Abstract
To understand the relationship between permanent cell cycle exit and differentiation the immortalized keratinocyte cell line, SIK and the squamous cell carcinoma, SCC9 were compared during differentiation induced by anchorage-deprivation. The SIK cells when placed in suspension culture promptly lost almost all ability to reinitiate growth by 2 days concomitantly expressing the differentiation specific proteins, transglutaminase (TGK) and involucrin. These cells rapidly underwent G1 cell cycle arrest with complete disappearance of phosphorylated RB. In contrast SCC9 cells neither showed TGK expression nor increase in involucrin. They decreased their colony-forming ability much more slowly, which coordinated well with a gradual decrease in phosphorylated RB, demonstrating the significant resistance to loss of colony-forming ability and cell cycle exit. In accordance, cyclin D1, a positive regulator of cyclin-dependent kinase (CDK) 4/6 which phosphorylates RB decreased drastically in anchorage deprived SIK but not in SCC9 cells. Endogenous cyclin D1 knockdown in SCC9 cells by siRNA enhanced loss of the colony-forming ability during anchorage-deprivation. Conversely enforced expression of cyclin D1 in SIK cells and in another immortalized keratinocyte cell line, HaCaT, partly prevented loss of their colony-forming abilities. Cyclin D1 overexpression antagonized Keratin 10 expression in suspended HaCaT cells. The result demonstrates the importance of cyclin D1 down regulation for proper initiation of keratinocyte differentiation.
Keywords: cyclin D1, keratinocytes, differentiation, permanent cell cycle exit, anchorage-deprivation
The epidermis is a stratified squamous epithelium that is characterized by four distinct layers: basal, spinous, granular and cornified. Cell proliferation takes place predominantly in the basal layer. As basal cells become committed to terminal differentiation and begin to migrate upwards to the suprabasal layer, they permanently exit from the cell cycle and lose their ability to proliferate, concomitantly starting to express differentiation specific proteins (Fuchs, 1990). Placing normal keratinocytes into suspension culture mimics this process (Drozdoff and Pledger, 1993; Green, 1977). Malignant human squamous cell carcinoma (SCC) lines were resistant to loss of colony-forming ability to some extent and partially defective in expression of differentiation specific proteins, depending on the cell lines (Scott et al., 1988; Rheinwald and Beckett, 1980).
Cell cycle progression of eukaryotic cells is driven by cyclin-dependent kinases (CDKs) associated with cyclins (Ekholm and Reed, 2000). D-type cyclins associated with CDK4/6, and cyclin E associated with CDK2, sequentially phosphorylate and inactivate the retinoblastoma tumor-suppressor protein (RB), thereby controlling the precise timing of G0/G1-S phase transition (Ewen et al., 1993). A number of CDK inhibitor proteins (CKIs), such as the Cip/Kip family proteins p21cip1, p27kip1 and p57kip2 and the Ink family proteins, p15ink4b, p16ink4a and p18ink4c regulate the activities of cyclin/CDK complexes by responding to extracellular signals (Vidal and Koff, 2000; Sherr and Roberts). In addition to functioning in the control of cell cycle progression, some cell cycle proteins were involved in the regulation of differentiation of various tissues and cell types (Devgan et al., 2006; Grossel and Hinds, 2006; Slomiany et al., 2006; Ogasawara et al., 2004; Matushansky et al., 2003; Di Cunto et al., 1998; Shih et al., 1998; Missero et al., 1996).
Extracellular signal-regulated kinases (ERK1/2) play a pivotal role in cell proliferation and cell cycle control (Meloche and Pouysségur, 2007; Roovers and Associan, 2000). Cell adhesion to the extracellular matrix and growth factors jointly stimulate sustained ERK activity which is associated with induction of cyclin D1 expression and G1- to S-phase progression in fibroblasts (Roovers et al., 1999). On the other hand sustained ERK activity is also associated with cell cycle arrest, differentiation, migration and survival in other cell types. For instance, in matrix-deprived normal and immortalized human keratinocytes, which are supposed to be growth arrested, ERK is activated and found to be required for enhanced cell survival (Jost et. al., 2001). Thus the consequences of ERK activation are diverse depending on the cell types and the circumstances.
Overexpression of cyclin D1 has long been associated with development and progression of human cancers. During the early G1 phase at the restriction point, the mitogenic signal stimulates cyclin D1 expression and elevates the cyclin D1 protein level through activation of ERK pathway (Matsushime et al, 1991). Physical association of cyclin D1 with the catalytic partners, CDK4 and CDK6 drives cell cycle progression further in the G1 phase. Consequently deregulation of cyclin D1 expression results in loss of restriction point control leading to uncontrolled cell growth and thus appears to contribute to cancer development (Sherr, 1996). Cyclin D1 also has been shown to have CDK-independent activity; cyclin D1 without complex formation with CDKs modulates the activities of several transcription factors and possibly contributes to tumor development and progression (Ewen et al., 2004; Fu et al., 2004).
Tight coupling of terminal differentiation and permanent cell cycle exit is essential for formation of a proper architecture of the epidermis, but the molecular mechanism of coupling is poorly understood. Previously cell cycle exit and initiation of differentiation were studied in various keratinocyte cells with a variety of differentiation system. (Hauser et al., 2004; Martinez et al., 1999; Paramio et al., 1998; Harvat et al., 1998; Ruesch and Laimins, 1998; Hauser et al., 1997). The studies established molecularly that G1 cell cycle arrest precedes initiation of differentiation. However, G1 cell cycle arrest is not always accompanied by permanent cell cycle exit. G1 arrested cells can reinitiate cell growth and reenter the cell cycle as has been demonstrated by growth hormone deprivation and treatment with certain drugs (Schnier et al., 1994; Kaur et al., 1992; Zetterberg and Larsson, 1985). In this work we assessed permanent cell cycle withdrawal by measuring colony forming ability when keratinocytes held in suspension were returned to the adherent culture and associated this ability with molecular aspects of cell cycle regulators. We found that the elevated level of cyclin D1 during anchorage-deprivation antagonized the loss of colony-forming ability and interfered with differentiation specific gene expression.
MATERIALS AND METHODS
Cell Lines and Culture
The spontaneously immortalized human epidermal line, SIK (Rice et al., 1993) and the human squamous carcinoma line, SCC9 (Rheinwald and Beckett, 1981) were cultured in DMEM and Ham’s F-12 media (2:1) supplemented with 5% fetal bovine serum (FBS), 0.4 µg/ml hydrocortisone using a feeder layer of lethally irradiated 3T3 cells. Media for SIK culture was supplemented with 10 ng/ml EGF and 10 ng/ml cholera toxin. Passage 19–23 of SIK cells were used for the experiments. The spontaneously immortalized human keratinocyte line, HaCaT, AAV-293 (Stratagene), 293T, NIH3T3 and HeLa cells were grown in DMEM supplemented with 10 % FBS and 2 mM L-glutamine. For suspension culture methylcellulose was added at the final concentration of 1.4% to the growth medium.
Suspension Culture and Colony Formation Assay
1–3×106 cells were suspended in 10 ml methylcellulose containing medium in a 50 ml tube and incubated for the indicated time periods. Cells were recovered by centrifugation following dilution of the medium with phosphate saline solution (PBS). Cell viability was determined by trypan-blue (4%) exclusion. Cell numbers were counted using a hematocytometer. 1×103 and 1×104 cells (SIK), 1×102 and 1×103 cells (SCC9) or 1×103 cells (HaCaT) were plated on a 60 mm dish and cultured at minimum 14 days. Colonies were stained with a solution of rhodamine B (1% v/v in ddH2O) and Nile Blue (0.5% v/v in ddH2O) (Rheinwald and Green, 1975) and colonies containing more than 20 cells were counted.
