Abstract
Previous studies of associative learning implicate higher-level cognitive processes in some forms of classical conditioning. An ongoing debate is concerned with the extent to which attention and awareness are necessary for trace but not delay eye-blink conditioning [Clark, R. E. & Squire, L. R. (1998) Science 280, 77–81; Lovibond, P. F. & Shanks, D. (2002) J. Exp. Psychol. Anim. Behav. Processes 28, 38–42]. In trace conditioning, a short interval is interposed between the termination of the conditioned stimulus (CS) and the onset of the unconditioned stimulus (US). In delay conditioning, the CS and US overlap. We here investigate the extent to which human classical fear conditioning depends on working memory. Subjects had to carry out an n-back task, requiring tracking an item 1 or 2 back in a sequentially presented list of numbers, while simultaneously being tested for their ability to associate auditory cues with shocks under a variety of conditions (single-cue versus differential; delay versus trace; no task versus 0-, 1-, and 2-back). Differential delay conditioning proved to be more resilient than differential trace conditioning but does show a reduction due to task interference similar in slope to that found in trace conditioning. Explicit knowledge of the stimulus contingency facilitates but does not guarantee trace conditioning. Only the single-cue delay protocol shows conditioning during the more difficult working memory task. Our findings suggest that the larger the cognitive demands on the system, the less likely conditioning occurs. A postexperimental questionnaire showed a positive correlation between conditioning and awareness for differential trace conditioning extinction.
Pavlovian conditioning is widely used to study associative learning in species ranging from mollusks to flies, rodents, monkeys, and humans (1–10). This form of learning involves the association of an initially neutral stimulus, the conditioned stimulus (CS), with a correlated meaningful stimulus, the unconditioned stimulus (US). An unresolved question concerns the extent to which certain forms of classical conditioning depend on higher-level cognitive processes, including selective attention, working memory, and awareness (11–17). Eye-blink conditioning is an associative learning paradigm where the role of explicit knowledge/awareness is being investigated. The paradigm involves the association of an eye blink (a somatic motor response) with a previously meaningless stimuli (CS).
Recent data showed that, in humans, associative trace conditioning of eye-blink responses requires awareness of the contingency between the CS (a tone) and the US (a puff of air to the eye), whereas this is not the case for delay conditioning (11, 18–20). In delay conditioning, the start of the US is temporally contiguous with the CS, whereas in trace conditioning, an interval is interposed between the end of the CS and the start of the US. Distracting subjects by having them perform a secondary task (for example, a verbal shadowing task) during a trace procedure prevents conditioning. Furthermore, subjects' ability to report the exact nature of the CS/US relationship (e.g., “I believe the tone came before the airpuff”) is greatly impaired with concurrent distraction during trace conditioning. Conversely, associative delay conditioning appears to be insensitive to distractors. Other experiments find that both trace and delay associative differential conditioning can be disrupted by tasks that demand sufficient attention, whereas this is not the case for single-cue conditioning paradigms (16, 17). In single-cue conditioning, only one CS is presented (paired with the US). In differential conditioning, two CSs are presented, one of which is correlated with US presentations (CS+), whereas the other is not (CS−).
We chose fear conditioning to replicate and extend these findings with human subjects on the basis of a conditioning protocol easily extendable to mice, animals for which well established molecular tools are available to manipulate genetically identifiable cell populations. Fear conditioning differs from eye-blink conditioning in its underlying neuronal implementation, due in part to the fact that the association involves an autonomic rather than a somatic motor response. Fear conditioning is easy to obtain in humans and rodents, is acquired in a fraction of the trials needed for eye-blink conditioning, and is tolerant to long trace periods, making it amenable to functional MRI investigations (21–24). Finally, the neural circuits underlying fear conditioning, in particular the lateral nucleus of the amygdala, hippocampus and prefrontal cortex, are being vigorously explored (25, 26). We use transient elevations in skin conductance [skin conductance response (SCR)] as our measure of autonomic arousal when testing responses to auditory stimuli which have been previously paired with a shock. At the same time, we distract our subjects with tasks of variable working memory load. We are engaged in a parallel effort to reproduce selected aspects of this work in mice (27).
Materials and Methods
Equipment.
Conditioning stimuli were presented and SCRs were recorded by using equipment from Contact Precision Instruments (www.psylab.com) controlled by psylab software. Silver/Silver Chloride electrodes filled with Med Associates paste TD-246 were used for shock presentation and recording skin conductance. CS presentations were mixed into stereo headphones. Distracting tasks were written in matlab (Mathworks, Natick, MA) by using the psychophysics toolbox (28). Analysis was carried out by using programs written in matlab as well as spss 10.
Subjects.
