected by the endpoints of mortality [15]–[16], lifespan [17]–[19], reproduction [20]–[21], and feeding [21]–[22] in C. elegans. Because the nematode behaviors can be easily monitored under the microscope, another sensitive endpoint, movement or locomotion behavior, was also monitored using a computer tracking system after metal exposure in nematodes [23]–[26]. In addition, Wang and Xing (2008) have examined the endpoints of head thrash, body bend, and basic movements in metal exposed nematodes, and indicated that the endpoints of head thrash, body bend, and forward turn can establish a fast and economic way to assess the presence of acute toxicity from heavy metal exposure [27]. More recently, it was reported that pre-treatment with mild metal exposure can activate the adaptive response to neurotoxicity of locomotion behavior induced by subsequent severe metal exposure in nematodes [28]. Moreover, pre-treatment with mild UV irradiation increases the resistance of nematodes to toxicity on locomotion behaviors from metal exposure [29].
Metallothioneins (MTs) are small (∼60 amino acid residues), cysteine-rich (∼30%) polypeptides that avidly bind 7–12 M of transition metal/M of protein via thiolate bonds, and exist in a wide range of organisms, including higher and lower eukaryotes, and even some prokaryotes [30]–[31]. MTs may be involved in the detoxication of heavy metals, such as cadmium (Cd) and mercury (Hg), the homoeostasis of essential metals such as zinc (Zn) and copper (Cu), and the protection against intracellular oxidative damage [32]–[33]. The MT transcription can be induced by metal exposure, ionizing radiation, heat-shock, and oxidative stress [33]–[35]. In C. elegans, two MT genes, designated mtl-1 and mtl-2, have been identified and characterized [32]. However, it is still largely unknown whether the MTs can regulate the formation of adaptive response in C. elegans.
Thus, in this study, we first investigated whether pre-treatment of heat shock can confer a protective effect against the neurobehavioral toxicity induced by subsequent metal exposure in nematodes. Moreover, we examined the possible important roles of C. elegans MTs in regulating the formation of cross-adaptation response to the neurobehavioral toxicity induced by subsequent metal exposure. Our data suggest that MTs are essential for the formation of cross-adaptation response to neurobehavioral toxicity induced by metal exposure in C. elegans.
Results
Locomotion behavior activities in heat-shock treated wild-type N2 nematodes
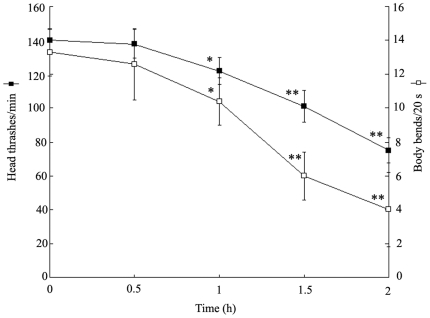
In the present study, the locomotion behavior activities were assessed by the endpoints of head thrash and body bend. As shown in Fig. 1, in wild-type nematodes, no obvious alterations of head thrash were recorded in nematodes treated with heat-shock for 0.5 h. The most significant (p<0.01) decreases of head thrashes were observed in heat-shock treated nematodes for 1.5 and 2 h at 36°C compared to control. Similarly, no remarkable changes of body bend were formed in nematodes treated with heat-shock for 0.5 h, and the very noticeable (p<0.01) reduction of body bends was also found in heat-shock treated nematodes for 1.5 and 2 h compared to control. Especially, only moderate, but significant (p<0.05) reduction of head thrash or body bend was observed in heat-shock treated nematodes for 1 h compared to control. Therefore, 1-h of heat-shock treatment will induce the mild reduction of locomotion behaviors in nematodes.
Figure 1. Neurobehavioral toxicity induced by heat-shock in wild-type N2 nematodes.
L4-stage larvae animals were heat stressed for 0.5, 1, 1.5, 2 h at 36°C. Bars represent means ± S.D. * p<0.05 vs 0 h; ** p<0.01 vs 0 h.
The alterations of stress response in heat-shock treated wild-type N2 nematodes
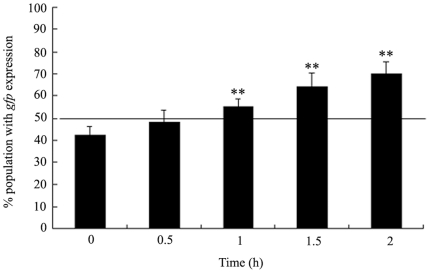
It was reasoned that if the stress exposure was toxic, it would thus result in a stress response as detected by the expression of HSP-16 expression [16]. Again, we explored one of the stable transgenic lines of hsp-16.2::gfp to investigate the stress response induced by heat-shock. As shown in Fig. 2, exposure to heat-shock for 0.5 h would not result in a significant induction of hsp-16.2::gfp expression (50% of a population, above the line), whereas treatment with both heat-shock for 1.5 and 2 h caused a sharp (p<0.01) elevation of hsp-16.2::gfp expression compared to control. Moreover, treatment with 1-h of heat-shock caused a moderate, but significant (p<0.01) induction of hsp-16.2::gfp expression compared to control. Thus, 1-h of heat-shock treatment will result in the mild stress response in nematodes.
Figure 2. Stress response in heat-shock treated wild-type N2 nematodes.
L4-stage larvae animals were heat stressed for 0.5, 1, 1.5, 2 h at 36°C. To evaluate the stress response, significant induction of hsp-16.2::gfp expression (50% of a population, above the line) was observed in wild-type N2 animals. Bars represent means ± S.D. * p<0.05 vs 0 h; ** p<0.01 vs 0 h.