Immunoblotting and Antibodies
Cells in adherent and in suspension culture were harvested by trypsinization and centrifugation. Cell extracts were prepared and protein amounts were determined as described elsewhere (Nishi et al., 2002). 20–40 µg of protein were separated by SDS–polyacrylamide gel electrophoresis followed by blotting onto a PVDF membrane (Millipore). Membranes were blocked with tris-buffered saline containing 4% nonfat dry milk and 0.1% tween 20. Antibody reactions were carried out in the same buffer as above. Immuno reactions were visualized with the ECL plus western Blotting Detection Reagents (Amersham) and ImageQuant (Molecular Dynamics) software was used for quantification. Anti-TGK and anti-involucrin antibodies were as described elsewhere (Thacher and Rice, 1985). Antibodies purchased are anti-p27kip1 and anti-RB antibodies from BD PharMingen, anti-p21cip1 and anti- actin antibodies from Santa Cruz Biotechnology, anti-cyclin D1 antibodies from BD PharMingen and Santa Cruz Biotechnology, anti-phospho-RB (Ser795) antibody from Cell Signalling Technology, anti-ERK1/2 and anti-phospho ERK1/2 from Biolabs and anti-keratin 10 from NeoMarkers. Horseradish peroxidase-conjugated secondary antibodies were purchased from Promega, Cell Signalling Technology and Santa Cruz Biotechnology.
Si RNA Transfection
SiRNA specific to cyclin D1 (SMARTpool® CCND1) was purchased from Dharmacon, Inc. Exponentially growing SCC9 cells were transfected with the siRNA pool using X-tremeGENE siRNA Transfection Reagent (Roche) following the manufacture’s protocol. Cells were subjected to the suspension experiment 48 h after transfection.
Construction of Cyclin D1 Expression Plasmid
Cyclin D1 cDNA was isolated by RT-PCR using total RNA from normal human epidermal cells (hEp). First strand cDNA was synthesized employing M-MLV reverse transcriptase (Promega), with a random hexamer as primer, and was amplified by PCR employing PfuUltra DNA polymerase (Stratagene). The cyclin D1 PCR primers used were 5’GCCGGATCCCATGGAACACCAGCTCCT-G3’ and 5’CCCGTCGACTCAGATGTCCACGTCCCGC3’. Cyclin D1 cDNA was first cloned into pCR2.1-TOPO vector (Invitrogen) following addition of a single deoxyadenosine to the 3’ ends of the cDNA utilizing a nontemplate-dependent terminal transferase activity of Tag polymerase (Promega). Then the correct cyclin D1 cDNA, confirmed by sequencing of both strands, was excised utilizing BamHI and SalI restriction sites created by the PCR primers and cloned into the pBabePuro retrovirus expression vector.
Recombinant AAV Virus Preparation and Infection
The AAV (adeno-associated virus) Helper-Free System (Stratagene) was employed to overproduce cyclin D1 in SIK cells: Cyclin D1 cDNA was excised from pBabePuro/cyclin D1 plasmid and subcloned into the pAAV-MCS vector at the Bam HI and SalI multiple cloning sites located downstream of CMV promoter. To produce recombinant virus particles AAV-293 cells were co-transfected with pAAV-MCS containing cyclin D1, pAAV-RC and pHelper plasmids using the calcium-phosphate transfection method. Four days after transfection, the virus containing solution was prepared and titrated employing HeLa cells, with virus containing a pAAV-LacZ plasmid as indicator following the manufacture’s protocol. For transient infection, SIK cells were exposed for 5–6 h to AAV permissive medium containing 40 mM hydroxyurea (HU) and 1 mM sodium butyrate. After removing the medium, cells were incubated with virus containing solution for 2 h at 37°C and then an equal volume of growth medium was added to the cell culture. 48 h after infection, cells were used for experiments, and cells infected with LacZ containing virus were used to determine infection rates.
Recombinant Retrovirus Preparation and Infection
The pBabePuro/cyclinD1 plasmid constructed as above was cotransfected with pCL-Ampho vector, which expresses an amphotropic envelope, into 293T cells using Lipofectamine Plus reagent (Life Technologies, Bethesda, MD, USA) following the manufacturer’s protocols. Two days after transfection, the culture supernatants were collected and used as viral stocks. Cells to be infected were plated at a density of 2 × 105 cells per 60-mm dish and cultured overnight at 37°C. Culture medium was removed after polybrene treatment (2 µg/ml) for 30 min, and the cells were then incubated with amphotropic retrovirus for 1 h at 37°C. One day after infection, cells were selected with puromycin (2 µg/ml) for 10 days, and drug-resistant colonies were pooled, expanded and used for assays.
RESULTS
Loss of Colony-Forming Ability and Expression of Differentiation Specific Genes
Unlike rodent epidermal cells, human skin cells are rather resistant to malignant transformation in vitro. Infection with simian virus-40 (SV40) or transfection with SV40 DNA created immortalized but nontumorigenic cell lines that showed altered growth properties and partial defects in differentiation (Lechner and Laimins, 1991; Steinberg and Defendi.1983). In contrast spontaneously immortalized human keratinocytes, such as SIK and HaCaT cell lines derived from cancer-prone patients were shown to be capable of undergoing a normal differentiation process even after multiple passages (<40 passages for SIK and >140 passages for HaCaT) (Rice et al., 1993; Boukamp et al., 1988). These cell lines provide an excellent system to study keratinocyte differentiation and permanent cell cycle exit.
The colony-forming abilities of SIK and SCC9 cells that were held in suspension culture for the indicated times were evaluated (Fig.1a). Following suspension culture, cells were replated in adherent culture and the colony-forming ability was assessed by the number of visible colonies formed. The SIK cells cultured in suspension promptly lost their ability to reinitiate growth. Their colony-forming ability decreased reduced by 94% within a day and further declined by almost 100% during prolonged incubation of 2 and 4 days in suspension culture. In contrast the colony-forming ability of the SCC9 cells decreased only by 67% within a day and even after 4 days the reduction was limited to 83% showing a significant resistance to loss of proliferation ability. The viability of cells held in suspension culture was higher in SIK cells than in SCC9 throughout the incubation time (average 71 % and 54.6 % for SIK and SCC9, respectively), demonstrating that the observed difference in colony-forming ability is not due to the difference in the cell survival rate. Expression of the differentiation specific proteins transglutaminase (TGK) and involucrin were examined by immunoblotting during incubation in suspension (Fig.1b). SIK cells expressed TGK within a day and the involucrin protein level increased dramatically during a 4-day period of suspension culture. In contrast the SCC9 cells neither showed TGK expression nor increase in involucrin protein in this period of suspension culture. This result showed that immortalized SIK cells underwent terminal differentiation in suspension culture and that transformed keratinocytes SCC9 have a defect in this process.
Figure 1.