Subjects were recruited from the California Institute of Technology and were paid $20 for their participation, based on informed consent. Their ages ranged from 18 to 31 years, with a mean of 21 years. The following uninformed differential conditioning groups consisted of six subjects each: (i) delay no task; (ii) delay 1-back; (iii) delay 2-back; (iv) trace no task; (v) trace 1-back; and (vi) trace 2-back. The following single-cue conditioning groups consisted of four subjects each: (i) delay no task; (ii) delay 2-back; (iii) uninformed trace no task; (iv) uninformed trace 0-back; (v) uninformed trace 2-back; (vi) informed trace no task; (vii) informed trace 0-back; and (viii) informed trace 2-back.
Procedure.
Skin-conductance electrodes were attached to the palmar surface of the second phalanx of the first and second fingers of the nondominant hand. Shocking electrodes were attached to the palmar surface of the third and fourth fingers of the dominant hand. Each individual's shock level was determined using a subjective rating protocol looking for a level that was “uncomfortable but not painful.” This shock level was used throughout the experiment.
After determining their shock level, subjects completed task training, the third of three sessions of ∼5 min to ensure the subject has reached plateau performance. Before conditioning, subjects were read instructions asking them to focus on either their visual task or the wall in front of them. Naive subjects had no previous specific knowledge of the experiment except that it was a “… learning and memory experiment that involves electric shocks.” Instructions were read to subjects in the informed groups; the instructions explicitly stated that an “electric shock shortly follows most presentations of a tone” and that “the tone generally predicts the occurrence of the electric shock.” The subjects were asked to confirm verbally that they “understand that the tone usually predicts the occurrence of the electric shock.” Subjects were given a postexperimental questionnaire to assess their knowledge of the CS/US relationship, similar to that used in ref. 11, and were debriefed. The questionnaire for differential conditioning included 17 questions to assess the subject's explicit knowledge of stimulus relationships. Subjects were not allowed to correct previous answers. The awareness index is a number between 0 and 17, corresponding to the number of correct responses. The higher the index, the more detailed the subject's ability to recall the presence or absence of a contingency relationship between stimuli.
The informed consent procedure was reviewed and approved by the California Institute of Technology committee for the protection of human subjects.
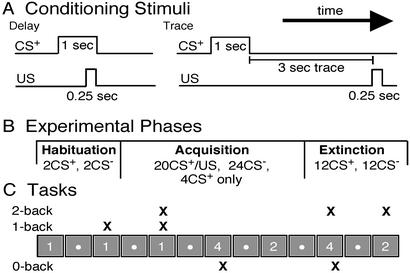
Conditioning Stimuli (Fig. 1A).
Figure 1.
(A) Delay conditioning consisted of a 0.25-sec-long electric shock that overlapped and coterminated with the 1-sec-long CS+ (tone or noise). In trace conditioning, the CS+ was followed 3 sec later by the US. (B) The conditioning protocol consisted of three phases: habituation, acquisition, and extinction. (C) Distraction tasks and conditioning procedures were performed concurrently. During a 0-back task, the subject pressed a key (marked by an X) whenever a predetermined number appeared (4 in this case). During a 1- or 2-back task, the subject pressed a key whenever the number matched the one before it or the one before the previous one, respectively.
The US used in these experiments was a 0.25-sec-long 60-Hz AC shock whose amplitude was determined by each subject. During differential conditioning, the CS+ and CS− were balanced between a 2-kHz tone (83 dB) or white noise (72 dB) and were always 1 sec in length. The 2-kHz tone was always used as the CS+ during single-cue conditioning. During delay conditioning, reinforced CS+ presentations coterminated with the US. Reinforced CS+ presentations during trace conditioning were followed by a shock 4 sec after the CS+ onset (leaving a 3-sec trace period).
Experimental Phases (Fig. 1B).
The learning procedure consisted of three phases: habituation, acquisition, and extinction. In the first phase, habituation, subjects received two presentations of the CS+ and two of the CS−, in that order, to familiarize them with both stimuli. During acquisition, subjects received 24 CS+ and 24 CS− presentations, a total of 48 trials. Twenty of the 24 CS+ presentations were reinforced with a US, whereas four were not reinforced to allow conditioning assessment. These four stimuli were positioned by randomly removing the US after one of the six CS+ presentations in each of the four blocks of 12 trials (six CS+, six CS−) during acquisition (excluding the first two CS+/US pairings in the experiment). During the extinction phase, subjects received 12 nonreinforced CS+ and 12 CS− presentations. CS+/CS− presentations occurred in random order, with the limiting factors being that no more than two presentations of a specific CS occurred in a row and that six of each occurred in each block of 12 trials. Intertrial intervals were uniformly distributed from 15 to 25 sec.