Pre-treatment with mild heat-shock activates the adaptive response to neurobehavioral toxicity induced by metal exposure in wild-type N2 nematodes
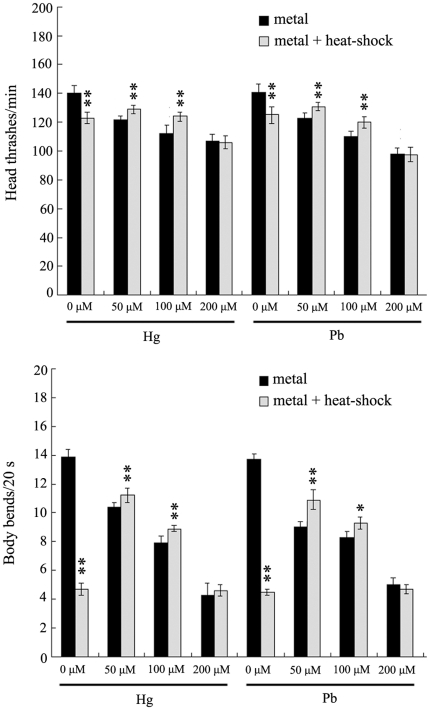
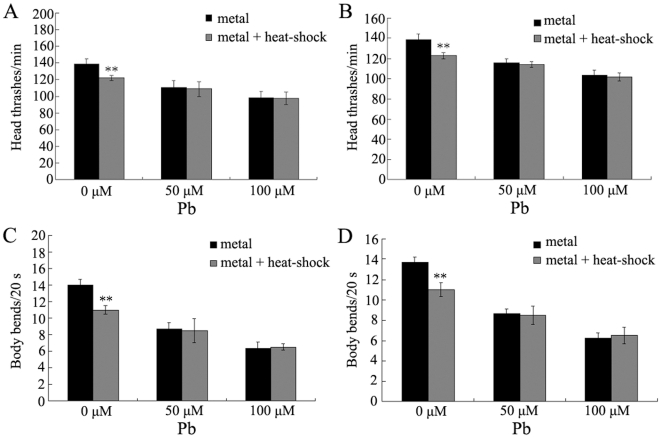
Previous studies have demonstrated that metal exposure would cause severe neurobehavioral toxicity in nematodes [22]–[26]. As shown in Fig. 3, in wild-type nematodes, exposure to metals of Hg and Pb at concentrations of 50, 100, and 200 µM will noticeably suppress the head thrashes and body bends compared to control. Moreover, pre-treatment with heat-shock for 1 h at L2-larva stage significantly (p<0.01) suppressed the decreases of head thrash and body bend formed in metal (Hg and Pb) exposed nematodes at the concentration of 50 µM. Similarly, pre-treatment with heat-shock for 1 h also markedly (p<0.01) inhibited the reductions of head thrash and body bend induced by metal exposure at the concentration of 100 µM. In contrast, pre-treatment with heat-shock for 1 h did not obviously influenced the occurrence of neurobehavioral defects formed in metal (Hg and Pb) exposed nematodes at the concentration of 200 µM compared to those without heat-shock pre-treatment. These data imply that pre-treatment with mild heat-shock can largely suppress the formation of neurobehavioral defects induced by metal exposure at the concentrations of 50 µM and 100 µM. We selected the concentrations of 50 µM and 100 µM to perform the following experiments on the adaptive response.
Figure 3. Effects of pre-treatment with mild heat-shock on neurobehavioral toxicity induced by metal exposure in wild-type N2 nematodes.
L2-stage larvae animals were heat stressed for 1 h at 36°C. The exposed metal concentrations were 50, 100, and 200 µM. Bars represent means ± S.D. * p<0.05 vs metal; ** p<0.01 vs metal.
Effects of pre-treatment with mild heat-shock on stress responses induced by metal exposure in wild-type N2 nematodes
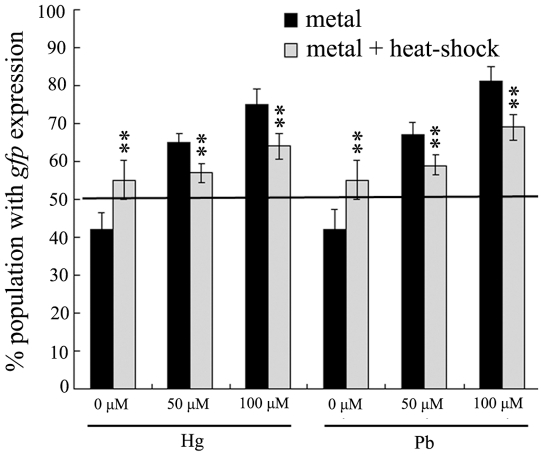
To examine the role of stress response in inducing the adaptation to metal toxicity after pre-treatment with mild heat-shock, we next investigated the effects of pre-treatment with mild heat-shock on the expression of hsp-16.2::gfp in metal exposed nematodes. hsp-16.2 is ubiquitously expressed throughout most somatic tissues, and the induced hsp-16.2::gfp expression signal at the pharyngeal bulb was strong and allowed a distinct identification of induced expression by stresses [16]. Previous study has indicated that a low level of background gfp expression in about 40% of the hsp-16.2::gfp transgenic nematodes was observed, although all the transgenic nematodes responded positively after metal exposure [16]. To distinguish the positive results from the background in the stress test, only stresses that could induce 50% of the transgenic nematodes to display strong hsp-16.2::gfp expression signal at the pharyngeal bulb were taken as having positive effect [16]. As shown in Fig. 4, metal (Hg and Pb) exposure at the concentrations of 50 µM and 100 µM induced the noticeable elevation of hsp-16.2::gfp expression. Furthermore, pre-treatment with heat-shock for 1 h at the L2-larva stage significantly reduced the percentage of population with hsp-16.2::gfp expression in examined metal exposed nematodes at the concentrations of 50 µM (p<0.01) and 100 µM (p<0.01) compared to those without heat-shock pre-treatment; however, no obvious alteration of the percentage of population with hsp-16.2::gfp expression was observed in metal exposed nematodes at the concentration of 200 µM after mild heat-shock pre-treatment (data not shown). Therefore, pre-treatment with mild heat-shock will largely suppress the metal-induced stress response in nematodes.
Figure 4. Effects of pre-treatment with mild heat-shock on the stress responses induced by metal exposure in wild-type N2 nematodes.
L2-stage larvae animals were heat stressed for 1 h at 36°C. The exposed metal concentrations were 50 and 100 µM. To evaluate the stress response, significant induction of hsp-16.2::gfp expression (50% of a population, above the line) was observed in wild-type N2 animals. Bars represent means ± S.D. * p<0.05 vs metal; ** p<0.01 vs metal.