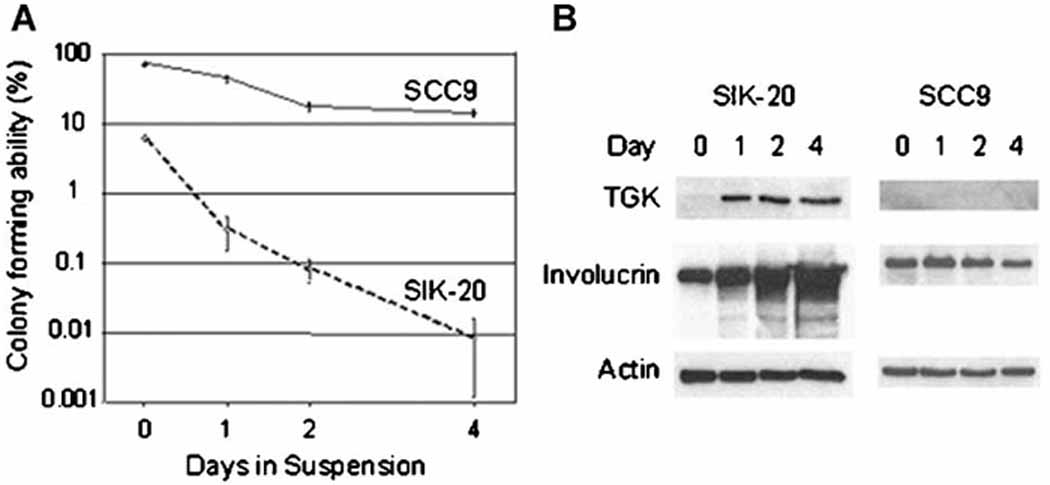
Loss of colony-forming ability and expression of differentiation specific proteins in SIK and SCC9 during differentiation induced by anchorage-deprivation: Cells were held in methylcellulose containing suspension culture and incubated at 37 °C for the indicated periods and were collected by centrifugation. (a) Cells were replated in triplicate dishes with a feeder layer, and colonies formed were stained and counted as described in Materials and Methods. Colony-forming abilities were expressed as the percentage of cells that formed colonies in total viable cells replated. (b) Cell extracts were prepared, separated by electrophoresis and subjected to immunoblotting using antibodies against TGK, involucrin and actin as internal control.
Expression of Cell Cycle Regulatory Proteins
The above result suggested that induction of G1 cell cycle arrest by anchorage-deprivation might be impaired in SCC9 cells prior to permanent cell cycle exit. To investigate the G1 cell cycle arrest in suspension the phosphorylation status of the retinoblastoma protein RB and expression of the negative cell cycle regulatory proteins, p21cip1, p27kip1 and p57kip2 were analyzed by immunoblotting (Fig.2a and 2b). To analyze RB phosphorylation we used two kinds of antibodies, anti-RB and anti-phospho-RB (Ser795). The former recognizes the RB protein as well as hyper-phosphorylated RB by cyclin A/E-CDK2. The latter exclusively detects RB phosphorylated at serine 795 by cyclin D-CDK4/6 and, therefore, reflects the cyclin D-CDK4/6 activity. The RB phosphorylation at serine 795 has been reported to be critical for inactivation of RB-mediated growth arrest (Connell-Crowley et al., 1997). Within a day after being transferred to suspension culture, SIK cells completely lost both hyper-phosphorylated and ser-795 phosphorylated RB, indicating that almost all of the cells rapidly underwent G1 cell cycle arrest. In contrast, in SCC9 cells both types of phosphorylation of RB gradually diminished over a 4-day period of suspension, suggesting a gradual G1 cell cycle arrest. This was confirmed by cell number count showing no increase but about 20% reduction of the initial inocula of SCC9 cells on the 4th day most probably due to appoptosis. To understand the different kinetics of loss of hyper-phosphorylated RB in these cell lines, expression of CDK2 inhibitors was examined. In SIK cells expression of p21cip1 transiently decreased upon anchorage-deprivation and returned to the initial level. In SCC9 cells p21cip1 continuously accumulated during suspension culture which may contribute to the decrease in RB hyper-phosphorylation in this cell line. The p27kip1 was dramatically increased and stayed high in SIK cells, while the increase in the p27kip1 level was slow and gradual in SCC9 cells. The pattern of p27kip1 expression was well coordinated with the kinetic of loss of RB hyper-phosphorylations both in SIK and SCC9 cell lines. In the p57kip2 immunoblot two polypeptides were detected. The levels of the slower migrating polypeptide, the molecular weight of which corresponded to that of p57kip2, remained unchanged during differentiation in both cell lines. The disappearance of the faster migration polypeptides coincided with the RB phosphorylation in both cell lines. However, the nature of this shorter polypeptide, except identification by the anti p57kip2 antibody, is unknown.
Figure 2.
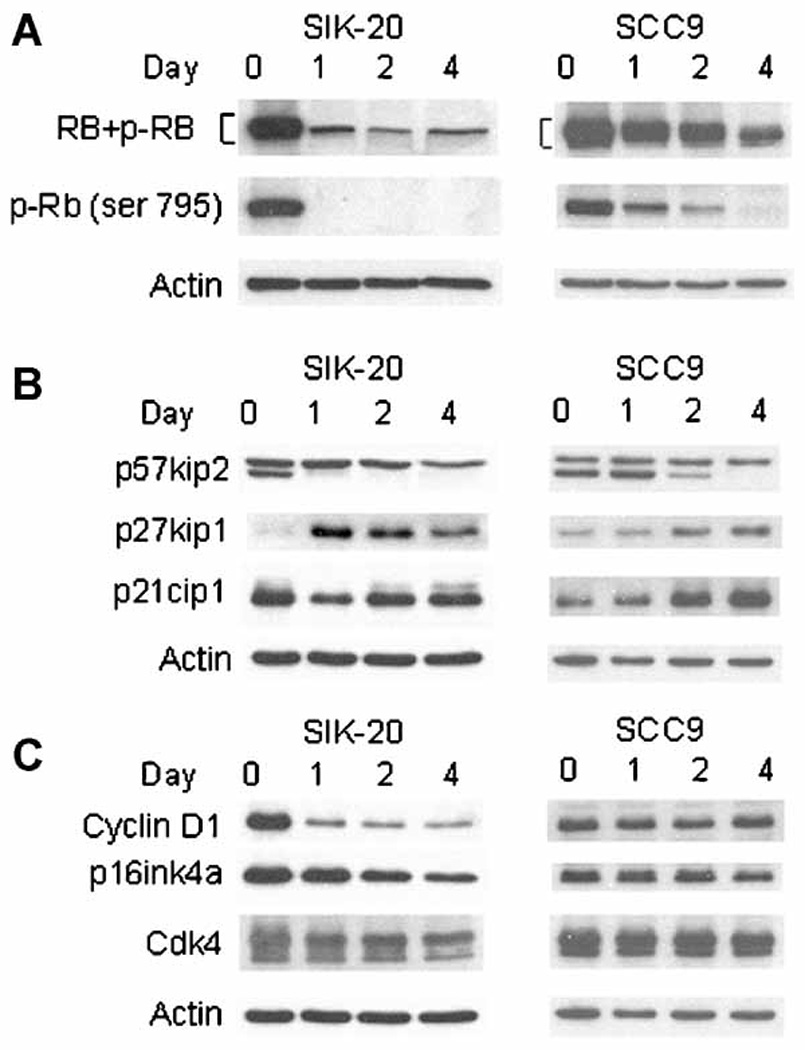
Expression of cell cycle regulator in SIK and SCC9 during differentiation: Cell extracts were prepared from cells held in suspension for indicated periods. (a) Immunoblotting was performed with the antibody against RB which recognizes the RB protein itself and the hyper-phosphorylated forms of RB, and the antibody against phospho-RB (Ser795). (b) Immunoblotting with antibodies against p57kip2, p27kip1, p21cip1 and (c) cyclin D1, p16ink4a, CDK4. Actin immunoblot was used as internal control.