Single-cue conditioning experiments were performed in a similar fashion by using a phantom CS−, marked periods of time that had no actual stimuli instead of an explicitly unpaired stimulus. The analysis protocol for single-cue conditioning was analogous to the differential protocol by using these phantom CSs−. Although when compared with a US-only control method, our procedure has the disadvantage of not controlling for unassociated stimulus SCR, it has several advantages. It allows a within-subject comparison, a more effective means of detecting conditioning. This method also avoids the pitfalls of using a US-only protocol where the US/CS− relationship is randomized or explicitly unpaired. The former may be associated with elevated CS− responses due to a generally elevated anxiety level. The latter tests the subject's ability to learn the anticorrelated relationship between the CS and US to enable suppression of the aforementioned general anxiety. It should be noted that our results show working memory tasks interfere with our single-cue trace conditioning protocol, adding validity to the idea that using the phantom CS− allows accurate and sensitive detection of conditioning.
Distracting Tasks (Fig. 1C).
To confirm that the conditioning protocols were effective, a group of subjects was not asked to perform a task (i.e., to simply stare at the wall) for each procedure. The degree to which conditioning depends on working memory was assessed by asking a group of subjects to perform an n-back memory task during a conditioning procedure. Subjects had to press a key every time a given number appeared (0-back), when the present number matched the one before it (1-back), or when it was identical to the one before the previous one (2-back). Only single-cue trace subjects were asked to perform the 0-back task. The 0-back task involves the same input and the same motor output, including frequency of response, as the 1- and 2-back tasks but depends only minimally on working memory.
The numeral 1, 2, 3, or 4 appeared at a constant rate that, for a 2-back task, was adjusted for each subject to achieve a performance of ∼85%. The mean rate of 2-back presentation was 1 Hz for differential subjects (88% correct), 1.33 Hz for uninformed single-cue subjects (84% correct), and 1.2 Hz for informed single-cue subjects (85% correct). All 1- and 0-back tasks were performed at a presentation rate of 1.33 Hz. The mean performance for subjects focusing on the 1-back task was 93.5%. The mean task performance for single-cue subjects in the 0-back group was 98% for uninformed subjects and 99% for informed subjects.
Analysis of SCR.
A skin-conductance response was measured as the maximal amplitude difference of more than 10 nS that occurred in a 1- to 4-sec window after the delay CS onset or in a 1- to 7-sec window after the trace CS onset. Valid responses were range-corrected by the largest-amplitude response for each subject (29). When there was no response, a 0-amplitude response was included in the analysis.
Habituation analysis was performed for differential conditioning by using a paired t test and a normalized ANOVA. No significant SCR differences were observed between the CSs, with one exception. Only the differential delay group performing no task showed a SCR difference (P < 0.05) using the normalized ANOVA. However, no difference was observed by using the paired t test. The discrepancy between these statistical tests, the robustness of the conditioning for this group, and the biased presentation order of the CSs led us to regard this difference as inconsequential.
All CS+ presentations were compared with adjacent CS− presentations. During acquisition, when there were two adjacent CS− presentations available for comparison to a CS+, one was chosen at random.
Reported P values for conditioning were ranked F statistics for bootstrapped ANOVAs (105 resamples per test). Four other tests were performed for confirmation: ranked F statistics for a permuted ANOVA (105 resamples); a square-root-corrected ANOVA; a permutation test (30) (105 resamples); and a paired t test (averaging each trial across subjects). These confirmation statistics yielded similar results with the exceptions noted below. Differential awareness correlations used a least-squares fit. Analysis of main factors and interactions were performed by using the General Linear Model univariate ANOVA in spss (Ver. 10, Macintosh). These tests used the mean CS+/CS− difference for each subject.
Trial Effects.
Trial effects were analyzed overall for acquisition and extinction phases of the experiment to assess the possible presence of consistent trends, such as a gambler's fallacy effect. Whether conditioning has occurred is assessed by comparing the results of the habituation analysis to the results of the acquisition/extinction phases of the experiment. In general, no CS+/CS− (or phantom CS−) difference is present during habituation. There is a significant difference (P < 0.05) between CS+ and CS− (or phantom CS−) responses during acquisition and extinction when conditioning has occurred. Learning is then demonstrated by the presence of this difference (reported in the results below).
Results
Differential Conditioning.
No task.