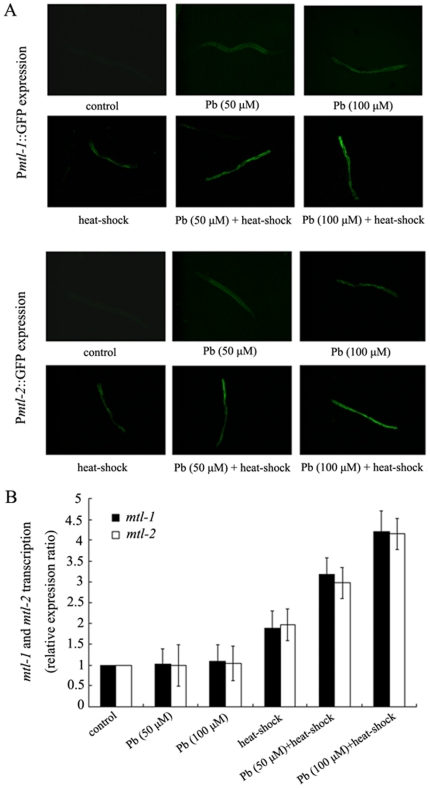
Alterations of MTs gene expression during the formation of cross-adaptation response to neurobehavioral toxicity induced by metal exposure
MTs are considered to be involved in the detoxification of and protection from some heavy metals, such as cadmium and mercury [31]–[32], [34]–[36]. The nematode C. elegans has two MTs, mtl-1 and mtl-2, with the similar functions [31], [34]–[35]. Moreover, it has been implied that the adaptation to elevated environmental metal concentrations may be achieved through the increased synthesis of MT or via the duplication of MT genes [37]–[38]. We further examined the possible alterations of MTs gene expression during the formation of cross-adaptation response to neurobehavioral toxicity induced by metal exposure. The two nematode strains, Pmtl-1::GFP and Pmtl-2::GFP, were constructed to have GFP protein fused to the promoters of mtl-1 and mtl-2, respectively. As shown in Fig. 5A, the GFP signal was nearly undetectable in control animals. The mild heat-shock treatment induced the moderate but significant increase of GFP signals in Pmtl-1::GFP and Pmtl-2::GFP transgenic strains. Exposure to 50 µM and 100 µM of Pb induced the slight increase of GFP signals in Pmtl-1::GFP and Pmtl-2::GFP transgenic strains. In contrast to these, after mild heat-shock treatment at the L2-larval stage, the GFP signals were sharply increased in 50 µM or 100 µM of Pb exposed Pmtl-1::GFP and Pmtl-2::GFP transgenic adult animals compared with those treated with mild heat-shock or Pb exposure alone. We have obtained 5 lines of Pmtl-1::GFP and 7 lines of Pmtl-2::GFP transgenic nematodes, and all showed the similar phenotypes (data not shown). The similar observations were obtained in Hg exposed nematodes after mild heat-shock pre-treatment (data not shown). These data imply that the formation of cross-adaptation response to neurobehavioral toxicity induced by metal exposure may be closely associated with the induction of mtl-1 and mtl-2 promoter activity and subsequent GFP gene expression. Similarly, as shown in Fig. 5B, exposure to 50 µM and 100 µM of Pb did not significantly increase the expression of mtl-1 and mtl-2 at the transcription level; however, after mild heat-shock treatment at the L2-larval stage, the mtl-1 or mtl-2 expression at the transcription level was obviously increased in 50 µM or 100 µM of Pb exposed nematodes compared with those treated with mild heat-shock or Pb exposure alone. We next selected the Pb to further investigate the roles of MTs in regulating the formation of cross-adaptation response to neurobehavioral toxicity induced by metal exposure in C. elegans.
Figure 5. Induction of metallothionein gene expression during the formation of cross-adaptation response to neurobehavioral toxicity induced by Pb exposure.
(A) Induction of green fluorescence protein in the Pmtl-1::GFP and Pmtl-2::GFP transgenic nematodes. (B) mtl-1 and mtl-2 transcription assay. Relative expression ratio (between mtl-1 or mtl-2 gene and ubq-1 reference gene) in treatments are normalized to the control. L2-stage larvae animals were heat stressed for 1 h at 36°C. The exposed metal concentrations were 50 and 100 µM. Bars represent means ± S.D.
Effects of pre-treatment with mild heat-shock on neurobehavioral toxicity induced by Pb exposure in mtl-1 and mtl-2 mutant nematodes
We further investigated the possible alterations of neurobehavioral toxicity in Pb (50 µM and 100 µM) exposed mtl-1 and mtl-2 mutants after pre-treatment with mild heat-shock. As shown in Fig. 6, mutations of mtl-1 and mtl-2 did not obviously affect the nematode locomotion behaviors compared to wild-type, and treatment with heat-shock for 1 h at the L2-larva stage caused a moderate but significant (p<0.01) decrease of head thrashes and body bends in mtl-1(tm1770) and mtl-2(gk125) mutant nematodes compared with those observed in wild-type nematodes. Moreover, after pre-treatment with mild heat-shock, no noticeable increase of head thrashes and body bends were detected in Pb exposed mtl-1(tm1770) and mtl-2(gk125) mutant nematodes compared with those without mild heat-shock pre-treatment. Therefore, mutations of mtl-1 and mtl-2 genes result in the alterations of cross-adaptation response to neurobehavioral toxicity induced by Pb exposure in nematodes. In addition, we did not observed the obvious increase of head thrashes and body bends in 200 µM of Pb exposed mtl-1(tm1770) and mtl-2(gk125) mutant nematodes after pre-treatment with mild heat-shock compared with those without mild heat-shock pre-treatment (data not shown).
Figure 6. Effects of pre-treatment with mild heat-shock on neurobehavioral toxicity in Pb exposed mtl-1(tm1770) and mtl-2(gk125) mutant nematodes.
(A) Effects of pre-treatment with mild heat-shock on head thrash in Pb exposed mtl-1(tm1770) mutant nematodes. (B) Effects of pre-treatment with mild heat-shock on head thrash in Pb exposed mtl-2(gk125) mutant nematodes. (C) Effects of pre-treatment with mild heat-shock on body bend in Pb exposed mtl-1(tm1770) mutant nematodes. (D) Effects of pre-treatment with mild heat-shock on body bend in Pb exposed mtl-2(gk125) mutant nematodes. L2-stage larvae animals were heat stressed for 1 h at 36°C. The exposed metal concentrations were 50 and 100 µM. Bars represent means ± S.D. ** p<0.01 vs metal.
Rescue assay on the deficits in adaptive response to neurobehavioral toxicity induced by Pb exposure formed in mtl-1 and mtl-2 mutant nematodes
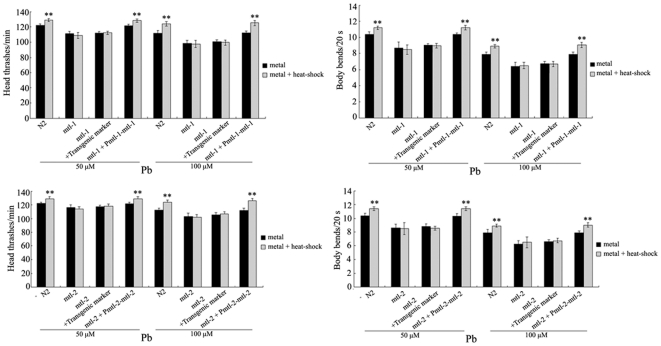
To confirm the important roles of mtl-1 and mtl-2 genes in regulating the adaptive response to neurobehavioral toxicity observed in mtl-1(tm1770) and mtl-2(gk125) mutants, we next performed the rescue experiments in nematodes. As shown in Fig. 7, transgene only with the vector of Pdop-1::rfp (Pdop-1, dop-1 promoter), which served as a transgenic marker, did not affect the formation of adaptive response to neurobehavioral toxicity induced by metal expression. The defects of adaptive response to neurobehavioral toxicity on head thrash or body bend induced by Pb exposure at the concentrations of 50 µM and 100 µM formed in mtl-1(tm1770) mutant were completely rescued by the expression of mtl-1 with the aid of its native promoter. Similarly, the defects of adaptive response to neurobehavioral toxicity on head thrash or body bend induced by Pb exposure at the concentrations of 50 µM and 100 µM formed in mtl-2(gk125) mutant were also completely rescued by the expression of mtl-2 with the aid of its native promoter. We have obtained 4 lines of Pmtl-1-mtl-1 and 6 lines of Pmtl-2-mtl-2 transgenic nematodes, and all showed the similar phenotypes (data not shown).