Since in these two cell lines we observed different kinetics of disappearance of cyclin D1-CDK4/6 related phospho-RB (ser-795) (Fig. 2a), the expression of cyclin D1, CDK4 and its major inhibitor p16ink4a was examined during anchorage-deprivation (Fig. 2c). The levels of CDK4 did not change in both cell lines. The p16ink4a levels slightly decreased in SIK cells whereas they remained unchanged in SCC9 cells, indicating that p16ink4a probably does not play a role in reduction of ser-795 phosphorylated RB. However, a rapid and drastic reduction in the cyclin D1 level was observed in suspended SIK cells suggesting a reduction in the cyclin D1-CDK4 activity and coordinating the prompt dephosphorylation of RB and the cell cycle exit. In contrast, the cyclin D1 level in SCC9 cells remained unchanged throughout the incubation in suspension, although almost all phosphorylated RB disappeared at the 4th day and cells were arrested in the G1 phase (Fig. 2a). The result showed that the expression of cyclin D1 appears to correlate with maintenance of colony-forming ability (Fig. 1a) but not cell cycle arrest during suspension culture.
ERK Activation during Anchorage-Deprivation
In normal and immortalized human keratinocytes held in suspension culture, ERK was activated and found to be required for enhanced survival (Jost et al., 2001). A sustained activation/phosphorylation of ERK1/2 in mitogen-stimulated fibroblasts has been associated with continued cyclin D1 expression and G1 cell cycle progression (Weber et al., 1997). Since we and others observed a drastic reduction of cyclin D1 in immortalized and normal cells in suspension (Fig. 2c and Hauser et al., 1997), we examined the levels of ERK activity in anchorage-deprived SIK and SCC9 cells by phospho-ERK1/2 immunoblotting (Fig. 3). In SIK cells ERK1 (p44) was immediately activated upon matrix-deprivation and stayed active for 4 days and ERK2 (p42) was active both in adherent and suspension cultures. Despite sustained ERK activation during suspension the level of cyclin D1 was shown to decrease dramatically in this cell line (Fig. 2c). In SCC9 cells both ERK1 and 2 were activated and the level of cyclin D1 remained unchanged in suspension culture. Thus in keratinocytes ERK activation during anchorage-deprivation is independent of the cyclin D1 level.
Figure 3.
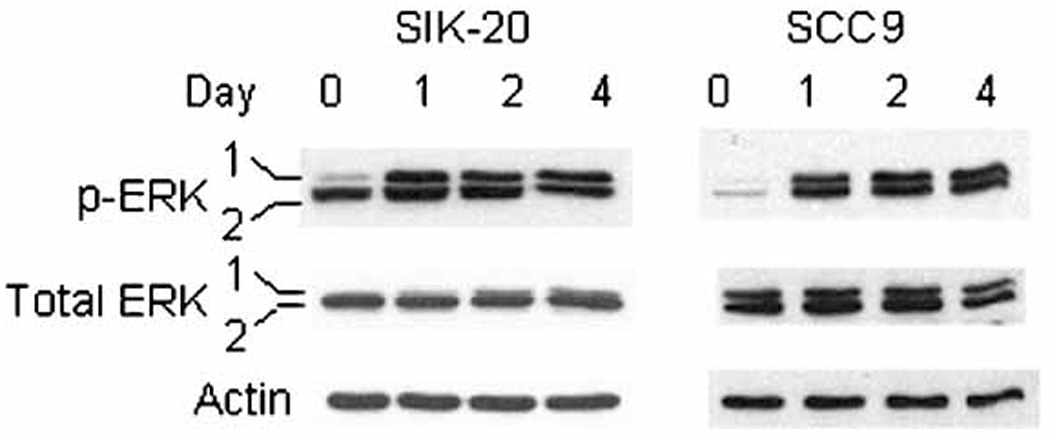
ERK1/2 activation in SIK and SCC9 during suspension culture: Cell extracts were prepared and immunoblotting was performed with antibodies against ERK1/2 and phospho-ERK1/2 which are activated forms of ERK1/2. Actin immunoblot was used as internal control.
Cyclin D1 Knockdown by siRNA Enhances Loss of Colony-Forming Ability but Does not Restore the Expression of Differentiation Specific Proteins in Anchorage-Deprived SCC9
To examine if the reduced endogenous cyclin D1 in SCC9 cells affects the colony-forming ability and differentiation specific gene expression during anchorage-deprivation, we introduced siRNA for cyclin D1 into these cells. Two days after transfection, the cyclin D1 level of siRNA transfected cells was 73% of that of mock transfected control cells (Fig. 4a). Following a 2 day period of suspension the cyclin D1 level of siRNA transfected cells was reduced further to 32%, although that of mock transfected control cells also decreased to 53 % as compared to each adherent culture in this experiment. Prior to anchorage-deprivation the colony-forming abilities of siRNA transfected and control SCC9 cells were 21.1% and 30.7%, respectively and dropped to 0.17% and 0.63% during suspension, respectively (Fig. 4b). As compared to each adherent culture, the reduction in colony-forming efficiency was 5% for siRNA transfected SCC9 cells and 11% for control cells, demonstrating about 50% less colony forming ability in the cells expressing about 40% less cyclin D1 as compared to control cells (Fig. 4a and 4c). Cell viability after transfection and during suspension culture was similar in these cells: 96.4–92.3% in siRNA transfected cells and 98.3–94.7% in mock transfected cells. The expression levels of differentiation specific proteins, keratin 10 and involucrin did not increase and TGK was not expressed during suspension culture in both siRNA and mock transfected SCC9 cells, indicating additional oncogenic alterations in the squamous cell carcinoma SCC9 (Fig.4a).
Figure 4.
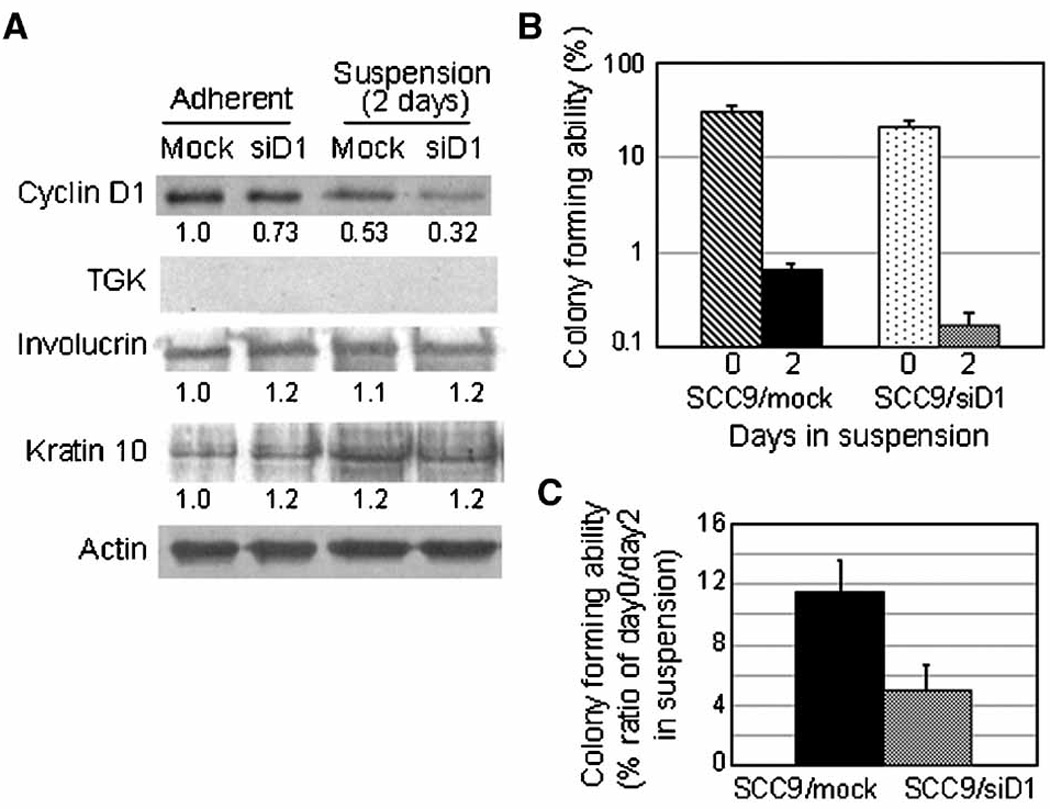
Knockdown of endogenous cyclin D1 in SCC9 and its effects on colony formation and differentiation during anchorage-deprivation: Two days after transfection with siRNA for cyclin D1 and no RNA (mock), cells were subjected to suspension culture. (a) Extracts from cells cultured in adherent (Adherent and Day 0) and from cells suspended for 2 days were prepared and subjected to immunoblotting with antibodies against cyclin D1, actin, TGK, involcurin and keratin 10. The expression levels of cyclin D1 as well as differentiation specific proteins were determined with the actin levels as control employing ImageQuant (Molecular Dynamics) software. (b) Cells held in suspension for 0 (control) and 2 days were replated in triplicate surface cultures, and 2 weeks later colonies were stained and counted. Colony-forming ability was expressed by the percentage of cells that formed colonies in total viable cells replated. (c) Colony-forming abilities of cells held in suspension for 2 days were normalized with that of each adherent culture.