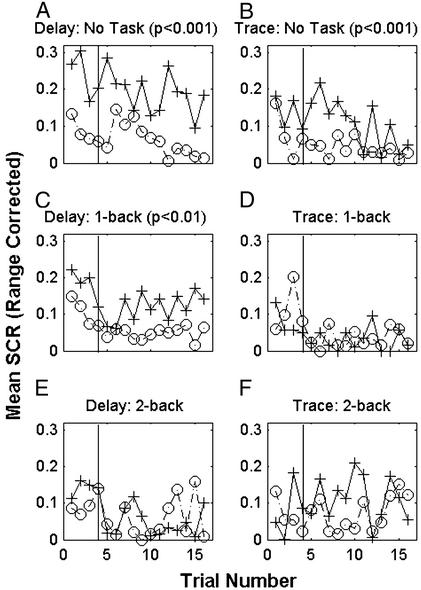
Differential conditioning relationships were first established for trace and delay paradigms, by using six subjects per group asked to perform no task during conditioning. The delay group (Fig. 2A) shows larger SCRs to the CS+ test trials than to adjacent CS− presentations (P < 0.001). The same is true of SCRs during trace conditioning (Fig. 2B, P < 0.001; paired t test, P < 0.01). No significant trial effects are present in either group. Thus, trace and delay differential protocols are sufficient to produce conditioning when performed alone, without distraction.
Figure 2.
Mean range-corrected SCRs to CS presentations for each trial. Thirty-six subjects (six per group) participated in either the differential delay (A, C, or E) or trace (B, D, or F) learning procedure without any task or while being distracted by a 1- or a 2-back task. Mean range-corrected SCRs to CS+ are shown in solid lines with cross markers. Mean range-corrected SCRs to CS− are indicated by dashed lines with circles. Significant conditioning exists during the delay procedure with no concurrent task and while performing the 1-back task. Only under the no-task condition did we find significant trace conditioning. The vertical line marks the last test trial during the acquisition phase.
Concurrent distracting task.
The n-back working memory task served as a distraction from the concurrently performed conditioning protocol. When six subjects performed the 1-back working memory task during differential delay conditioning (Fig. 2C), there is a statistically significant difference between responses to CS+ and CS− during conditioning (P < 0.01). However, when a 1-back working memory task was performed by six subjects during differential trace conditioning, there is no significant difference between SCRs to CS+ and SCRs to CS− (Fig. 2D). When subjects carried out the 2-back task, there is no significant difference between responses to CS+ and CS− for either delay (n = 6) or trace (n = 6) conditioning (Fig. 2 E and F). No significant trial effects are present.
Differential main effects.
A univariate ANOVA using the mean CS+/CS− differences for each subject showed that both the delay/trace difference and task level were significant main effects (P < 0.05 and P < 0.01, respectively). The delay/trace by task interaction was not significant but may have been lost in the floor effect between differential trace 1-back and differential trace 2-back.
Awareness of CS/US Contingency.
Correlations between awareness and CS+/CS− amplitude differences.
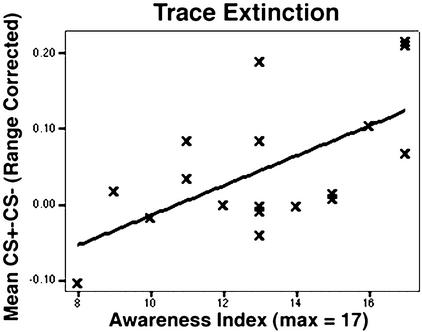
There is a positive correlation between the awareness index and strength of conditioning (mean [CS+ − CS−]) during extinction for the 18 subjects carrying out the differential trace learning procedure (Fig. 3). The correlation has an adjusted r2 value of 0.334 (Pearson coefficient = 0.611, P < 0.01). No significant correlations between contingency awareness and CS+/CS− difference are present for trace acquisition or for either acquisition or extinction during delay conditioning.
Figure 3.
Scatter plot of mean range-corrected differences between CS+ and CS− and the subject's awareness index. During differential trace extinction (Fig. 2 B, D, and F; trials 5–16), subjects show a linearly increasing relationship between average amplitude of response difference and postexperimental questionnaire score (adjusted r2 = 0.334, Pearson coefficient = 0.611, P < 0.01, n = 18). Subjects show no significant correlation between conditioning (average range-corrected CS+ to CS−) and awareness index during differential trace acquisition, differential delay acquisition, or differential delay extinction.
Differential conditioning task interference.
The 12 subjects who were not performing a task during differential conditioning (six delay, six trace) have an average awareness index of 15.2 (maximum 17). Twenty-four subjects who were performing a task during differential conditioning (trace and delay, 1- and 2-back, six subjects in each combination of conditions) have an average index of 13.4. A univariate ANOVA using the awareness questionnaire score to test factors that influence awareness shows significant main effects for both task (P < 0.05) and delay/trace (delay mean = 14.8, trace mean = 13.2, P < 0.05) with no significant interaction. In summary, the addition of both a task and a short trace interval reduces the subject's ability to report the CS/US contingency relationship in a postexperimental questionnaire.
Single-Cue Conditioning.
No task.