Figure 7. The defects of adaptive response to neurobehavioral toxicity induced by Pb exposure in mtl-1(tm1770) and mtl-2(gk125) mutants can be rescued by the expression of mtl-1 and mtl-2 with their native promoters.
L2-stage larvae animals were heat stressed for 1 h at 36°C. The exposed metal concentrations were 50 and 100 µM. The plasmids were injected as a mix at 20 ng/µl using Pdop-1::rfp as a transgenic marker. Bars represent means ± S.D. ** p<0.01 vs metal.
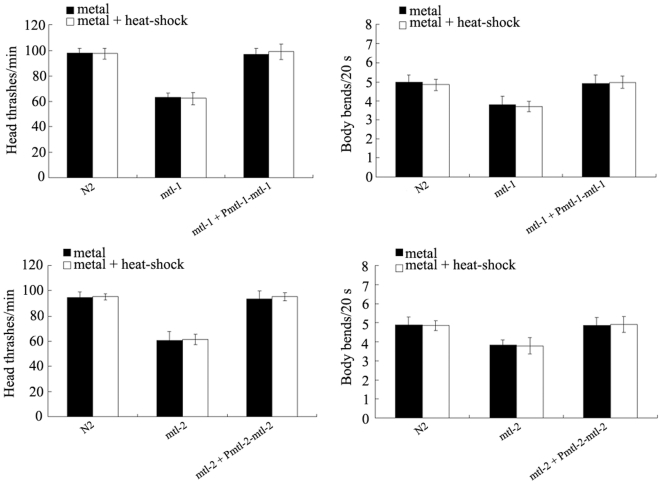
Interestingly, after mild heat-shock treatment at the L2-larval stage, we further observed the different phenotypes of locomotion behavior in 200 µM of Pb exposed mtl-1 and mtl-2 adult mutants. As shown in Fig. 8, the defects of adaptive response to neurobehavioral toxicity on head thrash or body bend induced by Pb exposure at the concentration of 200 µM formed in mtl-1(tm1770) mutant could not be rescued by the expression of mtl-1 with the aid of its native promoter. Similarly, the defects of adaptive response to neurobehavioral toxicity on head thrash or body bend induced by Pb exposure at the concentration of 200 µM formed in mtl-2(gk125) mutant could not also be rescued by the expression of mtl-2 with the aid of its native promoter. Therefore, the expression of mtl-1 and mtl-2 with their native promoters can not rescue the defects of adaptive response to neurobehavioral toxicity induced by exposure to 200 µM of Pb in mtl-1 and mtl-2 mutants.
Figure 8. The defects of adaptive response to neurobehavioral toxicity induced by exposure to 200 µM of Pb in mtl-1(tm1770) and mtl-2(gk125) mutants can not be rescued by the expression of mtl-1 and mtl-2 with their native promoters.
L2-stage larvae animals were heat stressed for 1 h at 36°C. The plasmids were injected as a mix at 20 ng/µl using Pdop-1::rfp as a transgenic marker. Bars represent means ± S.D.
Over-expression of MTL-1 or MTL-2 at the L2-larval stage suppresses the neurobehavioral toxicity induced by Pb exposure
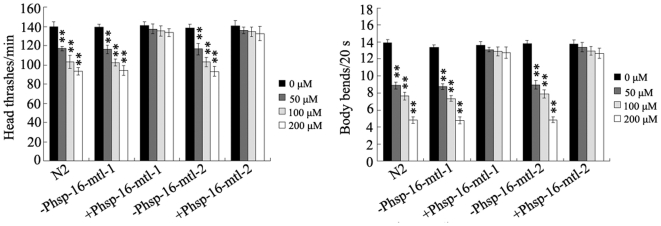
We finally investigated the effects of MTL-1 and MTL-2 over-expression on the neurobehavioral toxicity from Pb exposure. To induce the over-expression of MTL-1 and MTL-2 at the L2-larval stage, MTL-1 and MTL-2 were expressed in the wild-type N2 animals using a heat shock promoter. The transgenic animals were treated by heat-shock at the L2-larval stage, and the treated animals were further exposed to metal solutions at the L4-larval stage. Under our experimental conditions, the wild-type nematodes treated with heat-shock at 30°C for 4-hr showed normal locomotion behaviors (data not shown). As shown in Fig. 9, transgenes with Phsp-mtl-1 and Phsp-mtl-2 did not obviously influence the locomotion behaviors (head thrash and body bend) of Pb exposed nematodes without heat-shock treatment. In contrast, over-expression of MTL-1 and MTL-2 by heat-shock treatment at the L2-larval stage significantly suppressed the toxicity on locomotion behaviors from Pb exposure at all examined concentrations. More interestingly, the over-expression of MTL-1 and MTL-2 by heat-shock treatment at the L2-larval stage even noticeably inhibited the toxicity on locomotion behaviors from Pb exposure at the concentration of 200 µM.
Figure 9. Effects of MTL-1 and MTL-2 over-expression at the L2-larval stage on the neurobehavioral toxicity induced by Pb exposure.
–Phsp-16-mtl-1 and –Phsp-16-mtl-2, wild-type animals carrying Phsp-16-mtl-1 or –Phsp-16-mtl-2 without heat-shock treatment; –Phsp-16-mtl-1 and –Phsp-16-mtl-2, wild-type animals carrying Phsp-16-mtl-1 or –Phsp-16-mtl-2 treated with heat-shock (30°C, 4-hr) at the L2-larval stage. Bars represent means ± S.D. ** p<0.01 vs 0 µM.