Enforced-Expression of Cyclin D1 in SIK Cells Partially Prevents Reduction of Colony Formation
To corroborate the correlation between the cyclin D1 level and colony-forming ability, we transiently infected SIK cells with recombinant adeno-associated virus (AAV) particles containing a cyclin D1 expressing plasmid or an empty plasmid as control. The cyclin D1 expressing SIK cell population (SIK/D1) showed 20% more cyclin D1 than the control cell population (SIK/V) (Fig. 5a). Two days after infection, cells were transferred to methylcellulose containing medium and held in suspension for 2 days. The cyclin D1 levels in SIK/V and SIK-20/D1 were reduced to 65 % and 74% of their adherent cultures, respectively. The colony-forming efficiency of SIK-20/V was 0.6 % in adherent and decreased to 0.072% (12% of adherent culture) in suspension culture, and that of SIK-20/D1 decreased from 0.54 % in adherent to 0.094 % (17.4% of adherent culture) during suspension culture (Fig. 5b and 5c). This demonstrated a 1.3-fold increase of the colony-forming efficiency in suspension culture by cyclin D1 overexpression. In this experiment the virus infection rate was about 15% as evaluated by LacZ assay therefore only a small proportion of cells in the entire cell population that was subjected to the colony-forming assay were transiently overexpressing cyclin D1. Furthermore overexpressed cyclin D1 in addition to endogenous cyclin D1 appeared to be degraded during suspension culture. These conditions might have limited an increase in the colony formation by cyclin D1 enforced-expression in this experiment. There was no significant difference in cell viability during suspension culture (79.2 % for SIK-20/V and 82.6 % for SIK-20/D1). Cyclin D1 enforced-expression did not increase the colony-forming ability in adherent culture.
Figure 5.
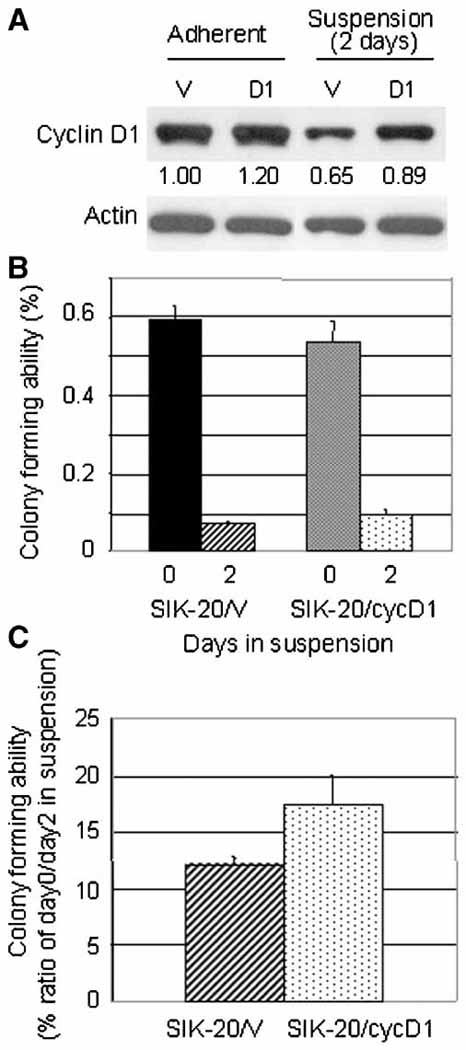
Transient overexpression of cyclin D1 in SIK cells and its effect on colony formation: SIK cells were transiently infected with AAV particles carrying empty vectors (SIK-20/V) and plasmids containing cDNA encoded for cyclin D1 (SIK-2/cycD1). 48 h after infection cells were subjected to anchorage-deprivation by incubating in suspension medium for 2 days. (a) Cyclin D1and actin immunoblot and the cyclin D1 levels quantified as Figure 4 are shown. (b) Colony forming abilities were shown as in Figure 4 and (c) their normalized values with each adherent culture are plotted.
Cyclin D1 Overexpression in HaCaT Cells Antagonizes Loss of Colony Formation and Prevents Increase in Keratin 10 Expression
To confirm the increase in colony-forming ability by cyclin D1 enforced-expression, we employed a retrovirus system to efficiently introduce the cyclin D1 gene. With another immortalized keratinocyte cell line HaCaT we succeeded to obtain pools of puromycin-resistant colonies, HaCaT/D1 which express cyclin D1 and control HaCaT/V which carries the empty vector. HaCaT/D1 showed 1.4 fold more cyclin D1 than HaCaT/V in adherent culture (Fig. 6a). The cyclin D1 expression levels in individual cells are low due to the nature of retrovirus gene introduction; however, almost 100% of the cells in the HaCaT/D1 population was expected to express cyclin D1. Importantly HaCaT cells, like SIK cells, demonstrated loss of colony-forming ability and rapid reduction in the cyclin D1 level during suspension culture and were shown to have the ability to differentiate. In HaCaT/V suspended for 2 days, the cyclin D1 level decreased to 55% compared to adherent cultures (Fig. 6a). HaCaT/D1 showed a similar reduction in the cyclin D1 level during suspension culture (61% of adherent culture), but it was 1.56-fold more than HaCaT/V in suspension culture. The colony-forming efficiencies of HaCaT/V and HaCaT/D1 in adherent culture were similar (18.2% and 19.3%, respectively). During 2 days incubation in suspension, the colony-forming abilities of HaCaT/V decreased to 7.2% (39.6% of adherent) and that of HaCaT/D1 decreased to 14.1 % (73.1% of adherent), demonstrating an almost 2-fold increase by cyclin D1 enforced-expression (Fig. 6b and 6c). The viability rates of cells suspended for 2 days were 70.0% and 62.3% for HaCaT/V and HaCaT/D1, respectively. To examine their state of differentiation, the expression levels of keratin 10, which is known to increase in the differentiating keratinocytes in early stages, was determined. Immunoblotting demonstrated a 2.1 to 2.6-fold increase in keratin 10 expression in HaCaT/V and no increase in HaCaT/D1 cells during 3 days in suspension culture, indicating interference with differentiation by enforced-expression of cyclin D1 (Fig. 6d). Thus the increased level of cyclin D1 in HaCaT cells partially prevented loss of their colony-forming ability and interfered with differentiation.