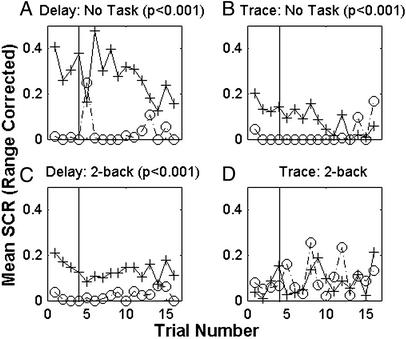
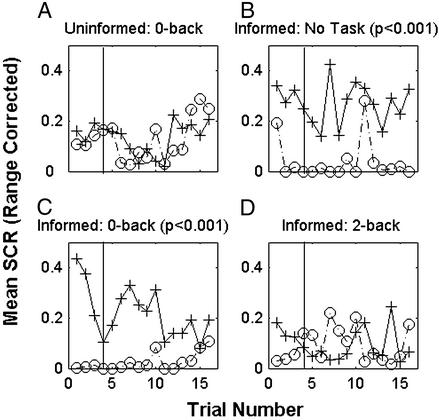
Single-cue conditioning relationships were established in a group of four delay subjects and four trace subjects who did not perform any distracting task during the conditioning protocol. Both groups (Fig. 4 A and B, respectively, n = 4 each) show significant differences between CS+ test trials and adjacent phantom CS− presentations (P < 0.001). No significant trial effects are present.
Figure 4.
Mean range-corrected SCRs to CS presentations for each trial. Sixteen subjects (four per group) participated in either single-cue delay (A or C) or trace (B or D) conditioning without any distraction or while carrying out a 2-back task. Mean range-corrected SCRs to CS+ are shown in solid lines with cross markers. Mean range-corrected SCRs to marked phantom CS− time points are indicated by dashed lines with circles. Significant conditioning exists for delay conditioning with no concurrent task and while performing the 2-back task. Significant trace conditioning is present only while no task is performed. The vertical line marks the last test trial presented during acquisition.
Concurrent distracting task.
A group of four single-cue delay subjects and a group of four single-cue trace subjects were asked to focus on the 2-back working memory task during conditioning (Fig. 4 C and D, respectively). The subjects that carried out the 2-back task during single-cue delay conditioning show greater SCRs to CS+ test trials than to phantom CS− trials (P < 0.001). The four subjects performing the same 2-back task during a trace conditioning protocol show no significant conditioning for the experiment. No significant trial effects are present. Although the 2-back task interferes with single-cue trace (Fig. 4D) and differential delay conditioning (Fig. 2E), there is still a significant CS difference in single-cue delay conditioning during the 2-back task.
Uninformed 0-back task.
A group of four subjects had to signal whenever a particular number appeared on the screen (0-back) during the single-cue trace conditioning procedure (Fig. 5A). There is no statistically significant difference between responses to the CS+ and the phantom CS− for this group. No significant trial effects are present. Although the 0-back task is a simple signal-detection task, there is no significant CS difference during single-cue trace conditioning.
Figure 5.
Mean range-corrected SCRs to CS presentations for each trial. Sixteen subjects (four per group) participated in either informed or uninformed single-cue trace conditioning without being distracted (no task) or while carrying out a 0- or a 2-back task. Mean range-corrected SCRs to CS+ are shown in solid lines with cross markers. Mean range-corrected SCRs to marked phantom CS− time points are indicated by dashed lines with circles. Significant conditioning is present for informed trace conditioning while subjects performed no task or a 0-back task. Significant uninformed trace conditioning is present only without a concurrent task (Fig. 2B). The vertical line marks the last test trial presented during acquisition.
Informed subjects.
For the group of four informed subjects not distracted by any additional task (Fig. 5B) and for the four performing the 0-back task (Fig. 5C), there are significant differences between responses to the CS+ and the phantom CS− during single-cue trace conditioning (P < 0.001). However, for the group of four informed subjects performing the 2-back task (Fig. 5D), there are no significant differences between responses to the CS+ and the phantom CS−. No significant trial effects are present in any group. Prior explicit knowledge of the stimulus contingency facilitates but does not guarantee single-cue trace conditioning.
Discussion
It is generally held in both eye-blink and fear conditioning that acquired trace and delay CS/US associations are distinct forms of learning. Although the key difference between the two is the interposition of a temporal gap between the end of the CS and the start of the US, they involve different neural circuits and obey different regularities. For instance, acquisition of trace but not delay conditioning depends critically on hippocampus and certain prefrontal structures (31–36). In addition, Clark and Squire (11) showed that differential trace eye-blink conditioning depends on CS/US contingency awareness, whereas this is not the case for delay conditioning (see also refs. 20, 37, and 38). This claim has been challenged. For example, Carrillo et al. (16) demonstrated that not only single-cue delay but also single-cue trace conditioning was unaffected by division of attention. They used a dual-task paradigm to study the ability of subjects to acquire eye-blink conditioning while their attention is concurrently engaged by watching a silent movie or verbal shadowing. Differential delay conditioning, however, is affected by the division of attention. Therefore, Carrillo et al. argue that the additional attentional demands imposed by the need to discriminate CS+ from CS− prevent delay conditioning from occurring when subjects have to perform a second task (see also refs. 17 and 39, and above results).