Discussion
The neurotoxicity from metal exposure on locomotion behaviors has been widely studied for many years. In this project, we selected the metals of Hg and Pb to study the adaptation response of nematodes to neurobehavioral toxicity induced by metal exposure after mild stress pre-treatment. These metals are ubiquitous toxicants that will result in a wide range of adverse health effects in humans, and inorganic metals from natural and man-made sources are released into air, soil and water. The severe deficits in locomotion behaviors have been observed in Hg and Pb exposed C. elegans [27], [39]. In this study, we provide evidence to raise such a notion that the neurobehavioral toxicity induced by metal exposure can be effectively prevented by pre-treatment with mild heat-shock, which provides the nematodes an important strategy to resist the neurotoxicity from metal exposure or other stresses. This conclusion is consistent with our previous observation that pre-treatment with mild UV irradiation increases the resistance of nematodes to toxicity on locomotion behaviors from metal exposure [29].
In the present study, we examined the possible roles of mild heat-shock in inducing the adaptive response to neurobehavioral toxicity from metal exposure. The locomotion behavior was assessed by the endpoints of head thrash and body bend, which have been successfully explored to evaluate the neurobehavioral toxicity induced by metal exposure [17]-[18]. The heat-shock is a commonly and extensively investigated stress in nematodes [9]. Previous studies usually explored the heat-shock for 2 h as a severe stress [9]. Our data suggest that treatment with heat-shock for 2 h could further induce severe deficits in locomotion behaviors, whereas treatment with heat-shock for 1 h could only cause mild toxicity on locomotion behaviors in exposed nematodes. Thus, we here selected the heat-shock for 1 h for the pre-treatment to study the adaptive response of nematode to neurobehavioral toxicity from metal exposure. Moreover, our previous studies suggest that exposure to more than 50 µM of metals often resulted in severe defects of locomotion behaviors; however, exposure to 2.5 µM of metals would induce moderate but significant decrease of locomotion behaviors (27). Therefore, the concentrations of 50, 100, and 200 µM were selected further to evaluate the neurobehavioral toxicity induced by metal exposure. In addition, all examinations were performed at larval stages (L2 or L4), since Darr and Fridovich (1995) indicated that the adaptation to oxidative stress exists in young, but not mature or old C. elegans [11]. Our previous study further suggested that exposure to Pb and Hg in L2-larvae can induce more severe deficits in locomotion behaviors than adult nematodes [23].
Our data suggest that pre-treatment with mild heat-shock will largely increase the resistance of nematodes to neurobehavioral toxicity induced by metal exposure, and suppress the formation of metal-induced stress response in nematodes, suggesting that pre-treatment with mild stresses can activate the adaptive response of nematodes to neurobehavioral toxicity from metal exposure by suppressing the severe stress response formed in metal exposed nematodes. Together with our previous observations [28]–[29], both the cross adaptive response and the non-cross adaptive response to neurobehavioral toxicity induced by metal exposure can be formed in nematodes. Thus, the induction of adaptive response to severe forms of exposure by suppressing the stress response should be a conserved mechanism after pre-treatment with mild stress. Furthermore, our data demonstrate that pre-treatment with severe stress can not effectively induce the adaptive response to neurobehavioral toxicity from metal exposure, and the adaptive response can also not be effectively activated by mild stress pre-treatment in nematodes with a mostly severe impairment from metal exposure. Especially, pre-treatment with severe heat-shock resulted in significant decrease of locomotion behavior (head thrashe and body bend) in metal exposed nematodes at different concentrations (D-Y Wang et al., personal communication), implying that the adaptive response might be activated but was not effective enough in protecting against the heavy metal toxicity. Thus, the suitable and mildly toxic pre-exposure is essential for the activation of adaptive response to neurobehavioral toxicity from metal exposure in nematodes.
Furthermore, our data suggest that MTs are essential for the formation of cross-adaptation response to neurobehavioral toxicity induced by metal exposure in C. elegans. MT proteins play a major role in metal detoxification in nematodes [34]–[36], [40]. It was also reported that the MT-null mice seemed more susceptible to Hg0-induced neurobehavioral toxicity than the wild-type, and the increased susceptibility to MT-null females to behavioral changes caused by prenatal Hg0 exposure is due to a greater retention of mercury [41]–[42]. The adaptive response to the neurobehavioral toxicity induced by metal exposure can not be detected in mtl-1 and mtl-2 mutant nematodes. The deficits in the formation of cross-adaptation response to neurobehavioral toxicity induced by exposure to examined metals at concentrations of 50 and 100 µM in mtl-1 and mtl-2 mutants could be further rescued by the expression of MTL-1 and MTL-2 with the aid of their own native promoters. These data imply that the adaptive response to neurobehavioral toxicity induced by metal exposure could not be effectively activated in mtl-1 and mtl-2 mutant nematodes. Similarly, the adaptive response to X-radiation exists in wild-type nematode that is also not present in rad-1 and rad-2 mutants with the properties of UV and ionizing radiation hypersensitive [1].
C. elegans MT1 is constitutively expressed in three cells of the posterior bulb of the pharynx but is also induced by metal exposure and heat-shock in intestinal cells, and MT2 expression occurs only in intestinal cells in metal exposed nematodes [32]–[34]. In addition, significant levels of intestinal cell transcription of C. elegans MTs are difficult to be observed in the absence of stress [32]–[34]. In the present study, our data demonstrate that the defects of adaptive response to neurobehavioral toxicity induced by metal exposure formed in mtl-1 and mtl-2 mutants could be not only completely rescued by the expression of mtl-1 or mtl-2 with the aid of its own promoter, but also largely rescued by the expression of mtl-1 or mtl-2 gene in the nervous system (D.-Y. Wang et al., personal communication). In contrast, expression of MTL-1 and MTL-2 in the muscle cells could not rescue the deficits in the formation of cross-adaptation response to neurobehavioral toxicity induced by metal exposure in the mtl-1 and mtl-2 mutant nematodes (D.-Y. Wang et al., personal communication). These data imply that mtl-1 and mtl-2 genes may function cell non-autonomously in regulating the formation of cross-adaptation response to neurobehavioral toxicity induced by metal exposure in nematodes.