Figure 6.
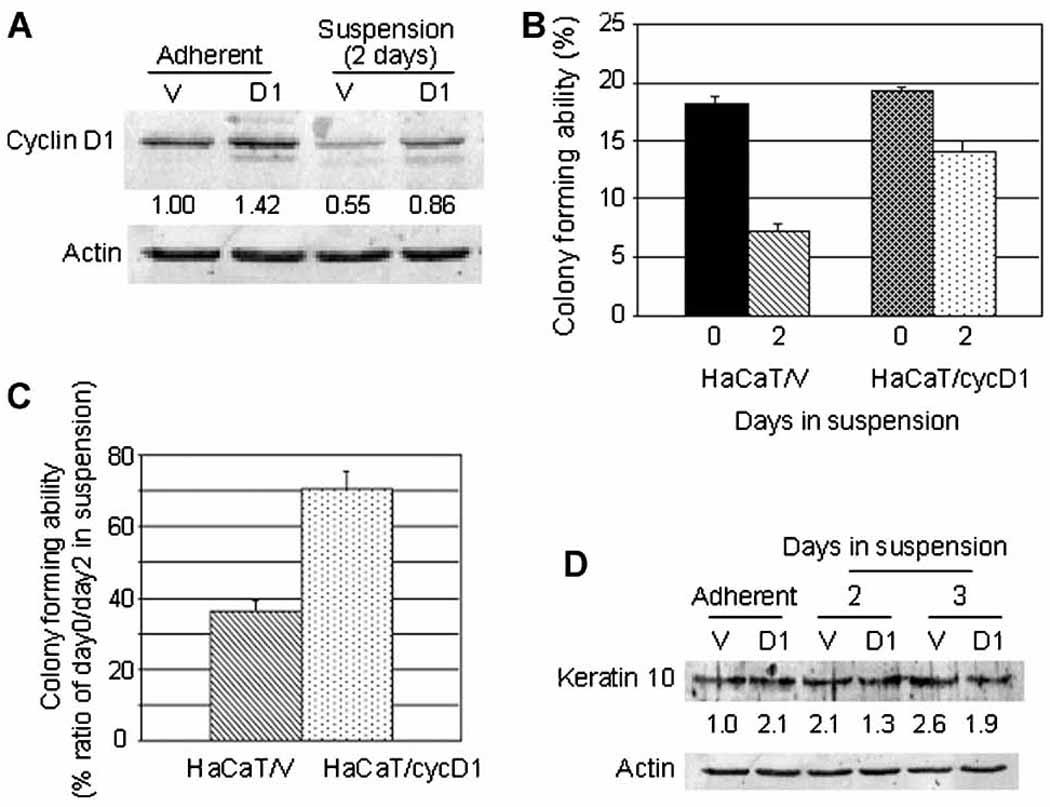
Stable overexpression of cyclin D1 in HaCaT cells and its effects on colony forming abilities and differentiation: The stable HaCaT cell line which overexpresses cyclin D1 (HaCaT/cyc D1) and the control cell line (HaCaT/V) were isolated by employing a retrovirus system as described in Materials and Methods. Cells were subjected to suspension and analyzed as described in Figure 4. (a) Cyclin D1 and actin immunoblot and the cyclin D1 levels quantified as in Figure 4 are shown. (b) Colony-forming abilities were shown as Figure 4 and (c) their normalized values with each adherent culture are plotted. (d) Keratinocyte differentiation marker protein keratin 10 immunoblot and the quantified keratin 10 levels using actin as internal control are shown. This experiment was repeated three times and a representative result is shown.
DISCUSSION
To understand the relationship between permanent cell cycle exit and differentiation on the molecular basis, we compared immortalized SIK cells and transformed SCC9 cells during anchorage-deprivation induced differentiation. In differentiating immortalized SIK cells we found a remarkable correlation between rapid cell cycle exit demonstrated by dephosphorylation of RB accompanied by increased expression of differentiation specific proteins and by a sharp decline of colony-forming ability. These observations clearly indicated that almost all SIK cells held in suspension exited the cell cycle irreversibly and were committed to differentiation. In contrast, SCC9 cells, although slowly underwent G1 cell cycle arrest as demonstrated by gradual dephosphorylation of RB, did not express differentiation specific proteins. This cell cycle arrest was unexpected because many malignant SCCs were reported to be anchorage-independent for growth (Mulherkar et al., 1997). However, despite the cell cycle arrest, 33 % (1 day in suspension) to 17 % (4 days in suspension) of arrested SCC9 cells reinitiated cell growth when they were returned to the adherent culture, indicating that those cells did not permanently exit the cell cycle.
The increased levels of cyclin E-CDK2 inhibitors, p27kip1 in SIK cells and p27kip1 and p21cip1 in SCC9 cells in suspension appeared to be responsible for the cell cycle arrest as demonstrated by the disappearance of cyclin E-CDK2 dependent hyper-phosphorylation of RB. However, a drastic decrease in the cyclin D1 level is probably responsible for loss of RB phosphorylation at ser-795, which is cyclin D1-CDK4/6 dependent, and primarily for G1 cell cycle arrest in suspended SIK cells. On the contrary, the reason for the dephosphorylation (ser-795) of RB is unclear in SCC9 since the level of cyclin D1, CDK4 and p16ink4a remained unchanged during suspension in this cell line, suggesting possible involvement of other cyclin D-CDK4/6 inhibitors, such as p15ink4b and p18ink4c.
Anchorage-independent growth has been associated with cyclin D1 expression and with malignant transformation (Li et al., 2003; Kawada et al., 1999; Resnitzky, 1997). Growth of normal as well as immortalized cells, such as HaCaT and NIH3T3 are anchorage-dependent, and downregulation of cyclin D1 has been demonstrated under anchorage-deprived conditions (Leslie et al., 2006; Jost et al., 2001; Zhu et al., 2000; Hauser et al., 1997). These observations might suggest that downregulation of cyclin D1 and subsequent cell cycle arrest are merely a cellular response to the loss of signals from the extra cellular matrix. However, in primary mouse keratinocytes induced to differentiate by raising Ca2+ concentrations in the adherent culture, cyclin D1 and cyclin D2 were downregulated, which was notably suppressed by expression of the V-Ha-Ras oncogene in these cells (Martinez et al., 1999). This suggests that the downregulation of cyclin D1 is not simply a response to anchorage-deprivation but might be a necessary process for permanent cell cycle exit and proper differentiation in keratinocytes. In further support, the cyclin D1 overexpressing immortalized HaCaT cells failed to form a well-stratified and orderly differentiated epidermis-like epithelium when they were subjected to organotypic co-culture (Burnworth et al., 2006). The primary keratinocytes derived from cyclin D1 overexpressing transgenic mice were resistant to Ca2+-induced terminal differentiation (Yamamoto et al., 2002). These observations suggest that deregulation of the cyclin D1 might interfere with the strictly ordered differentiation processes. To test this hypothesis, we knocked down endogenous cyclin D1 in SCC9 cells by introducing siRNA and found that during a 2 day period of anchorage-deprivation, without affecting cell viabilities, loss of colony-forming ability was 50% enhanced as compared to mock transfected SCC9 cells. However, the expression of differentiation specific proteins was neither induced nor increased suggesting that the squamous cell carcinoma SCC9 might carry additional oncogenic alterations that interfere with the differentiation process. Conversely, we overexpressed cyclin D1 transiently by the AAV system in SIK cells and stably by the retrovirus system in HaCaT cells. Without increasing the cell survival rate, enforced cyclin D1 expression in SIK cells and HaCaT cells resulted in a 1.3-and a 2-fold increase in the colony-forming efficiency, respectively, as compared to their control cells following a 2 day period of suspension. Coordinately an increase in the expression of the differentiation marker protein, keratin 10 was suppressed by cyclin D1 enforced expression in suspended HaCaT cells. However, the increase in colony-forming abilities observed in both cell lines did not exceed the intrinsic colony-forming abilities (about 0.6% for SIK and about 18% for HaCaT), which were measured by replating cells in adherent culture without subjecting them to suspension. The possible reasons might be: 1) expression of cyclin D1 was not high enough due to the low AAV infection rate in SIK (10–15%) and due to the nature of the retrovirus gene introduction which resulted in low level expression in HaCaT, 2) degradation of overexpressed cyclin D1 in suspension, the mechanism of which remains to be investigated, 3) involvement of additional factors. In support of the third possibility, cyclin D1 overexpressing HaCaT cells, in which CDK4 and p21cip1 were additionally upregulated, were found to exhibit severe defects in differentiation, demonstrated by stronger impairment in architecture of epithelium in organotypic co-culture, as compared to cells overexpressing only cyclin D1 (Burnworth et al., 2006).