In this paper, we present experiments on fear conditioning. Fear conditioning differs from eye-blink conditioning in that it depends on the amygdala for both delay and trace conditioning, whereas eye-blink conditioning shows a similar pattern of dependence on the cerebellum (26). Our experimental paradigm involves association between tones or noises as CSs and electric shocks as USs. As a measure of autonomic conditioning, we use increases in skin conductance in a comparatively young population (college students). We choose fear conditioning because it can easily be adapted to rodents, allowing the use of molecular and genetic tools to study the underlying neuronal substrates of conditioning.
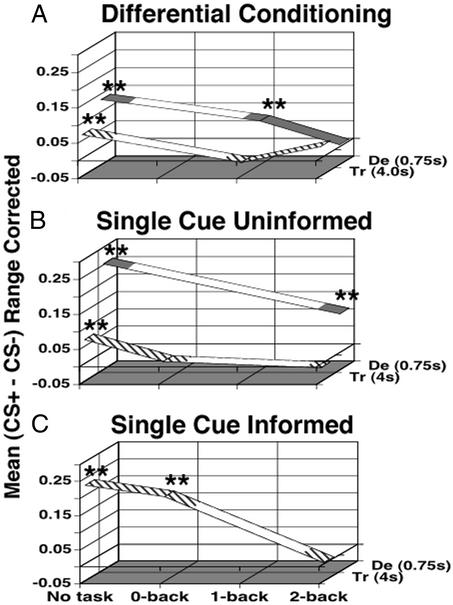
The general pattern of our findings is that the extent of associative autonomic conditioning depends on the cognitive load involved. The larger the demands on the system, the less conditioning occurs. We use the mean CS+/CS− difference for each group as a measure of strength of conditioning. This measure of conditioning is plotted in Fig. 6 for each of our experiments. Fig. 6 represents the transition from uninformed differential (Fig. 6A) to uninformed single cue (Fig. 6B, removing the second anticorrelated CS), and then the addition of explicit knowledge of the CS+/US relationship in the informed single-cue condition (Fig. 6C). Task difficulty increases from left to right on the horizontal axis. The axis into the plane of the paper separates the trace and delay groups by the stimulus onset asynchrony (SOA) between the CS+ and US (trace SOA = 4 s, delay SOA = 0.75 s). Moving in Fig. 6 from bottom to top (Fig. 6 C to A), from left to right, or out of the plane of the paper all result in an increase in overall conditioning complexity for the subject. Moving in any of these directions, away from the origin, the plotted measure of conditioning decreases, supporting the hypothesis that as conditioning complexity increases, the amplitude/probability of conditioning decreases. This is reflected in a univariate ANOVA, where the main effects single/differential, delay/trace, task level, and informed/uninformed are all significant. The only significant interaction is between single/differential and delay/trace. The lack of a significant delay/trace task effect could be due to a floor effect, because the conditioning amplitude has reached zero for trace conditioning protocols in the first level where a concurrent task has been added. We are not making any claims about the uniqueness of this representation. Others are possible and might prove advantageous.
Figure 6.
Summary of our data plotted in a 3D space capturing the contingencies of our protocol. The vertical axis marks the group average for each subject's average range-corrected and normalized CS+/CS− difference. The horizontal axis marks the task difficulty. The axis into the plane of the paper marks the group as trace or delay using the difference in CS/US onset (stimulus onset asynchrony, SOA) in seconds. In addition, the line for trace is hatched, whereas the line for the delay group is solid. **, significant conditioning at P < 0.01. Areas of the lines that are not filled in are meant to assist the stability of the figure, not to imply any prediction about the magnitude of conditioning in that area. (A) Mean group differences for differential subjects. (B) Mean group differences for uninformed single-cue subjects. (C) Mean group differences for single-cue informed subjects. Our results indicate the higher the cognitive load, the smaller the CS+/CS− difference.
It should be noted that Fig. 6 is compatible with the existence of secondary tasks that do not interfere with trace conditioning in naive subjects. A similar plot might also prove beneficial in summarizing the eye-blink conditioning literature.