During the formation of cross-adaptation response to neurobehavioral toxicity induced by metal exposure, we further observed that the induction of mtl-1 and mtl-2 promoter activity and subsequent GFP gene expression were sharply increased in 50 µM or 100 µM of metal exposed Pmtl-1::GFP and Pmtl-2::GFP transgenic adult animals after mild heat-shock treatment at the L2-larval stage compared with those treated with mild heat-shock or metal exposure alone. Previous study from Ma et al. (2009) further supported our observations on the expression of Pmtl-1::GFP and Pmtl-2::GFP in Pb and Hg exposed nematodes, and help to explain our data presented in this study [43]. These data strongly imply that the normal formation of cross-adaptation response to neurobehavioral toxicity induced by metal exposure may require the enough accumulation of MTs protein in animal tissues. Nevertheless, expression of MTL-1 and MTL-2 did not rescue the defects of adaptive response to neurobehavioral toxicity induced by exposure to 200 µM of Pb in mtl-1 and mtl-2 mutants. Moreover, we observed that over-expression of MTL-1 and MTL-2 at the L2-larval stage significantly suppressed the toxicity on locomotion behaviors from Pb exposure at all examined concentrations. In contrast, expression of proteins not related with stress response and adaptive response, such as MOD-5, a serotonin transporter, and EAT-4, a glutamine transporter, at the L2-larval stage did not influence the toxicity on locomotion behaviors from Pb exposure at all examined concentrations (D.-Y. Wang et al., personal communication). Therefore, pre-treatment with mild stress may also activate some unknown mechanism, which will further enhance the MTs accumulation while the animals are further exposure to very severe stresses.
In conclusion, pretreatment with mild heat-shock will activate the resistance of nematodes to neurobehavioral toxicity from metal exposure, and MT genes are required for the formation of cross-adaptation response to neurobehavioral toxicity induced by metal exposure in nematodes.
Materials and Methods
Reagents
The metal concentrations used in this study were referred to our previous description [17]–[18]. Three concentrations of HgCl2, and Pb(NO3)2 solutions were used in the current work, and they were 50 µM, 100 µM, and 200 µM, respectively. Metal concentrations of exposed solutions were analyzed by atomic absorption spectrophotometry (AAS; Pye-Unicam model SP9, Cambridge, UK). All the chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Strain preparation
Nematodes used in the present study were wild-type Bristol (N2), mutants of VC128 [mtl-2(gk125)], FX01770[mtl-1(tm1770)], and transgenic strains of Ex(Pmtl-1::GFP), Ex(Pmtl-1::GFP), Ex(Pmtl-1-mtl-1), Ex(Pmtl-2-mtl-2) and KC136 [hsp-16.2::gfp]. KC136 [hsp-16.2::gfp], an integrated transgenic line [16], is the gift from Dr. King L. Chow. They were maintained on nematode growth medium (NGM) plates seeded with E. coli OP50 at 20°C as described [44]. Gravid nematodes were washed off the plates into centrifuge tubes, and were lysed with a bleaching mixture (0.45 M NaOH, 2% HOCl). Age synchronous populations of L2- or L4-larval animals were obtained by the collection as described [45]. The collected nematodes were washed with double-distilled water, followed by washing with modified K medium once (50 mM NaCl, 30 mM KCl, 10 mM NaOAc, pH 5.5) [46]. Metal exposures were performed on L4-larval nematodes in 12-well sterile tissue culture plates, and all exposures were 12-h long and were carried out in 20°C incubator in the presence of food.
Heat-shock experiments
Approximately 500 L4-stage larvae animals were heat stressed for 0.5, 1, 1.5, 2 h at 36°C, and approximately 500 L2-stage larvae animals were heat stressed for 1 h or 2 h at 36°C and afterwards further maintained at 20°C. All assays were replicated more than three times.
Head thrash frequency
To assay the head thrashes, nematodes were washed with the double-distilled water, followed by washing with modified K medium. Every nematode was transferred into a microtiter well containing 60 µl of modified K medium on the top of agar. After a 1-min recovery period, the head thrashes were counted for 1-min. A thrash was defined as a change in the direction of bending at the mid body. Fifty nematodes were examined per treatment.
Body bend frequency
To assay the body bends, nematodes were picked onto a second plate and scored for the number of body bends in an interval of 20 sec. A body bend was counted as a change in the direction of the part of the nematodes corresponding to the posterior bulb of the pharynx along the y axis, assuming that the nematode was traveling along the x axis. Fifty nematodes were examined per treatment.
Analysis of KC136 transgenic strain
To analyze the changes of hsp-16.2 expression patterns, the treated KC136 animals were allowed to settle for 10 min, and then pipetted onto an agar pad on a glass slide, mounted and observed for the fluorescent signals with a fluorescent microscope. Observations of the green fluorescent protein (GFP) at the pharyngeal bulb were recorded and color images were taken for the documentation of results with Magnafire® software (Olympus, Irving, TX, USA). The stress response was evaluated by the percentage of population with gfp expression. More than 50 nematodes were counted for the statistical analysis.
DNA construct
The promoter fragments of mtl-1 and mtl-2 genes were selected according to the reference [35] and the suitable restriction enzyme sites for further sub-cloning into the pPD95_75 vector. The mtl-1 (1197bp, PstI-XbaI) and mtl-2 (551bp, XbaI/XhoI) promoter fragments were first sub-cloned into the pPD95_75 vector, and the mtl-1 and mtl-2 full length cDNA were further inserted into sites of BamHI/KpnI and XhoI/EcoRI of the pPD95_75 vector behind the fragments of Pmtl-1 and Pmtl-2, respectively, to obtain the plasmids of Pmtl-1-mtl-1 and Pmtl-2-mtl-2. The mtl-1 and mtl-2 full length cDNA were sub-cloned into the sites of BamHI/KpnI of the vector of pPD49_78 to obtain the plasmids of Phsp-16-mtl-1 and Phsp-16-mtl-2. Transgenic worms were generated as previously described [47]. The plasmids were injected as a mix at 20 ng/µl using Pdop-1::rfp as a transgenic marker, and the anticipated transgenic animals were identified with strong red signals [48]. The Pmtl-1 and Pmtl-2 plasmids were injected into the wild-type N2 nematodes to obtain the Pmtl-1::GFP and Pmtl-2::GFP transgenic animals.
To perform the rescue experiments, the constructs of Pmtl-1-mtl-1 and Pmtl-2-mtl-2 plasmids were injected into the mtl-1 and mtl-2 mutants, respectively. To perform the over-expression experiments, the constructs of Phsp-16-mtl-1 and Phsp-16-mtl-2 plasmids were injected into the wild-type N2 nematodes, respectively. Adult nematodes carrying Phsp-16-mtl-1 and Phsp-16-mtl-2 were placed on the NGM plates (0 hr), allowed to lay eggs for 4 hr at 20°C, and removed from the plates. The nematodes were heat shocked at 30°C for 4 hr, beginning at 40 hr (L2-larval stage), after the start of egg laying. The nematodes were cultured at 20°C again and subjecting to the locomotion behavior assay.