As has been found for some cell cycle proteins, cyclin D1 also has cell cycle or CDK-independent functions. Recently the transcription factor C/EBPβ has been identified as a principal effector of the cyclin D1 CDK-independent transcription modulator activity (Lamb et al., 2003). C/EBPβ is known to have essential roles in various physiological processes including differentiation, and deregulated cyclin D1 expression is suspected to disrupt the C/EBPβ-coordinated transcription activities specific to differentiation and contribute to tumor development and progression. Considering our observation of malignant SCC9, which failed to downregulate cyclin D1 upon induction of differentiation, it is highly possible that cyclin D1 participates in blockage of differentiation specific transcriptional events and partially prevents cells from permanent cell cycle exit, which in turn help cancer cells remain in a proliferation status.
ACKNOWLEDGMENTS
We thank Drs. David P. Richman and Frederic A. Gorin for their critical reading of the manuscript and for valuable discussion. This work was supported by the National Institute of Health (NIH-PHS P42 ES04699 to R. H. R.) and by the Ministry of Education, Science, Sports, and Culture of Japan (a Grant-in Aid for Scientific Research/Grant No. 19590388 to H. I.).
REFERENCES
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnworth B, Popp S, Stark HJ, Steinkraus V, Bröcker EB, Hartschuh W, Birek C, Boukamp P. Gain of 11q/cyclin D1 overexpression is an essential early step in skin cancer development and causes abnormal tissue organization and differentiation. Oncogene. 2006;25:4399–4412. doi: 10.1038/sj.onc.1209474. [DOI] [PubMed] [Google Scholar]
- Connell-Crowley L, Harper JW, Goodrich DW. Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol Biol Cell. 1997;8:287–301. doi: 10.1091/mbc.8.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devgan V, Nguyen BC, Oh H, Dotto GP. p21WAF1/Cip1 suppresses keratinocyte differentiation independently of the cell cycle through transcriptional up-regulation of the IGF-I gene. J Biol Chem. 2006;281:30463–30470. doi: 10.1074/jbc.M604684200. [DOI] [PubMed] [Google Scholar]
- Di Cunto F, Topley G, Calautti E, Hsiao J, Ong L, Seth PK, Dotto GP. Inhibitory function of p21Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science. 1998;280:1069–1072. doi: 10.1126/science.280.5366.1069. [DOI] [PubMed] [Google Scholar]
- Drozdoff V, Pledger WJ. Commitment to differentiation and expression of early differentiation markers in murine keratinocytes in vitro are regulated independently of extracellular calcium concentrations. J Cell Biol. 1993;123:909–919. doi: 10.1083/jcb.123.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm SV, Reed SI. Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol. 2000;12:676–684. doi: 10.1016/s0955-0674(00)00151-4. [DOI] [PubMed] [Google Scholar]
- Ewen ME, Lamb J. The activities of cyclin D1 that drive tumorigenesis. Trends Mol Med. 2004;10:158–162. doi: 10.1016/j.molmed.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Ewen ME, Sluss HK, Sherr CJ, Matsushime H, Kato J, Livingston DM. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Epidermal differentiation: the bare essentials. J Cell Biol. 1990;111:2807–2814. doi: 10.1083/jcb.111.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Minireview: Cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- Green H. Terminal differentiation of cultured human epidermal cells. Cell. 1977;11:405–416. doi: 10.1016/0092-8674(77)90058-7. [DOI] [PubMed] [Google Scholar]
- Grossel MJ, Hinds PW. From cell cycle to differentiation: an expanding role for cdk6. Cell Cycle. 2006;5:266–270. doi: 10.4161/cc.5.3.2385. [DOI] [PubMed] [Google Scholar]
- Harvat BL, Wang A, Seth P, Jetten AM. Up-regulation of p27Kip1, p21WAF1/Cip1 and p16Ink4a is associated with, but not sufficient for, induction of squamous differentiation. J Cell Sci. 1998;111:1185–1196. doi: 10.1242/jcs.111.9.1185. [DOI] [PubMed] [Google Scholar]
- Hauser PJ, Agrawal D, Flanagan M, Pledger WJ. The role of p27kip1 in the in vitro differentiation of murine keratinocytes. Cell Growth Differ. 1997;8:203–211. [PubMed] [Google Scholar]
- Hauser P, Ma L, Agrawal D, Haura E, Cress WD, Pledger WJ. Efficient down-regulation of cyclin A-associated activity and expression in suspended primary keratinocytes requires p21(Cip1) Mol Cancer Res. 2004;2:96–104. [PubMed] [Google Scholar]
- Jost M, Huggett TM, Kari C, Rodeck U. Matrix-independent survival of human keratinocytes through an EGF receptor/MAPK-kinase-dependent pathway. Mol Biol Cell. 2001;12:1519–1527. doi: 10.1091/mbc.12.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Stetler-Stevenson M, Sebers S, Worland P, Sedlacek H, Myers C, Czech J, Naik R, Sausville E. Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86-8275. J Natl Cancer Inst. 1992;84:1736–1740. doi: 10.1093/jnci/84.22.1736. [DOI] [PubMed] [Google Scholar]
- Kawada M, Kuwahara A, Nishikiori T, Mizuno S, Uehara Y. NA22598, a novel antitumor compound, reduces cyclin D1 levels, arrests cell cycle at G1 phase, and inhibits anchorage-independent growth of human tumor cells. Exp Cell Res. 1999;249:240–247. doi: 10.1006/excr.1999.4467. [DOI] [PubMed] [Google Scholar]
- Lamb J, Ramaswamy S, Ford HL, Contreras B, Martinez RV, Kittrell FS, Zahnow CA, Patterson N, Golub TR, Ewen ME. A mechanism of cyclin D1 action encoded in the patterns of gene expression in human cancer. Cell. 2003;114:323–334. doi: 10.1016/s0092-8674(03)00570-1. [DOI] [PubMed] [Google Scholar]
- Lechner MS, Laimins LA. Human epithelial cells immortalized by SV40 retain differentiation capabilities in an in vitro raft system and maintain viral DNA extrachromosomally. Virology. 1991;185:563–571. doi: 10.1016/0042-6822(91)90526-h. [DOI] [PubMed] [Google Scholar]
- Leslie K, Lang C, Devgan G, Azare J, Berishaj M, Gerald W, Kim YB, Paz K, Darnell JE, Albanese C, Sakamaki T, Pestell R, Bromberg J. Cyclin D1 is transcriptionally regulated by and required for transformation by activated signal transducer and activator of transcription 3. Cancer Res. 2006;66:2544–2552. doi: 10.1158/0008-5472.CAN-05-2203. [DOI] [PubMed] [Google Scholar]