In Fig. 6, there are several interesting points to note. First, similar to results shown by others in eye-blink conditioning (16, 17, 39), differential delay conditioning is susceptible to interference tasks. Second, it should be noted that although reduced, single-cue delay conditioning still occurred during the difficult 2-back task. Third, all of the distracting tasks tested so far interfere with the trace fear conditioning protocol in our naive subject pool. This is even the case for the 0-back task under single-cue trace conditioning, a simple signal detection task, pressing a button whenever the target appeared in a string of numbers, with minimal attentional and working memory demands (subjects had to remember only a single target number during the 20-min conditioning procedure). Fourth, it is only when we briefed subjects ahead of time about the nature of the experiment that we could reliably induce trace conditioning under a 0-back task. We conjecture that providing this information focused their attention onto the CS/US relationship and boosted learning.
The evaluation of the postexperimental questionnaire showed a correlation (r2 = 0.395) between differential trace subjects' awareness scores and conditioning during the extinction phase. We found no significant correlation in the acquisition phase, nor did we find a correlation for either phase of delay conditioning. The correlation found establishes a link between explicit knowledge of the CS/US relationship and the expression of trace fear conditioning during extinction. It is different from the explicit knowledge/conditioning correlations reported in ref. 11, because our correlation occurs in fear conditioning and is true for the extinction phase as opposed to acquisition. A challenge for the future will be to develop on-line measures of CS/US contingency awareness (12, 40).
One might expect that subjects who are aware of the stimulus contingency would show a gambler's fallacy effect, where the differential response amplitude during extinction phase increases for a number of extinction trials. Such a pattern was reported during eye-blink conditioning (37). We failed to find any significant trend in response slope. In fact, it is likely that if higher awareness scores cause stronger conditioning, this may lead to more than one response strategy (for example, higher initial responses with rapid extinction or gambler's fallacy). Our results also show a reduction in awareness in those groups who were performing a task compared with the no-task controls.
Two possible nonexclusive explanations for our results are the following. (i) Explicit knowledge of the CS/US relationship is necessary for the expression of more complex types of conditioning. When that explicit knowledge cannot be acquired, conditioning cannot be established. This is supported by the fact that task performance reduces both the awareness index and the efficacy of differential conditioning. In addition, explicit prior knowledge of the CS/US relationship compensates for some of the interference in single-cue trace conditioning caused by concurrent task performance. (ii) It is possible that concurrent task performance suppresses amygdala activity and therefore the establishment of a conditioned fear response. Medial prefrontal cortex stimulation in rodents shows suppression of the basolateral complex of the amygdala (41). Furthermore, the n-back task shows increased brain activity, as assayed by fMRI, in human prefrontal areas that could be linked to suppression of normal brain activity under adverse conditions (42). Either of these observations could explain fear conditioning interference by concurrent task performance. Our ongoing investigations of associative fear conditioning in both mice and people using various distracting tasks should prove useful in better understanding conditioning and its neuronal basis.
Acknowledgments
We thank D. Anderson, M. Fanselow, C. J. Han, C. O'Tuathaigh, and J. R. Manns for input and assistance throughout the development of this work. This research was supported by the William T. Gimbel Discovery Fund in Neuroscience at California Institute of Technology, the Keck Foundation, the National Institute of Mental Health, and by the Engineering Research Centers program of the National Science Foundation.
Abbreviations
- CS
conditioned stimulus
- US
unconditioned stimulus
- SCR
skin conductance response
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Baer P E, Fuhrer M J. Mem Cognit. 1982;10:135–140. doi: 10.3758/bf03209214. [DOI] [PubMed] [Google Scholar]
- 2.Thompson R F, Krupa D J. Annu Rev Neurosci. 1994;17:519–549. doi: 10.1146/annurev.ne.17.030194.002511. [DOI] [PubMed] [Google Scholar]