Reverse transcription-polymerase chain reaction
Total RNA was extracted using RNeasy Mini Kit (Qiagen). Total nematode RNA (∼1 µg) was reverse-transcribed using cDNA Synthesis kit (Bio-Rad Laboratories), and real-time PCR was performed using the primers for the target genes of mtl-1 (forward primer, 5′-AGCTCAATTTGACTGCTGAA-3′; reverse primer, 5′-GAAACATTTTAATGAGCCGC-3′) and mtl-2 (forward primer, 5′-CTGCCAGTGAGAAGAAATGC-3′; reverse primer, 5′-CGAACAATATCAATTAGTAGGAATTTG-3′), and reference gene of ubp-1 (forward primer, 5′-CACTTGGTTCTTCGTCTTAG-3′; reverse primer, 5′-CCTCCTTGTCTTGAATCTTG-3′). Real-time PCR was run at the optimized annealing temperature of 58°C. The relative quantification of the mtl-1 or mtl-2 gene in comparison to the reference ubq-1 gene was determined using the method described [49], and the final results were expressed as the relative expression ratio (between target gene and reference gene) in the treatments as compared to the ratio in the control.
Statistical analysis
All data in this article were expressed as means ± SD. Graphs were generated using Microsoft Excel (Microsoft Corp., Redmond, WA). One-way analysis of variance (ANOVA) followed by a Dunnett's t-test was used to determine the significance of the differences between the groups. The probability levels of 0.05 and 0.01 were considered statistically significant.
Acknowledgments
Some strains used in this study were provided by the Caenorhabdits Genetics Center (funded by the NIH National Center for Research Resource, USA) and the Mitani laboratory (Tokyo Women's Medical University School of Medicine, Japan). We thank Dan Chase for the pCL40 (Pdop-1::rfp) vector, and King L. Chow for the KC136 strain.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the grants from the National Natural Science Foundation of China (No. 30870810) and the Program for New Century Excellent Talents in University of China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yanase S, Hartman P S, Ito A, Ishii N. Oxidative stress pretreatment increases the X-radiation resistance of the nematode Caenorhabditis elegans. Mutat Res. 1999;426:31–39. doi: 10.1016/s0027-5107(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 2.Samson L, Cairns J. A new pathway for DNA repair in Escherichia coli. Nature. 1977;267:281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- 3.Samson L, Schwartz JL. Evidence for an adaptive DNA repair pathway in CHO and human skin fibroblast cell lines. Nature. 1980;287:861–863. doi: 10.1038/287861a0. [DOI] [PubMed] [Google Scholar]
- 4.Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223:594–597. doi: 10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- 5.Domínguez I, Panneerselvam N, Escalza P, Natarajan AT, Cortés F. Adaptive response to radiation damage in human lymphocytes conditioned with hydrogen peroxide as measured by the cytokinesis-block micronucleus technique. Mutat Res. 1993;301:135–141. doi: 10.1016/0165-7992(93)90036-u. [DOI] [PubMed] [Google Scholar]
- 6.Morgan RW, Christan MF, Jacobson FS, Storz G, Ames BN. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci USA. 1986;83:8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cypser JR, Johnson TE. Hormesis extends the correlation between stress resistance and life span in long-lived mutants of Caenorhabditis elegans. Hum Exp Toxicol. 2001;20:295–296. doi: 10.1191/096032701701548070. [DOI] [PubMed] [Google Scholar]
- 8.Cypser JR, Johnson TE. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J Gerontol A Biol Sci Med. 2002;57:B109–B114. doi: 10.1093/gerona/57.3.b109. [DOI] [PubMed] [Google Scholar]
- 9.Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci USA. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanase S, Yasuda K, Ishii N. Adaptive responses to oxidative damage in three mutants of Caenorhabditis elegans (age-1, mev-1 and daf-16) that affect life span. Mech Ageing Dev. 2002;123:1579–1587. doi: 10.1016/s0047-6374(02)00093-3. [DOI] [PubMed] [Google Scholar]
- 11.Darr D, Fridovich I. Adaptation to oxidative stress in young, but not in mature or old, Caenorhabditis elegans. Free Radic Biol Med. 1995;18:195–201. doi: 10.1016/0891-5849(94)00118-4. [DOI] [PubMed] [Google Scholar]
- 12.Wang D-Y, Xing X-J. Pre-treatment with mild UV irradiation suppresses reproductive toxicity induced by subsequent cadmium exposure in nematodes. Ecotoxicol Environ Safety. 2010;73:423–429. doi: 10.1016/j.ecoenv.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Riddle DL, Blumenthal T, Meyer BJ, Priess JR. Plainview, New York: C. ELEGANS II, Cold Spring Harbor Laboratory Press ; 1997. [PubMed] [Google Scholar]
- 14.Peredney CL, Williams PL. Utility of Caenorhabditis elegans for assessing heavy metal contamination in artificial soil. Arch Environ Contam Toxicol. 2000;39:113–118. doi: 10.1007/s002440010086. [DOI] [PubMed] [Google Scholar]
- 15.Ura K, Kai T, Sakata S, Iguchi T, Arizono K. Aquatic acute toxicity testing using the nematode Caenorhabditis elegans. J Health Sci. 2002;48:583–586. [Google Scholar]
- 16.Chu KW, Chow KL. Synergistic toxicity of multiple heavy metals is revealed by a biological assay using a nematode and its transgenic derivative. Aquat Toxicol. 2002;61:53–64. doi: 10.1016/s0166-445x(02)00017-6. [DOI] [PubMed] [Google Scholar]
- 17.Wang D-Y, Shen L–L, Wang Y. The phenotypic and behavioral defects can be transferred from zinc exposed nematodes to their progeny. Environ Toxicol Pharmacol. 2007;24:223–230. doi: 10.1016/j.etap.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Xie W, Wang D-Y. Transferable properties of multi-biological toxicity caused by cobalt exposure in Caenorhabditis elegans. Environ Toxicol Chem. 2007;26:2405–2412. doi: 10.1897/06-646R1.1. [DOI] [PubMed] [Google Scholar]
- 19.Harada H, Kurauchi M, Hayashi R, Eki T. Shortened lifespan of nematode Caenorhabditis elegans after prolonged exposure to heavy metals and detergents. Ecotoxicol Environ Safe. 2007;66:378–383. doi: 10.1016/j.ecoenv.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Dhawan R, Dusenbery DB, Williams PL. Comparison of lethality, reproduction, and behavior as toxicological endpoints in the nematode Caenorhabditis elegans. J Toxicol Environ Health A. 1999;58:451–462. doi: 10.1080/009841099157179. [DOI] [PubMed] [Google Scholar]
- 21.Anderson GL, Boyd WA, Williams PL. Assessment of sublethal endpoints for toxicity testing with the nematode Caenorhabditis elegans. Environ Toxicol Chem. 2001;20:833–838. [PubMed] [Google Scholar]