- Lipinski MM, Jacks T. The retinoblastoma gene family in differentiation and development. Oncogene. 1999;18:7873–7882. doi: 10.1038/sj.onc.1203244. [DOI] [PubMed] [Google Scholar]
- Li YJ, Song R, Korkola JE, Archer MC, Ben-David Y. Cyclin D1 is necessary but not sufficient for anchorage-independent growth of rat mammary tumor cells and is associated with resistance of the Copenhagen rat to mammary carcinogenesis. Oncogene. 2003;22:3452–3462. doi: 10.1038/sj.onc.1206411. [DOI] [PubMed] [Google Scholar]
- Martinez LA, Chen Y, Fischer SM, Conti CJ. Coordinated changes in cell cycle machinery occur during keratinocyte terminal differentiation. Oncogene. 1999;18:397–406. doi: 10.1038/sj.onc.1202300. [DOI] [PubMed] [Google Scholar]
- Matsushime H, Roussel MF, Ashmun RA, Sherr CJ. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- Matushansky I, Radparvar F, Skoultchi AI. CDK6 blocks differentiation: coupling cell proliferation to the block to differentiation in leukemic cells. Oncogene. 2003;22:4143–4149. doi: 10.1038/sj.onc.1206484. [DOI] [PubMed] [Google Scholar]
- Meloche S, Pouysségur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- Missero C, Di Cunto F, Kiyokawa H, Koff A, Dotto GP. The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev. 1996;10:3065–3075. doi: 10.1101/gad.10.23.3065. [DOI] [PubMed] [Google Scholar]
- Mulherkar R, Goud AP, Wagle AS, Naresh KN, Mahimkar MB, Thomas SM, Pradhan SA, Deo MG. Establishment of a human squamous cell carcinoma cell line of the upper aero-digestive tract. Cancer Lett. 1997;118:115–121. doi: 10.1016/s0304-3835(97)00241-3. [DOI] [PubMed] [Google Scholar]
- Nishi K, Schnier JB, Bradbury ME. Cell shape change precedes staurosporine-induced stabilization and accumulation of p27kip1. Exp Cell Res. 2002;280:233–243. doi: 10.1006/excr.2002.5637. [DOI] [PubMed] [Google Scholar]
- Ogasawara T, Kawaguchi H, Jinno S, Hoshi K, Itaka K, Takato T, Nakamura K, Okayama H. Bone morphogenetic protein 2-induced osteoblast differentiation requires Smad-mediated down-regulation of Cdk6. Mol Cell Biol. 2004;24:6560–6568. doi: 10.1128/MCB.24.15.6560-6568.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramio JM, Laín S, Segrelles C, Lane EB, Jorcano JL. Differential expression and functionally co-operative roles for the retinoblastoma family of proteins in epidermal differentiation. Oncogene. 1998;17:949–957. doi: 10.1038/sj.onc.1202031. [DOI] [PubMed] [Google Scholar]
- Resnitzky D. Ectopic expression of cyclin D1 but not cyclin E induces anchorage-independent cell cycle progression. Mol Cell Biol. 1997;17:5640–5647. doi: 10.1128/mcb.17.9.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald JG, Green H. Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma. Cell. 1975;6:317–330. doi: 10.1016/0092-8674(75)90183-x. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultures from human squamous cell carcinomas. Cancer Res. 1981;41:1657–1663. [PubMed] [Google Scholar]
- Rice RH, Steinmann KE, deGraffenried LA, Qin Q, Taylor N, Schlegel R. Elevation of cell cycle control proteins during spontaneous immortalization of human keratinocytes. Mol Biol Cell. 1993;4:185–194. doi: 10.1091/mbc.4.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roovers K, Assoian RK. Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays. 2000;22:818–826. doi: 10.1002/1521-1878(200009)22:9<818::AID-BIES7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Ruesch MN, Laimins LA. Human papillomavirus oncoproteins alter differentiation-dependent cell cycle exit on suspension in semisolid medium. Virology. 1998;250:19–29. doi: 10.1006/viro.1998.9359. [DOI] [PubMed] [Google Scholar]
- Schnier JB, Gadbois DM, Nishi K, Bradbury EM. The kinase inhibitor staurosporine induces G1 arrest at two points: effect on retinoblastoma protein phosphorylation and cyclin-dependent kinase 2 in normal and transformed cells. Cancer Res. 1994;54:5959–5963. [PubMed] [Google Scholar]
- Scott RE, Wilke MS, Wille JJ, Jr, Pittelkow MR, Hsu BM, Kasperbauer JL. Human squamous carcinoma cells express complex defects in the control of proliferation and differentiation. Am J Pathol. 1988;133:374–380. [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Shih HH, Tevosian SG, Yee AS. Regulation of differentiation by HBP1, a target of the retinoblastoma protein. Mol Cell Biol. 1998;18:4732–4743. doi: 10.1128/mcb.18.8.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany P, Baker T, Elliott ER, Grossel MJ. Changes in motility, gene expression and actin dynamics: Cdk6-induced cytoskeletal changes associated with differentiation in mouse astrocytes. J Cell Biochem. 2006;99:635–646. doi: 10.1002/jcb.20966. [DOI] [PubMed] [Google Scholar]
- Steinberg ML, Defendi V. Transformation and immortalization of human keratinocytes by SV40. J Invest Dermatol. 1983;81:131s–136s. doi: 10.1111/1523-1747.ep12540905. [DOI] [PubMed] [Google Scholar]
- Thacher SM, Rice RH. Keratinocyte-specific transglutaminase of cultured human epidermal cells: relation to cross-linked envelope formation and terminal differentiation. Cell. 1985;40:685–695. doi: 10.1016/0092-8674(85)90217-x. [DOI] [PubMed] [Google Scholar]
- Vidal A, Koff A. Cell-cycle inhibitors: three families united by a common cause. Gene. 2000;247:1–15. doi: 10.1016/s0378-1119(00)00092-5. [DOI] [PubMed] [Google Scholar]
- Weber JD, Raben DM, Phillips PJ, Baldassare JJ. Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem J. 1997;326:61–68. doi: 10.1042/bj3260061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Ochiya T, Takeshita F, Toriyama-Baba H, Hirai K, Sasaki H, Sasaki H, Sakamoto H, Yoshida T, Saito I, Terada M. Enhanced skin carcinogenesis in cyclin D1-conditional transgenic mice: cyclin D1 alters keratinocyte response to calcium-induced terminal differentiation. Cancer Res. 2002;62:1641–1647. [PubMed] [Google Scholar]
- Zetterberg A, Larsson O. Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1985;82:5365–5369. doi: 10.1073/pnas.82.16.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Scharf E, Assoian RK. Induction of anchorage-independent growth by transforming growth factor-beta linked to anchorage-independent expression of cyclin D1. J Biol Chem. 2000;275:6703–6706. doi: 10.1074/jbc.275.10.6703. [DOI] [PubMed] [Google Scholar]