- 3.Mackintosh N J. Conditioning and Associative Learning. Oxford, U.K.: Clarendon; 1983. [Google Scholar]
- 4.Gallistel C R. The Organization of Learning. MIT Press, Cambridge, MA: Bradford; 1990. [Google Scholar]
- 5.Pearce J M, Redhead E S, Aydin A. Q J Exp Psychol B. 1997;50:273–294. doi: 10.1080/713932660. [DOI] [PubMed] [Google Scholar]
- 6.Kocorowski L H, Helmstetter F J. Neurobiol Learn Mem. 2001;75:149–163. doi: 10.1006/nlme.2000.3963. [DOI] [PubMed] [Google Scholar]
- 7.Connolly J B, Roberts I J, Armstrong J D, Kaiser K, Forte M, Tully T, O'Kane C J. Science. 1996;274:2104–2107. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- 8.Tully T. Nat Neurosci. 1998;1:543–545. doi: 10.1038/2780. [DOI] [PubMed] [Google Scholar]
- 9.Eichenbaum H. Science. 1997;277:330–332. doi: 10.1126/science.277.5324.330. [DOI] [PubMed] [Google Scholar]
- 10.Squire L R, Kandel E R. From Mind to Molecules. Freeman, NY: Scientific American Library; 1999. [Google Scholar]
- 11.Clark R E, Squire L R. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- 12.Lovibond P, Shanks D. J Exp Psychol Anim Behav Processes. 2002;28:38–42. [PubMed] [Google Scholar]
- 13.Hilgard E R, Campbell R K, Sears W N. Am J Psychol. 1937;51:498–506. [Google Scholar]
- 14.Dawson M E, Furedy J J. Psychophysiology. 1976;13:50–53. doi: 10.1111/j.1469-8986.1976.tb03336.x. [DOI] [PubMed] [Google Scholar]
- 15.Ohman A, Soares J J. J Exp Psychol Gen. 1998;127:69–82. doi: 10.1037//0096-3445.127.1.69. [DOI] [PubMed] [Google Scholar]
- 16.Carrillo M C, Gabrieli J D E, Disterhoft J F. Psychobiology. 2000;28:293–302. [Google Scholar]
- 17.Knuttinen M G, Power J M, Preston A R, Disterhoft J F. Behav Neurosci. 2001;115:747–757. doi: 10.1037//0735-7044.115.4.747. [DOI] [PubMed] [Google Scholar]
- 18.Clark R E, Squire L R. Psychol Sci. 1999;10:14–18. [Google Scholar]
- 19.Manns J R, Clark R E, Squire L R. Learn Mem. 2000;7:267–272. doi: 10.1101/lm.33400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manns J R, Clark R E, Squire L R. Hippocampus. 2000;10:181–186. doi: 10.1002/(SICI)1098-1063(2000)10:2<181::AID-HIPO7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 21.LaBar K S, Gatenby J C, Gore J C, LeDoux J E, Phelps E A. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 22.Buchel C, Morris J, Dolan R J, Friston K J. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 23.Buchel C, Dolan R J, Armony J L, Friston K J. J Neurosci. 1999;19:10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knight D C, Smith C N, Stein E A, Helmstetter F J. NeuroReport. 1999;10:3665–3670. doi: 10.1097/00001756-199911260-00037. [DOI] [PubMed] [Google Scholar]
- 25.Fendt M, Fanselow M S. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 26.Medina J F, Repa J C, Mauk M D, LeDoux J E. Nat Rev Neurosci. 2002;3:122–131. doi: 10.1038/nrn728. [DOI] [PubMed] [Google Scholar]
- 27.Han C J, VanTrigt L, Mongeau R, Anderson D J, Koch C. Society for Neurology. 2001. p. 531.17. [Google Scholar]
- 28.Brainard D H. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 29.Lykken D T. Psychophysiology. 1972;9:373–379. doi: 10.1111/j.1469-8986.1972.tb03222.x. [DOI] [PubMed] [Google Scholar]
- 30.Efron B, Tibshirani R J. An Introduction to the Bootstrap. 2nd Ed. New York: Chapman & Hall; 1998. [Google Scholar]
- 31.Kim J J, Fanselow M S. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 32.Phillips R G, LeDoux J E. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 33.Maren S, Aharonov G, Fanselow M S. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 34.Weible A P, McEchron M D, Disterhoft J F. Behav Neurosci. 2000;114:1058–1067. doi: 10.1037//0735-7044.114.6.1058. [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin J, Skaggs H, Churchwell J, Powell D A. Behav Neurosci. 2002;116:37–47. [PubMed] [Google Scholar]
- 36. Quinn, J. J., Oommen, S. S., Morrison, G. E. & Fanselow, M. S. (2003) Hippocampus, in press. [DOI] [PubMed]
- 37.Clark R E, Manns J R, Squire L R. Psychol Sci. 2001;12:304–308. doi: 10.1111/1467-9280.00356. [DOI] [PubMed] [Google Scholar]
- 38.Manns J R, Clark R E, Squire L R. J Exp Psychol Anim Behav Processes. 2002;28:32–37. [PubMed] [Google Scholar]
- 39.Mayer M J, Ross L E. J Exp Psychol. 1969;81:469–474. doi: 10.1037/h0027901. [DOI] [PubMed] [Google Scholar]
- 40.LaBar K S, Disterhoft J F. Hippocampus. 1998;8:620–626. doi: 10.1002/(SICI)1098-1063(1998)8:6<620::AID-HIPO4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Rosenkranz J A, Grace A A. J Neurosci. 2001;21:4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pochon J B, Levy R, Fossati P, Lehericy S, Poline J B, Pillon B, Le Bihan D, Dubois B. Proc Natl Acad Sci USA. 2001;99:5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]