- 22.Boyd WA, Cole RD, Anderson GL, Williams PL. The effects of metals and food availability on the behavior of Caenorhabditis elegans. Environ Toxicol Chem. 2003;22:3049–3055. doi: 10.1897/02-565. [DOI] [PubMed] [Google Scholar]
- 23.Xing X-J, Guo Y-L, Wang D-Y. Using the larvae nematode Caenorhabditis elegans to evaluate neurobehavioral toxicity to metallic salts. Ecotoxicol Environ Safety. 2009;72:1819–1823. doi: 10.1016/j.ecoenv.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Anderson GL, Cole RD, Williams PL. Assessing behavioral toxicity with Caenorhabditis elegans. Environ Toxicol Chem. 2004;23:1235–1240. doi: 10.1897/03-264. [DOI] [PubMed] [Google Scholar]
- 25.Wang D-Y, Wang Y. Phenotypic and behavioral defects caused by barium exposure in nematode Caenorhabditis elegans. Arch Environ Contam Toxicol. 2008;54:447–453. doi: 10.1007/s00244-007-9050-0. [DOI] [PubMed] [Google Scholar]
- 26.Wang D–Y, Wang Y. Nickel sulfate induces numerous defects in Caenorhabditis elegans that can also be transferred to progeny. Environ Pollut. 2008;151:585–592. doi: 10.1016/j.envpol.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Wang D-Y, Xing X-J. Assessment of locomotion behavioral defects induced by acute toxicity from heavy metal exposure in nematode Caenorhabditis elegans. J Environ Sci. 2008;20:1132–1137. doi: 10.1016/s1001-0742(08)62160-9. [DOI] [PubMed] [Google Scholar]
- 28.Wang D-Y, Xing X-J. Pre-treatment with mild metal exposure suppresses the neurotoxicity on locomotion behavior induced by the subsequent severe metal exposure in Caenorhabditis elegans. Environ Toxicol Pharmacol. 2009;28:459–464. doi: 10.1016/j.etap.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Wang D-Y, Liu P-D, Xing X-J. Pre-treatment with mild UV irradiation increases the resistance of nematodes Caenorhabditis elegans to toxicity on locomotion behaviors from metal exposure. Environ Toxicol Pharmacol. 2010;29:213–222. doi: 10.1016/j.etap.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Slice LW, Freedman JH, Rubin CS. Purification, characterization, and cDNA cloning of a novel metallothionein-like, cadmium-binding protein from Caenorhabditis elegans. J Biol Chem. 1990;265:256–263. [PubMed] [Google Scholar]
- 31.Hughes SL, Bundy JG, Want EJ, Kille P, Stürzenbaum SR. The metabolomic responses of Caenorhabditis elegans to cadmium are largely independent of metallothionein status, but dominated by changes in cystathionine and phytochelatins. J Proteome Res. 2009;8:3512–3519. doi: 10.1021/pr9001806. [DOI] [PubMed] [Google Scholar]
- 32.Imagawa M, Onozawa T, Okumura K, Osada S, Nishihara T, et al. Characterization of metallothionein cDNAs induced by cadmium in the nematode Caenorhabditis elegans. Biochem J. 1990;268:237–240. doi: 10.1042/bj2680237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moilanen LH, Fukushige T, Freedman JH. Regulation of metallothionein gene transcription. J Biol Chem. 1999;274:29655–29665. doi: 10.1074/jbc.274.42.29655. [DOI] [PubMed] [Google Scholar]
- 34.Barsyte D, Lovejoy DA, Lithgow GJ. Longevity and heavy metal resistance in daf-2 and age-1 long-lived mutants of Caenorhabditis elegans. FASEB J. 2001;15:627–634. doi: 10.1096/fj.99-0966com. [DOI] [PubMed] [Google Scholar]
- 35.Swain SC, Keusekotten K, Baumeister R, Stürzenbaum SR. C. elegans metallothioneins: new insights into the phenotypic effects of cadmium toxicosis. J Mol Biol. 2004;341:951–959. doi: 10.1016/j.jmb.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 36.Palmiter RD. The elusive function of metallothioneins. Proc Natl Acad Sci USA. 1998;95:158428–158430. doi: 10.1073/pnas.95.15.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephan W, Rodriguez VS, Zhou B, Parsch J. Molecular evolution of the metallothionnein gene Mtn in the melanogaster species group: results from Drosophila ananassae. Genetics. 1994;138:135–143. doi: 10.1093/genetics/138.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knapen D, Redeker ES, Inacio I, De Coen W, Verheyen E, et al. New metallothionein mRNAs in Gobio gobio reveal at least three gene duplication events in cyprinid metallothionein evolution. Comp Bio-Chem Physiol C Toxicol Pharmacol. 2005;140:347–355. doi: 10.1016/j.cca.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Wang D-Y, Yang P. The multi-biological defects caused by lead exposure exhibit transferable properties from exposed parents to their progeny in Caenorhabditis elegans. J Environ Sci. 2007;19:1367–1372. doi: 10.1016/s1001-0742(07)60223-x. [DOI] [PubMed] [Google Scholar]
- 40.Hughes S, Stürzenbaum SR. Single and double metallothionein knockout in the nematode C. elegans reveals cadmium dependent and independent toxic effects on the life history traits. Environ Pollut. 2007;145:395–400. doi: 10.1016/j.envpol.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida M, Watanabe C, Horie K, Satoh M, Sawada M, et al. Neurobehavioral changes in metallothionein-null mice prenatally exposed to mercury vapor. Toxicol Lett. 2005;155:361–368. doi: 10.1016/j.toxlet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida M, Watanabe C, Kishimoto M, Yasutake A, Satoh M, et al. Behavioral changes in metallothionein-null mice after the cessation of long-term, low-level exposure to mercury vapor. Toxicol Lett. 2006;161:210–218. doi: 10.1016/j.toxlet.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Ma H, Glenn TC, Jagoe CH, Jones KL, Willams PL. A transgenic strain of the nematode Caenorhabditis elegans as a biomonitor for heavy metal contamination. Environ Toxicol Chem. 2009;28:1311–1318. doi: 10.1897/08-496.1. [DOI] [PubMed] [Google Scholar]
- 44.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donkin S, Williams PL. Influence of developmental stage, salts and food presence on various end points using Caenorhabditis elegans for aquatic toxicity testing. Environ Toxicol Chem. 1995;14:2139–2147. [Google Scholar]
- 46.Williams PL, Dusenbery DB. Aquatic toxicity testing using the nematode Caenorhabditis elegans. Environ Toxicol Chem. 1990;9:1285–1290. [PubMed] [Google Scholar]
- 47.Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequence. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chase DL, Pepper JS, Koelle MR. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci. 2004;7:1096–1103. doi: 10.1038/nn1316. [DOI] [PubMed] [Google Scholar]
- 49.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]